Abstract
Osteoporosis is a major health problem in both elderly women and men. Epidemiological evidence has shown an association between tea consumption and the prevention of age-related bone loss in elderly women and men. Ingestion of green tea and green tea bioactive compounds may be beneficial in mitigating bone loss of this population and decreasing their risk of osteoporotic fractures. This review describes the effect of green tea or its bioactive components on bone health, with an emphasis on: (i) the prevalence and etiology of osteoporosis, (ii) the role of oxidative stress and antioxidants in osteoporosis, (iii) green tea composition and bioavailability, (iv) the effects of green tea and its active components on osteogenesis, osteoblastogenesis, and osteoclastogenesis from human epidemiological, animal, as well as cell culture studies, (v) possible mechanisms explaining the osteo-protective effects of green tea bioactive compounds, (vi) other bioactive components in tea that benefit bone health, and (vii) a summary and future direction of green tea and bone health research and the translational aspects. In general, tea and its bioactive components might decrease the risk of fracture by improving bone mineral density (BMD) and supporting osteoblastic activities while suppressing osteoclastic activities.
Keywords: tea polyphenols, antioxidant, bone mineral density, osteoporosis, molecular mechanism, human, rat, cell culture
1. Introduction
Osteoporosis is a degenerative bone disease characterized by low bone mass and microarchitectural deterioration of bone tissue that leads to bone fragility and an increased susceptibility to fractures, especially in the hip, spine, and wrist [1]. Osteoporosis research has also reported some gender disparities. Women are four times more likely than men to develop osteoporosis because of a decrease in their estrogen levels after menopause in conjunction with generally lighter and thinner bones [2]. The rapid decrease in bone mineral density (BMD) that occurs in the first 3 to 5 years immediately following menopause and the slower decrease that continues throughout the remainder of a woman's life markedly increase the risk of hip or vertebral fracture, which is a major cause of morbidity and mortality in older women [2, 3]. Over half of postmenopausal women will experience a bone fracture as the result of osteoporosis [4]. Similarly, one out of four osteoporosis patients is male and 30% of hip fractures occur in men [5]. The pathogenesis of osteoporosis in men is still poorly understood; it has been reported that approximately one third of osteoporotic men have an idiopathic disease [6].
Hip fracture is the most severe consequence of osteoporosis, leading to reduced activities of daily living, lowered quality of life, and increased mortality of patients [7, 8]. As the population ages worldwide, osteoporosis has become a serious health threat in many countries [7, 8]. It is estimated that almost 44 million American women and men aged 50 and older have osteoporosis and low bone mass. By the year 2010, it is estimated that over 52 million women and men in this same age category will be affected, and, if current trends continue, the number will climb to over 61 million by 2020 [9]. The economic costs due to hip fractures have increased tremendously in the past decade and are predicted to grow [10-12].
To predict the risk of fractures, clinical application of bone densitometry, such as dual-energy x-ray absorptiometry, is generally used to measure BMD at the spine, hip, and femoral neck. This method is also used to monitor the natural progression of diseases that affect BMD, or the therapeutic response to osteoporosis-specific treatments 1 to 2 years after beginning treatment [13-16]. Low areal BMD is the most important risk factor for hip fractures [17].
Recent research has suggested that BMD is positively associated with tea consumption, which may optimize bone health. The bioactive components in tea may benefit bone health in terms of maintaining higher BMD [18-23] and reducing the risk of fracture [24, 25]. Specifically, green tea appeared to benefit bone health more than other kinds of tea (e.g., black, oolong), which may be due to decreased oxidative stress [26, 27], increased activity of antioxidant enzymes [26], and decreased expression of proinflammatory mediators [26, 27]. In this review, we discuss the beneficial osteo-protective effects of green tea and its bioactive components. In addition, the possible mechanisms of osteo-protection of green tea along with its bioactive components are discussed.
2. Role of Oxidative Stress and Antioxidants in Osteoporosis
Both osteoblastic and osteoclastic cells regulate bone metabolism, and both cell types are involved in the development of osteoporosis [28]. Osteoblasts are bone-forming cells located near the surface of the bone that produce cytokines. Cytokines, including macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL), are both essential for osteoclast differentiation, function, and survival [29, 30]. Osteoclasts are bone-resorbing multinucleated cells that become tightly attached to mineralized bone surfaces through their integrins and form resorption lacuna by secreting protons, proteases, and superoxide through ruffled borders [31-34]. Bone resorption by activated osteoclasts with subsequent deposition of a new matrix by osteoblasts causes the formation of bone structure and bone remodeling [28]. Imbalance between bone formation and bone resorption is the key pathophysiological event in many metabolic bone disorders in adult humans, including osteoporosis, a result of bone loss [35].
Oxidative stress is a pivotal pathogenic factor for age-related bone loss [36] in mice [37] and rats [26], leading to an increase in osteoblast and osteocyte apoptosis, among other changes, and a decrease in osteoblast numbers and the rate of bone formation via Wnt/β-catenin signaling [37]. Recent studies showed that oxidative stress inhibited osteoblastic differentiation [38, 39] via extracellular signal-regulated kinases (ERK) and ERK-dependent nuclear factor-κB signaling pathways [40]. Osteoblasts can produce antioxidants, such as glutathione peroxidase, to protect against ROS [41], as well as transforming growth factor-β (TGF-β), which is involved in a reduction of bone resorption [42]. ROS are also involved in bone resorption with a direct contribution of osteoclast-generated superoxide to bone degradation [43, 44] and oxidative stress increases differentiation and function of osteoclasts [45].
Several lines of evidence suggest a tight association between oxidative stress and the pathogenesis of osteoporosis in humans. For instance, Polidori et al. [46] reported that osteoporosis due to increased oxidative stress occurred, particularly in severe osteoporotic syndrome in relatively young males (mean age, 33 years old). A marked decrease in plasma antioxidants was also found in aged osteoporotic women [47]. There is also a biochemical link between increased oxidative stress and reduced BMD in men and women 55 years and older [48]. Dietary antioxidant (i.e., ascorbic acid) intake has a beneficial effect on BMD in postmenopausal women [49]. Since oxidative stress can contribute to bone loss, it is important to elucidate the role of antioxidants like green tea in mitigating bone loss during the development of osteoporosis.
3. Green Tea Composition and Bioavailability
Drinking green tea and/or ingesting green tea bioactive compounds may mitigate bone loss in elderly women and men, thereby decreasing their risk of osteoporotic fractures. Tea, the dried leaves of the Camellia sinensis species of the Theaceae family, is a popular beverage with an annual production of three billion kilograms. Of the tea produced worldwide, 78% is black tea, which is usually consumed in Western countries; 20% is green tea, which is commonly consumed in Asian countries; and 2% is Oolong tea, which is produced mainly in southern China [50]. Green tea is made by drying fresh leaves (by frying or roasting) at high temperatures to inactivate the oxidizing enzymes. Green tea is a non-oxidized/non-fermented product that contains several polyphenolic components, also called catechins or tea polyphenols, including (−) epigallocatechin gallate (EGCG), (−) epicatechin gallate (ECG), (−) epicatechin (EC), and (−) epigallocatechin (EGC) [50]. EGCG is the most abundant catechin and it has received the most attention from researchers. The tea catechins account for approximately 30% to 40% of the extractable solids of dried green tea leaves [51].
Tea catechins vary across tea tree and preparations, especially for EGCG and ECG contents. Tea with large leaves usually have less EGCG compared tea with small leaves. Tea should be consumed around 50°C. For better quality control, current chemoprevention or intervention trials usually use dry tea extracts in capsules that can be chemically measured for the exact concentration of each tea component. A traditional dose for human studies is 400 to 1500 mg tea per day according to published studies. The composition of green tea is quite stable in it's dry condition. The shelf life for EGCG, EC, ECG, and EGC as well as the mixture is longer than 1 year in a dry condition and can be oxidized in 30 minutes in water.
The bioavailability of EGCG or catechins, however, is relatively low due to its short half-life by nature. EGCG's short half-life in vivo, which ranges from 1.87 to 4.58 hours from a 50 to 1600 mg dose (≅ 0.7 to 23 mg/kg body weight, based on 70 kg body weight) [52], might be overcome by repeated administration because of its reported low toxicity and high tolerance by human subjects, even when given in doses as high as 1600 mg (≅ 23 mg/kg body weight) [52]. Drinking one cup of green tea could lead to a level of EGCG of 1 μmol/L in the circulation [24, 53, 54]. Studies have also demonstrated the maximum achievable in vivo EGCG concentrations [52, 55]. For example, a 1600 mg oral dose of EGCG under fasting conditions has been reported to achieve a maximum human plasma level of 7.6 μmol/L [52]. This level is eight times higher than the highest reported daily intake from tea [52], making it likely that only pharmaceutically prepared formulations of green tea could reach plasma levels of the catechin equal to those used in an in vitro study. The distribution of catechins concentration is dependent on tissue sites. It is likely that concentrations of catechins at tissue sites are higher than in the blood. For example, 400 to 1000 times greater concentrations of EGCG in the oral cavity, as compared with plasma, have been obtained when a green tea solution (1.2 g of green tea solid per 200 ml of water) is held in the mouth without swallowing [55]. Furthermore, Watkins laboratory reported in a mouse study [56] that tea catechins can accumulate in long bones (e.g., femur and tibia) with continuous tea consumption as short as 16 days, although this short-term period had no impact on BMD.
4. Beneficial Effects of Tea on Bone Health
The health benefits of tea consumption in preventing cancers and cardiovascular diseases have been intensively investigated [57]. However, limited information is available about the protective effect of consumption of green tea or its bioactive components on bone health. In this section, we summarize the impact of tea or green tea and its bioactive components on bone health, including human, animal, and cellular studies.
4.1. Human studies
In terms of BMD, a positive [19, 20, 22, 23, 58, 63, 65, 67, 132], a weak inversion [62], or no correlation [68, 70] between tea drinking and osteoporosis has been reported by a number of human studies (findings summarized in Table 1). However, published results on BMD and tea consumption were based on cross-sectional or retrospective studies and are therefore inconsistent [64], which may compromise the quality of evidence. For instance, Hegarty et al. [20] reported that after adjustment for age and body mass index, the mean BMD of older women (aged 65 to 75 years in the United Kingdom, N = 1,256) at the lumbar spine, greater trochanter, and Ward's triangle was significantly higher in tea drinkers (N = 1,134) than in non tea drinkers (N = 122). Differences at the femoral neck were not significant between tea drinkers and non tea drinkers. These findings were independent of smoking status, use of hormone replacement therapy, coffee drinking, or the addition of milk to the tea. Hegarty's study also found that the magnitude of the effect of drinking tea was notable. Tea drinkers had approximately 5% higher mean BMD at various sites than non tea drinkers (Table 1, reference 20). This effect was equivalent to about half of the difference in BMD observed in women using hormone replacement therapy compared with women who did not use such therapy, or a decrease in age of approximately 5 years, and was associated with a decline in fracture risk of approximately 10% to 20% [20].
Table 1.
Epidemiological studies of tea drinking and bone health
| Mode | Study design | Number of subjects | cups/day | Endpoint(s) | Reference |
|---|---|---|---|---|---|
| Bone mineral density (BMD) | |||||
| Tea drinking | Older women in United Kingdom | N = 1,256 (65−77 yr) | FFQ | (i) An increase in BMD at lumbar, spine, trochanter, and Ward's triangle in tea drinkers | 20 |
| 122 non-tea drinkers | 0 (n=122) | ||||
| Cross-sectional study | 1134 tea drinkers | 1−3 (n=438) | (ii) No changes in BMD at femoral neck in tea drinkers | ||
| 4−6 (n=567) | (iii) ≅ 5% higher BMD in tea drinker than non-tea drinkers | ||||
| >6 (n=129) | |||||
| Tea drinking | Postmenopausal women in USA | N = 4,979 | FFQ | (i) A significant trend of increased total BMD with a higher level of tea consumption | 58 |
| (50−79 yr) | <1 (n=3,683) | ||||
| Prospective study (4.1 yr follow-up) | 1 (n=566) | (ii) No difference in BMD between hip and lumbar spine | |||
| 2−3 (n=588) | |||||
| ≥4 (n=142) | |||||
| Tea drinking | Postmenopausal women in Canada | N = 62 (62.9±6 yr) | 7-d FR | (i) 10% increase in BMD at lumbar spine in tea drinkers than non-tea drinkers | 22 |
| 0−12 | |||||
| Cross-sectional study | (ii) 14% increase in BMD at femoral neck in tea drinkers than non-tea drinkers | ||||
| Tea drinking | Elderly women in Australia | N = 1027 | FFQ | (i) 2.8% increase in BMD at total hip and trochanter in tea drinkers than non-tea drinkers | 23 |
| 172 non-tea drinkers | 0−5 | ||||
| Cross-sectional study | 855 tea drinkers | (ii) No changes in BMD at neck/intertrochanter | |||
| Tea drinking | Elderly women in Australia | N = 164 (70−85 yr) | 24-hr dietary recall | (i) Tea drinkers lost 1.6% BMD at hip | 23 |
| 42 non-tea drinkers | (ii) Non-tea drinkers lost 4.0% BMD at hip | ||||
| Prospective study (5 yr follow-up) | 122 tea drinkers | ||||
| Tea drinking | Perimenopausal Women in Demark | N = 2,016 (45−58 yr) (50.1±2.8 yr) | FR | (i) Protective effect on femoral neck and lumbar spine (L2-l4) when T-scores > −0.75 | 65 |
| 0−30 | |||||
| Cross-sectional study | (ii) No association when T-scores ≤ −0.75 | ||||
| Green tea | Elderly women in Japan | N = 632 (≥ 60 yr) | FFQ | T-scores at lumbar spine for non-green tea drinkers (−2.17±2.08) vs. green tea drinkers (−1.59±2.70) | 19 |
| 52 non-green tea drinkers | < 5 days/wk | ||||
| Cross-sectional study | 580 green tea drinkers | ≥ 5 days/wk | |||
| Tea drinking | Postmenopausal Women in Turkey | N = 724 (57.6±9.6 yr) | FFQ | (i) Habitual tea drinking may have a positive effect on BMD | 63 |
| non-tea drinkers | 0−2 | ||||
| Cross-sectional study (IPPOT study) | tea drinkers | ≥ 2 | (ii) T-scores for non-tea drinkers: −1.09±1.66 vs. tea drinkers: −1.51±1.68 | ||
| 42.5% normal | |||||
| 27.2% osteopenia | |||||
| 30.2% osteoporosis | |||||
| Tea drinking | Asian elderly | N = 1,037 (≥ 30 yr) (51.8±13.8 yr) | Years of tea | (i) 6−10 yr: an increase BMD in lumbar spine | 132 |
| Cross-sectional study | 0 yr (n=532) | (ii) >10 yr: an increase in BMD in total body, lumbar spine, hip | |||
| 497 men, 540 women | 1−5 yr (n=226) | ||||
| non-habitual tea drinkers | 6−10 yr (n=152) | ||||
| vs. habitual tea drinkers | >10 yr (n=124) | ||||
| Tea drinking | Chinese women | N = 1,432 (≥ 15 yr) | NP | Tea drinking may protect low BMD | 67 |
| Cross-sectional study | |||||
| Tea drinking | Pre- and peri-Menopausal women in USA | N = 281 (50−60 yr) | FFQ | (i) Inverse association between ultradistal BMD and tea intake | 62 |
| (52.5±1.7 yr) | NP | ||||
| (ii) No associations between midshaft BMD and tea intake. | |||||
| Cross-sectional study | |||||
| Tea drinking | Healthy men in Greek | N = 300 (18−30 yr) | FFQ | No association between tea consumption and BMD or BMC at radius | 68 |
| Cross-sectional study | 0−2 | ||||
| Tea drinking | Healthy men in Turkey | N = 70 (45−65 yr) | FFQ | No difference in BMD at proximal femur and lumbar | 70 |
| Cross-sectional study | 17 normal | up to 20 | |||
| 30 osteopenia | |||||
| 23 osteoporosis | |||||
| Risk of Fractures | |||||
| Tea drinking | Women in European | N = 5,618 (≥ 50 yr) | FFQ | Tea consumption was associated with a decrease in risk of hip fractures | 24 |
| Case-control study (MEDOS study) | 3,532 controls (77.7±8.8 yr) | Never | |||
| 2,086 cases (78.1±9.4 yr) | Sometimes | ||||
| 1−2 | |||||
| ≥ 3 | |||||
| Tea drinking | Men in European | N = 1,862 (≥ 50 yr) | FFQ | Tea consumption was associated with a decrease in risk of hip fractures for any tea consumption at any age | 25 |
| Case-control study (MEDOS study) | 1,132 controls (74.1±10.1 yr) | Never | |||
| 730 cases (73.9±10.6 yr) | Sometimes | ||||
| 1−2 | |||||
| ≥ 3 | |||||
| Tea drinking | Postmenopausal Women | N = 363 | FFQ | Tea consumption (≥ 7 cups/day) to be significant protective factors of osteoporosis risk | 61 |
| 185 normal (55.7±6.0) | NP | ||||
| Case-control study | 178 osteopenia (58.2±7.1) | ||||
| Tea drinking | Postmenopausal women in USA | N = 91,465 (50−79 yr) | FFQ | (i) Effect of habitual tea drinking on BMD was small | 58 |
| <1 (n=68,188) | |||||
| Cohort study | 1 (n=11,363) | (ii) No association between tea drinking and risk of fractures at hip/forearm/wrist | |||
| Cross-sectional study | 2−3 (n=9,480) | ||||
| (Women's Health Initiative Observational Study) | ≥ 4 (n=2,434) | ||||
| Tea drinking | Women in Swedish | N = 31,527 (40−76 yr) | FFQ | No association between consumption of tea and incidence of osteoporotic fractures | 60 |
| Prospective study (10.3 yr follow-up) | <1 (n=2,520) | ||||
| 1 (n=4,128) | |||||
| 2−3 (n=18,703) | |||||
| ≥ 4 (n=5,887) | |||||
| Tea drinking | Postmenopausal women in USA | N = 4 (50−67 yr) | FR | (i) No association between increased BMD and a reduced risk of fractures | 69 |
| 10−40 | |||||
| Case report | (ii) Toxic serum fluoride levels (> 15 μmol/L) | ||||
FFQ, food frequency questionnaires; FR, food record; BMD, bone mineral density; NP, not provided.
A positive relation between tea drinking, regardless of the type of tea, and BMD has also been reported among postmenopausal women in the United States (age 50 to 79 years) [58], Canada (62.9 ± 6 yr) [22], Australia (70 to 85 years) [23], Demark (45 to 58 years) [65], and Japan (71.8 ± 7.5 yr) [19, 66], as well as among older Asian men (51.8 ± 13.8 yr) [21] and women (≥ 15 yr) [21, 67]. In a cross-sectional study (N = 632 women age ≥ 60 yr), Muraki et al. [19] reported that at an osteoporosis outpatient clinic, patients with the habit of green tea drinking had significantly higher BMD at the lumbar spine than those without the habit, after adjusting for age, body mass index, and other variables related to lifestyle. A prospective analysis over 4 years from an Australian study [23] suggested that tea drinking is associated with the preservation of hip structure in elderly women (see Table 1).
Not all studies have reported positive results for tea drinking. A weak inverse relationship between tea consumption and BMD of the ultradistal radius was found in a study among pre- and perimenopausal women (N = 281, 50 to 60 years of age) in the United States [62]. Kyriazopoulos et al. [68] reported there was no association between tea consumption and BMD or bone mineral content in young Greek men (N = 300, 18 to 30 years of age). Moreover, Hallanger et al. [69] reported that obsessive tea drinking (10 to 40 cups/day containing fluoride up to 56 mg/day) (N = 4) could lead to toxic concentrations of serum fluoride (> 15 μmol/L), in which increased BMD is not associated with a reduced risk of fractures. However, Saitoglu et al. [70] reported that there was no difference in BMD at the proximal femur and lumbar spine among healthy Turkish tea drinkers (N = 70, 45 to 65 years of age), regardless of bone mineral status (normal, osteopenia, or osteoporosis).
With respect to bone fracture, studies offer conflicting data on the role of tea drinking (Table 1). Results from the Mediterranean Osteoporosis Study showed that drinking up to 3 cups of tea per day was associated with a 30% reduction in the risk of hip fractures in both women (N = 5,618) [24] and men (N = 1,862) [25] over 50 years of age. Keramat et al. [61] demonstrated that tea drinking (≥ 7 cups per day) increased protective factors associated with osteoporosis risk in postmenopausal women (N = 717). Based on a longitudinal follow-up study, Chen et al. [58] concluded that the current level of tea consumption in the United States results in such a weak effect on BMD that it is unlikely to have any significant impact on fracture risk at the hip and forearm/wrist among postmenopausal women (N = 91,465, 50 to 70 years of age). A limitation of Chen's study included a lack of information on decaffeinated tea consumption and nondaily tea drinking in the studied group, which may mask an association between tea consumption and BMD or fractures. Hallstrom et al. [60] further reported that during a mean follow-up of 10.3 years, there was no association between consumption of tea up to 4 cups per day and incidence of osteoporotic fractures in women in Sweden (N = 31,527, 40 to 76 years of age).
These discrepancies among published findings may be due to experimental design differences (hospital-based or longitudinal); inconsistent definitions of tea intake categories; and incomplete adjustments of the confounding lifestyle characteristics that affect BMD or fracture risk, such as exercise, alcohol intake, smoking, and the intake of other nutrients. The differences in measured menopausal status and skeletal sites evaluated may also contribute to the discrepancies. In addition, these studies’ lack of quantitative biomarkers for tea ingestion further hinders the validity of their conclusions [71]. Therefore, animal models are adopted to investigate the effect of green tea on bone health, which effectively eliminate possible confounding factors as well as allow for evaluation of bioavailability, efficacy, and related mechanisms through simulating the human consumption of green tea for targeted populations.
4.2. Animal studies
Although there is conflicting information from human studies, animal studies support that green tea may benefit bone health (Table 2) in terms of mitigating bone loss due to aging, aging plus estrogen deficiency, or chronic inflammation, thereby improving clinical symptoms of rheumatoid arthritis, normalizing bone metabolic disorders, and impacting trace element metabolism.
Table 2.
Animal studies of supplementation of green tea or green tea polyphenols and bone health
| Bioactive | Animal model | Dose | Duration | Endpoint(s)/Mechanism(s) | Reference Compound |
|---|---|---|---|---|---|
| GTP | sham and OVX | 80, 400 mg/kg BW | 16 weeks | ↑ femur BMDa | 26, 112 |
| 14-mo-old female rats | ↑ serum OC, ↓ serum TRAP, ↓ urinary Ca | ||||
| ↑ BV/TV, BFR, Tb.N and Tb.Th of proximal tibiab | |||||
| ↓ Tb.Sp and erosion of proximal tibiab | |||||
| Via ↓8-OHdG, ↑GPX activity | |||||
| GTP | chronic LPS-infected | 400 mg/kg BW | 12 weeks | ↑ femur BMDa | 27, 79 |
| 3-mo-old female rats | ↑ serum OC, ↓serum TRAP | ||||
| ↑ BV/TV, Tb.N and Tb.Th of femur and tibiab | |||||
| ↓ eroded surface and osteoclastic number of tibia shiftsb | |||||
| Via ↓ 8-OHdG, COX-2, and TNF-α production | |||||
| EGCG | antibody-induced | 20 mg/kg BW | 15 days | ↓ bone resorption activityc | 85 |
| arthritic model | intraperitoneally | ↓ osteoclast differentiation by TRAP stainc | |||
| 6 wk-old male mice | ↔ osteoclast cell viabilityc | ||||
| Via ↓ expression of NF-ATC1 | |||||
| Green tea catechin | chronic Cd-poisoned | 250 mg, 500 mg/kg BW** | 20 weeks | normalized bone metabolic disorders in BMDa, BMCa, bone Ca, urinary DYD, serum OC caused by Cd intoxication | 87 |
| young male rats * | |||||
| Green tea extract | hindlimb suspension | 1500 mg/kg BW** | 16 days | Tea catechins accumulated in femur, tibia | 56 |
| 15-wk-old male mice | ↔ femur BMDa | ||||
| Green tea | Young rats* | 0, 350, 1170, 3500 mg/kg BW** | 2 weeks | ↑ Zn in tibia | 88 |
| ↔ Fe, Cu, and Al in tibia | |||||
| Green tea extract | 12-mo-old SD rats | 400, 800, 1600 mg/kg BW | 7 weeks | ↑ Mn and Al in tibia | 89 |
| ↔ Ca and Fe in tibia | |||||
| Via modulating apparent absorption | |||||
| Green tea decoction | male rats* | 10 g/Kg BW** | 6 weeks | ↓ Fe in serum, liver, femur, kidney, heart | 90 |
| ↑ Zn in serum, liver, femur, kidney, heart | |||||
| ↑ Se in serum |
measured by DEXA
measured by histomorphometry
measured by histology.
Abbreviation: BFR, bone formation rate; BMC, bone mineral content; BMD, bone mineral density; BV/TV, bone total volume; BW, body weight; Cd, cadmium; COX-2, cyclooxygenase-2; DYD, deoxypyridinoline; GTP, green tea polyphenols; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; OC, osteocalcin; TRAP, tartrate-resistant acid phosphatase; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; TNF-α, tumor necrosis factor-α.
information on the age of rats or mice not available.
Since there are no information available, we estimated that each rat consumed 30 g feed, 25 mL water every day, and 300 g average body weight. We estimated that each mice consumed 30 g feed /100 g body weight every day and 30 g average body weight.
4.2.1. Green Tea Polyphenols (GTP) mitigate aging-induced and aging-plus-estrogen-deficiency-induced bone loss
Estrogen deficiency is another factor that results in oxidative stress to bone compartments during bone remodeling in elderly women. Estrogen is a phenolic compound that shares structural similarities with well-known lipophilic antioxidants, such as α-tocopherol, which enables estrogen to detoxify accumulated ROS [72]. Estrogen decreases oxidative stress in bone cells, and the loss of this sex steroid accelerates the involution of the skeleton by increasing oxidative stress [73]. Studies showed that estrogen deficiency leads to bone loss by lowering thiol antioxidant defenses in osteoclasts [74]. Hydrogen peroxide is the ROS responsible for estrogen-deficiency-induced bone loss [41]. Increasing concentrations of intracellular antioxidants, such as glutathione [74] or catalase [75], prevents bone loss due to estrogen deficiency.
Shen et al. [26] reported that both aging (sham) and aging-plus-estrogen-deficiency (ovariectomized) resulted in bone loss in 15-month-old female rats as shown by a decreased bone formation biomarker serum osteocalcin (OC) and increased bone resorption biomarker serum tartrate-resistant acid phosphatase (TRAP) and urinary calcium. Compared with the non-GTP supplemented groups, GTP supplementation (400 mg/kg body weight) resulted in a higher value of OC and lower values for TRAP and urinary calcium. Furthermore, the same study showed beneficial effects of GTP supplementation in preserving BMD in both cancellous and cortical bone compartments of sham and ovariectomized rats. GTP supplementation resulted in increased trabecular volume, thickness, number, and bone formation of the proximal tibia, periosteal bone formation rate of tibia shaft, and cortical thickness and area of the femur; and decreased trabecular separation and bone erosion of the proximal tibia and endocortical bone eroded surface of tibia shaft, resulting in a larger net bone volume.
In addition, it was found that the positive effects on bone parameters, such as femur BMD, trabecular number of the proximal tibia, bone formation rate, and eroded surface/bone surface at proximal tibia, were observed with both low (80 mg/kg body weight) and high doses (400 mg/kg body weight) of GTP supplementation. These findings demonstrate that GTP supplementation markedly improved femoral BMD, as well as the microarchitecture of trabecular and cortical bone in the tibia and femur, which were negatively impacted by aging in the middle-aged female rats. However, GTP could not completely prevent bone loss due to aging plus estrogen deficiency in ovariectomized rats.
From the same study [26], the impact of GTP in bone mass in ovariectomized 15-month-old rats (estrogen deficiency) may be independent of estrogen and showed no changes in serum estradiol concentration. Interestingly, GTP supplementation on the sham-operated groups (estrogen adequacy) only showed an impact on uterine weight, not serum estradiol concentration, and therefore may be associated with EGCG's [76, 77] and ECG's [76, 78] weak binding affinity for estrogen receptor(s) (ER), especially ER-α and ER-β.
4.2.2. GTP mitigates chronic-inflammation-induced bone loss
Green tea polyphenols have been found to counteract inflammation-induced bone loss in a recent animal study [27, 79]. Shen et al. [27, 79] reported that chronic administration of lipopolysaccharide to 3-month-old female rats using time-release pellets for 90 days resulted in a significant increase in the inflammation index in the animals as determined by the total white blood cell count, accompanied by a significant bone loss. Bone loss was demonstrated by decreases in BMD, accompanied by lowered trabecular volume fraction, number, and thickness in the proximal tibia, and increased eroded surface and osteoclast number in the endocortical tibial shafts. In addition, lipopolysaccharide also resulted in a lower value for OC and a higher value for TRAP when compared with those treated with placebo. Supplementation of GTP (400 mg/kg body weight) in the drinking water significantly increased BMD, serum OC, and trabecular volume fraction and number in both the femur and tibia, but decreased serum TRAP, eroded surface, and osteoclast number in endocortical tibial shafts. This study demonstrated that GTP supplementation in drinking water for 12 weeks prevented trabecular bone loss through increased bone turnover by lipopolysaccharide.
4.2.3. EGCG improves clinical symptoms of rheumatoid arthritis
Rheumatoid arthritis (RA), a chronic inflammatory disorder, is characterized by cellular infiltration and proliferation of the synovium, leading to the progressive destruction of the joints through the interaction between infiltrating cells and mediators [80, 81]. Local bone erosion is one of the essential pathologic features of RA and is clinically related to functional outcome [82]. Osteoclasts appear to play a major role in bone erosion in RA joints [83, 84]. In a model of RA and osteoporosis, Morinobu et al. [85] demonstrated that EGCG decreased bone resorption activity and osteoclast-specific gene expression without affecting cell viability of osteoclasts, and EGCG treatment ameliorated clinical symptoms and reduced histologic scores in arthritic mice.
4.2.4. Green tea catechin normalizes bone metabolic disorders due to cadmium toxicity
Bone metabolic disorders, such as kidney malfunction, calcium absorption disorders, and osteoporosis, are a major result of chronic cadmium toxicity [86]. During cadmium toxicity, cadmium directly interferes with the function of 1,25-dihydroxycholecalciferol in the intestinal cells, lowers calcium resorption by inhibiting the synthesis of calcium binding protein, and increases calcium excretion due to kidney malfunction, which subsequently causes the loss of bone minerals [86]. Green tea catechin is known for its detoxifying benefits in that catechin binds with metal ions to form an insoluble complex-ionic salt to remove heavy metals. Choi et al. [87] reported that in cadmium-poisoned rats, green tea catechin reduced bone metabolic disorders by suppressing bone turnover rate as well as normalizing BMD, bone mineral content, and bone calcium content at the vertebra, pelvis, tibia, and femur.
4.2.5. Influence of green tea on trace element metabolism
Several compounds found in teas (i.e., polyphenols, methylxanthines, and aluminum) may interact with the utilization of trace elements. Greger et al. [88] reported that ingestion of green tea, which contains lower molecular weight polyphenols than black tea, tended to have less effect on liver copper and plasma ceruloplasmin levels in young rats than ingestion of black tea. From the same study [88], green tea elevated hematocrits in both normal rats and rats that had been repleted with iron for several weeks, yet initially slowed the iron repletion in iron-depleted anemic rats. This observation suggests that the ingestion of tea had a minimal direct effect on iron utilization and absorption as well as iron deposition in the tibia. The small changes in hematocrits induced by the ingestion of tea might relate to changes in copper metabolism. However, ingestion of tea seemed to have no effect on zinc or aluminum absorption. Zeyuan et al. [89] showed that tea and its water extracts had the following effects:
Increased absorption of manganese and copper and the content of manganese in the tibia.
Increased content of aluminum in the tibia, but caused no changes in the apparent absorption rates of aluminum.
Inhibited absorption of calcium and iron without affecting calcium and iron in the tibia.
Decreased apparent absorption rates of zinc with little improvement in the content of 11 zinc in the tibia of 12-month-old SD rats.
Similar findings were reported by Hamdaoui [90] on tea's impact on iron and zinc of male rats, and that green tea decoction (i) reduced the concentration of iron in serum, liver, spleen, and femur; (ii) increased the concentration of zinc in serum, kidney, heart, and femur; and (iii) increased serum selenium and whole blood glutathione peroxidase activity of rats. The effect of green tea on iron status may be beneficial in some cases. Evidence suggests that the reduction of iron absorption, especially in patients with low iron requirements, may protect tissue against damage caused by oxygen free radicals and iron-dependent metal lipid peroxidation [91]. Indeed, the cytoprotective effects of GTP against lipid peroxidation arise not only from their antioxidant properties, including the scavenging of oxygen radicals and lipid radicals, but also from their iron-chelating activity.
4.3. Cellular studies
In addition to human and animal studies, the abilities of green tea bioactive components to increase and/or maintain indices of bone formation and to suppress indices of bone resorption were observed in cellular studies (Table 3).
Table 3.
Effect of green tea bioactive compounds on bone health and related molecular mechanisms
| Bioactive | Model | Dose used in medium | Dose duration | Endpoint(s)/Mechanism(s) | Reference compounds |
|---|---|---|---|---|---|
| Bone formation (osteoblastogenesis) | |||||
| (+)-catechin | MC3T3-E1 cells | 10−4−10−5 mol/L | 48 hours | ↑ ALP activity, ↑ osteoblast survial | 87 |
| ↓ apoptosis of osteoblasts | |||||
| via ↓ IL-6 and TNF-α production | |||||
| EGCG | SaOS-2 cells | 1−5 μmol/L | 8 days | ↑ ALP activity | 93 |
| 17 days | ↑ formation of mineralized bone nodules including area and number | ||||
| via ↓ Runx2 expression | |||||
| EGCG | D1 cells | 10 μmol/L | up to 4 weeks | ↑ALP activity, ↑mineralization | 94 |
| via ↑mRNA expression of Runx2, osterix, osteocalcin, ALP | |||||
| EGCG | 3T3-E1 cells | 10 μmol/L | 14 days | ↑ALP activity | 94 |
| EGCG | antler progenitor cells | 25 μmol/L | 24 hours | ↑ ALP activity | 96 |
| via Wnt pathway | |||||
| EGCG | rat femoral tissue | 10−4−10−7 mol/L | 24 hours | ↔calcium content | 98 |
| 10−4 mol/L | ↓ ALP activity | ||||
| EGCG | MC3T3-E1 cells | 30 μmol/L | 60 min | ↓ TGF-β-stimulated HSP27 induction | 137 |
| via ↓ SAPK/JNK pathway | |||||
| EGCG | MC3T3-E1 cells | 100 μmol/L | 60 min | ↓ PGD2-induced HSP27 induction | 140 |
| via ↓ p44/p42 MAPK pathway | |||||
| EGCG | MC3T3-E1 cells | 10−100 μmol/L | 60 min | ↑ PGF2α-induced VEGF synthesis | 143 |
| via ↑ SAPK/JNK activation | |||||
| EGCG | NRG cells infected with Staphylococcus aureus | 272 μmol/L | 4 hours | ↓ IL-6 production | 161 |
| via ↓ RNAKL expression | |||||
| 48 hours | ↓ osteomyelitis (osteoblast infection) | ||||
| via ↓ inflammation | |||||
| Bone resorption (osteoclastogenesis) | |||||
| (+) catechin | embryonic mouse calvaria | 0.1−1 mmol/L | 18 hours | ↓ development of bone resoprtion induced by PTH or retinoic acid | 105 |
| via collagen-stabilizing properties of catechin | |||||
| EGCG | RAW264.7 cells | 50 μmol/L | 24 hours | ↓ survival of osteoclasts | 151 |
| ↑ apoptosis of osteoclasts | |||||
| via activation of caspase-3 | |||||
| GTP | SaOS-2 cells | 43−130 μmol/L | 48 hours | ↑ apoptosis of SAOS-2 | 109 |
| via caspase-3 activation with ↓NF-κB | |||||
| EGCG | RAW 264.7 cells | 10−100 μmol/L | 2 hours | ↓ osteoclastic differentiation | 110 |
| via ↓ RANKL-induced NF-κB transcriptional and nuclear translocation | |||||
| EGCG | DC cells | 10−100 μmol/L | 24 hours | ↓ maturation of DC | 172 |
| via ↓ MAPK and NF-κB activation | |||||
| EGCG | osteoclast-like | 25−100 μmol/L | 24 hours | ↑ apoptosis of osteoclasts | 107, 108 |
| multinucleated cells | ↔ oastoblasts | ||||
| via Fenton reaction | |||||
| EGCG | bone marrow with primary osteoclasts | 20 μmol/L | up to 3 days | ↓ formation of osteoclasts | 106 |
| via ↓ expression of MMP-9 | |||||
| EGCG | MC3T3-E1 cells | 10−100 μmol/L | 60 min | ↓ ET-1-induced IL-6 synthesis | 158 |
| via ↓ p44/p42 MAPK pathway | |||||
| EGCG | MC3T3-E1 cells | 100 μmol/L | 60 min | ↓ FGF-2-stimulated IL-6 synthesis | 159 |
| via ↓ p44/p42 MAPK pathway | |||||
| EGCG | MC3T3-E1 cells | 30 μmol/L | 60 min | ↓ PGDF-BB-stimulated IL-6 synthesis | 160 |
| via ↓ SAPK/JNK expression | |||||
Abbreviation: ALP, alkaline phosphatase activity; DC, dendritic cells; EGCG, (−) epigallocatechin gallate; ET-1, endothelin-1; FGF, fibroblast growth factor; HSP27, heat shock protein 27; IL-6, interleukin-6; NF-κB, receptor activator of nuclear factor-κB; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinases; PGD2, prostaglandin D2; ; PGDF-BB, platelet-derived growth factor-BB; PTH, parathyroid hormone; Runx2, Runt-related transcription factor-2; SAPK/JNK, stress-activated protein kinase/c-Jun N-terminal kinase; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; RANKL, receptor activator of nuclear factor-κB.
4.3.1. Green tea bioactive components favor bone formation
The phenotype of mature osteoblasts is characterized by their ability to synthesize and secrete molecules of the extracellular matrix. The beneficial effect of EGCG on bone formation has been demonstrated by increasing alkaline phosphatase activity (ALP, a mature osteoblast phenotype) at both the gene expression and protein levels in osteoblastic-like cells, such as MC3T3-E1 cells [87], osteogenic sarcoma, and SaOS-2 cells [93], followed by increased formation of mineralized bone (as shown on both von Kossa and Alizarin red staining) [93]. Similar observations were also reported in mouse bone marrow cells, D1 cells, and 3T3-E1 cells [94], human stem cells [95], and antler progenitor cells [96]. Studies showed that catechin caused a significant elevation of osteoblastic survival as well as a decrease of osteoblastic apoptosis [87], resulting in stimulating cell proliferation and differentiation of osteoblasts [97]. Yamaguchi and Jie [98] reported that the effect of EGCG on bone calcification is biphasic at a high concentration. EGCG (10−4 M) appeared to inhibit ALP activity in ex vivo femoral-diaphyseal and -metaphyseal tissues, while a low concentration of EGCG (10−7 M) had no effect on bone calcium content.
4.3.2. Green tea bioactive components suppress bone resorption
Bone is resorbed mainly by multinucleated osteoclasts. These are formed by fusion of preosteoclasts [99], which are derived from hematopoietic stem cells in the presence of cytokines, such as M-CSF [100, 101] and RANKL [29, 102]. These cytokines are expressed by osteoblasts/stromal cells and modulate the function and survival of osteoclasts [29, 101, 103]. Cytokines and signals from integrins also induce the formation of polarized cell structures in osteoclasts that participate in bone resorption [104]. After a period of bone resorption, some osteoclasts die by apoptosis. Thus, regulation of these processes could potentially lead to control of bone resorption. Because enhanced bone resorbing activity and/or recruitment of osteoclasts is responsible for the pathogenesis of bone diseases like osteoporosis, agents that inhibit differentiation or induce apoptosis in osteoclasts could be used as a prophylactic or therapeutic agent for treatment of such diseases [102-104].
Using embryonic mouse calvaria, Delaiss [105] reported that (−)-catechin inhibited the development of bone resorption. Other cellular studies have extended this finding, observing that EGCG: (i) significantly inhibited the survival of differentiated osteoclasts [106] and increased the apoptosis of osteoclasts [107-109]; (ii) induced cell death of osteoclasts in terms of single-strand DNA damage, without affecting osteoblastic cells in a cocultured system of osteoblasts and osteoclasts [107-108]; and (iii) inhibited the differentiation of osteoclasts [110] and the formation of osteoclasts [106, 108, 110]. In an animal model of RA, EGCG treatment suppressed osteoclast differentiation in vitro in a dose-dependent manner, as judged by TRAP-positive multinucleated cell counts [85] (Table 2).
5. Possible mechanisms of green tea on osteo-protection
There are five main possible mechanisms through which green tea protects bone health: (1) by mitigating bone loss through anti-oxidative stress action, (2) by mitigating bone loss through anti-inflammatory action, (3) by enhancing osteoblastogenesis, (4) by suppressing osteoclastogenesis, and (5) probably through osteoimmunological action.
5.1. Mitigating bone loss through anti-oxidative stress action
The most widely recognized properties of GTP are their anti-oxidative activities, owing to their ability to capture and detoxify ROS [111]. Recent studies [26, 112] addressed whether GTP supplementation would improve cellular antioxidant enzymes and/or diminish oxidative stress damage, and also whether the impact of GTP supplementation on the anti-oxidative defense system would have a beneficial effect on bone mass and microarchitecture. In one study, after GTP supplementation (400 mg/kg body weight), liver glutathione peroxidase activity, which might prevent oxidative damage during skeletal remodeling, increased in both 15-month-old sham and ovariectomized rats. This is the first study [26, 112] to provide strong evidence of GTP's bone-mass-conservation effect due to its antioxidant capacity.
In a model of chronic-inflammation-induced bone loss, GTP mitigated the loss of BMD due to GTP's antioxidative ability (as demonstrated by a decreased urinary 8-hydroxydeoxyguanosine level) [26]. In another study, EGCG in GTP decreased the formation of oxidative-stress-induced calcium stone deposit formation in rats due to EGCG's anti-oxidative effects [113].
5.2. Mitigating bone loss through anti-inflammatory action
Due to its anti-inflammatory activity, GTP has also been proven to be beneficial in the prevention and treatment of a number of inflammatory diseases. A low-grade systemic chronic inflammation occurring in atherosclerosis leading to inflammation can also result in systemic bone loss. In the development of atherosclerosis, normal bone remodeling can be disrupted and bone loss can be caused by chronic elevation of pro-inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-β, γ-interferon (γ-IFN), and prostaglandin (PG) E2 [114-116]. In general, these mediators act directly on bone or indirectly to increase osteoclastogenesis, prevent osteoclast apoptosis [117], and/or inhibit osteoblastic activity [118].
There is evidence to indicate a possible interaction between bone and the heart vessel, where a common pathophysiological mechanism is shared in both bone loss and atherosclerosis [79, 114, 119]. Shen et al. [79] reported that lipopolysaccharide (an inducer of chronic inflammation) not only caused low BMD in rats, but also induced a higher degree of fibrosis in heart vessels (a marker of atherosclerosis) of young rats. GTP supplementation in the drinking water (400 mg/kg body weight) was shown to mitigate bone loss and lessen the degree of fibrosis in the heart vessels of young rats. This protective role of GTP may in part be attributed to decreased inflammation [79].
Accumulating evidence indicates that EGCG's impact on osteoporosis is most likely mediated through its ability to inhibit cyclooxygenase-2 (COX-2), lipoxygenase (LOX), and inducible nitric oxide synthase (iNOS), predominately at the transcriptional level and, to a certain extent, the posttranslational level, although the specific regulation is not fully established [120, 121]. At both the cellular and molecular levels, EGCG regulates a number of signaling pathways, including the eicosanoid pathway involving COX-2 and LOX in human colon mucosa and colon tumors [120]. The protective effect of EGCG is due to its ability to decrease lipid peroxidation, oxidative stress, and the production of NO radicals by inhibiting the expression of iNOS [121].
5.3. Increasing osteoblast numbers, osteoblastogenesis, and bone formation
Green tea bioactive components may be beneficial to bone health by promoting an increase in osteoblast numbers and activity of osteoblasts. The evidence suggests that the components in green tea support osteoblastogenesis by increasing osteoblastic survival, proliferation, differentiation, and bone formation (Table 3).
5.3.1. Improving the survival of osteoblasts through inhibiting TNF-α and IL-6 production
Suppressing the production of TNF-α and IL-6 by osteoblasts may increase osteoblast survival. Both cytokines can mediate the effects of many stimulators of bone resorption (i.e., parathyroid hormone, PTH and IL-1) [122, 123]. TNF-α inhibits bone formation, collagen synthesis, and ALP activity in osteoblasts [124]. IL-6 promotes the recruitment of osteoclast precursors and their subsequent differentiation into mature osteoclasts. Both TNF-α and IL-6 could modulate the life span of osteoblasts via apoptosis, thus regulating bone metabolism in certain pathologic conditions such as periarticular osteoporosis found in patients with RA [125]. In an in vitro study, TNF-α or IL-6 acted on murine osteoblasts and induced apoptosis of these cells [126]. Choi et al. [87] reported that (+)-catechin can promote survival and ALP activity in osteoblastic MC3T3-E1 cells by inhibiting apoptosis of osteoblasts through a reduction in TNF-α and IL-6 production.
5.3.2. Enhancing bone mineralization through Runx2-mediated mechanism
Green tea bioactive components may affect bone strength by enhancing bone mineralization through the Runt-related transcription factor-2 (Runx2)-mediated mechanism. Runx2 (also known as core binding factor a1) regulates differentiation of osteoblasts from multipotent mesenchymal stem cells [127, 128]. Runx2 enhances osteoblast differentiation at early stages but inhibits osteoblast maturation at later stages [129] by interacting with osteogenic genes.
In a murine bone marrow mesenchymal stem cell line D1, Chen et al. [94] reported that after 48 hours of EGCG treatment (1 and 10 μmol/L), the mRNA expression of Runx2, osterix, osteocalcin, and ALP was increased. After a long-term 4-week treatment of EGCG, mineralization was confirmed via von Kossa and Alizarin Red S stain [94]. These authors also reported that EGCG increased ALP activities in 3T3-E1 cells after treatment for 14 days [94]. EGCG's stimulatory effect on ALP activity and the mineralization on D1-cell culture further confirmed its post-transcriptional influences on osteogenesis through blocking the synthesis of Runx2 protein. However, depending on the types of cell line, Vali et al. [93] reported that Runx2 expression decreased after 48 hours of EGCG treatment; this reduction was observed with 1 μM and reached a maximal inhibitory effect with 5 μM in a human osteoblast-like cell line (SaOS-2). EGCG may enhance the differentiation of osteoblasts to progress to the maturation level, thereby leading to increase the mineralization of bone matrix and bone formation in general [93].
5.3.3. Increasing osteoblastic activity through Wnt Signaling
Green tea bioactive components may increase osteoblastic activity through activating the Wnt signaling pathway. The Wnt pathway is now known to control bone development and bone mass acquisition at all skeletal sites [130, 131], and Wnt signaling drives differentiation of osteo-chondrogenic progenitor cells toward the osteoblast lineage. When the Wnt ligand binds to its receptor (Frizzled) and co-receptor (low-density lipoprotein receptor-related protein [LRP)5/6] [160-162], the signal turns on the Wnt pathway [133], giving the power of Wnt signaling to stimulate osteoblastic differentiation.
In an antler progenitor cell culture, Mount et al. [96] evaluated the effect of EGCG on canonical Wnt signaling on antler progenitor cell differentiation using ALP as a marker. Although ALP is normally used as a marker of the osteoblast phenotype, its activity has also been shown to increase with chondrocyte terminal differentiation [134]. EGCG induced apoptosis of antler progenitor cells; therefore, EGCG increased ALP activity, probably through activating β-catenin of Wnt signaling [96].
5.3.4. Inducing osteoblastogenesis by suppressing the HSP27-mediated mechanism
Green tea bioactive components can favor osteoblastogenesis via heat shock protein (HSP) 27-mediated mechanisms that are involved in bone modulators, such as transforming growth factor-beta (TGF-β) and PGD2. During osteoblastogenesis, down-regulation of osteoblastic proliferation is accompanied by a transient increase in the HSP27 mRNA expression [135].
TGF-β is one of the most abundant cytokines in the bone matrix and plays a major role in the development and maintenance of the skeleton, affecting bone cartilage and bone metabolism [136]. Hayashi [137] first demonstrated that EGCG significantly suppressed the TGF-β-stimulated induction of HSP27 through the suppression of the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathway in the osteoblast-like MC3T3-E1 cells. However, EGCG had little effect on the TGF-β-stimulated phosphorylation of ERK1/2 and p38 mitogen-activated protein (MAP) kinase in osteoblast-like MC3T3-E1 cells. In addition, EGCG scarcely affected the TGF-β-induced Smad2 phosphorylation, suggesting that EGCG does not act at a point upstream of Smad2-mediated signaling in osteoblasts.
PGD2 is also a potent regulator of osteoblastic functions [138, 139]. Yamauchi [140] reported that EGCG suppressed the PGD2-stimulated induction of HSP27 via inhibition of p44/p42 MAP kinase, but not p38 MAP kinase or SAPK/JNK in these cells. Although the physiological significance of HSP27 in osteoblasts has not yet been clarified, it is probable that EGCG-induced suppression of the SAPK/JNK or p44/p42 MAP kinase cascades in osteoblasts contributes to the modulation of osteoblastic cell function toward bone formation at least in part by specifically down regulating HSP27 induction.
5.3.5. Enhancing bone formation through activating the VEGF-mediated mechanism
Green tea bioactive component EGCG can enhance osteoblastogenesis via the vascular endothelial growth factor (VEGF)-mediated mechanism that is involved in PGF2α stimulation. VEGF is a heparin-binding angiogenic growth factor displaying high specificity for vascular endothelial cells. VEGF is involved in trabecular bone formation and expansion of the hypertrophic chondrocyte zone in the epiphyseal growth plate of mice [141]. Therefore, the inactivation of VEGF led to complete suppression of blood vessel invasion concomitant with impaired trabecular bone formation and expansion of the hypertrophic chondrocyte zone in the mouse tibial epiphyseal growth plate [141].
In addition to VEGF, PGF2α is known as a potent bone-resorptive agent; it stimulates the proliferation of osteoblasts and inhibits their differentiation [142]. PGF2α has also been shown to stimulate VEGF synthesis through protein kinase C (PKC)-dependent activation of p44/p42 MAP kinase in osteoblast-like MC3T3-E1 cells [143]. Tokuda et al. [143] reported that EGCG upregulates PGF2α-stimulated VEGF synthesis via amplifying activation of SAPK/JNK, but neither p44/p42 MAP kinase nor p38 MAP kinase, in osteoblasts.
5.4. Suppressing osteoclastogenesis and osteoclastic activity
The bioactive components in green tea appear to decrease the actions of osteoclasts in vivo (Table 2) and reduce osteoclastogenesis in cell culture (Table 3). The effects of green tea include suppressing bone resorption, increasing apoptosis of osteoclasts, and inhibiting the formation of osteoclasts.
5.4.1. Inhibiting bone resorption by stabilizing collagen
Collagen is the main organic constituent of the extracellular matrix of bone [144]. The basic building block of the bone matrix fiber network is type I collagen, a triple-helical molecule containing α1(I) and α2(I) chains. The removal of collagen by tissue collagenase [145] and cystine-proteinases [146] is a necessary step for bone resorption. Therefore, an increase in the resistance of collagen to the action of collagenase can prevent collagen degradation, which reduces bone resorption.
Delaisse et al. [105] reported that the (+)-catechin inhibited bone resorption and prevented osteoclast activation by acting on bone collagen. In this study, the inhibitory effect of (+)-catechin may have been due to its oxidation products that bind to the thin layer of unmineralized collagen to separate resting osteoblasts from the mineralized matrix and prevent their removal by osteoblast-secreted collagenase. This could be sufficient to inhibit the whole process of bone resorption by preventing osteoclast activation.
5.4.2. Increasing apoptosis of osteoclasts through caspase activation-dependent mechanisms
The number of osteoclasts in an organism depends on the relative rates of osteoclastogenesis and apoptosis. Apoptosis of osteoclasts is regulated by the activation of caspases [147, 148]. There are two main pathways leading to the activation of caspase-3 (a key executioner), including the mitochondria (receptor-independent) pathway and the death receptor with its ligand pathway (i.e., TNF and Fas ligand) [149, 150]. Yun [151] reported that EGCG significantly inhibited the survival of osteoclasts differentiated from RAW 264.7 cells and induced the apoptosis of osteoclasts as shown by DNA fragmentation. EGCG-induced apoptosis in RAW 264.7 cell-derived osteoclasts was mediated in part through the activation of caspase-3. ECGC stimulates the activation of caspase-3, the elevation of caspase-3 activity, and the cleavage of pro-caspase-3.
The activation of NF-κB in cells provides cell survival signals and protects cells from apoptosis [152, 153], whereas the inhibition of NF-κB activity in cells dramatically reduces cell growth [153, 154]. Hafeez [109] demonstrated that high-dose GTP effectively reduced cell proliferation and induction of apoptosis via decreasing nuclear DNA binding of NF-κB/p65 and lowering of NF-κB/p65 and p50 levels in the cytoplasm and nucleus of human osteosarcoma SaOS-2 cells. GTP inhibits NF-κB activation through suppressing inhibitor of kappaB kinase activation and increasing phosphorylation of IκB-α in SaOS-2 cells. Inhibition of NF-κB decreases Bcl-2 protein expression and increases the levels of Bax, thus shifting the Bax/Bcl-2 ratio in favor of apoptosis. From the same study, GTP activated both caspase-3 and -8. These findings suggest that GTP may induce osteoclasts’ apoptosis by involving a caspase-dependent mechanism with downregulation of NF-κB [109].
5.4.3. Increasing apoptosis of osteoclasts through the Fenton reaction
Hydroxyl radicals generated via the Fenton reaction from hydrogen peroxide (H2O2) and ferrous ions are produced by a variety of cells, including osteoclastic cells. The resulting hydroxyl radicals are reactive and may initiate and propagate the degenerative reaction in cell membranes known as lipid peroxidation. Such lipid peroxidation would contribute to oxidative stress in cells. Nakagawa et al. [107] first demonstrated that EGCG triggers the Fenton reaction to form highly reactive hydroxyl radicals from H2O2 and Fe2+ [H2O2 + Fe2+ → ·OH + OH− + Fe3+], and the resulting hydroxyl radical activates caspase-3 activity and induces single-strand DNA breakage (a hallmark of cell death) in osteoclasts. These results indicate that the Fenton reaction is primarily involved in EGCG-induced osteoclastic cell death.
5.4.4 Inhibiting the formation of osteoclasts via the MMP pathway
Green tea bioactive components can suppress the formation of osteoclasts by inhibiting the release of matrix metalloproteinases (MMPs) by osteoblasts. Both collagenase (i.e., MMP-1 and MMP-13) and gelatinase A (MMP-2) and B (MMP-9) have been considered the principal MMPs in the digestion of bone collagen by osteoblasts [155, 156]. In a co-culture system of mouse bone marrow cells and calvarial primary osteoblastic cells, Yun et al. [106] demonstrated that EGCG inhibited the expression of Porphyromonas gingivalis-induced MMP-9 mRNA of osteoblasts, not MMP-2 and MMP-13, and EGCG inhibited osteoclastic formation, but had no inhibitory effect on the cell viability of either the co-culture system or primary osteoblastic cells. These findings suggest that EGCG may inhibit the alveolar bone resorption that occurs in periodontal diseases by inhibiting the expression of MMP-9 in osteoblasts and the formation of osteoclasts.
5.4.5 Suppressing bone resorption by inhibiting IL-6 production
Green tea bioactive component EGCG can suppress bone resorption by inhibiting IL-6 production by osteoblasts. In bone metabolism, IL-6, the most potent osteoclastogenic factor, stimulates bone resorption and induces osteoclast formation [157]. Tokuda et al. [158] reported that EGCG suppresses endothelin-1-induced IL-6 synthesis in osteoblasts via an inhibition of p44/p42 MAP kinase activation in osteoblastic-like MC3T3-E1 cells, and the inhibitory effect is exerted at a point between PKC and Raf-1 in the endothelin-1 signaling cascade. These same authors [159] also reported that EGCG inhibits basic-fibroblast-growth-factor-2-induced IL-6 synthesis at least partly via attenuation of the p44/p42 MAP kinase pathway and the p38 MAP kinase pathway in osteoblasts. Takai et al. [160] found that EGCG significantly reduced the IL-6 synthesis and IL-6 mRNA expression induced by platelet-derived growth factor-BB through suppression of the SAPK/JNK pathway in osteoblast-like MC3T3-E1 cells. Moreover, Ishida [161] reported that EGCG controls inflammatory bone resorption in chronic osteomyelitis by inhibiting the production of IL-6 and RANKL in osteoblasts infected with S. aureus.
5.5. Modulating osteoimmunological activity
Observation of accelerated bone loss caused by inflammatory diseases, such as RA, contributed enormously to the emergence of the field of osteoimmunology. Excessive bone loss is not only present in inflammatory diseases but also in autoimmune diseases and cancer. The prevalent skeletal disorder, osteoporosis, is associated with alterations in the immune system [162]. Although GTP has not been evaluated from the aspect of osteoimmunology, we speculate that there are two ways that GTPs may modulate osteoimmunological activity: first, by inhibiting differentiation of osteoclasts through RANKL signaling, and second, by modulating the production of cytokines by immune cells. Our speculation can be a possible new application to explore how GTP may influence bone health through osteoimmunological activity.
5.5.1. Inhibiting differentiation of osteoclasts through RANKL signaling
Green tea bioactive components can suppress osteoclastic differentiation via RANKL signaling. The differentiation of the macrophage polykaryon into osteoclasts is principally regulated by three cytokines: RANKL, macrophage-colony stimulating factor (M-CSF), and osteoprotegerin (OPG) [163, 164]. During osteoclast development under RANKL stimulation, the nuclear factor of activated T cells c1 (NF-ATc1) is up-regulated, and this up-regulation is considered to be a master regulator for inducing osteoclast-specific genes such as TRAP, calcitonin receptor (CTR), carbonic anhydrase II, cathepsin K, and αv and β3 integrins [165].
One example of how EGCG is involved in suppressing osteoclasts via RANKL is what happens in the joints of RA patients. Under arthritic conditions, factors such as RANKL and inflammatory cytokines produced by T cells, synovial fibroblasts, and activated macrophages, facilitate osteoclast formation [166]. Thus, suppressing osteoclast development or inhibiting RANKL and M-CSF expression in inflamed joints is an option for reducing bone erosion in RA joints. In an experimental arthritis model using mice, Morinobu et al. [85] reported that EGCG reduced the generation of TRAP-positive multinucleated cells, bone resorption activity, and osteoclast-specific gene expression without affecting cell viability. EGCG down-regulated RANKL-induced expression of NF-ATc1, but not of NF-κB, c-FOS, and c-Jun, resulting in blocking differentiation of monocytes into osteoclasts, as judged by decreased bone resorption. In addition, EGCG inhibited the mRNA expression of TRAP, CTR, carbonic anhydrase II, cathepsin K, and αv and β3 integrins that are induced by RANKL. Lin et al. [110] also recently reported that EGCG significantly suppressed the RANKL-induced differentiation of osteoclasts and pit formation in murine RAW 264.7 cells (a murine preosteoclast cell line) and bone marrow macrophages (precursor of osteoclasts) at the early stage of osteoclastogenesis. EGCG blocked RANKL signaling by significantly reducing RANKL-induced NF-κB transcriptional activity and the nuclear transport of NF-κB, a transcription factor known to be essential for the development of osteoclasts.
5.5.2. Modulating the production of cytokines by immune cells
EGCG has modulating effects on cytokine production by immune cells. An increased production of mononuclear cell immune cytokine products (such as IL-1, IL-6, IL-12, and TNF-1α) contributes to the postmenopausal enhancement of bone resorption. In addition to changes of cytokine production by monocytes, T-cell abnormalities have been reported in patients with osteoporosis [167]. Eghbali-Fatourechi [168] reported that the surface expression of RANKL on marrow stromal cells, B cells, and T cells was significantly higher in early postmenopausal women when compared with premenopausal or estrogen-treated women. These findings suggest that upregulation of RANKL on stromal cells and lymphocytes in the bone marrow could mediate increased bone resorption due to estrogen deficiency. Wu et al. [169] reported that EGCG upregulated the production of T-helper cell cytokines, IL-12, and TNF-α, which are important for antimicrobial cell-mediated immunity in murine alveolar macrophage cell lines (MH-S cells). Matsunaga et al. [170] found that EGCG downregulated the production of IL-10, an IL associated with Th2 helper cells that is important in humoral antibody-based immunity. Matsunaga et al. [170] also showed that EGCG stimulated the production of IFN-γ by macrophage cells.
Besides T-cells, EGCG also has an impact in dendritic cells (DC) and antigen-presenting cells, which play key roles as the immune sentinels by initiating T-cell responses against microbial pathogens and tumors [171]. In a model of primary murine bone marrow-derived DCs, EGCG has an inhibitory effect on production of the TNF-α and IL-12 in stimulated DCs. Ahn et al. [172] reported that EGCG suppressed the LPS-induced phenotypic and functional maturation of murine DCs through inhibition of expression of MAPK (ERK, p38, and JNK) and of NF-κB activation. The cytokine modulatory effects of EGCG on pro-inflammatory cytokines appears to be host-cell specific. For example, EGCG decreased LPS-induced TNF-α production in a dose-dependent manner in the murine macrophage cell line, RAW 264.7, and similarly inhibited LPS-induced TNF-α production in elicited BALB/c mouse peritoneal macrophages, effects attributed in part by blocking NF-κB activation [173].
6. Other bioactive components in tea that benefit bone health
Besides the catechins, tea is also an important source of flavonoids, caffeine, and dietary fluoride [174]. Fluoride intake can alleviate osteoporotic progression [175]. Fluoride concentration in tea brewed in fluoride-free water ranges from negligible to four parts per million, depending on the type and amount of tea used. Three or more cups of tea daily would be expected to increase fluoride intake by up to 4 mg daily. Therefore, the relatively high fluoride content of the tea leaves may enhance the protective effect of tea on BMD. However, overdose of fluoride from excessive tea drinking (10 to 40 cups/day containing fluoride up to 56 mg/day) had a detrimental effect on BMD [69].
Tea is a potential source of flavonoids, including phytoestrogen, isoflavone [176], and lignans [177], which are reported to have several biological actions, including a weak estrogenic effect [178]. Tea-derived flavonoids and lignans may improve BMD [105, 179, 180], particularly in older women with low concentrations of endogenous estrogen. Compared with American and European women, Japanese women have a diet that is higher in isoflavonoids [181]; high dietary isoflavonoid intake and its subsequent estrogenic effect may be a reason for the infrequent occurrence of hot flashes and other menopausal symptoms in Japanese women [181]. Relatively weak estrogenic effects of isoflavonoids in tea may not have a noticeable effect on BMD in premenopausal women, who have high amounts of endogenous estrogen, such as predominated in the group studied by Hernandez et al. [182], or in men, in whom androgens predominate, but may be important in maintaining BMD in older women who have low levels of endogenous estrogen [22].
Another bioactive component of tea is caffeine. Some studies suggest that caffeine intake is inversely related to BMD [62, 182], but these findings were not supported by other studies [183, 184]. This discrepancy may be due partially to the addition of milk to coffee, which could ameliorate the adverse effects of coffee drinking [20, 182]. Although the caffeine content of the tea leaf is higher (2% to 3% versus 1%) compared with that of roasted coffee, tea is diluted more for drinking. The average caffeine content of a tea beverage in the United States is about 30 to 45 mg/cup, while that of a coffee beverage is about 60 to 129 mg/cup [185]. Hence, an adverse effect of caffeine from tea on BMD may be less significant. However, it is possible that at higher tea intakes, caffeine may attenuate the benefit of other bioactive components of tea.
7. Summary and future research
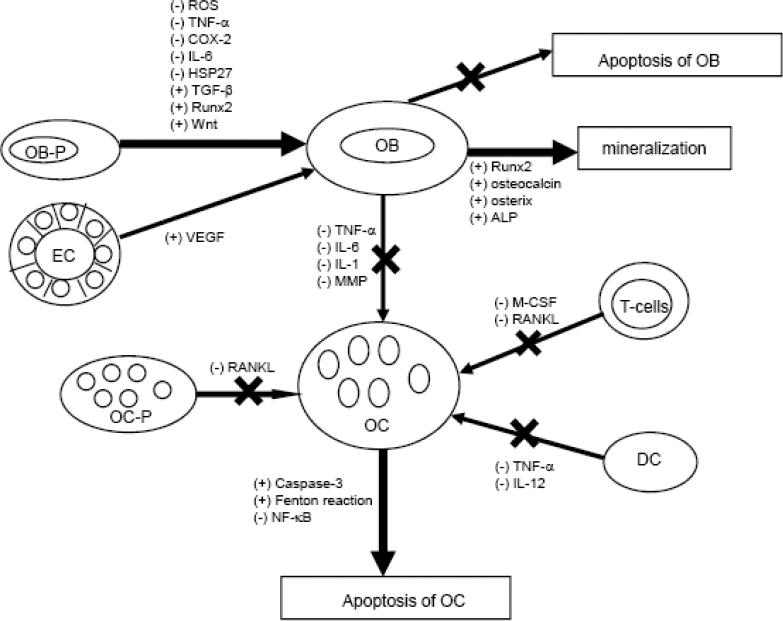
Osteoporosis is the result of an imbalance in the ratio with more resorption than formation. Enhancing the activity of osteoblasts, plus reducing that of the osteoclasts, may help restore the balance in bone metabolism and limit bone loss in the development of osteoporosis. There is mounting evidence that green tea contains many bioactive ingredients that support some protection against osteoporosis. This is supported by data from in vitro, ex vivo, and in vivo animal studies and human epidemiological findings. The beneficial effects of tea bioactive products appear to be mediated through antioxidant or anti-inflammatory pathways and their related signaling pathways in the various cells comprised of bone compartments (Figure 1).
Figure 1.
Possible actions (stimulatory or inhibitory) of green tea bioactive component (EGCG) in osteoblasts (OB) and osteoclasts (OC). Green tea bioactive components appear to promote bone formation by decreasing oxidative stress (ROS) and pro-inflammatory mediators (TNF-α, COX-2), and by increasing OB activity and survival (HSP27, TGFβ, Runx2, Wnt), resulting in enhanced mineralization (Runx2, osteocalcin, osterix, ALP). Green tea bioactive components suppress bone resorption by inhibiting OC formation (MMP) via increasing OC apoptosis (caspases, Fenton) that results in suppressing osteoclastogenesis. In addition, EC is involved in stimulating osteoblastogenesis, while T-cells and DC are involved in suppressing osteoclastogenesis.
These significant beneficial effects on bone suggest that GTP may serve as an effective dietary supplement to prevent BMD loss in patients with low bone mass. It is worthy to point out that even though green tea and its metabolites are found to be useful in treating bone loss, there is still a gap in our knowledge that needs to be filled in regard to the translation of findings in animal observations and how this is applied to human populations. Evidence from all animal studies only shows an increase in BMD without testing bone strength and anti-fracture capacity; these animal data mainly focus on long bones, while the published human data are for spine and hip. In addition, there is still limited data supporting the BMD increment and anti-fracture effect of green tea from longitudinal studies. In future human studies, green tea and its active ingredients should be given for long-term periods, the bioavailability should be monitored via validated biomarkers, and efficacy in terms of bone mass and micro-architecture should be evaluated through advanced imaging technology in order to ensure their possible benefits in treating osteoporosis.
8. Acknowledgment
The preparation of this review was supported by NIH/NCCAM grant R21AT003735, the Laura W. Bush Institute for Women's Health (CLS), and NIH/NCI grant CA90997 (JSW). The authors thank Ryan K. Boettger, Angela Eaton, and Cynthia R. Davidson for their editorial contribution.
Abbreviation
- ALP
alkaline phosphatase
- BMD
bone mineral density
- COX
cyclooxygenase
- EC
(−) epicatechin
- CTR
calcitonin receptor
- DC
dendritic cells
- ECG
(−) epicatechin gallate
- EGC
(−) epigallocatechin
- EGCG
(−) epigallocatechin gallate
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinases
- GTP
green tea polyphenols
- H2O2
hydrogen peroxide
- HSP
heat shock protein
- γ-IFN
γ-interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LOX
lipoxygenase
- MAP
mitogen-activated protein
- M-CSF
macrophage-colony stimulating factor
- MMP
matrix metalloproteinases
- NF-ATc1
nuclear factor of activated T cells c1
- NF-kB
receptor activator of nuclear factor-kB
- OC
osteocalcin
- PG
prostaglandin
- PKC
protein kinase C
- PTH
parathyroid hormone
- RA
rheumatoid arthritis
- RANKL
receptor activator of nuclear factor-kB ligand
- ROS
reactive oxygen species
- Runx2
Runt-related transcription factor-2
- SAPK
stress-activated protein kinase
- TGF-β
transforming growth factor-beta
- TNF-α
tumor necrosis factor-α
- TRAP
tartrate-resistant acid phosphatase
- VEGF
vascular endothelial growth factor
Abbreviation
- (−)
inhibitory effect
- (+)
stimulatory effect
- “×”
indicates blocking the pathway
- ALP
alkaline phosphatase
- COX-2
cyclooxygenase-2
- DC
dendritic cells
- EC
endothelial cells
- HSP27
heat shock protein 27
- γ-IFN
γ-interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LOX
lipoxygenase
- M-CSF
macrophage-colony stimulating factor
- MMP
matrix metalloproteinases
- NF-κB
receptor activator of nuclear factor-κB
- OB-P
osteoblast precursor
- OB
osteoblast
- OC-P
osteoclast precursor
- OC
osteoclast
- PGE2
prostaglandin E2
- RANKL
receptor activator of nuclear factor-κB ligand
- ROS
reactive oxygen species
- Runx2
Run-related transcription factor-2
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Institutes of Health Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online 2000 March 27−29. 17:1–36. [PubMed] [Google Scholar]
- 2.Looker AC, Johnston CC, Jr, Wahner HW, Dunn WL, Calvo MS, Harris TB, et al. Prevalence of low femoral bone density in older U.S. women from NHANES III. J Bone Miner Res. 1995;10:796–802. doi: 10.1002/jbmr.5650100517. [DOI] [PubMed] [Google Scholar]
- 3.National Osteoporosis Foundation . American's bone health: the state of osteoporosis and low bone mass in our nation. National Osteoporosis Foundation; Washington, DC: 2002. pp. 1–55. [Google Scholar]
- 4.Boonen S, Dejaeger E, Vanderschueren D, Venken K, Bogaerts A, Verschueren S, Milisen K. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: a geriatric perspective. Best Pract Res Clin Endocrinol Metab. 2008;22(5):765–85. doi: 10.1016/j.beem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 5. [April 9, 2009];Osteoporosis in Men. http://www.nof.org/men/index.htm.
- 6.Bone Health and Osteoporosis: A Report of the Surgeon General. U.S. Department of Health and Human Services; Rockville, MD: 2004. The Frequency of Bone Disease. pp. 69–87. U.S.D.H.H.S. Chapter 4. [Google Scholar]
- 7.Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22(5):671–85. doi: 10.1016/j.beem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Boonen S, Singer AJ. Osteoporosis management: impact of fracture type on cost and quality of life in patients at risk for fracture I. Curr Med Res Opin. 2008;24(6):1781–8. doi: 10.1185/03007990802115796. [DOI] [PubMed] [Google Scholar]
- 9. http://www.nof.org/advocacy/prevalence/index.htm.
- 10.Konnopka A, Jerusel N, König HH. The health and economic consequences of osteopeni- and osteoporosis-attributable hip fractures in Germany: estimation for 2002 and projection until 2050. Osteoporosis Int. 2008 doi: 10.1007/s00198-008-0781-1. [DOI] [PubMed] [Google Scholar]
- 11.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005−2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 12.Ray NF, Chan JK, Thamer M, Melton LJ. Medical expenditure for the treatment of osteoporotic fractures in the United States in 1995 report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 13.Miller LJ, 3rd, Crowson CS, O'Fallon WM, Wahner HW, Riggis BL. Relative contributions of bone density, bone turnover, and clinical risk factors to long-tern fracture prediction. J Bone Miner Res. 2003;18(2):312–8. doi: 10.1359/jbmr.2003.18.2.312. [DOI] [PubMed] [Google Scholar]
- 14.Hui SL, Slemenda CW, Johnston CC., Jr. Age and bone mass predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–9. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measurements of bone mineral density predict the occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1549. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer DC, Gluer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK, et al. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. Arch Int Med. 1997;157:629–34. [PubMed] [Google Scholar]
- 17.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, et al. Appendicular bone density and age predict hip fracture in women. JAMA. 1990;263:665–8. [PubMed] [Google Scholar]
- 18.Hamdi Kara I, Aydin S, Gemalmaz A, Aktürk Z, Yaman H, Bozdemir N, et al. Habitual tea drinking and bone mineral density in postmenopausal Turkish women: investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study). Int J Vitam Nutr Res. 2007;77(6):389–97. doi: 10.1024/0300-9831.77.6.389. Erratum in: Int J Vitam Nutr Res. 2008 May;78(3):following 166.
- 19.Muraki S, Yamamoto S, Ishibashi H, Oka H, Yoshimura N, Kawaguchi H, et al. Diet and lifestyle associated with increased bone mineral density: cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J Orthop Sci. 2007;12(4):317–20. doi: 10.1007/s00776-007-1143-0. [DOI] [PubMed] [Google Scholar]
- 20.Hegarty VM, May HM, Khaw KT. Tea drinking and bone mineral density in older women. Am J Clin Nutr. 2000;71(4):1003–7. doi: 10.1093/ajcn/71.4.1003. [DOI] [PubMed] [Google Scholar]
- 21.Wu CH, Yang YC, Yao WJ, Lu FH, Wu JS, Chang CJ. Epidemiological evidence of increased bone mineral density in habitual tea drinkers. Arch Intern Med. 2002;162:1001–6. doi: 10.1001/archinte.162.9.1001. [DOI] [PubMed] [Google Scholar]
- 22.Hoover PA, Webber CE, Beaumont LF, Blake JM. Postmenopausal bone mineral density: relationship to calcium intake, calcium absorption, residual estrogen, body composition, and physical activity. Can J Physiol Pharmacol. 1996;74(8):911–7. [PubMed] [Google Scholar]
- 23.Devine A, Hodgson JM, Dick IM, Prince RL. Tea drinking is associated with benefits on bone density in older women. Am J Clin Nutr. 2007;86(4):1243–7. doi: 10.1093/ajcn/86.4.1243. [DOI] [PubMed] [Google Scholar]
- 24.Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker J, et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res. 1995;10:1802–15. doi: 10.1002/jbmr.5650101125. [DOI] [PubMed] [Google Scholar]
- 25.Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS Study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9:45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 26.Shen CL, Wang P, Guerrieri J, Yeh J, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporosis Int. 2008;19(7):979–90. doi: 10.1007/s00198-007-0527-5. [DOI] [PubMed] [Google Scholar]
- 27.Shen CL, Yeh JK, Liu X-Q, Dunn DM, Stoecker BJ, Wang P, et al. Effect of green tea polyphenols on chronic inflammation-induced bone loss in female rats. FASEB J. 2008;22:314.3. [Google Scholar]
- 28.Nijweide PJ, Burger EH, Feyen JH. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66(4):855–86. doi: 10.1152/physrev.1986.66.4.855. Review. [DOI] [PubMed] [Google Scholar]
- 29.Ando K, Mori K, Rédini F, Heymann D. RANKL/RANK/OPG: key therapeutic target in bone oncology. Curr Drug Discov Technol. 2008;5(3):263–8. doi: 10.2174/157016308785739857. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–9. doi: 10.1016/j.abb.2008.03.027. Review. [DOI] [PubMed] [Google Scholar]
- 31.Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13(1):66–80. doi: 10.1210/edrv-13-1-66. Review. [DOI] [PubMed] [Google Scholar]
- 32.Steinbeck MJ, Appel WH, Jr, Verhoeven AJ, Karnovsky MJ. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J Cell Biol. 1994;126(3):765–72. doi: 10.1083/jcb.126.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darden AG, Ries WL, Wolf WC, Rodriguiz RM, Key LL., Jr Osteoclastic superoxide production and bone resorption: stimulation and inhibition by modulators of NADPH oxidase. J Bone Miner Res. 1996;11(5):671–5. doi: 10.1002/jbmr.5650110515. [DOI] [PubMed] [Google Scholar]
- 34.Yavropoulou MP, Yovos JG. Osteoclastogenesis- Current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8(3):204–16. [PubMed] [Google Scholar]
- 35.Fazzalari NL. Bone remodeling: A review of the bone microenvironment perspective for fragility fracture (osteoporosis) of the hip. Semin Cell Dev Biol. 2008;19(5):467–72. doi: 10.1016/j.semcdb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46(11):1550–5. doi: 10.1515/CCLM.2008.302. Review. [DOI] [PubMed] [Google Scholar]
- 37.Manolagas SC. De-fense! De-fense! De-fense: scavenging H2O2 while making cholesterol. Endocrinology. 2008;149(7):3264–6. doi: 10.1210/en.2008-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mody N, Parhami F, Saraflan TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–19. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 39.Fatokun AA, Stone TW, Smith RA. Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur J Pharmacol. 2008;587(1−3):35–41. doi: 10.1016/j.ejphar.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Bai X-C, Lu D, Bai J, Zheng H, Ke ZY, Li XM, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem Biophys Res Commun. 2004;314(1):197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 41.Dreher I, Schütze N, Baur A, Hesse K, Schneider D, Köhrle J, et al. Selenoproteins are expressed in fetal human osteoblast-like cells. Biochem Biophys Res Commun. 1998;245:101–7. doi: 10.1006/bbrc.1998.8393. [DOI] [PubMed] [Google Scholar]
- 42.Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ. A role for TGF-β in osteoclast differentiation and survival. J Cell Sci. 2000;113:2445–53. doi: 10.1242/jcs.113.13.2445. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, Madyastha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–8. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 44.Sontakke AN, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta. 2002;318(1−2):145–8. doi: 10.1016/s0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 45.Garrett JR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–9. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polidori MC, Stahl W, Eichler O, Niestroj I, Sies H. Profles of antioxidants in human plasma. Free Radic Biol Med. 2001;30:456–462. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 47.Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: reslts of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–7. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 48.Basu K, Michaëlsson H, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288:275–9. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 49.Morton DJ, Barrett-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. J Bone Miner Res. 2001;16:135–40. doi: 10.1359/jbmr.2001.16.1.135. [DOI] [PubMed] [Google Scholar]
- 50.Yang CS, Landau JM. Effects of tea consumption on nutrition and health. J Nutr. 2000;130:2409–12. doi: 10.1093/jn/130.10.2409. [DOI] [PubMed] [Google Scholar]
- 51.Cooper R, Moore DJ, Morre DM. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. J Alternative and Complentary Medicine. 2005;11(3):521–8. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 52.Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. Journal of International Medical Research. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 53.Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitam Horm. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. Review. [DOI] [PubMed] [Google Scholar]
- 54.van het Hof KH, Wiseman SA, Yang CS, Tijburg LB. Plasma and lipoprotein levels of tea catechins following repeated tea consumption. Proc Soc Exp Biol Med. 1999;220(4):203–9. doi: 10.1046/j.1525-1373.1999.d01-34.x. [DOI] [PubMed] [Google Scholar]
- 55.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133(12):4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 56.Neilson AP, Li Y, Hannon K, Ferruzzi MG, Watkins BA. Accumulation of catechins in bone and liver of mice fed gree ntea while under physical stress. FASEB J. 2006;20:A570. [Google Scholar]
- 57.Schneider C, Segre T. Green tea: potential health benefits. Am Fam Physician. 2009;79(7):591–4. [PubMed] [Google Scholar]
- 58.Chen X, Pettinger MB, Ritenbaugh C, LaCroix AZ, Robbins J, Caan BJ, et al. Habitual Tea Consumption and Risk of Osteoporosis: A Prospective Study in the Women's Health Initiative Observational Cohort. Am J Epidemiol. 2003;158:772–81. doi: 10.1093/aje/kwg214. [DOI] [PubMed] [Google Scholar]
- 59.Siddiqui IA, Afaq F, Adhami VM, Ahmad N, Mukhtar H. Antioxidants of the beverage tea in promotion of human health. Antioxid Redox Signal. 2004;6(3):571–82. doi: 10.1089/152308604773934323. [DOI] [PubMed] [Google Scholar]
- 60.Hallström H, Wolk A, Glynn A, Michaëlsson K. Coffee, tea and caffeine consumption in relation to osteoporotic fracture risk in a cohort of Swedish women. Osteoporos Int. 2006;17(7):1055–64. doi: 10.1007/s00198-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 61.Keramat A, Patwardhan B, Larijani B, Chopra A, Mithal A, Chakravarty D, et al. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord. 2008;27(9):28. doi: 10.1186/1471-2474-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hernández-Avila M, Stampfer MJ, Ravnikar VA, Willett WC, Schiff I, Francis M, et al. Caffeine and other predictors of bone density among pre- and perimenopausal women. Epidemiology. 1993;4(2):128–34. doi: 10.1097/00001648-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Hamdi Kara I, Aydin S, Gemalmaz A, Aktürk Z, Yaman H, Bozdemir N, et al. Habitual tea drinking and bone mineral density in postmenopausal Turkish women: investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study). Int J Vitam Nutr Res. 2007;77(6):389–97. doi: 10.1024/0300-9831.77.6.389. [DOI] [PubMed] [Google Scholar]
- 64.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 65.Vestergaard P, Hermann AP, Gram J, Jensen LB, Eiken P, Abrahamsen B, et al. Evaluation of methods for prediction of bone mineral density by clinical and biochemical variables in perimenopausal women. Maturitas. 2001;40(3):211–20. doi: 10.1016/s0378-5122(01)00240-7. [DOI] [PubMed] [Google Scholar]
- 66.Kasamatsu T, Yoshimura N, Morioka S, Sugita K, Hashimoto T. A population survey on bone mineral density in a fishing village in Wakayama prefecture. (Part 1) Distribution of bone mineral density by sex and age based on a representative sample of the community. Nippon Eiseigaku Zasshi. 1996;50(6):1084–92. doi: 10.1265/jjh.50.1084. Japanese. [DOI] [PubMed] [Google Scholar]
- 67.Hong X, Lü H, Yang J, Li Z. An analysis on the forearm bone mass density of rural female and the environmental risk factors. Wei Sheng Yan Jiu. 2001;30(4):227–30. [PubMed] [Google Scholar]
- 68.Kyriazopoulos P, Trovas G, Charopoulos J, Antonogiannakis E, Galanos A, Lyritis G. Lifestyle factors and forearm bone density in young Greek men. Clin Endocrinol (Oxf) 2006;65(2):234–8. doi: 10.1111/j.1365-2265.2006.02581.x. [DOI] [PubMed] [Google Scholar]
- 69.Hallanger Johnson JE, Kearns AE, Doran M, et al. Fluoride-related bone disease associated with habitual tea consumption. Mayo Clin Proc. 2007;82:719–24. [PubMed] [Google Scholar]
- 70.Saitoglu M, Ardicoglu O, Ozgocmen S, Kamanli A, Kaya A. Osteoporosis risk factors and association with somatotypes in males. Arch Med Res. 2007;38(7):746–51. doi: 10.1016/j.arcmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Wang JS, Luo H, Wang P, Tang L, Yu J, Huang T, et al. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem Toxicol. 2008;46(1):232–40. doi: 10.1016/j.fct.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Behl C. Estrogen can protect neurons: modes of action. J Steroids Biochem Mol Biol. 2003;83:195–7. doi: 10.1016/s0960-0760(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 73.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–23. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146(2):728–35. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 76.Kuruto-Niwa R, Inoue S, Ogawa S, Muramatsu M, Nozawa R. Effects of tea catechins on the ERE-regulated estrogenic activity. J Agric Food Chem. 2000;48(12):6355–61. doi: 10.1021/jf0008487. [DOI] [PubMed] [Google Scholar]
- 77.Shenouda NS, Zhou C, Browning JD, Ansell PJ, Sakla MS, Lubahn DB, et al. Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr Cancer. 2004;49(2):200–8. doi: 10.1207/s15327914nc4902_12. [DOI] [PubMed] [Google Scholar]
- 78.Goodin MG, Fertuck KC, Zacharewski TR, Rosengren RJ. Estrogen receptor-mediated actions of polyphenolic catechins in vivo and in vitro. Toxicol Sci. 2003;69(2):354–61. doi: 10.1093/toxsci/69.2.354. [DOI] [PubMed] [Google Scholar]
- 79.Shen CL, Yeh JK, Stoecker BJ, Samathanam C, Graham S, Dunn DM, et al. Green tea polyphenols protects bone microarchitecture in female rats with chronic inflammation-induced bone loss. JBMR. 2008;23:s458. [Google Scholar]
- 80.Lee JD, Kim SY, Kim TW, Lee SH, Yang HI, Lee DI, et al. Anti-inflammatory effect of bee venom on type II collagen-induced arthritis. Am J Chin Med. 2004;32(3):361–7. doi: 10.1142/S0192415X04002016. [DOI] [PubMed] [Google Scholar]
- 81.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. Review. [DOI] [PubMed] [Google Scholar]
- 82.Schett G, Smolen JS. New insights in the mechanism of bone loss in arthritis. Curr Pharm Des. 2005;11(23):3039–49. doi: 10.2174/1381612054865046. Review. [DOI] [PubMed] [Google Scholar]
- 83.Pettit AR, Ji H, von Stechow D, Müller R, Goldring SR, Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Redlich K, Hayer S, Maier A, Dunstan CR, Tohidast-Akrad M, Lang S, et al. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 2002;46(3):785–92. doi: 10.1002/art.10097. [DOI] [PubMed] [Google Scholar]
- 85.Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, et al. (−)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008;58(7):2012–8. doi: 10.1002/art.23594. [DOI] [PubMed] [Google Scholar]
- 86.Kazantzis G. Cadmium, osteoporosis and calcium metabolism. Biometals. 2004;17(5):493–8. doi: 10.1023/b:biom.0000045727.76054.f3. Review. [DOI] [PubMed] [Google Scholar]
- 87.Choi EM, Hwang JK. Effects of (+)-catechin on the function of osteoblastic cells. Biol Pharm Bull. 2003;26(4):523–6. doi: 10.1248/bpb.26.523. [DOI] [PubMed] [Google Scholar]
- 88.Greger JL, Lyle BJ. Iron, copper and zinc metabolism of rats fed various levels and types of tea. J Nutr. 1988;118(1):52–60. doi: 10.1093/jn/118.1.52. [DOI] [PubMed] [Google Scholar]
- 89.Zeyuan D, Bingying T, Xiaolin L, Jinming H, Yifeng C. Effect of green tea and black tea on the metabolisms of mineral elements in old rats. Biol Trace Elem Res. 1998;65(1):75–86. doi: 10.1007/BF02784115. [DOI] [PubMed] [Google Scholar]
- 90.Hamdaoui MH, Chahed A, Ellouze-Chabchoub S, Marouani N, Ben Abid Z, Hédhili A. Effect of green tea decoction on long-term iron, zinc and selenium status of rats. Ann Nutr Metab. 2005;49(2):118–24. doi: 10.1159/000084745. [DOI] [PubMed] [Google Scholar]
- 91.Samman S, Sandström B, Toft MB, Bukhave K, Jensen M, Sørensen SS, et al. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr. 2001;73(3):607–12. doi: 10.1093/ajcn/73.3.607. [DOI] [PubMed] [Google Scholar]
- 92.Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76(6):899–910. Review. [PubMed] [Google Scholar]
- 93.Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem. 2007;18(5):341–7. doi: 10.1016/j.jnutbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 94.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005;16(12):2039–45. doi: 10.1007/s00198-005-1995-0. [DOI] [PubMed] [Google Scholar]
- 95.Bickford PC, Tan J, Shytle RD, Sanberg CD, El-Badri N, Sanberg PR. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006;15(1):118–23. doi: 10.1089/scd.2006.15.118. [DOI] [PubMed] [Google Scholar]
- 96.Mount JG, Muzylak M, Allen S, Althnaian T, McGonnell IM, Price JS. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Dev Dyn. 2006;235(5):1390–9. doi: 10.1002/dvdy.20742. [DOI] [PubMed] [Google Scholar]
- 97.Mizutani A, Sugiyama I, Kuno E, Matsunaga S, Tsukagoshi N. Expression of matrix metalloproteinases during ascorbate-induced differentiation of osteoblastic MC3T3-E1 cells. J Bone Miner Res. 2001;16(11):2043–9. doi: 10.1359/jbmr.2001.16.11.2043. [DOI] [PubMed] [Google Scholar]
- 98.Yamaguchi M, Jie Z. Effect of polyphenols on calcium content and alkaline phosphatase activity in rat femoral tissues in vitro. Biol Pharm Bull. 2001;24(12):1437–9. doi: 10.1248/bpb.24.1437. [DOI] [PubMed] [Google Scholar]
- 99.Takami M, Woo JT, Nagai K. Osteoblastic cells induce fusion and activation of osteoclasts through a mechanism independent of macrophage-colony-stimulating factor production. Cell Tissue Res. 1999;298(2):327–34. doi: 10.1007/s004419900092. [DOI] [PubMed] [Google Scholar]
- 100.Felix R, Cecchini MG, Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990;127(5):2592–4. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- 101.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345(6274):442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 102.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat Rec. 1989;224(2):317–24. doi: 10.1002/ar.1092240220. Review. [DOI] [PubMed] [Google Scholar]
- 104.Duong LT, Lakkakorpi P, Nakamura I, Rodan GA. Integrins and signaling in osteoclast function. Matrix Biol. 2000;19(2):97–105. doi: 10.1016/s0945-053x(00)00051-2. Review. [DOI] [PubMed] [Google Scholar]
- 105.Delaissé JM, Eeckhout Y, Vaes G. Inhibition of bone resorption in culture by (+)-catechin. Biochem Pharmacol. 1986;35(18):3091–4. doi: 10.1016/0006-2952(86)90391-6. [DOI] [PubMed] [Google Scholar]
- 106.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (−)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004;39(5):300–7. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 107.Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, et al. Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun. 2002;292(1):94–101. doi: 10.1006/bbrc.2002.6622. [DOI] [PubMed] [Google Scholar]
- 108.Nakagawa H, Hasumi K, Takami M, Aida-Hyugaji S, Woo JT, Nagai K, et al. Identification of two biologically crucial hydroxyl groups of (−)-epigallocatechin gallate in osteoclast culture. Biochem Pharmacol. 2007;73(1):34–43. doi: 10.1016/j.bcp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Hafeez BB, Ahmed S, Wang N, Gupta S, Zhang A, Haqqi TM. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol Appl Pharmacol. 2006;216(1):11–9. doi: 10.1016/j.taap.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 110.Lin RW, Chen CH, Wang YH, Ho ML, Hung SH, Chen IS, et al. (−)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem Biophys Res Commun. 2009 Jan 14; doi: 10.1016/j.bbrc.2009.01.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 111.Weisburger JH. Tea and health: the underlying mechanisms. Proc Soc Exp Biol Med. 1999;220(4):271–5. doi: 10.1046/j.1525-1373.1999.d01-46.x. Review. [DOI] [PubMed] [Google Scholar]
- 112.Shen CL, Yeh JK, Stoecker BJ, Chyu MC, Wang JS. Green tea polyphenols mitigate deterioration of bone microarchitecture in middle-aged female rats. Bone. 2009;44(4):684–90. doi: 10.1016/j.bone.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 113.Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol. 2005;173(1):271–5. doi: 10.1097/01.ju.0000141311.51003.87. [DOI] [PubMed] [Google Scholar]
- 114.Romas E, Gillespie MT, Martin TG. Involvement of receptor activator of NF-κB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–6. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 115.Miyaura C, Inada M, Matsumoto C, Ohshiba T, Uozumi N, Shimizu T, et al. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med. 2003;197:1303–10. doi: 10.1084/jem.20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tatakis DN. Interleukin-1 and bone metabolism: a review. J Peridontol. 1993;64:416–31. [PubMed] [Google Scholar]
- 117.Johnson RA, Boyce BF, Mundy GR, Roodman GD. Tumoros producting human tumor necrosis factor induced hypercalcemia and osteoclastic bone resorptio nin nude mice. Endocrinology. 1989;124:1424–7. doi: 10.1210/endo-124-3-1424. [DOI] [PubMed] [Google Scholar]
- 118.Wallach S, Avioli LV, Feinblatt JD, Carstens JH., Jr Cytokines and bone metabolism. Calcif Tissue Int. 1993;53:293–6. doi: 10.1007/BF01351830. [DOI] [PubMed] [Google Scholar]
- 119.Perez-Edo L, ez-Perez A, Marinoso L, Valles A, Serrano S, Carbonell J. Bone metabolism and histomorphometric changes in rheumatoird arthritis. Scand J Rheumatol. 2002;31:285–90. doi: 10.1080/030097402760375188. [DOI] [PubMed] [Google Scholar]
- 120.Hong J, Smith TJ, Ho CT, August DA, Yang CS. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem Pharmacol. 2001;62(9):1175–83. doi: 10.1016/s0006-2952(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 121.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7(2):135–44. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- 122.Blair HC, Athanasou NA. Recent advances in osteoclast biology and pathological bone resorption. Histol Histopathol. 2004;19(1):189–99. doi: 10.14670/HH-19.189. [DOI] [PubMed] [Google Scholar]
- 123.Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49–60. doi: 10.1016/j.cytogfr.2003.10.005. Review. [DOI] [PubMed] [Google Scholar]
- 124.Robinson LJ, Borysenko CW, Blair HC. Tumor necrosis factor family receptors regulating bone turnover: new observations in osteoblastic and osteoclastic cell lines. Ann N Y Acad Sci. 2007;1116:432–43. doi: 10.1196/annals.1402.025. [DOI] [PubMed] [Google Scholar]
- 125.Moreland LW, Curtis JR. Systemic Nonarticular Manifestations of Rheumatoid Arthritis: Focus on Inflammatory Mechanisms. Semin Arthritis Rheum. 2008 Nov 18; doi: 10.1016/j.semarthrit.2008.08.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 126.Centrella M, McCarthy TL, Canalis E. Tumor necrosis factor-alpha inhibits collagen synthesis and alkaline phosphatase activity independently of its effect on deoxyribonucleic acid synthesis in osteoblast-enriched bone cell cultures. Endocrinology. 1988;123(3):1442–8. doi: 10.1210/endo-123-3-1442. [DOI] [PubMed] [Google Scholar]
- 127.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 128.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 129.Schroeder TM, Jensen ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today. 2005;75(3):213–25. doi: 10.1002/bdrc.20043. Review. [DOI] [PubMed] [Google Scholar]
- 130.Rawadi G. Wnt signaling and potential applications in bone diseases. Curr Drug Targets. 2008;9(7):581–90. doi: 10.2174/138945008784911778. Review. [DOI] [PubMed] [Google Scholar]
- 131.Johnson ML. The high bone mass family--the role of Wnt/Lrp5 signaling in the regulation of bone mass. J Musculoskelet Neuronal Interact. 2004;4(2):135–8. Review. [PubMed] [Google Scholar]
- 132.Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277(44):41762–9. doi: 10.1074/jbc.M207850200.Erratum in: J Biol Chem 2005;280(35):31340.
- 133.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19(1):71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115(Pt 24):4809–18. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 135.Shakoori AR, Oberdorf AM, Owen TA, Weber LA, Hickey E, Stein JL, et al. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem. 1992;48(3):277–87. doi: 10.1002/jcb.240480308. [DOI] [PubMed] [Google Scholar]
- 136.Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26(6):743–74. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 137.Hayashi K, Takai S, Matsushima-Nishiwaki R, Hanai Y, Kato K, Tokuda H, et al. (−)-Epigallocatechin gallate reduces transforming growth factor beta-stimulated HSP27 induction through the suppression of stress-activated protein kinase/c-Jun N-terminal kinase in osteoblasts. Life Sci. 2008;82(19−20):1012–7. doi: 10.1016/j.lfs.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 138.Yamaguchi DT, Green J, Merritt BS, Kleeman CR, Muallem S. (1989) Modulation of osteoblast function by prostaglandins. Am J Physiol. 1989;257(5 Pt 2):F755–61. doi: 10.1152/ajprenal.1989.257.5.F755. [DOI] [PubMed] [Google Scholar]
- 139.Koshihara Y, Kawamura M. Prostaglandin D2 stimulates calcification of human osteoblastic cells. Biochem Biophys Res Commun. 1989;159(3):1206–12. doi: 10.1016/0006-291x(89)92238-9. [DOI] [PubMed] [Google Scholar]
- 140.Yamauchi J, Takai S, Matsushima-Nishiwaki R, Hanai Y, Doi T, Kato H, et al. (−)-epigallocatechin gallate inhibits prostaglandin D2-stimulated HSP27 induction via suppression of the p44/p42 MAP kinase pathway in osteoblasts. Prostaglandins Leukot Essent Fatty Acids. 2007;77(3−4):173–9. doi: 10.1016/j.plefa.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 141.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 142.Hakeda Y, Shiokawa M, Mano H, Kameda T, Raisz LG, Kumegawa M. Prostaglandin F2alpha stimulates tyrosine phosphorylation and mitogen-activated protein kinase in osteoblastic MC3T3-E1 cells via protein kinase C activation. Endocrinology. 1997;138(5):1821–8. doi: 10.1210/endo.138.5.5107. [DOI] [PubMed] [Google Scholar]
- 143.Tokuda H, Harada A, Hirade K, Matsuno H, Ito H, Kato K, et al. Incadronate amplifies prostaglandin F2α-induced vascular endothelial growth factor synthesis in osteoblasts. Enhancement of MAPK activity J Biol Chem. 2003;278:18930–7. doi: 10.1074/jbc.M209159200. [DOI] [PubMed] [Google Scholar]
- 144.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Delaissé JM, Eeckhout Y, Sear C, Galloway A, McCullagh K, Vaes G. A new synthetic inhibitor of mammalian tissue collagenase inhibits bone resorption in culture. Biochem Biophys Res Commun. 1985;133(2):483–90. doi: 10.1016/0006-291x(85)90932-5. [DOI] [PubMed] [Google Scholar]
- 146.Delaissé JM, Eeckhout Y, Vaes G. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem Biophys Res Commun. 1984;125(2):441–7. doi: 10.1016/0006-291x(84)90560-6. [DOI] [PubMed] [Google Scholar]
- 147.Grütter MG. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000;10(6):649–55. doi: 10.1016/s0959-440x(00)00146-9. Review. [DOI] [PubMed] [Google Scholar]
- 148.Okahashi N, Koide M, Jimi E, Suda T, Nishihara T. Caspases (interleukin-1beta-converting enzyme family proteases) are involved in the regulation of the survival of osteoclasts. Bone. 1998;23(1):33–41. doi: 10.1016/s8756-3282(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 149.Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115(10):2665–72. doi: 10.1172/JCI26252. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, Moskowitz RW, et al. Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem Biophys Res Commun. 2000;270(3):793–7. doi: 10.1006/bbrc.2000.2536. [DOI] [PubMed] [Google Scholar]
- 151.Yun JH, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH. (−)-Epigallocatechin gallate induces apoptosis, via caspase activation, in osteoclasts differentiated from RAW 264.7 cells. J Periodontal Res. 2007;42(3):212–8. doi: 10.1111/j.1600-0765.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- 152.Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22(21):3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 153.Haefner B. NF-kappa B: arresting a major culprit in cancer. Drug Discov Today. 2002;7(12):653–63. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 154.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004;23(14):2507–22. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 155.Heath JK, Atkinson SJ, Meikle MC, Reynolds JJ. Mouse osteoblasts synthesize collagenase in response to bone resorbing agents. Biochim Biophys Acta. 1984;802:151–4. doi: 10.1016/0304-4165(84)90046-1. [DOI] [PubMed] [Google Scholar]
- 156.Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, et al. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998;139(3):1338–45. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 157.Blair HC, Robinson LJ, Zaidi M. Osteoclast signalling pathways. Biochem Biophys Res Commun. 2005;328(3):728–38. doi: 10.1016/j.bbrc.2004.11.077. Review. [DOI] [PubMed] [Google Scholar]
- 158.Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Hosoi T, Harada A, et al. (−)-Epigallocatechin gallate suppresses endothelin-1-induced interleukin-6 synthesis in osteoblasts: inhibition of p44/p42 MAP kinase activation. FEBS Lett. 2007;581(7):1311–6. doi: 10.1016/j.febslet.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 159.Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Yamauchi J, Harada A, et al. (−)-Epigallocatechin gallate inhibits basic fibroblast growth factor-stimulated interleukin-6 synthesis in osteoblasts. Horm Metab Res. 2008;40(10):674–8. doi: 10.1055/s-2008-1073164. [DOI] [PubMed] [Google Scholar]
- 160.Takai S, Matsushima-Nishiwaki R, Adachi S, Natsume H, Minamitani C, Mizutani J, et al. (−)-Epigallocatechin gallate reduces platelet-derived growth factor-BB-stimulated interleukin-6 synthesis in osteoblasts: suppression of SAPK/JNK. Mediators Inflamm. 2008;2008:291808. doi: 10.1155/2008/291808. Epub 2009 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ishida I, Kohda C, Yanagawa Y, Miyaoka H, Shimamura T. Epigallocatechin gallate suppresses expression of receptor activator of NF-kappaB ligand (RANKL) in Staphylococcus aureus infection in osteoblast-like NRG cells. J Med Microbiol. 2007;56(Pt 8):1042–6. doi: 10.1099/jmm.0.47029-0. [DOI] [PubMed] [Google Scholar]
- 162.Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2007;143(1):31–48. doi: 10.1159/000098223. Review. [DOI] [PubMed] [Google Scholar]
- 163.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–57. doi: 10.1210/edrv.20.3.0367. Review. [DOI] [PubMed] [Google Scholar]
- 164.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14(11):628–38. doi: 10.1016/j.tcb.2004.09.016. Review. [DOI] [PubMed] [Google Scholar]
- 165.Takayanagi H, Kim S, Taniguchi T. Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res. 2002;4(Suppl 3):S227–32. doi: 10.1186/ar581. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sato K, Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol. 2006;18(4):419–26. doi: 10.1097/01.bor.0000231912.24740.a5. Review. [DOI] [PubMed] [Google Scholar]
- 167.Fujita T, Matsui T, Nakao Y, Watanabe S. T lymphocyte subsets in osteoporosis. Effect of 1-alpha hydroxyvitamin D3. Miner Electrolyte Metab. 1984;10(6):375–8. [PubMed] [Google Scholar]
- 168.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111(8):1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu H, Zhu B, Shimoishi Y, Murata Y, Nakamura Y. (−)-Epigallocatechin-3-gallate induces up-regulation of Th1 and Th2 cytokine genes in Jurkat T cells. Arch Biochem Biophys. 2008 Dec 27; doi: 10.1016/j.abb.2008.12.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 170.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. In vitro therapeutic effect of epigallocatechin gallate on nicotine-induced impairment of resistance to Legionella pneumophila infection of established MH-S alveolar macrophages. J Infect Dis. 2002;185(2):229–36. doi: 10.1086/338449. [DOI] [PubMed] [Google Scholar]
- 171.Granucci F, Zanoni I, Feau S, Ricciardi-Castagnoli P. Dendritic cell regulation of immune responses: a new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J. 2003;22(11):2546–51. doi: 10.1093/emboj/cdg261. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahn SC, Kim GY, Kim JH, Baik SW, Han MK, Lee HJ, et al. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and NF-kappaB. Biochem Biophys Res Commun. 2004;313(1):148–55. doi: 10.1016/j.bbrc.2003.11.108. [DOI] [PubMed] [Google Scholar]
- 173.Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128(12):2334–40. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- 174.Gulati P, Singh V, Gupta MK, Vaidya V, Dass S, Prakash S. Studies on the leaching of fluoride in tea infusions. Sci Total Environ. 1993;138(1−3):213–21. doi: 10.1016/0048-9697(93)90416-4. [DOI] [PubMed] [Google Scholar]
- 175.Vestergaard P, Jorgensen NR, Schwarz P, Mosekilde L. Effects of treatment with fluoride on bone mineral density and fracture risk--a meta-analysis. Osteoporos Int. 2008;19(3):257–68. doi: 10.1007/s00198-007-0437-6. [DOI] [PubMed] [Google Scholar]
- 176.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342(8878):1007–11. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 177.Mazur WM, Wähälä K, Rasku S, Salakka A, Hase T, Adlercreutz H. Lignan and isoflavonoid concentrations in tea and coffee. Br J Nutr. 1998;79(1):37–45. doi: 10.1079/bjn19980007. [DOI] [PubMed] [Google Scholar]
- 178.Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol. 1993;44(1):37–43. [PubMed] [Google Scholar]
- 179.de Aloysio D, Gambacciani M, Altieri P, Ciaponi M, Ventura V, Mura M, et al. Bone density changes in postmenopausal women with the administration of ipriflavone alone or in association with low-dose ERT. Gynecol Endocrinol. 1997;11(4):289–93. doi: 10.3109/09513599709152548. [DOI] [PubMed] [Google Scholar]
- 180.Whelan AM, Jurgens TM, Bowles SK. Natural health products in the prevention and treatment of osteoporosis: systematic review of randomized controlled trials. Ann Pharmacother. 2006;40(5):836–49. doi: 10.1345/aph.1G226. [DOI] [PubMed] [Google Scholar]
- 181.Adlercreutz H, Honjo H, Higashi A, Fotsis T, Hämäläinen E, Hasegawa T, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54(6):1093–100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 182.Barrett-Connor E, Chang JC, Edelstein SL. Coffee-associated osteoporosis offset by daily milk consumption. The Rancho Bernardo Study. JAMA. 1994;271(4):280–3. doi: 10.1001/jama.1994.03510280042030. [DOI] [PubMed] [Google Scholar]
- 183.Sakamoto W, Nishihira J, Fujie K, Iizuka T, Handa H, Ozaki M, et al. Effect of coffee consumption on bone metabolism. Bone. 2001;28(3):332–6. doi: 10.1016/s8756-3282(00)00444-0. [DOI] [PubMed] [Google Scholar]
- 184.Lloyd T, Johnson-Rollings N, Eggli DF, Kieselhorst K, Mauger EA, Cusatis DC. Bone status among postmenopausal women with different habitual caffeine intakes: a longitudinal investigation. J Am Coll Nutr. 2000;19(2):256–61. doi: 10.1080/07315724.2000.10718924. [DOI] [PubMed] [Google Scholar]
- 185.Debry G, Nehlig A. [Coffee, source of performance or dependence?] Rev Infirm. 1994;(1):55–62. [PubMed] [Google Scholar]