Abstract
The neurohormonal control of body weight involves a complex interplay between long-term adiposity signals (e.g., leptin), and short-term satiation signals (e.g., amylin). In diet-induced obese (DIO) rodents, amylin/leptin combination treatment led to marked, synergistic, fat-specific weight loss. To evaluate the weight-lowering effect of combined amylin/leptin agonism (with pramlintide/metreleptin) in human obesity, a 24-week, randomized, double-blind, active-drug-controlled, proof-of-concept study was conducted in obese or overweight subjects (N = 177; 63% female; 39 ± 8 years; BMI 32.0 ± 2.1 kg/m2; 93.3 13.2 kg; mean ± s.d.). After a 4-week lead-in period with pramlintide (180 μg b.i.d. for 2 weeks, 360 μg b.i.d. thereafter) and diet (40% calorie deficit), subjects achieving 2–8% weight loss were randomized 1:2:2 to 20 weeks of treatment with metreleptin (5 mg b.i.d.), pramlintide (360 μg b.i.d.), or pramlintide/metreleptin (360 μg/5 mg b.i.d.). Combination treatment with pramlintide/metreleptin led to significantly greater weight loss from enrollment to week 20 (−12.7 ± 0.9%; least squares mean ± s.e.) than treatment with pramlintide (−8.4 ± 0.9%; P < 0.001) or metreleptin (−8.2 ± 1.3%; P < 0.01) alone (evaluable, N = 93). The greater reduction in body weight was significant as early as week 4, and weight loss continued throughout the study, without evidence of a plateau. The most common adverse events with pramlintide/metreleptin were injection site events and nausea, which were mostly mild to moderate and decreased over time. These results support further development of pramlintide/metreleptin as a novel, integrated neurohormonal approach to obesity pharmacotherapy.
INTRODUCTION
Over the past decade, it has become increasingly evident that the control of energy homeostasis and body weight involves complex interactions between the central nervous system and peripheral neurohormonal signals from adipose tissue, pancreatic islets, and the gastrointestinal system (1-4). These interactions include the integration of long-term adiposity signals, such as leptin and insulin, and short-term satiation signals, such as amylin, peptide YY, incretins, and cholecystokinin.
Leptin, a neurohormone that is predominantly secreted by adipocytes and binds to receptors in the hypothalamus, plays a key role in regulating long-term energy homeostasis. Leptin-deficient humans exhibit severe hyperphagia and profound obesity, and leptin replacement with recombinant methyl-human leptin (metreleptin) leads to an almost complete reversal of this phenotype (5-7). In nonleptin-deficient obesity, however, metreleptin monotherapy, even at high pharmacological doses, has failed to produce meaningful weight loss (8).
Amylin, a neuroendocrine peptide hormone that is co-secreted with insulin from pancreatic β-cells and binds to receptors in the hindbrain, contributes to short-term energy regulation (1,9,10). In contrast to leptin, the amylin analog pramlintide has been shown in several clinical trials to increase satiation, reduce food intake, and elicit durable weight loss in obese individuals (11-13).
To harness the therapeutic potential of individual neurohormones, it is important to understand how they interact with one another, both during the dynamic phase of weight loss and in the weight-reduced state (3,4). For instance, circulating leptin levels fall rapidly in response to diet-induced weight loss, triggering a host of counter-regulatory metabolic, autonomic, and hormonal responses aimed at defending the initial body weight (14,15). Restoration of leptin concentrations to preweight-loss concentrations, via administration of metreleptin, has been shown to mitigate weight-loss counter-regulation (16-18). A diet-induced fall in leptin may also dampen the anorexigenic effect of hindbrain satiation signals (19,20). Conversely, there is growing recognition that hindbrain signals may exert important effects on hypothalamic leptin signaling. For instance, amylin treatment significantly enhanced leptin-mediated p-STAT3 signaling in the ventromedial hypothalamus of leptin-resistant diet-induced obese (DIO) rats (21).
In a comprehensive series of preclinical studies, we showed that combination treatment with amylin and leptin led to marked, synergistic reductions in food intake (up to 45%) and body weight (up to 15%) in DIO rats (21,22). The weight loss observed with combined amylin/leptin receptor agonism was fat-specific and not accompanied by the reductions in energy expenditure and fat oxidation usually observed with caloric restriction or pair-feeding (22). In a translational clinical research study, we examined the weight-lowering effect of combined amylin and leptin agonism (with pramlintide/metreleptin) in human obesity. Limited data on the primary efficacy from this study were reported in conjunction with the preclinical studies (22). Here, we report the comprehensive analysis of this clinical trial, including categorical weight loss, excess weight loss, rate of weight loss, lipoprotein and glycemic profiles, and safety and tolerability findings.
METHODS AND PROCEDURES
Subjects
The enrolled study population included 177 obese (BMI ≥30 kg/m2 and ≤35 kg/m2; 81% of enrolled population) or overweight (BMI ≥ 27 kg/m2 and <30 kg/m2; 19% of enrolled population) men (18–55 years) and women (18–45 years). If overweight, subjects were required to have abdominal obesity (waist circumference >102 cm (male) or >88 cm (female)). Additional inclusion criteria included not having clinically significant abnormalities during physical examination, electrocardiogram, or in clinical laboratory test values. Female subjects could not be pregnant or lactating and were required to practice appropriate contraception throughout the study. Exclusion criteria included type 2 diabetes mellitus, a fasting serum triglyceride concentration ≥400 mg/dl, a body weight change of ≥3 kg during the 2 months before screening, smoking, current or recent (within 2 months) use of prescription or nonprescription weight-loss agents, and current enrollment or plans to enroll in a diet, weight loss, or exercise program.
Trial design and treatments
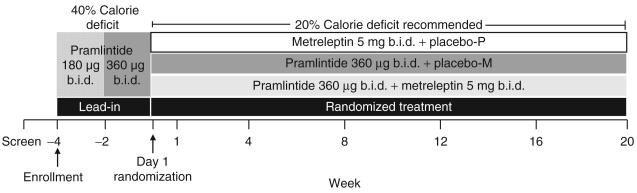
This was a 24-week, randomized, double-blind, active-drug-controlled, multicenter study conducted in the United States (12 sites) between October 2006 and September 2007. The study consisted of a 4-week lead-in period and a 20-week randomized treatment period (Figure 1). During the 4-week single-blind lead-in period, enrolled subjects were instructed to follow a 40% caloric deficit diet and self-administer pramlintide (180 μg b.i.d. for the first 2 weeks, 360 μg b.i.d. thereafter). The caloric deficit was calculated based on each subject's daily caloric requirement according to the Mifflin equation (23) with a lowest limit of 1,000kcal/day. Dietary guidelines including menu plans were provided. Subjects who lost 2–8% of their body weight at the end of the lead-in period were randomized (day 1) 1:2:2 to 20 weeks of double-blind treatment with metreleptin (5mg b.i.d.) + placebo for pramlintide (designated as the metreleptin arm), pramlintide (360 μg b.i.d.) + placebo for metreleptin (designated as the pramlintide arm), or pramlintide (360 μg b.i.d.) + metreleptin (5mg b.i.d.) (designated as the pramlintide/metreleptin arm). At the time the study was conducted, availability of appropriate manufactured placebo vials was limited, precluding the inclusion of a stand-alone placebo arm. Randomization was stratified by gender, enrollment BMI (≥27 to ≤30kg/m2 vs. >30 to ≤35kg/m2), and the percentage of weight loss at the end of the lead-in period (≥2 to <5% vs. ≥5 to ≤8%). At the time of randomization (day 1) and at week 1, subjects were counseled to maintain a 20% caloric deficit (with a lowest limit of 1,200 kcal/day) during the 20-week treatment period without any structured reinforcement during subsequent visits. Menu plans to maintain the energy deficit were provided if requested. Study medications were self-administered subcutaneously as separate injections 15 min before the morning and evening meals. After screening, subjects visited the study site twice during the lead-in period (weeks −4 and −2), at randomization (day 1), and at weeks 1, 4, 8, 12, 16, and 20.
Figure 1.
Study design. After screening, subjects were enrolled at week −4 and underwent a 4-week lead-in period. Subjects achieving a 2–8% body weight loss during the lead-in were randomized 1:2:2 on day 1 to treatment with metreleptin, pramlintide, or pramlintide/metreleptin for 20 weeks.
The study protocol was approved by the institutional review boards of the 12 study sites. All subjects provided written informed consent before study initiation. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki (1964), including all amendments through the South Africa revision (1996).
Study endpoints
The primary study endpoints were the absolute change in body weight and all treatment-emergent adverse events. The primary comparison was between the pramlintide group and the pramlintide/metreleptin group. The smaller metreleptin group served as a reference arm. Other efficacy endpoints included the percent change in body weight, the percent change in excess body weight, the categorical percent body weight loss (e.g., ≤5%, ≤10%), and the overall (day 1 to week 20), initial (day 1 to week 12), and late (weeks 12–20) rates of absolute change in body weight. Pharmacodynamic endpoints included changes in fasting metabolic parameters (glucose, insulin, lipids, leptin), and Homeostasis Model Assessment of Insulin Resistance. Patient reported outcomes included subscale scores of the Hospital Anxiety and Depression Scale questionnaire (24).
Safety assessments included incidence and intensity of treatment-emergent adverse events, evaluation of concomitant medications, vital signs, physical examination findings, electrocardiograms, and clinical laboratory measures.
Statistical analyses
There were four analysis populations. The enrolled population included all subjects who received at least one dose of pramlintide during the lead-in period or who had nonmissing vital signs data at week −4. The randomized population included all enrolled subjects who lost 2–8% of their body weight between enrollment and day 1 and were randomly assigned to treatment medication. The intent-to-treat (ITT) population included all randomized subjects who received at least one injection of any component of the randomized study medication. The evaluable population included all ITT subjects who completed week 16 study procedures and adequately complied with the protocol. Analyses of safety and tolerability were conducted in the enrolled and ITT populations. Changes in body weight were analyzed in both the evaluable and ITT populations. Missing efficacy data for the ITT population were also imputed using the last observation carried forward method. All other endpoints were analyzed in the evaluable population.
A total of 87 evaluable subjects (17 metreleptin, 35 pramlintide, 35 pramlintide/metreleptin) were considered sufficient to detect a difference of 2.5 kg (with common s.d. of 3.5 kg) in absolute body weight change between the pramlintide and pramlintide/metreleptin arms, with 83% power using a two-sided t-test at the 0.05 significance level.
Absolute changes in body weight from enrollment were analyzed using analysis of covariance including a factor for treatment, stratification factors used in randomization, and enrollment weight as a covariate. Percent changes from enrollment in body weight were analyzed using analysis of variance including a factor for treatment and stratification factors used in randomization. Excess body weight was calculated as the body weight (kg) at a given visit minus 24.9 kg/m2 × height (m2), or 0, whichever was greater. Demographic data are presented as mean ± s.d. (or percentage). Pharmacokinetic data and lipids, blood glucose and insulin data are presented as mean ± s.e. All other parameters are presented as least squares mean ± s.e. unless otherwise indicated.
RESULTS
Enrollment demographics and disposition
The enrollment demographics of the three treatment arms were well matched (Table 1). Of the 177 enrolled subjects, 139 were eligible for randomization at the end of the 4-week lead-in period. Nine percent of enrolled subjects were withdrawn due to insufficient (<2%) lead-in weight loss, and 0% of subjects experienced weight loss >8% during the lead-in period. Within the randomized population (N = 139), withdrawal rates (30–34%) and reasons for withdrawal were generally similar among the three treatment arms (Table 1).
Table 1.
Enrollment demographics and subject disposition
| Enrolled (N = 177) | Metreleptin (ITT n = 27) |
Pramlintide (ITT n = 56) |
Pramlintide/Metreleptin (ITT n = 56) |
|
|---|---|---|---|---|
| Demographics | ||||
| Sex (% female) | 63 | 63 | 63 | 63 |
| Race (W/B/H/O, %) | 79/14/4/3 | 81/19/0/0 | 82/13/4/2 | 88/5/4/4 |
| Age (years) | 38.6 ± 8.4 | 40.5 ± 8.1 | 38.3 ± 9.1 | 38.5 ± 8.4 |
| Weight (kg) | 93.3 ± 13.2 | 93.8 ± 14.3 | 91.7 ± 11.1 | 93.9 ± 12.8 |
| Excess body weight (kg) | 20.7 ± 6.9 | 21.0 ± 7.2 | 19.2 ± 6.3 | 20.8 ± 6.9 |
| BMI (kg/m2) | 32.0 ± 2.1 | 32.0 ± 2.1 | 31.5 ± 2.0 | 32.0 ± 2.1 |
| Disposition | ||||
| Not randomized, reason (n (%)) | 38 (21) | |||
| Insufficient lead-in weight loss | 16 (9) | |||
| Adverse event | 5 (3) | |||
| Withdrawal of consent | 11 (6) | |||
| Other | 6 (4) | |||
| Randomized (N) | 139 | 27 | 56 | 56 |
| Withdrew during randomized treatment, reasona (n (%)) |
8 (30) | 19 (34) | 18 (32) | |
| Adverse event | 3 (11) | 3 (5) | 5 (9) | |
| Withdrawal of consent | 2 (7) | 6 (11) | 8 (14) | |
| Protocol violation | 0 (0) | 2 (4) | 1 (2) | |
| Loss to follow-up | 3 (11) | 8 (14) | 4 (7) | |
| Evaluableb (N) | 93 | 19 | 38 | 36 |
Data are presented as mean ± s.d. Numbers may not add up to 100% because of rounding.
ITT, intent-to-treat; W/B/H/O, white/black/Hispanic/other.
Withdrawal data through week 20.
Evaluable population defned as week 16 completers meeting drug compliance.
Body weight
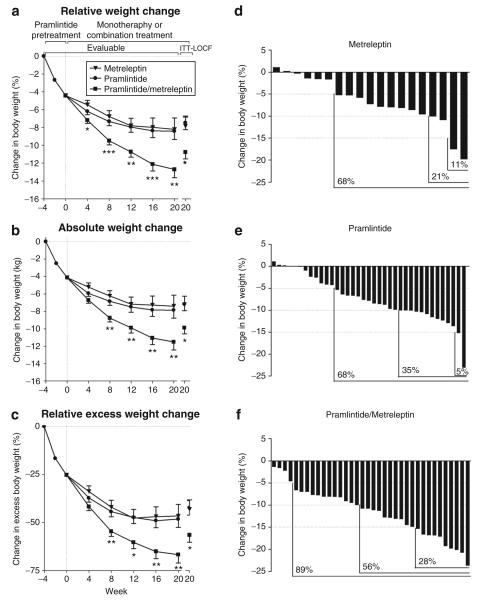
During the 4-week lead-in, evaluable subjects lost 4.4 ± 0.2% (4.1 ± 0.2 kg) of their body weight. After randomization, weight loss continued in all treatment arms and then plateaued at approximately week 12 in the metreleptin and pramlintide monotherapy arms (Figure 2a,b). In contrast, subjects treated with pramlintide/metreleptin experienced continuous weight loss throughout the study, and by week 20 these subjects lost significantly (P < 0.01) more body weight from enrollment (−12.7 ± 0.9%, −11.5 ± 0.9 kg) than subjects treated with metreleptin (−8.2 ± 1.3%, −7.4 ± 1.3 kg) or pramlintide (−8.4 ± 0.9%, −7.9 ± 0.9 kg) alone (Figure 2a,b). The difference in percent body weight change between the pramlintide/metreleptin and pramlintide arms was significant as early as week 4 and increased in magnitude throughout the study. Analyses of the absolute rate of weight change during the later phase (weeks 12–20) of the study demonstrated the lack of a weight-loss plateau in the pramlintide/metreleptin arm (Table 2). Analysis of the ITT–last observation carried forward population confirmed the statistical robustness of the results, with pramlintide/metreleptin-treated subjects experiencing significantly (P < 0.05) more weight loss at week 20 than subjects treated with metreleptin or pramlintide alone (Figure 2).
Figure 2.
Change in body weight. (a) Percent change and (b) absolute change in body weight from enrollment during the lead-in and randomized treatment phases (evaluable population: metreleptin n = 19, pramlintide n = 38, pramlintide/metreleptin n = 36, LS mean ± s.e.). Percent and absolute changes from enrollment to week 20 in body weight for the ITT-LOCF population are also shown (metreleptin n = 27, pramlintide n = 55, pramlintide/metreleptin n = 55). (c) Percent change in excess body weight for the evaluable and ITT-LOCF populations. (a–c) *P < 0.05, **P < 0.01, ***P < 0.001 for pramlintide/metreleptin vs. both monotherapies. (d–f) Percentage of patients (evaluable) experiencing ≥5, ≥10, and ≥15% weight loss from enrollment to week 20. ITT, intent-to-treat; LOCF, last observation carried forward; LS, least squares.
Table 2.
Rate of weight change
| Metreleptin (N = 19) |
Pramlintide (N = 38) |
Pramlintide/ Metreleptin (N = 36) |
|
|---|---|---|---|
| Overall rate of weight change (kg/week) |
−0.16 ± 0.06** | −0.17 ± 0.04*** | −0.36 ± 0.04***,## |
| Day 1 to week 12 | −0.23 ± 0.06*** | −0.25 ± 0.04*** | −0.46 ± 0.04***,### |
| Week 12 to week 20 | −0.02 ± 0.08 | −0.05 ± 0.06 | −0.20 ± 0.06***,# |
Data are for the evaluable population and are presented as LS mean ± s.e.
P < 0.01,
P < 0.001 compared to a rate of zero;
P < 0.05,
P < 0.01,
P < 0.001 compared to monotherapies.
In relative terms, evaluable subjects in the pramlintide/metreleptin arm lost approximately two-thirds of their excess body weight by week 20 (−67 p 4%), while subjects treated with metreleptin or pramlintide alone lost less than half of their excess weight (−47 p 6% and −48 p 4%, respectively; ITT-last observation carried forward: pramlintide/metreleptin, −57 p 4%; metreleptin, −43 p 5%; pramlintide, −42 p 4%; Figure 2c). In categorical terms, a greater proportion of subjects treated with pramlintide/metreleptin lost ≥5, ≥10, or ≥15% body weight compared to subjects treated with either monotherapy (Figure 2d–f).
Pharmacodynamics and pharmacokinetics
Although the mean concentrations of glucose and lipids were within the normal range at enrollment, the greater weight loss in the pramlintide/metreleptin arm was accompanied by trends toward greater reductions from enrollment to week 16 in fasting triglycerides (−8%), total cholesterol (−9%), low-density lipoprotein cholesterol (−8%), glycemia (−4 mg/dl), insulinemia (−22%), and insulin resistance (Homeostasis Model Assessment of Insulin Resistance, −25%) (Table 3).
Table 3.
Fasting lipoprotein and glycemic control parameters
| Metreleptin (N = 19) |
Pramlintide (N = 38) |
Pramlintide/ Metreleptin (N = 36) |
|
|---|---|---|---|
| Triglycerides at enrollment (mg/dl) |
139 ± 19 | 130 ± 10 | 128 ± 10 |
| Change in triglycerides | 5.7 ± 13.5 | −15.6 ± 6.9 | −19.2 ± 9.6 |
| Total cholesterol at enrollment (mg/dl) |
188 ± 7 | 194 ± 6 | 193 ± 7 |
| Change in total cholesterol | 2.7 ± 5.8 | −10.8 ± 3.3 | −20.6 ± 4.3 |
| HDL at enrollment (mg/dl) |
50 ± 3 | 50 ± 2 | 48 ± 2 |
| Change in HDL | 1.7 ± 1.5 | 1.6 ± 1.2 | −0.6 ± 1.3 |
| LDL at enrollment (mg/dl) |
116 ± 7 | 121 ± 5 | 121 ± 6 |
| Change in LDL | 1.1 ± 5.1 | −5.5 ± 2.9 | −13.3 ± 3.0 |
| Blood glucose at enrollment (mg/dl) |
88 ± 2 | 90 ± 2 | 90 ± 1 |
| Change in blood glucose | 0.7 ± 1.7 | −2.5 ± 1.4 | −3.9 ± 1.3 |
| Serum insulin at enrollment (μIU/ml) |
10.2 ± 2.0 | 10.4 ± 1.1 | 10.7 ± 0.9 |
| Change in insulin | −0.6 ± 1.1 | −0.7 ± 0.8 | −2.8 ± 0.6 |
| HOMA-IR at enrollment |
2.4 ± 0.6 | 2.4 ± 0.3 | 2.2 ± 0.2 |
| Change in HOMA-IR | −0.10 ± 0.29 | −0.25 ± 0.26 | −0.63 ± 0.12 |
Data are for the evaluable population, are presented as mean ± s.e., and are for changes from enrollment to week 16.
HDL, high-density lipoprotein; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; LDL, low-density lipoprotein.
Weight loss during the 4-week lead-in was accompanied by a mean 29% reduction in fasting plasma leptin concentrations (from 25 p 14 to 18 p 12 ng/ml; mean p s.d., P < 0.001). Treatment with metreleptin alone and pramlintide/metreleptin increased predose leptin concentrations by ~13- to 16-fold over enrollment concentrations at week 16. Combination treatment with pramlintide/metreleptin did not appear to alter the pharmacokinetics of either drug.
Safety and tolerability
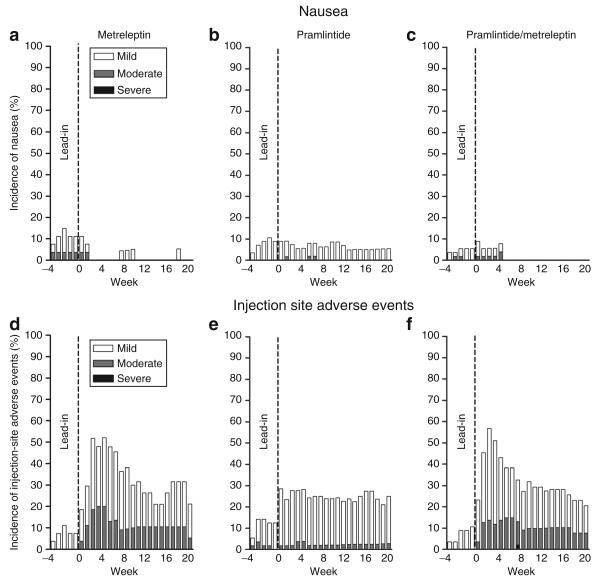
The most common side effects with pramlintide/metreleptin treatment were injection site adverse events and nausea, which were generally mild to moderate and decreased over time (Figure 3). Nausea led to four withdrawals during the lead-in period and one withdrawal in the pramlintide/metreleptin arm. Injection site events led to one withdrawal in each treatment arm postrandomization. These side effects are consistent with observations from previous studies of pramlintide or metreleptin used as monotherapy (8,13). Other adverse events occurring with a frequency ≥5% in any group are provided in Table 4. There were no reported adverse events of anxiety or depression, and there was no evidence of worsening in Hospital Anxiety and Depression Scale scores in any group.
Figure 3.
(a–c) Incidence and intensity of nausea and (d–f) injection site adverse events over time in the ITT population. ITT, intent-to-treat.
Table 4.
Treatment-emergent adverse events
| Metreleptin (N = 27) |
Pramlintide (N = 56) |
Pramlintide + Metreleptin (N = 56) |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Injection site adverse events |
18 (66.7) | 25 (44.6) | 33 (58.9) |
| Nausea | 7 (25.9) | 8 (14.3) | 7 (12.5) |
| Nasopharyngitis | 2 (7.4) | 7 (12.5) | 3 (5.4) |
| Headache | 2 (7.4) | 4 (7.1) | 3 (5.4) |
| Diarrhea | 0 (0.0) | 3 (5.4) | 2 (3.6) |
| Influenza | 1 (3.7) | 3 (5.4) | 2 (3.6) |
| Constipation | 1 (3.7) | 0 (0.0) | 3 (5.4) |
| Hypersensitivity | 2 (7.4) | 0 (0.0) | 1 (1.8) |
| Streptococcal pharyngitis |
0 (0.0) | 3 (5.4) | 1 (1.8) |
| Vomiting | 2 (7.4) | 1 (1.8) | 1 (1.8) |
| Back pain | 0 (0.0) | 3 (5.4) | 0 (0.0) |
Events occuring at an incidence ≥5% in any group.
Data are for the ITT population and include events occurring during the lead-in and postrandomization periods. n is the number of subjects experiencing a given adverse event. Subjects experiencing multiple episodes of a given adverse event are counted once. Percentages are based on the number of ITT subjects (N) in the treatment group.
ITT, intent-to-treat.
DISCUSSION
Before the discovery of leptin in 1994, it was not uncommon for obesity to be regarded primarily as a psychological/behavioral problem, and the difficulty of obese individuals with losing weight was often attributed to lack of discipline and willpower. Today, it is widely recognized that energy homeostasis is governed by a complex neurohormonal feedback system between the periphery and the central nervous system. This involves the interaction of long-term (tonic) adiposity signals (e.g., leptin, insulin) and short-term (episodic) satiation signals (e.g., amylin, cholecystokinin, peptide YY) with a network of hypothalamic, mesolimbic, and hindbrain circuits (1,3,4,25). To date, the translation of this scientific knowledge into innovative therapies for obesity has remained elusive.
We hypothesized that a combinatorial regimen comprising a long-term adiposity signal and a short-term satiation signal might be a promising avenue to achieve clinically meaningful weight loss. Evidence to support this integrated neurohormonal approach to obesity emerged with the observation that combined amylin/leptin agonism elicits marked, synergistic, fat-specific weight loss in DIO rats (21,22).
This translational research study constitutes the first clinical proof-of-concept for this combinatorial neurohormonal approach, demonstrating that combined amylin/leptin agonism elicited enhanced weight loss in human obesity. Specifically, pramlintide/metreleptin combination treatment in obese and overweight humans led to significantly greater weight loss than that achieved with either treatment alone. Subjects receiving pramlintide/metreleptin lost almost 13% of their initial body weight over 24 weeks, compared with only ~8% in subjects receiving either pramlintide or metreleptin. At study end, weight loss plateaued in subjects treated with monotherapy, but not in subjects treated with the combination. This is particularly noteworthy considering that the 20% caloric deficit diet recommendation was communicated only during the first week postrandomization and was not reinforced during the remaining 19 weeks of treatment. When efficacy was analyzed using the more conservative ITT–last observation carried forward approach, subjects treated with pramlintide/metreleptin still experienced an average weight loss of ~11% from enrollment that was significantly greater than that achieved with pramlintide or metreleptin alone.
In a previous obesity clinical study with life style intervention, subjects completing 6 months of treatment with 360 μg pramlintide b.i.d. experienced an average 7–8% weight loss from baseline, corresponding to a placebo-corrected weight loss of ~5–6% (refs. 12,13). Previous metreleptin monotherapy trials have failed to show significant weight loss with doses up to twofold those used in this study (ref. 8, and data on file, Amylin Pharmaceuticals, Inc.), although there was a suggestion of more meaningful weight loss at very high, poorly tolerated doses of metreleptin (0.3 mg/kg per day). Studies with pegylated, long-acting leptin analogs also failed to produce significant weight loss (26). There are no published reports of clinical studies of leptin use in combination with other weight-loss agents. Although differences in study design preclude direct efficacy comparisons between pramlintide/metreleptin therapy and available oral weight-loss medications, the 13% weight loss from baseline observed with pramlintide/metreleptin therapy appears to compare quite favorably to the results from other obesity pharmacotherapy trials, where weight loss from baseline is typically within the 5–10% range (27).
The subjects in this initial proof-of-concept study were moderately obese, nondiabetic, and in many cases normolipidemic and normotensive at baseline. The effects of pramlintide/metreleptin-mediated weight loss on obesity-related cardiovascular risk factors and comorbid conditions could therefore not be definitively addressed. Nevertheless, the weight loss in pramlintide/metreleptin-treated subjects was accompanied by a clear trend toward improvement of insulin sensitivity and lipoprotein profile. Considering the antisteatotic, antidiabetic, and insulin-sensitizing effect of leptin agonism in patients with severe lipodystrophy (28-30), future studies with pramlintide/metreleptin in overweight/obese subjects with type 2 diabetes, nonalcoholic fatty liver disease, and/or hyperlipidemia are warranted.
Although this study established a robust clinical proof-of-concept for the pramlintide/metreleptin combination, it did not elucidate the mechanism(s) for the enhanced weight loss observed. However, studies in DIO rats point to a set of complementary mechanisms. Amylin/leptin treatment led to a marked, synergistic reduction in food intake, and weight loss was not accompanied by a counter-regulatory decrease in energy expenditure or fat oxidation (22). As monotherapies in obese humans, pramlintide increased satiation (31) and reduced food intake and binge-eating tendencies (11,31), whereas metreleptin almost completely mitigated the fall in energy expenditure and other counter-regulatory responses to diet-induced weight loss (16,17). Further studies are therefore warranted to determine the mechanisms of weight loss and, potentially, weight-loss maintenance with pramlintide/metreleptin combination treatment.
A number of limitations need to be considered when interpreting the results of this study. The lack of a stand-alone placebo arm makes it difficult to determine the effect of the dietary intervention, and the true, overall magnitude of weight loss with pramlintide/metreleptin. Furthermore, the pramlintide lead-in period, and 2–8% weight-loss inclusion criterion, makes it difficult to determine the effect of prior pramlintide treatment and/or weight loss on the efficacy of pramlintide/metreleptin combination therapy during the 20-week treatment period. However, in DIO rats, combined amylin/leptin agonism consistently leads to synergistic weight loss, with or without amylin pretreatment (21,22). Other limitations included the relatively narrow BMI range (27–35 kg/m2) and the use of a single dose of pramlintide and metreleptin. These issues are all being addressed in a placebo-controlled, factorial design phase 2b dose-ranging study with pramlintide/metreleptin.
Pramlintide/metreleptin combination treatment was generally well tolerated. Consistent with the well-characterized side effect profile of pramlintide and metreleptin, nausea and injection site events, respectively, were the most common adverse events (8,13). Mild to moderate nausea occurred in 11% of randomized subjects during the lead-in period, and was not a major reason for withdrawal. Of note, nausea in the pramlintide/metreleptin arm completely subsided after 5 weeks of treatment, indicating that the 12.7% weight loss in that group was not due to poor tolerability. Compared to previous studies (8) with metreleptin at higher doses (≥10 mg b.i.d.), injection site adverse events appeared to be less frequent and less severe with the 5 mg b.i.d. regimen employed in this study. This is consistent with observations in patients with congenital leptin deficiency or severe lipodystrophy, in which chronic metreleptin treatment at lower doses is well-tolerated (28). Importantly, given the experience with many small molecule anorectics, there were no reported adverse events of anxiety, depression, suicidality, or cognitive impairment, nor were there any adverse changes in vital signs and/or clinical chemistry.
Considering the poor long-term success with dietary interventions and the modest efficacy and lingering safety concerns associated with many anorectic small molecule agents, there is a strong need for new approaches that translate scientific advances into innovative therapies offering durable, clinically meaningful weight loss with minimal risk of serious side effects. Although obesity has traditionally been treated with oral agents, injectable regimens that have a favorable safety profile and offer medically compelling efficacy may have considerable potential. Injectable therapies have already become an important part of the therapeutic armamentarium for other chronic diseases, such as type 2 diabetes and rheumatoid arthritis (32,33). From an endocrinological and scientific perspective, a combination neurohormonal approach appears both rational and promising, given that peptide hormones play a key, physiological role in the control of food intake and body weight, and are typically devoid of off-target toxicities. The results of this study provide an initial clinical proof-of-concept for an integrated neurohormonal approach to obesity pharmacotherapy and warrant further development of pramlintide/metreleptin as a weight-loss regimen.
ACKNOWLEDGMENTS
We thank the subjects, investigators, and study site staff, as well as Colleen Burns, Lily Chen and Shereen McIntyre for their assistance with data analysis and data management, Szecho Lin for project management, Cathy Serrano for quality control, and Stephan Miller for editorial assistance.
REFERENCES
- 1.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14(Suppl 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 3.Roth JD, Mack C, Parkes DG, Kesty NC, Weyer C. Integrated neurohormonal approaches to the treatment of obesity: the amylin agonist pramlintide and its interactions with leptin and PYY signaling pathways. In: Bray GA, Bouchard C, editors. Handbook of Obesity Clinical Applications. Informa Healthcare USA, Inc.; New York City, NY: 2008. pp. 341–361. [Google Scholar]
- 4.Chen HC, Roth JD, Schroeder BE, Weyer C. Role of islet-, gut-, and adipocyte-derived hormones in the central control of food intake and body weight: implications for an integrated neurohormonal approach to obesity pharmacotherapy. Curr Diabetes Rev. 2008;4:79–91. doi: 10.2174/157339908784220741. [DOI] [PubMed] [Google Scholar]
- 5.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 6.Farooqi IS, Matarese G, Lord G, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montague CT, Farooqi IS, Whitehead J, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 9.Lutz TA. Pancreatic amylin as a centrally acting satiating hormone. Curr Drug Targets. 2005;6:181–189. doi: 10.2174/1389450053174596. [DOI] [PubMed] [Google Scholar]
- 10.Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465–471. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Smith SR, Blundell JE, Burns C, et al. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am J Physiol Endocrinol Metab. 2007;293:E620–E627. doi: 10.1152/ajpendo.00217.2007. [DOI] [PubMed] [Google Scholar]
- 12.Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977–2983. doi: 10.1210/jc.2006-2003. [DOI] [PubMed] [Google Scholar]
- 13.Smith SR, Aronne LJ, Burns C, et al. Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care. 2008;31:1816–1823. doi: 10.2337/dc08-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 15.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 18.Kissileff HR, Thornton JC, Torres M, et al. Maintenance of reduced body weight in humans is associated with leptin-reversible changes in appetite-related ratings [abstract]; Presented at the Society for Neuroscience Annual Meeting; San Diego, California. 3–7 November 2007. [Google Scholar]
- 19.Morton GJ, Blevins JE, Williams D, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton GJ, Matsen M, Figlewicz D, Schwartz MW. Leptin signaling in the ventral tegmental area enhances the satiety response to cholecystokinin. Diabetes. 2007;56:A403. [Google Scholar]
- 21.Roth JD, Roland BL, Cole R, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trevaskis JL, Coffey T, Cole R, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149:5679–5687. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- 23.Frankenfield DC, Rowe WA, Smith JS, Cooney RN. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103:1152–1159. doi: 10.1016/s0002-8223(03)00982-9. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hukshorn CJ, Saris WH, Westerterp-Plantenga M, et al. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 27.Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–184. doi: 10.1124/pr.59.2.2. [DOI] [PubMed] [Google Scholar]
- 28.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 29.Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92:532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 30.Javor ED, Cochran EK, Musso C, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 31.Chapman I, Parker B, Doran S, et al. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia. 2005;48:838–848. doi: 10.1007/s00125-005-1732-4. [DOI] [PubMed] [Google Scholar]
- 32.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 33.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–712. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]