Abstract
Sepsis remains a prevalent clinical challenge and the underlying pathophysiology is still poorly understood. To investigate the complex molecular mechanisms of sepsis, various animal models have been developed, the most frequently used being the cecal ligation and puncture (CLP) model in rodents. In this model, sepsis originates from a polymicrobial infectious focus within the abdominal cavity, followed by bacterial translocation into the blood compartment, which then triggers a systemic inflammatory response. A requirement of this model is that it is performed with high consistency to obtain reproducible results. Evidence is now emerging that the accompanying inflammatory response varies with the severity grade of sepsis, which is highly dependent on the extent of cecal ligation. In this protocol, we define standardized procedures for inducing sepsis in mice and rats by applying defined severity grades of sepsis through modulation of the position of cecal ligation. The CLP procedure can be performed in as little as 10 min for each animal by an experienced user, with additional time required for subsequent postoperative care and data collection.
INTRODUCTION
Sepsis and sepsis-associated multiorgan failure remain as major challenges for both scientists and clinicians. Despite extensive research in the past, the pathophysiology of sepsis in humans is still poorly understood, and hospitalization and mortality rates of septic patients have significantly increased in the United States between 1993 and 2003 (see ref. 1). As a matter of fact, the sepsis syndrome is responsible for as many deaths as acute myocardial infarction in the United States, and sepsis is ranked as the tenth leading cause of death in the United States2. To study the underlying mechanisms of sepsis and the associated systemic inflammatory response, several experimental animal models have been developed, all of which attempt to mimic pathophysiological changes typically seen in septic patients3.
Cecal ligation and puncture in rodents has become the most widely used model for experimental sepsis and is currently considered as the gold standard in sepsis research3-6. Having been developed more than 30 years ago, the CLP model is considered to be a realistic model for the induction of polymicrobial sepsis in experimental settings to study the underlying mechanisms of sepsis3,4,7. In brief, CLP features ligation below the ileocecal valve after midline laparotomy, followed by needle puncture of the cecum. As the cecum is an endogenous source of bacterial contamination, perforation of the cecum results in bacterial peritonitis, which is followed by translocation of mixed enteric bacteria into the blood compartment. At the onset of sepsis, bacteremia then triggers systemic activation of the inflammatory response, subsequent septic shock, multiorgan dysfunction and, finally, death. When the CLP model is used in rodents, they show disease patterns with typical symptoms of sepsis or septic shock, such as hypothermia, tachycardia and tachypnea.
Despite its clinical relevance and widespread use in sepsis research, one of the major concerns of the CLP model is consistency. In the past, various versions of CLP have been used, some of which differ substantially from its original description in 1979 (see ref. 8). One of the advantages of the CLP model is that it can be adapted to induce sepsis with a range of severity, as described below. It is possible to tailor the severity for investigating acute as well as chronic sepsis9,10. However, this feature is also its major weakness, as it is important that this model be used with high consistency to obtain reliable and reproducible results. On the basis of the characteristics of the model itself, the outcome after CLP is closely associated with several factors during the procedure. It has been suggested that the length of the cecum ligated is a major determinant of mortality and that elevated serum levels of proinflammatory cytokines were linked to increasing length of cecum ligation11. Other factors with an impact on the mortality following CLP are the size of needle used for the puncture and the number of punctures12. In a recent study, different severity grades of CLP-induced sepsis have been used to investigate the functional roles of C5a receptors (C5aR and C5L2) in experimental sepsis13. Most strikingly, it has been demonstrated that the underlying pathophysiology is based on the severity grade of disease. These findings fortify the importance that the procedure of CLP has to be performed with high consistency to be able to correctly and reproducibly evaluate findings obtained from animals subjected to CLP. Unlike needle size and the number of punctures, details of cecal ligation are not routinely described in experimental studies, although such information represents the most important determinants for variability in the CLP model6,11.
In conclusion, it is an indispensable necessity that the CLP model is performed with high consistency, as the underlying inflammatory response and the outcome (survival rate) vary with the severity grade of sepsis. In a recent publication, we demonstrated that therapeutic interventions that showed highly protective effects in mid-grade sepsis were ineffective in a more severe form of sepsis13. Therefore, the model of CLP needs to be carried out in a standardized and uniform manner to create a controlled setting with a minimized number of variables that influence the outcome. Standardized length of cecal ligation represents a reliable and reproducible tool to design the severity grade of experimental sepsis. As shown in our most recent studies, using defined severity grades of CLP-induced sepsis allows researchers to obtain important functional insights about the inflammatory response that otherwise may be masked by heterogeneity of sepsis severity13. Moreover, standardized performance of the CLP procedure facilitates the comparison of experimental results between laboratory groups.
In this protocol, we define standardized procedures to induce acute sepsis in mice and rats by CLP, and we characterize distinct locations for cecal ligation that correlate with predictable outcomes. Figures 1 and 2 illustrate the surgical procedures and the positions for needle puncture of the cecum to achieve high- or low-grade mortality (Figs. 2 and 3) to address scientific questions (e.g., how a therapeutic intervention effectively improves survival and the time frame after CLP in which an intervention is still effective). In the hands of an experienced investigator, the CLP procedure can be done in less than 10 min for each animal. Additional time is required for postoperative care and specimen and data collection.
Figure 1.
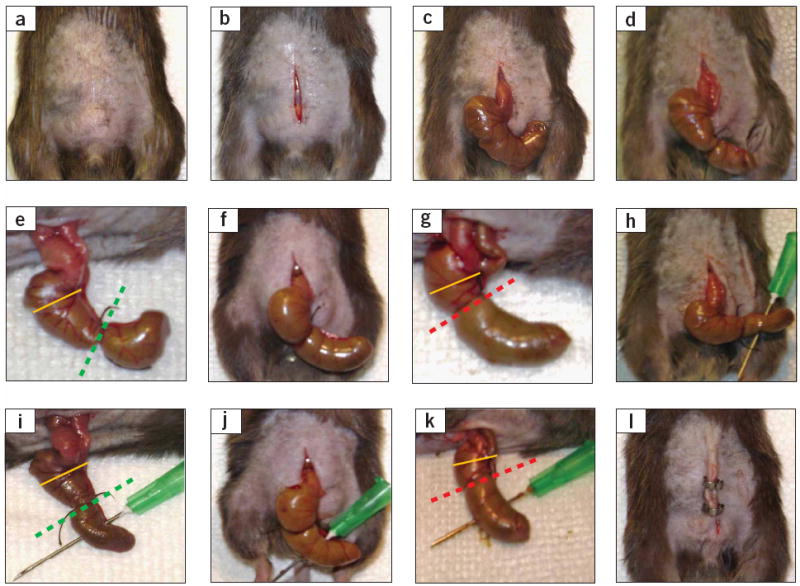
Critical steps in the CLP procedure in mice. (a) Disinfection of the abdominal area after shaving (Step 4). (b) Skin midline incision (Step 6). (c) Exposure of the cecum, which can be mostly found in the lower left area of the abdominal cavity (Step 8). (d,e) Ligation of the cecum at designated positions as the major determinant of sepsis severity (Step 10). For the induction of mid-grade sepsis resulting in survival rates of ~ 40%, the cecum is ligated (indicated by dotted green line) at half the distance between distal pole and the base of the cecum (black dotted line). (f,g) High-grade sepsis (100% lethality) comprises ligation of 75% of the cecum (dotted red line). The basis of the cecum is indicated by the yellow line. (h,i) Cecal puncture (‘through-and-through’) from mesenteric toward antimesenteric direction after medium ligation (Step 11). (j,k) Needle puncture of the cecum under the conditions of high-grade sepsis (large ligation; Step 11). (l) Wound closure by applying simple running sutures to the abdominal musculature and metallic clips to the skin (Steps 14 and 15).
Figure 2.

Anatomical locations for cecal ligation of rodents and map of arteries and ileocecal valve. (a,b) Schematic illustration of characterized positions of cecal ligation to induce mid-grade sepsis (medium ligation; dotted green line) or high-grade sepsis (large ligation; dotted red line) in (a) mice and (b) rats. The yellow line represents the basis of the cecum immediately below the ileocecal valve, which should be used as a reference to estimate the length of the cecum to be ligated. A major difference between mice and rats is that in rats a membrane stretches at the mesenteric site of the cecum (yellow area in b), which needs to be dissected before cecal ligation and which is not found in mice. In addition to medium and large ligation, small ligation (dotted blue line) comprising 10% of the cecum is presented for rats in (b).
Figure 3.
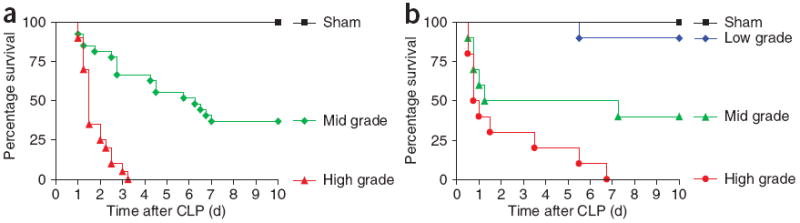
Survival rates after CLP in rodents as a function of rite of cecal ligation. (a,b) Survival rates after induction of different severity grades of sepsis by CLP in (a) mice (n ≥ 20) and (b) rats (n ≥ 10). Corresponding positions of cecal ligation are displayed in Figure 1d–g or Figure 2 (schematic), respectively. Green curves indicate the outcome after medium ligation (mid-grade sepsis), whereas red curves represent the settings of high-grade sepsis. Black curves reflect the outcome in sham animals, which underwent a similar procedure as CLP animals, including anesthesia and abdominal midline incision, with the only difference that the cecum in shams remained unmanipulated.
Experimental design
CLP procedure
Cecal puncture involves a through and through puncture with a needle (into the cecum on one side and through the cecal wall on the opposite side). As described above, the number of punctures and the needle size determine the mortality rate12. The effects on mortality in mice after CLP as a function of needle size and number of punctures of the cecum and other variables, such as fluid resuscitation, broad-spectrum antibiotics and parental nutrition, have been described in detail in earlier publications12,14,15.
Controls
Sham animals should undergo exactly the same procedures (Steps 1–9 and 13–18) except for the CLP (Steps 10–12), which are omitted in sham animals. Experimental protocols should include sham animals, in which there should be no mortality.
Furthermore, the procedure to induce sepsis should be carried out at the same time of the day because of circadian rhythm effects on the inflammatory response8.
Anesthetics and analgesics
We routinely use a combination of ketamine and xylazine as the anesthetic/analgesic agents. As indicated, isoflurane can be used as the anesthetic agent, especially as it induces good relaxation of skeletal muscles, but its use requires a hood or specially ventilated room to prevent the agent from spreading in room air.
Variations of the CLP model
For instance, fluid resuscitation and broad-spectrum antibiotic therapy have been used in rodents subjected to CLP, resulting in improved survival16,17. Another intervention, intended to simulate abdominal sepsis and its treatment in humans, involves post-CLP laparotomy with excision of the necrotic cecum and peritoneal washing. Under these conditions, survival is improved as a function of when the cecal resection was instituted after CLP: the earlier the resection is done, the better the outcome is18,19. It depends on the focus of interest (e.g. mimicking the clinical situation versus investigating the basic pathogenesis of sepsis) whether any of these variations should be employed.
MATERIALS
REAGENTS
C57BL/6 male mice (25–30 g) (Jackson Labs, cat. no. 000664)
Long–Evans rats (300–325 g) (Harlan) ! CAUTION Experiments must comply with national and institutional regulations concerning the use of animals for research purposes. Owing to substantial strain differences in both rats and mice, other strains of rats or mice are not interchangeable. Furthermore, because most genetically manipulated (knockout) mice are on a C57BL/6 background, this strain is most commonly used.
Ketamine (80–100 mg per kg body weight) (Fort Dodge IA, cat. no. 0856-2013-01)
Xylazine (5–15 mg per kg body weight) (Ben Venue Lab, cat. no.139-236)
Buprenorphin (0.05 mg per kg body weight) (Reckitt Benckiser Pharmaceutical, cat. no. 12496-0757-1)
Sterile alcohol prep pads (Fisher, cat. no. 06-669-62)
Sterile saline solution (0.9% (wt/vol) saline; LabChem)
EQUIPMENT
Protective equipment ! CAUTION Use latex gloves, face mask and surgical gown as protective equipment both for the human operator and to keep the surgical field reasonably aseptic.
Electric trimmer (Fisher, cat. no. 9364374)
Styrofoam pad
Razor blades (Fisher, cat. no. 12-640)
Gauze pads (4 inch × 4 inch; Fisher, cat. no. 19-160-277)
21 G (mice) or 18 G (rats) needles (Becton Dickinson, cat. no. 14-821-13K/14-818-13K)
26 G needles (Becton Dickinson, cat. no. 22-004-265)
Black-braided silk nonabsorbable surgical spool suture 4-0 (mice)/3-0 (rats) USP (Roboz Surgical SUT, cat. no.1066-31)
Wax-coated braided silk nonabsorbable surgical sutures 6-0 (mice)/4-0 (rats) USP with cutting needle (Ethicon SUT, cat. no. 1234-13)
Metallic wound closure clips (Michel Roboz Surgical, cat. no. RS-9272)
Syringes (1 and 10 ml; Becton Dickinson, cat. no. 14-820-11)
Surgical instruments: dissection scissors, microdissection scissors, straight surgical forceps, straight anatomical forceps and needle holder (Roboz Surgical)
PROCEDURE
Preoperative setup
-
Weigh animals to determine the amount of anesthetics to be used.
! CAUTION Use latex gloves, face mask and surgical gown as protective equipment both for the human operator and to keep the surgical field reasonably aseptic.
! CAUTION Experiments must comply with national and institutional regulations concerning the use of animals for research purposes.
Anesthetize the animals with ketamine (80–100 mg per kg body weight i.p.) and xylazine (5–15 mg per kg body weight i.p.); other anesthesia strategies, such as inhalational anesthetics (isoflurane), may be considered as well.
Monitor the intensity of anesthesia by toe pinch using tweezers. Adequate anesthesia should result in no response of extremity (e.g., no flexion of extremity).
Shave lower quadrants of the abdomen using an electric trimmer and disinfect the area with alcohol prep pads (Fig. 1a).
Place animals onto Styrofoam pads on their backs, with heads oriented away from the operator. With adequate anesthesia, no restraints are needed.
Exposure
-
6
Make longitudinal skin midline incision with a scalpel, being careful not to penetrate into the peritoneal cavity. After the initial incision, use small scissors to extend the incision and to gain entry into the peritoneal cavity (mice, 1.5–2 cm; rats, 3–4 cm; Fig. 1b).
-
7
Identify linea alba (midlines white fascia) of the abdominal musculature and dissect it for intermuscular incision and incision of fascial and peritoneal layers as shown in Figure 1b.
-
8
Locate the cecum by using blunt anatomical forceps to isolate the cecum and exteriorize it (Fig. 1c), leaving the remainder of the small and large bowel within the peritoneal cavity. It is critical not to breach or damage the mesenterial blood vessels (Fig. 1c; Fig. 2a and b). In the majority of cases, the cecum is found on the left side of the abdomen20.
-
9
Rats only: carefully dissect the mesentery of the cecum (yellow area in Fig. 2b).
▲ CRITICAL STEP Be careful not to damage the cecal branch of the ileocecal artery to avoid severe bleeding complications.
-
10
Ligate the cecum at the designated position for the desired severity grade (Fig. 1d–g; Fig. 2a and b). Make sure not to ligate the ileocecal valve so that intestinal continuity is maintained. For sham animals, skip Steps 10–12.
▲ CRITICAL STEP The position of the ligation determines the intensity of sepsis.
? TROUBLESHOOTING
-
11
Before cecal perforation, push the cecal contents gently toward the distal cecum, and at the time of cecal puncture, gently aspirate any trapped air or gases. Perforate the cecum by single through-and-through puncture midway between the ligation and the tip of the cecum in a mesenteric-to-antimesenteric direction (Fig. 1h–k). Be careful to avoid puncturing blood vessels.
-
12
After removing the needle, extrude a small amount (droplet) of feces from both the mesenteric and antimesenteric penetration holes to ensure patency.
▲ CRITICAL STEP Make sure that the amount of extruded cecal content is limited (small droplet) and the same in all animals to guarantee consistency.
-
13
Relocate the cecum into the abdominal cavity without spreading feces from the cecum onto the abdominal wall wound margins.
Closure and postoperative care
-
14
Close the peritoneum, fasciae and abdominal musculature by applying simple running sutures.
-
15
Close the skin by using metallic clips or applying simple interrupted sutures (Fig. 1l).
-
16
Resuscitate animals by injecting prewarmed normal saline (37 °C; 5 ml per 100 g body weight) subcutaneously.
▲ CRITICAL STEP Without postoperative fluid resuscitation, animals are not able to demonstrate the early, hyperdynamic phase of sepsis7,8. Further, it is important that the saline solution is prewarmed (37 °C) to avoid iatrogenic hypothermia, which most likely affects the outcome of CLP.
-
17
Inject buprenorphin (0.05 mg per kg body weight s.c.) for postoperative analgesia. Repeat every 6 h for at least 2 d.
-
18
Place mice or rats back in cages in a temperature-controlled room (22 °C) with 12-h light and dark cycles and monitor them every 6 h. Mice or rats are returned to cages immediately at the end of the surgical procedures where access to water and food is available.
● TIMING
CLP procedure: approximately 10 min for each animal
Postoperative monitoring: every half hour, for at least 2 h, and postoperative analgesia (Step 17) every 6 h for at least 2 d
? TROUBLESHOOTING
Step 10
As emphasized above, the position of cecal ligation is the major determinant of mortality and severity of disease in the CLP model11. However, it is often difficult to determine the appropriate length of cecum to be ligated, as the size of the mouse cecum may vary between individual animals (even between accurately age- and weight-matched littermates). In this case, it is important that the ratio between the ligated cecum (distance between the distal pole and the ligation) and the distance from the ligation to the basis of the cecum (immediately below the ileocecal valve) is consistent (50%:50% for ‘mid-grade’ sepsis; 75%:25% for ‘high-grade’ sepsis; Step 10 and Fig. 2).
Owing to differences of various mouse and rat strains in the susceptibility for sepsis and other infectious diseases21, it is conceivable that, if different strains of animals are used, the mortality rates might slightly differ despite the fact that the CLP procedure is performed in the same standardized manner as described above. Another reason for variability of sepsis results may lie in differences in the volumes of extruded stool8. This sometimes imposes a difficulty, as the texture of cecal content can also vary considerably between animals.
ANTICIPATED RESULTS
The model of CLP is widely used and known to closely mimic the pathophysiology of septic human patients. Accordingly, polymicrobial sepsis is associated with an early hyperdynamic phase and a late hypodynamic phase. Similar to septic human patients, rodents with sepsis induced by CLP respond to fluid resuscitation and antibiotics22,23. It is important to note that the rodent strain determines the susceptibility of animals to the response to CLP21. Rodents after CLP have positive blood cultures containing mixed enteric organisms that are detectable as early as 6 h after CLP24. While animals subjected to CLP appear healthy in the initial phase after the procedure, they begin to show clinical signs of sepsis at around 12 h following CLP, featuring malaise, fever, chills, piloerection, generalized weakness and reduced gross motor activity. Lethality begins at 18–24 h after CLP, indicating that CLP under the conditions described above represents a rapidly lethal model of acute sepsis. The overall lethality is closely related to the conditions under which CLP is used, and the position of the ligation is considered as a major determinant of mortality.
To determine the survival rates for the characterized positions of cecal ligation in this protocol, we used mice of C57BL/6 background or Long–Evans rats. The IACUC group of the University of Michigan Medical School approved all of the experimental protocols. In mice, medium cecal ligation (green dotted line in Fig. 2a), which comprises 50% of the cecum, results in a survival rate of 40% (referred to as mid-grade sepsis; Fig. 3a). Using this mid-grade form of sepsis, lethality seems to emerge in an early and a late phase: about 40% of mice die within the first 72 h, whereas another critical phase regarding mortality (another 20% of overall lethality) occurs between days 5 and 7. Most strikingly, when 75% of the cecum is being ligated (large; red dotted line in Fig. 2a), usually all mice die within 4 d after CLP induction, which we define as high-grade sepsis. Usually, in both severity grades, the onset of sepsis occurs at around 12 h after CLP. The main part of lethality proceeds within the first 48 h, whereas the frequency of death events decreases with time, which correlates with the position of the cecal ligation (medium versus large), as does the overall outcome.
Survival rates in rats are similar to those in mice. Medium ligation (green dotted line in Fig. 2b) results in a survival rate of 40%, whereas in high-grade sepsis (by large ligation; red dotted line in Fig. 2b), all rats succumb to the lethal consequences of sepsis (Fig. 3b). Under the conditions of high-grade sepsis, none of the rats survive beyond day 7. In either severity grade, no death events are usually denoted beyond day 7. As also shown in Figure 3b, the survival rate of sham-operated rats is 100%, whereas when the cecum was ligated in close proximity to the distal pole (comprising 10% of the cecum; blue dotted line in Fig. 2b), survival is 90%.
Acknowledgments
This work was supported by NIH grants GM-61656, GM-029507 and HL-31963 (to P.A.W.).
References
- 1.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 2.Kochanek KD, Smith BL. Deaths: preliminary data for 2002. Natl Vital Stat Rep. 2004;52:1–47. [PubMed] [Google Scholar]
- 3.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 4.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA. Rodent models of intra-abdominal infection. Shock. 2005;24(Suppl 1):19–23. doi: 10.1097/01.shk.0000191386.18818.0a. [DOI] [PubMed] [Google Scholar]
- 6.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 7.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard WJ, et al. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 9.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukoc Biol. 2004;75:408–412. doi: 10.1189/jlb.0503214. [DOI] [PubMed] [Google Scholar]
- 10.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singleton KD, Wischmeyer PE. Distance of cecum ligated influences mortality, tumor necrosis factor-alpha and interleukin-6 expression following cecal ligation and puncture in the rat. Eur Surg Res. 2003;35:486–491. doi: 10.1159/000073387. [DOI] [PubMed] [Google Scholar]
- 12.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 13.Rittirsch D, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 15.Heuer JG, et al. Cecal ligation puncture with total parenteral nutrition: a clinically relevant model of the metabolic, hormonal, and inflammatory dysfunction associated with critical illness. J Surg Res. 2004;121:178–186. doi: 10.1016/j.jss.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Smith EF, III, et al. Fluid resuscitation improves survival of endotoxemic or septicemic rats: possible contribution of tumor necrosis factor. Pharmacology. 1993;46:254–267. doi: 10.1159/000139053. [DOI] [PubMed] [Google Scholar]
- 17.Law M, Remick D. Fluid resuscitation and antibiotics enhance survival by unknown mechanisms. Shock. 2006;25:6. [Google Scholar]
- 18.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latifi SQ, O’Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–4446. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coria-Avila GA, Gavrila AM, Menard S, Ismail N, Pfaus JG. Cecum location in rats and the implications for intraperitoneal injections. Lab Anim (NY) 2007;36:25–30. doi: 10.1038/laban0707-25. [DOI] [PubMed] [Google Scholar]
- 21.Godshall CJ, Scott MJ, Peyton JC, Gardner SA, Cheadle WG. Genetic background determines susceptibility during murine septic peritonitis. J Surg Res. 2002;102:45–49. doi: 10.1006/jsre.2001.6319. [DOI] [PubMed] [Google Scholar]
- 22.Hollenberg SM, et al. Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med. 2001;164:891–895. doi: 10.1164/ajrccm.164.5.2010073. [DOI] [PubMed] [Google Scholar]
- 23.Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10:110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Flierl MA, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 22:2198–2205. doi: 10.1096/fj.07-105221. Epub 2008 Feb 25. [DOI] [PubMed] [Google Scholar]


