Summary
A nitric oxide (NO)/cyclic GMP (cGMP) signaling pathway is thought to play an important role in mammalian vasodilation during hypoxia. We show that Drosophila utilizes components of this pathway to respond to hypoxia. Hypoxic exposure rapidly induced exploratory behavior in larvae and arrested the cell cycle. These behavioral and cellular responses were diminished by an inhibitor of NO synthase and by a polymorphism affecting a form of cGMP-dependent protein kinase. Conversely, these responses were induced by ectopic expression of NO synthase. Perturbing components of the NO/cGMP pathway altered both tracheal development and survival during prolonged hypoxia. These results indicate that NO and protein kinase G contribute to Drosophila’s ability to respond to oxygen deprivation.
Introduction
Oxygen plays a key metabolic role, and organisms respond and adapt to limitations in oxygen supply. Small fluctuations in oxygen levels typically pose no threat to cellular well-being. In mammals, transient restrictions in blood supply or increased physical activity can create periods of localized hypoxia. Mammals deal with such hypoxia by a variety of responses, including a metabolic shift to glycolysis (an anaerobic process) to decrease oxygen requirements and increases in ventilation and hematocrit to promote oxygen uptake and distribution (for review, see Guillemin and Krasnow, 1997). Local vasodilation also promotes blood flow to areas experiencing a high demand for oxygen. Nitric oxide (NO) regulates vasodilation (Ignarro et al., 1987; Palmer et al., 1987; Furchgott, 1988). NO levels are thought to rise at sites that are hypoxic due to increased respiratory demand or poor perfusion (Stamler et al., 1997). NO is highly reactive and acts only locally in biological tissues (Meulemans, 1994). One effector of NO activity is soluble guanylyl cyclase (sGC), which contains a heme moiety to which NO can bind (Gerzer et al., 1981). Stimulation of sGC in the smooth muscle cells of vessel walls activates synthesis of cyclic GMP (cGMP), which stimulates protein kinase G (PKG) and a downstream cascade leading to relaxation of the smooth muscle, vasodilation, and increased local blood flow (Arnold et al., 1977; Gerzer et al., 1981; Pfeifer et al., 1998).
Although much effort has gone into understanding the mechanisms that mammals utilize to respond to oxygen deprivation, hypoxia has been little studied in model metazoan systems. The ability of diverse organisms to sense and respond to oxygen levels suggests that such responses appeared early in evolution and might involve primitive mechanisms that predate the divergence of metazoan phyla. In this case, the response to hypoxia in a model experimental organism such as Drosophila melanogaster should resemble that in other species. Drosophila feeds on the yeasts and molds that often grow anaerobically on rotting fruit. Consequently, Drosophila eggs and larvae compete with microbes for limited supplies of oxygen in fermenting fruit. Because of this, one might expect Drosophila to have a well-developed response to hypoxia.
We show that Drosophila embryos and larvae can survive extended periods of hypoxia. Embryos and larvae exhibit rapid and reversible responses to hypoxia that include developmental arrest, a block of the cell cycle, and behavioral changes in larvae. We demonstrate that these responses are, at least in part, triggered by components of a signal transduction pathway involving NO and PKG. These components also appear to modulate the growth of tracheal tubes, which comprise the oxygen delivery system in Drosophila. Parallels can be drawn between the use of NO and PKG in Drosophila’s response to hypoxia and the use of NO in mammals to induce vasodilation in response to hypoxia.
Results
cGMP-Dependent PKG Contributes to a Behavioral Response to Hypoxia
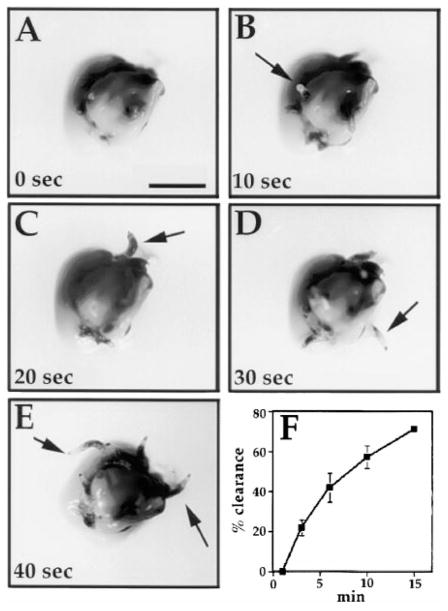
While testing the ability of early third instar larvae to survive exposure to 1% oxygen (about 5% of normal atmospheric levels), we noticed a striking behavioral response. Typically, larvae feed on yeast paste with only their posterior ends protruding from the food. This leaves the two posterior spiracles, the openings of the larval tracheal system, exposed to the outside atmosphere. Within seconds of oxygen deprivation, larvae stopped feeding, backed up slightly, and then exited the yeast (Figures 1A–1E). Subsequently, over about a 2 min period, larval motility increased but was largely confined to the surface of the yeast. In a third phase of the response, the larvae left the yeast and wandered onto a clean agar surface, occasionally escaping the petri dish. After 15 min of 1% oxygen, nearly 75% of the larvae left the yeast (Figure 1F). Prolonged exposure to 1% oxygen (>30 min, not shown) resulted in almost a complete cessation of motility. It is unclear whether the ultimate cessation of motility constitutes another phase of the behavioral response or a depletion of energy reserves.
Figure 1. Hypoxia Induces Exploratory Behavior in Larvae.
(A–E) Exposure of early third instar larvae to hypoxia (1% O2) resulted in a rapid behavioral response in which the larvae ceased feeding and extracted themselves from the food. Images were taken at 10 s intervals following onset of hypoxia (A–E). The panels show a mound of yeast in which the larvae are largely buried. Initially, only their posterior ends protrude (arrows in [B] and [C]), but they turn around as they prepare to leave the food (arrows in [D] and [E] show larvae with their more pointed anterior ends protruding). Scale bar is 5 mm.
(F) After 15 min of hypoxia, approximately 75% of the larva had left the food. Percents given are the average of three experiments.
Osborne et al. (1997) identified the molecular basis for a behavioral polymorphism (Sokolowski, 1980; de Belle et al., 1989). Sitters (fors) are less motile than rovers (forR) while on food. This difference in foraging behavior is due to the allele state at a single locus, for (de Belle et al., 1989; Osborne et al., 1997). The gene dg2, which encodes one of two cGMP protein-dependent kinases (PKG) in Drosophila, is located at the for locus (Kalderon and Rubin, 1989; Osborne et al., 1997). The fors stock contains an undefined polymorphism that eliminates expression of at least one dg2 transcript, and these stocks have slightly reduced PKG activity (Osborne et al., 1997). Thus, a partial reduction in PKG results in a behavioral change detected as decreased movement of larvae on a food source.
We suspected that roving behavior might be related to the vigorous exploratory behavior observed during hypoxia. Larvae on a food source (yeast) are likely to suffer a reduction in oxygen availability due to metabolic competition with yeast, and roving might be a response to the reduced oxygen availability. Accordingly, the failure of fors larvae to rove might result from a reduced behavioral response to hypoxia.
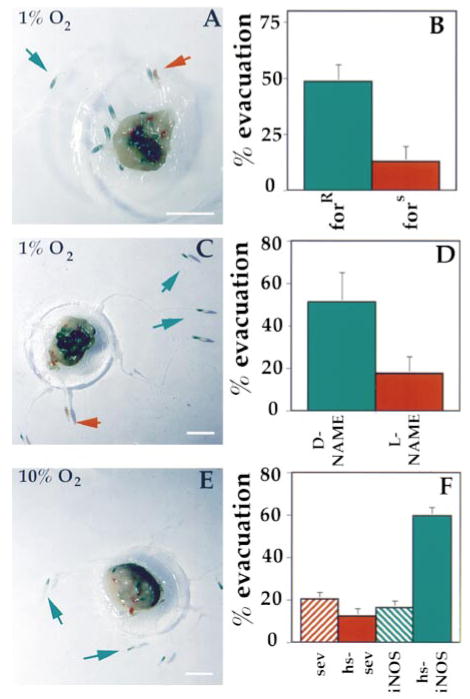
We examined the influence of the for alleles on the response to hypoxia. Third instar larvae raised under noncrowded conditions were transferred onto a pile of yeast paste located at the center of a grape agar plate. Larvae were allowed to settle for approximately 10 to 15 min. Under these conditions, we observed no obvious difference in behavior between forR and fors larvae. Dishes were sealed and gassed with 1% oxygen through a hole in the lid. The initial response to hypoxia, cessation of feeding and movement to the surface, occurred in both stocks, although fors larvae responded more slowly. We scored the latter response, movement away from the food, by counting larvae 5 mm or more from the yeast after 10 min of hypoxia: 49% of forR larvae migrated away from the yeast, compared to 13% of fors larvae (Figures 2A and 2B). Results comparable to those observed with fors were also obtained with fors2, a radiation-induced allele (not shown). These results indicate that PKG activity contributes to the exploratory response to hypoxia; however, during a prolonged mild hypoxia, fors larvae eventually left the food (data not shown).
Figure 2. NO and PKG Are Involved in Hypoxia-Induced Motility.
(A and B) Larvae carrying different for alleles have altered PKG levels (see text) and behavioral responses to hypoxia. (A) shows an experiment in which equal number of fors and forR larvae were subjected to 1% oxygen for 10 min. The larvae were previously fed colored food so that forR larvae are green (green arrows) and fors larvae are red (red arrows). (B) summarizes the results of three experiments in which an average of 48% of forR larvae left the yeast after 10 min of hypoxia (48.7% ± 7.5%) compared to 13% of fors larvae (13.2% ± 6.7%).
(C and D) Larvae fed NOS inhibitor are deficient in hypoxia-induced motility. (C) is an example of an experiment in which an equal number of larvae fed the NOS inhibitor L-NAME (red larvae, red arrows) or the inactive isomer D-NAME (green larvae, green arrows) were subjected to 10 min of 1% oxygen. (D) summarizes the results of three trials in which 52% (52.3% ± 14.1%) of the D-NAME-fed larvae left the yeast after 10 min, compared to 18% of the L-NAME-fed larvae (18.2% ± 8.5%).
(E and F) Induced expression of a NOS transgene results in hypersensitivity to hypoxia. (E) is an example of an experiment in which an equal number of either wild-type larvae (red larvae, red arrows) or larvae containing a hs-iNOS transgene (green larvae, green arrows) were subjected to heat shock for 20 min at 37°C, allowed to recover for 60 min, and then subjected to 10% O2 for 15 min. (F) shows the average of three trials in which wild-type and nonheat-shocked iNOS stocks showed low percentages of motility at 10% O2 (wt = 20.3% ± 3.2%; hs – wt = 12.3% ± 3.5%; iNOS = 16.3% ± 3.2%), whereas heat-shocked iNOS showed an increased sensitivity to hypoxia with nearly 60% (59.3% ± 4%) of the larvae leaving the yeast after 15 min of 10% O2. The scale bars in (A), (C), and (E) are 5 mm.
Nitric Oxide Synthase Also Contributes to Hypoxia-Induced Behavior
NO activates PKG in mammals where it has recognized roles in the response to hypoxia. We investigated whether NO might similarly mediate responses to hypoxia in Drosophila. To do this, we examined the ability of an inhibitor of nitric oxide synthase (NOS) to blunt the behavioral response to hypoxia. Early third instar larvae that had been fed either L-NAME, an inhibitor of NOS, or D-NAME, the inactive isomer of L-NAME, were tested as described above. After 10 min of 1% oxygen, only 18% of the larvae fed L-NAME cleared the yeast pile, compared to 53% of the larvae fed D-NAME (Figures 2C and 2D). This suggests that NOS activity contributes to the induction of exploratory behavior by hypoxia.
We used an inducible NOS transgene to increase NOS activity. Larvae carrying heat shock–inducible NOS (hs-iNOS) were either heat shocked for 20 min at 37°C to induce iNOS expression or left at 25°C as a control. As a second control, wild-type larvae lacking the transgene were subjected to a similar regime. After 60 min of recovery, oxygen was reduced to 10% for 15 min. This modest hypoxia induced only a low percentage of roving in the controls (wild type [wt] = 20%; hs – wt = 12%; iNOS = 16%; Figures 2E and 2F). In contrast, 60% of the larvae in the heat-shocked iNOS stock cleared the yeast (Figures 2E and 2F). Thus, induced iNOS made the larvae hypersensitive to reductions in oxygen levels. These results suggest that NO and PKG play an important role in the ability of larvae to respond to hypoxia.
Identification of Regions Expressing NOS Activity
Regions of the larvae that specialize in responding to hypoxia should express significant levels of the activities involved. We used two methods to define possible foci of function: a histochemical stain (diaphorase staining) for NOS activity, and DAF2/DA, a fluorescein derivative that increases in fluorescence when bound by NO (Kojima et al., 1998). We observed generalized diaphorase and DAF2/DA staining at a low level (not shown) and local regions of high staining. As previously reported, diaphorase staining detects higher NOS activity in the imaginal discs (Kuzin et al., 1996; Figure 3A). In addition, the CNS (optic lobes and ventral nerve cord; Figure 3A) and sections of the gut stained well for diaphorase (not shown). The highest amount of diaphorase staining was observed in the spiricular glands of the posterior spiracles and the spiricular pouch of the anterior spiracles (Figures 3B and 3C, respectively). DAF2/DA also stained the pouch of tissue surrounding the anterior spiracles as well as neuronal-like processes within the pouch (Figure 3D). This staining near the openings of the tracheal system is interesting because the location is consistent with a possible role in governing the opening (eversion) of the spiracles to increase access to oxygen.
Figure 3. Localization of NO and NOS Activity in Larvae.
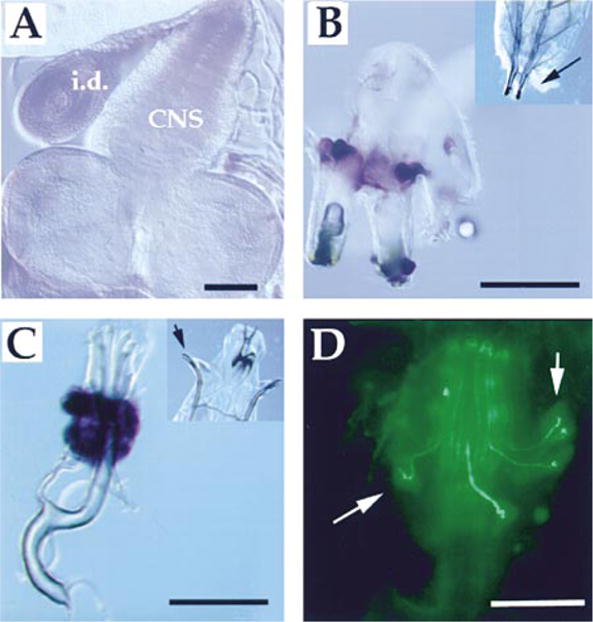
(A–C) NOS activity is present in the larval CNS and spiracles. Diaphorase staining (dark blue) was utilized to visualize the presence of NOS activity in various tissues following dissection of third instar larvae. (A) shows a disc (labeled i.d.) and the larval CNS (labeled CNS), both of which are lightly stained. (B) shows posterior spiracles that have been dissected with attached cuticle and trachea. Large cells at the base of the posterior spiracles stain intensely. (C) shows anterior spiracles that have been dissected from a larva with some surrounding cuticle. A structure immediately proximal to the end of the tracheal tubes is heavily stained. The inset at the top right in (B) shows the posterior portion of a larva with an arrow indicating the posterior spiracles; in (C), the anterior portion of a larva is shown with an arrow indicating the anterior spiracles.
(D) shows an anterior spiracle stained with DAF2/DA; arrows indicate neuronal-like processes and cell bodies that also stain intensely with the dye. Scale bar is 50 μm.
NO and PKG Contribute to Survival during Hypoxia
The behavioral responses we observed presumably allow larvae to escape local hypoxia. Embryos, however, that are nonmotile and larvae that are exposed to a more general hypoxia do not have the option to escape. We tested the capacity of embryos and larvae to endure prolonged hypoxia. Early syncytial embryos are quite sensitive to hypoxia, but the ability to survive periods of hypoxia improves during cellularization and gastrulation (Foe and Alberts, 1985). We found that 8-hr-old embryos can survive hypoxia for up to 8 days and larvae can survive for several days if protected from dehydration (J. A. W. and P. H. O., unpublished data; Figure 4). Perhaps NO and PKG contribute to the ability of larvae and embryos to endure hypoxia.
Figure 4. The dg2 Gene Is Involved in Surviving Prolonged Hypoxia.
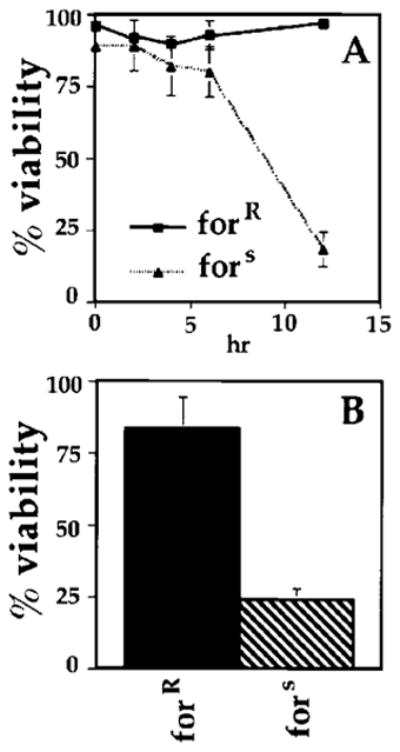
(A) The dg2 gene is required in embryos for survival during prolonged hypoxia. Stage 12 forR and fors embryos were subjected to hypoxia for either 2, 4, 6, or 12 hr and then removed and allowed to develop at 25°C on grape agar plates for 30 hr. The percent viability was determined by the number of larvae that hatched. Viability of forR embryos: 0 hr, 96%; 2 hr, 92% ± 2.8%; 4 hr, 90% ± 2.9%; 6 hr, 93% ± 5%; 12 hr, 97% ± 2.9%. Viability of fors embryos: 0 hr, 89%; 2 hr, 89% ± 8.7%; 4 hr, 82% ± 8.6%; 12 hr, 18% ± 5.8%. Percentages are the average of three experiments.
(B) The dg2 gene is required in larvae for survival during prolonged hypoxia. Early third instar forR and fors larvae were subjected to hypoxia for 6 hr, after which they were removed and allowed to develop on grape agar plates containing yeast. The percent viability was determined by the number of flies that eclosed. Viability of forR larvae, 83% ± 11.1%; fors larvae, 24% ± 4.0%. Percentages are the average of three experiments.
To test for an involvement of PKG, we abruptly exposed fors or forR embryos (stage 14) to severe hypoxia for different periods and followed the hatching and eclosion under normoxic conditions. Both types of embryos survived 2 to 6 hr of hypoxia (Figure 4A); however, while fors embryos survived 12 hr of hypoxia poorly (25% viability), forR embryos survived well (88% viability vs. about 95% for no treatment; Figure 4A). We conclude that PKG contributes to the survival of embryos exposed to hypoxia.
We also tested the involvement of NO and PKG in the survival of larvae. When fors larvae were kept hypoxic for 6 hr, they showed little sign of motility upon return to normoxic conditions, and they rarely pupated or eclosed (20%; Figure 4B). In contrast, forR larvae rapidly reacquired motility after 6 hr of hypoxia, and 80% eclosed (Figure 4B). Larvae fed L-NAME also showed diminished viability after hypoxia (not shown). It should be noted that the larvae in these experiments were subjected to severe and immediate hypoxia (<0.5% oxygen within 1 min). When less severe conditions were imposed (a decrease to 1.5% oxygen over 45 min), little difference between fors and forR larvae was observed. Thus, it appears that NO and PKG contribute to the ability of Drosophila to endure suddenly imposed hypoxia.
Both Hypoxia and Activation NOS or PKG Block S Phase
Drosophila eggs ordinarily require about 24 hr to hatch. Hypoxic embryos, however, can arrest for days and then resume development. Presumably, surviving this arrest requires coordinate blocks to many embryonic processes, including cell proliferation. We examined the influence of hypoxia on the well-defined cell cycles of the Drosophila embryo (Foe, 1989). Cellularized embryos undergoing S phase of cell cycle 15 (3.5 hr old, stage 8 embryos) were placed in media equilibrated with either 1% or 22% oxygen via a bubbler. After 5 min, BrdU was added, and 5 min later the embryos were fixed and stained for BrdU incorporation, a measure of DNA replication. Hypoxia blocked BrdU incorporation (Figure 5A vs. 5B). Since S phase length in cycle 15 (45 min) is longer than the treatment, the arrest indicates that the ongoing S phase is blocked. This block was reversed upon 5 min of reoxygenation (not shown). Different cells of the embryo progress through cell cycle 15 according to a strict developmental schedule, giving rise to stage-specific stereotyped patterns of S phase cells (Edgar and O’Farrell, 1990). Hypoxia did not appear to disrupt these patterns; upon reoxygenation, S phase resumed in the same domains as when it was blocked. Thus, hypoxia induces a rapid and reversible arrest of S phase.
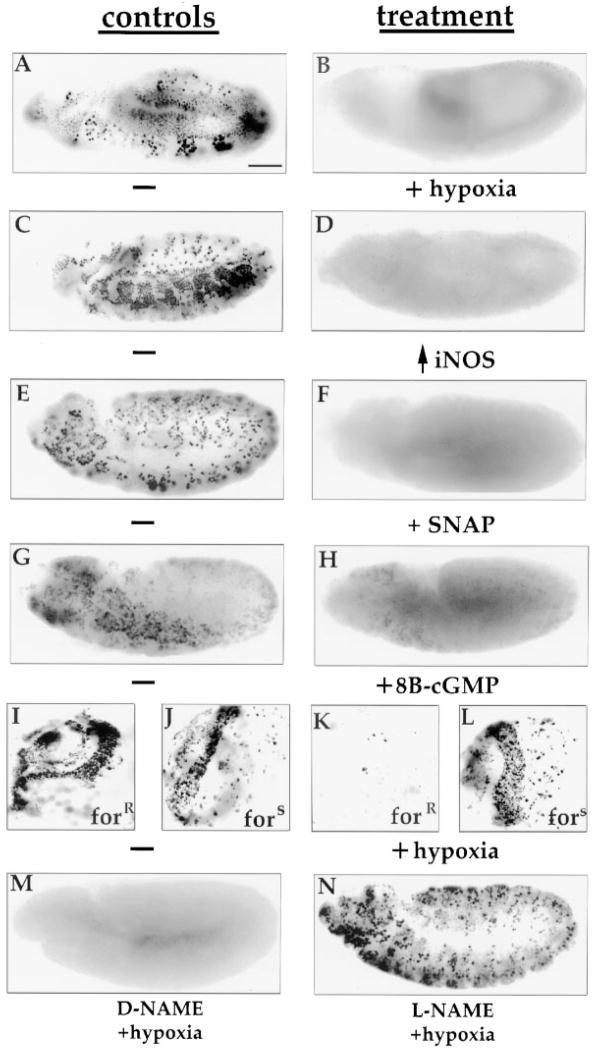
Figure 5. NO and PKG Are Utilized in a Hypoxic Block to S Phase.
(A and B) Hypoxia blocks S phase. Wild-type Sevelen embryos (stage 8) show BrdU incorporation into S phase cells during a 5 min labeling period (A). This incorporation is blocked when embryos are labeled from 5 to 10 min following imposition of hypoxia.
(C and D) The hypoxic block to S phase was mimicked by the ectopic expression of iNOS. Embryos (stage 10) carrying a hs-iNOS transgene were exposed to heat shock for 30 min at 37°C, allowed to recover, and then assayed for S phase using BrdU (D). Control embryos treated similarly (not shown) or nonheat-shocked transgenic embryos (C) had typical patterns of BrdU incorporation (Edgar and O’Farrell, 1990).
(E and F) The addition of the NO donor SNAP to stage 12 embryos resulted in a complete inhibition of BrdU incorporation. Embryos were exposed to 5 mM SNAP for 15 min prior to labeling with BrdU (F). Control embryos were left untreated (E).
(G and H) The addition of 8Br-cGMP to stage 14 embryos also resulted in a reduction of S phase as assayed by BrdU incorporation. Embryos were incubated in 5 mM 8Br-cGMP for 60 min prior to treatment with BrdU to detect S phase (H). Control embryos were similarly treated but incubated with 5 mM GMP (G).
(I–N) Inhibition of NO or PKG either genetically or pharmacologically resulted in a failure to block S phase during hypoxia. CNS (optic lobes shown) dissected from forR larvae showed a block to S phase when exposed to hypoxia (I and K), whereas CNS dissected from fors larvae continued to progress through S phase under hypoxia (J and L). Stage 11/12 embryos (360–480 min AED) that were incubated in 0.1 M D-NAME prior to hypoxia showed a block to S phase during hypoxia (M), whereas incubation of embryos in 0.1 M L-NAME allowed for continued BrdU incorporation during hypoxia (N). Scale bar is 50 μm. In (A–H) and (M and N), anterior is to the left and dorsal toward the top.
It was previously shown that the ectopic expression of NOS in Drosophila imaginal discs arrests the cell cycle (Kuzin et al., 1996). To test whether expression of iNOS might similarly induce an arrest of the embryonic cycles, the hs-iNOS transgene was induced in embryos during S phase of cycle 15 (30 min at 37°C). BrdU incorporation was almost completely blocked 40 min after heat shock (compare Figure 5C to 5D). Within 2 hr after iNOS expression, robust BrdU incorporation was again observed at positions consistent with the normal spatiotemporal pattern of S phase (not shown). Incubation of embryos with SNAP, a chemical NO donor, also blocked BrdU incorporation (compare Figure 5E to 5F). These results suggest that NO is capable of arresting S phase progression.
Since cGMP mediates the activation of PKG in the NOS/PKG pathway, we tested whether the hydrolysis-resistant cGMP analog 8Br-cGMP might mimic the effects of hypoxia. Incubation of embryos with 5 mM 8Br-cGMP had a slight or negligible effect on BrdU incorporation in early (stage 8) embryos (not shown; see Discussion) but substantially inhibited incorporation of older embryos (stage 14; Figure 5F). As a control, we added GMP, which had no effect (Figure 5E). Thus, accumulation of NO or a cGMP analog can block or diminish DNA replication.
Inhibition of NOS or PKG Alleviates the Block to S Phase Imposed by Hypoxia
To test the role of NOS activity in the response to hypoxia, we used the NOS inhibitor L-NAME. Incubation of embryos with L-NAME had no noticeable effect on BrdU incorporation under normoxic conditions (not shown). BrdU incorporation in L-NAME-treated embryos continued under hypoxic conditions, although the levels of incorporation were diminished compared to normoxic embryos and the pattern of incorporation appeared slightly altered (Figure 5N). The hypoxic response was intact in embryos incubated in the control D-NAME (Figure 5M). This result suggests that NO is at least partially required for hypoxia-induced arrest of S phase.
To determine whether hypoxia also arrests the cell cycle in larval stages, we cultured dissected CNS in media equilibrated with 1% or 22% oxygen and tested for BrdU incorporation. Hypoxia blocked incorporation in forR CNS cells undergoing S phase (compare Figure 5I to 5K). In contrast, hypoxia failed to completely block S phase in CNS obtained from fors larvae (compare Figure 5J to 5L). A comparable result was obtained when larval CNS were treated with L-NAME prior to hypoxia (not shown). While this result suggests that an effective arrest of S phase requires the forR allele, hypoxia did reduce BrdU incorporation in fors CNS, suggesting that fors larvae are only partially deficient in this response. In contrast to larvae, fors embryos retained the ability to block S phase under hypoxia (not shown). These results show that hypoxia induces cell cycle arrest in both embryos and larvae and suggest an involvement of NO in these responses.
NO and PKG Are Involved in Larval Tracheal Development
Whereas severe hypoxia can arrest development, low oxygen levels can modify it. Development of the tracheal system shares many similarities with the development of the mammalian circulatory system (for review, see Manning and Krasnow, 1993). In both, the terminal branches elongate and ramify as growth increases oxygen demand and creates slight hypoxia (Locke, 1958). Perhaps NO and PKG contribute to this response to low oxygen.
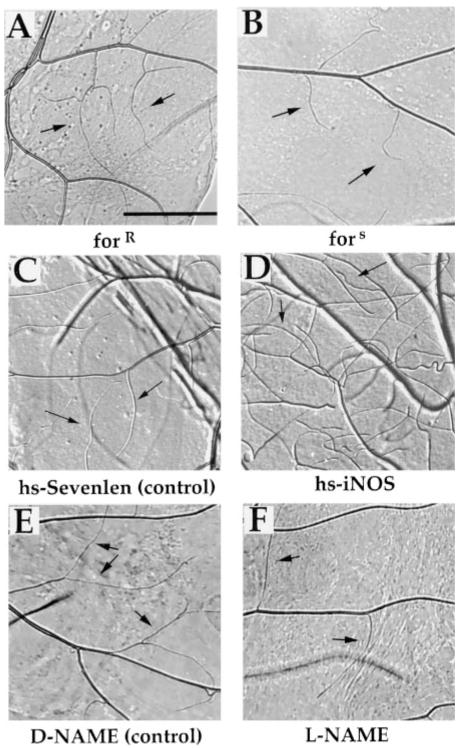
If NO and PKG stimulate tracheal ramification in low oxygen, it might be expected that terminal ramifications will decrease if these components are inhibited and increase if their expression is stimulated. Consistent with this, fors larvae, which are genetically compromised for PKG activity, and L-NAME-treated larvae, in which NOS activity is inhibited, appeared to have less frequent and shorter terminal ramifications (shown for the gut in Figure 6; compare A vs. B and E vs. F). In contrast, induction of iNOS appeared to have the opposite effect, increasing the number and the length of terminal branches (Figures 6C and 6D). These results provide one example in which NO and PKG modulate an aspect of development.
Figure 6. NO and PKG Promote Tracheal Branching in Larvae.
(A and B) fors larvae are deficient in tertiary tracheal branching. Anterior midguts immediately posterior to the proventriculus were dissected from early third instar forR (A) and fors (B) larvae and mounted in PBS with 5% Ficoll. Arrows indicate tertiary branches. Scale bar is 50 μm.
(C and D) Ectopic expression of iNOS increases tertiary tracheal branching. Early second instar Sevelen (C) and hs-iNOS (D) larvae were subjected to 30 min of heat shock at 37°C and then allowed to age for 12 hr followed by another 30 min heat shock. Twelve hours later, third instar larvae midguts were dissected and mounted as described in (A) and (B). Arrows indicate tertiary branches.
(E and F) Inhibition of NOS blocks tertiary tracheal branching. Wild-type larvae were fed from hatching either 0.5 M of D-NAME (E) or L-NAME (F). Midguts from early third instar larvae were dissected and mounted as described in (A and B). Arrows indicate tertiary branching.
Discussion
In most organisms, oxygen plays a key and often limiting role in energy metabolism. It can limit the performance of an athlete or the growth of a microorganism. Since restriction of metabolism has profound effects on cellular functions, the ability to adapt to hypoxia should aid in survival. It is likely that such adaptive mechanisms are widespread and possible that they are evolutionarily conserved. We have presented evidence that nitric oxide and a cGMP-dependent protein kinase contribute to Drosophila’s ability to react to hypoxia. We show that manipulation of NO and PKG either genetically or pharmacologically affects behavioral, cellular, and developmental responses to hypoxia, suggesting that NO and PKG play an important role in modulating the hypoxic response in Drosophila.
Hypoxia Invokes a Behavioral Response in Larvae
Larvae exhibit a rapid behavioral response when exposed to hypoxia (Figure 1). Wild-type larvae have a reduced response to hypoxia when they have been treated with an inhibitor of NOS, L-NAME, but are hypersensitive if expression of a NOS transgene is induced (Figure 2). These results indicate that NO contributes to the behavioral response to hypoxia. We also have shown that fors larvae, which have lower levels of PKG activity, have an attenuated or slowed response to hypoxia (Figure 2). Based on these results, analogies can be drawn to well-studied signaling pathways in mammalian cells that use both NO and PKG (Figure 7). In insects, it has been demonstrated that specific neurons containing high levels of NOS activity can induce cGMP accumulation in nearby cells that express soluble guanylyl cyclase (Truman et al., 1996; Gibbs and Truman, 1998; Wildemann and Bicker, 1999). Based on these precedents and our observations, we speculate that low oxygen levels result in increased levels of NO in “sensor” cells, which then communicate to nearby neuronal cells to modify behavior. Based on their position and the very high level of NO and NOS activity, the cells surrounding the anterior and posterior openings of the tracheal tubes (spiracles) are candidates for these sensor cells (Figure 3).
Figure 7. NO and PKG Play Multiple Roles in the Response to Hypoxia in Drosophila.
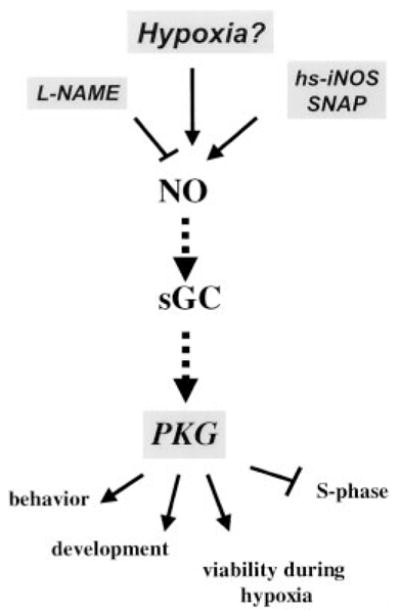
A model suggesting how NO and PKG might be utilized by Drosophila to sense and respond to hypoxia. Unknown upstream factors might sense oxygen levels and affect levels of NO. Alternatively, the pathway might be activated at different levels in response to hypoxia. Our data suggest that the NO/PKG pathway influences multiple responses (depicted at the bottom of the figure) to hypoxia in Drosophila. The responses were altered by genetically or pharmacologically activating or inhibiting the pathway (manipulations are shown boxed in gray). Note that the pathway shown (dashed line) is inferred from the pathway established in other systems.
The Role of NO/PKG in Surviving Hypoxia
Embryos and, in certain situations, larvae might be exposed to periods of prolonged hypoxia. Nitric oxide and PKG contribute to embryonic/larval survival during prolonged periods of oxygen deprivation (Figure 4). Many responses are likely to contribute to the survival. When embryos are exposed to hypoxia, they arrest and remain unchanged until oxygen is returned. Diverse processes such as cell proliferation, tissue movements, patterning, and differentiation must be arrested during this diapause. Death of the organism during hypoxia might reflect a failure to coordinate the arrest of these diverse events. The same issues might influence survival of individual cells. Hypoxia has been shown to lead to programmed cell death in a number of mammalian tissue types (Shimizu et al., 1995; Yao et al., 1995; Schmaltz et al., 1998). Genetic dissection of the programs contributing to survival upon exposure to hypoxia might provide a route to uncover the events important for both cellular and organismal survival.
Hypoxia Blocks S Phase
We found that hypoxia blocked S phase, as assayed by BrdU incorporation, in embryos as well as larval brains. Overexpression of iNOS or incubation with a NO donor phenocopied this response, whereas the block was bypassed when embryos were preincubated in the NOS inhibitor L-NAME prior to hypoxic exposure (Figure 5). While this suggests that NO is involved in the response, it is not clear that PKG is involved in the arrest of S phase in the early embryo (S phase 14 and 15). fors embryos showed no obvious defect in the hypoxic block of S phase 14 or 15, and 8Br-cGMP had little effect on blocking BrdU incorporation. In contrast, 8Br-cGMP suppressed S phase in later-stage embryos, and the hypoxia-induced S phase block in CNS requires the more active allele of for. Either the hypoxic response in the early embryo relies on a PKG isozyme that is not affected by the for allele or the cell cycle arrest in the early embryo involves a different mechanism. We tentatively suggest that NO can arrest the cell cycle independent of PKG in the early embryo but that the later mechanism relies substantially on PKG.
How might NO effect a block on the cell cycle? While the block to S phase in the CNS was relatively slow (>20 min, Figure 5), the block to S phase in the early embryo was very rapid (<5 min, Figure 5). The speed of arrest of the embryonic S phase indicates that the block affects ongoing S phases. In mammalian cells, NO can reversibly block S phase by cGMP-independent mechanisms, apparently by inhibiting ongoing S phase (Sciorati et al., 1997). Possible targets for NO are ribonucleotide reductase, which has been shown to be nitrosylated by NO, and Rb, which can be activated by NO (Lepoivre et al., 1994; Roy et al., 1995; Ishida et al., 1997). The features of this block resemble those in the hypoxia-dependent block observed in early Drosophila embryos (Figure 5).
Hypoxia can also arrest mammalian cells in G1/G0. Hypoxia or prolonged treatment with NO has been reported to block cells in a G0-type state (Graeber et al., 1994; Ho et al., 1996; Ishida et al., 1997; Poluha et al., 1997). Since early embryonic cell cycles lack a G1 phase, we suggest that mechanisms analogous to those arresting the cell cycle in G1/G0 in mammalian cells only come into play in cycle 17 and later, when this cell cycle phase first appears.
NO and PKG Influence Tracheal Development
Nitric oxide and PKG also contribute to long-term developmental responses to hypoxia in Drosophila. The development of terminal branches in larvae is not stereotyped and is thought to be induced in response to oxygen need (Locke, 1958; Manning and Krasnow, 1993). The terminal branches, which extend and ramify during larval growth, provide oxygen to internal tissues. This larval elaboration of the tracheal tubes appears to be reduced in both fors larvae and larvae fed L-NAME and appears to be enhanced by induction of NOS (Figure 6). These results indicate that production of NO influences the development of terminal branches. The enhancement of terminal tracheal branching in response to NO production might reflect an involvement of NO in the developmental response to hypoxia.
The development of tertiary branches in larvae is analogous to the process of tumor angiogenesis in mammals, in which hypoxia promotes vascular growth (Shweiki et al., 1992). The induction of angiogenesis is mediated, at least in part, by induction of growth factors. Vascular endothelial growth factor (VEGF) is one of the main proteins involved in promoting angiogenesis, and it is upregulated by the transcriptional regulator hypoxia-inducible factor (HIF) in response to hypoxia (Forsythe et al., 1996). HIF is involved in activating a broad range of genes during hypoxia, some of which might make contributions to angiogenesis. One of the HIF-induced genes is the inducible form of NOS, iNOS (Wang and Semenza, 1993; Semenza et al., 1994; Wang et al., 1995; Forsythe et al., 1996). Homologs of HIF have been identified in Drosophila, and it will be interesting to see whether they play a role in tracheal development in response to hypoxia and whether they also stimulate the NO pathway (Nambu et al., 1996; Sonnenfeld et al., 1997).
Possible Mechanisms for NO and PKG Involvement in the Response to Hypoxia
Our results implicate the involvement of NO and PKG in the response to hypoxia in Drosophila (Figure 7). This suggests that the responses we observed are induced by a regulatory pathway resembling the one characterized in mammals. In the mammalian nervous system, this signaling process is triggered by calcium release, which stimulates the calcium/calmodulin–dependent activity of neuronal NOS. This results in NO production, which then stimulates cGMP synthesis in adjacent cells containing soluble guanylyl cyclase (Bredt and Snyder, 1994). In insects, this type of pathway is thought to play a specific role in signaling between specialized cells of the nervous system (Truman et al., 1996; Gibbs and Truman, 1998; Wildemann and Bicker, 1999). Perhaps a similar signaling pathway is utilized in the more global responses observed during hypoxia.
By virtue of its ability to stimulate vasodilation, NO has been implicated in the mammalian response to hypoxia. There is, however, no known mechanism by which hypoxia can induce a rapid (transcription-independent) increase in NOS activity. Furthermore, the synthesis of NO by NOS requires oxygen as a substrate, and rates of NO synthesis by NOS have been shown to decline under hypoxia (Kantrow et al., 1997; Whorton et al., 1997). Nonetheless, our results suggest that NOS makes a positive contribution in the response to hypoxia (Figure 7). Perhaps there is sufficient oxygen to support NOS-catalyzed generation of NO during hypoxia. Alternatively, the activity of NOS prior to hypoxia might contribute to the release of NO during hypoxia. The NO produced by NOS under normoxic conditions rapidly reacts with molecular oxygen, resulting in the accumulation of NO2−. It has been suggested that hypoxia stimulates the release of NO from preformed stores of NO2−, which may be established by the earlier action of NOS (Reutov and Sorokina, 1998). It has recently been shown that mammalian xanthine oxidase reduces NO3−/NO2− to NO under hypoxic conditions (Millar et al., 1997; Zhang et al., 1998). Thus, under hypoxic conditions, accumulated NO2− might be reduced through the action of xanthine oxidase or other related enzymatic activities, resulting in the release of NO. Such a mechanism might have a general role in sensing oxygen levels.
While our findings implicate NO and PKG in the response to hypoxia, we do not know their exact roles in the process. By analogy to the known roles of NO and PKG, they might act as direct transducers of the signal or they may function as facilitators of a separately transduced signal. We presently favor a model in which NO and PKG are involved in a signal tranduction pathway that is related to but perhaps different from the recognized NO signal transduction pathway.
Experimental Procedures
Stocks
Sevelen was used as a wild-type stock. forR and fors stocks contain a naturally occurring polymorphism in dg2 (Osborne et al., 1997). fors2 is a radiation-induced allele of dg2 that phenotypically behaves like fors (Osborne et al., 1997). The hs-iNOS stock was provided by Cahir O’Kane (Kuzin et al., 1996).
Behavioral Assays and Localization of NO and NOS Activity
Assays of hypoxia-induced motility were performed on early third instar larvae. Larvae were raised under well-fed, noncrowded conditions. For the assay, larvae were transferred to a yeast pile at the center of a 15 × 100 mm grape agar plate and allowed to settle for 10 to 15 min. Plates were then sealed with parafilm, and the desired gas was introduced through a hole in the lid. Positive motility was scored as larvae that moved 5 mm from the yeast. For inhibition studies, larvae were fed from hatching either 0.5 M L-NAME or D-NAME (Sigma) added into a yeast plus food coloring mix. Ectopic expression of NOS was achieved by heat shocking larvae carrying the hs-iNOS transgene for 20 min at 37°C. Heat-shocked larvae were allowed to recover 60 min prior to the assay. Diaphorase staining to determine NOS activity was performed as described by Kuzin et al. (1996). For determination of NO, dissected tissues were incubated with 10 μm DAF2/DA (Cal-Biochem) in either PBS or Schneider’s media for 60 min prior to analysis.
Long-Term Survival of Hypoxia
To study the long-term effect of hypoxia on Drosophila embryos, stage 14 embryos were dechorionated and placed into degassed Schneider’s media in 1.5 ml screw-cap microfuge tubes. Tubes were topped with N2 and then placed into glass jars. The jars were topped with N2 and then sealed. For survival studies using larvae, early third instar larvae were selected and placed into microfuge tubes containing a piece of Kimwipe (Kimberly-Clark) moistened in PBS to prevent desiccation. Tubes were topped with N2 and sealed and then placed into glass jars that were also topped with N2 and sealed. Upon release from hypoxia, both embryos and larvae were allowed to develop on grape agar plates at 25°C. Percent viability was determined as number of adults that eclosed.
Examination of S Phase
To determine the effect of hypoxia on embryonic S phase, wild-type Sevelen embryos were aged 200 min after egg deposition (AED; stage 8). Dechorionated and octane-permeabilized embryos were subjected to hypoxia in degassed Schneider’s media. For experiments examining S phase during hypoxia, a degassed solution of BrdU (1 mM final, Sigma) was added to the hypoxic embryos. Embryos were reoxygenated by the addition of oxygenated Schneider’s media. Incorporation of BrdU was assayed by immunofluorescence microscopy. For experiments looking at the effect of NOS on S phase, embryos carrying the hs-iNOS transgene were subjected to a 30 min heat shock at 37°C followed by a 20 min recovery and the addition of BrdU. S-nitroso-N-acetylpenicillamine (SNAP, Cal-Biochem) was used at 5 mM in Schneider’s media for 15 min prior to the addition of BrdU. To assay the effect of activating PKG on S phase, embryos were incubated for 60 min in a 5 mM solution of either GMP or 8-Bromo cGMP (Sigma) followed by the addition of BrdU.
To assay the effect of inhibiting NOS activity on the hypoxic block to S phase (stage 11/12, 360–480 hr AED), embryos were incubated in either 0.1 M L-NAME or D-NAME for 120 min prior to hypoxic exposure. Experiments looking at the effect of hypoxia on S phase in CNS derived from either forR or fors larvae were performed on CNS from early third instar larvae. Dissected CNS were subjected to 45 min of hypoxia in degassed Schneider’s media followed by BrdU labeling as described above.
Examination of Trachea
To examine tertiary tracheal branching, sections of the midgut just posterior to the proventriculus were dissected from wandering third instar larvae. Dissected tissue was placed on a slide in minimal liquid (5 μl; PBS + 5% Ficoll, Sigma) over which a coverslip was placed. Tissues were viewed using DIC optics within 5 min, after which time the trachea became less distinct due to influx of liquid.
Acknowledgments
We wish to thank Cahir O’Kane for the hs-iNOS stock and Marla Sokolowski for providing the fors, fors2, and forR stocks as well as valuable advice and discussion. We also wish to thank Ira Herskowitz, Mary Maxon, and members of the O’Farrell lab for critical reading of the manuscript. This research was supported by a Sandler Award and NIH grant to P. H. O. and fellowships to J. A. W. from the NIH and the Cancer Coordinating Research Committee. J. A. W. is an Abbott Fellow of the Life Science Research Foundation.
References
- Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994;13:301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Hilliker AJ, Sokolowski MB. Genetic localization of foraging (for): a major gene for larval behavior in Drosophila melanogaster. Genetics. 1989;123:157–163. doi: 10.1093/genetics/123.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, O’Farrell PH. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell. 1990;62:469–480. doi: 10.1016/0092-8674(90)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe VE. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development. 1989;107:1–22. [PubMed] [Google Scholar]
- Foe VE, Alberts BM. Reversible chromosome condensation induced in Drosophila embryos by anoxia: visualization of interphase nuclear organization. J Cell Biol. 1985;100:1623–1636. doi: 10.1083/jcb.100.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF. Studies on relaxation of rabbit aorta by sodium nitrate: basis for the proposal that the acid-activatable component of the inhibitory factor is inorganic nitrate and the endothelium-derived relaxing factor is nitric oxide. In: Vanhoutte PM, editor. Mechanisms of Vasodilatation. New York: Raven; 1988. pp. 401–414. [Google Scholar]
- Gerzer R, Bohme E, Hofmann F, Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981;132:71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- Gibbs SM, Truman JW. Nitric oxide and cyclic GMP regulate retinal patterning in the optic lobe of Drosophila. Neuron. 1998;20:83–93. doi: 10.1016/s0896-6273(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ, Jr, Giaccia AJ. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Ho YS, Wang YJ, Lin JK. Induction of p53 and p21/WAF1/CIP1 expression by nitric oxide and their association with apoptosis in human cancer cells. Mol Carcinog. 1996;16:20–31. doi: 10.1002/(SICI)1098-2744(199605)16:1<20::AID-MC4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Sasaguri T, Kosaka C, Nojima H, Ogata J. Induction of the cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) by nitric oxide-generating vasodilator in vascular smooth muscle cells. J Biol Chem. 1997;272:10050–10057. doi: 10.1074/jbc.272.15.10050. [DOI] [PubMed] [Google Scholar]
- Kalderon D, Rubin GM. cGMP-dependent protein kinase genes in Drosophila. J Biol Chem. 1989;264:10738–10748. [PubMed] [Google Scholar]
- Kantrow SP, Huang YC, Whorton AR, Grayck EN, Knight JM, Millington DS, Piantadosi CA. Hypoxia inhibits nitric oxide synthesis in isolated rabbit lung. Am J Physiol. 1997;272:1167–1173. doi: 10.1152/ajplung.1997.272.6.L1167. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Kuzin B, Roberts I, Peunova N, Enikolopov G. Nitric oxide regulates cell proliferation during Drosophila development. Cell. 1996;87:639–649. doi: 10.1016/s0092-8674(00)81384-7. [DOI] [PubMed] [Google Scholar]
- Lepoivre M, Flaman JM, Bobe P, Lemaire G, Henry Y. Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide. Relationship to cytostasis induced in tumor cells by cytotoxic macrophages. J Biol Chem. 1994;269:21891–21897. [PubMed] [Google Scholar]
- Locke M. The co-ordination of growth in the tracheal system in insects. Q J Microsc Sci. 1958;99:373–391. [Google Scholar]
- Manning G, Krasnow MA. Development of the Drosophila tracheal system. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 609–685. [Google Scholar]
- Meulemans A. Diffusion coefficients and half-lives of nitric oxide and N-nitroso-L-arginine in rat cortex. Neurosci Lett. 1994;171:89–93. doi: 10.1016/0304-3940(94)90612-2. [DOI] [PubMed] [Google Scholar]
- Millar TM, Stevens CR, Blake DR. Xanthine oxidase can generate nitric oxide from nitrate in ischaemia. Biochem Soc Trans. 1997;25:528S. doi: 10.1042/bst025528s. [DOI] [PubMed] [Google Scholar]
- Nambu JR, Chen W, Hu S, Crews ST. The Drosophila melanogaster similar bHLH-PAS gene encodes a protein related to human hypoxia-inducible factor 1 alpha and Drosophila single-minded. Gene. 1996;172:249–254. doi: 10.1016/0378-1119(96)00060-1. [DOI] [PubMed] [Google Scholar]
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluha W, Schonhoff CM, Harrington KS, Lachyankar MB, Crosbie NE, Bulseco DA, Ross AH. A novel, nerve growth factor-activated pathway involving nitric oxide, p53, and p21WAF1 regulates neuronal differentiation of PC12 cells. J Biol Chem. 1997;272:24002–24007. doi: 10.1074/jbc.272.38.24002. [DOI] [PubMed] [Google Scholar]
- Reutov VP, Sorokina EG. NO-synthase and nitrite-reductase components of nitric oxide cycle. Biochemistry. 1998;63:874–884. [PubMed] [Google Scholar]
- Roy B, Lepoivre M, Henry Y, Fontecave M. Inhibition of ribonucleotide reductase by nitric oxide derived from thionitrites: reversible modifications of both subunits. Biochemistry. 1995;34:5411–5418. doi: 10.1021/bi00016a012. [DOI] [PubMed] [Google Scholar]
- Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorati C, Nistico G, Meldolesi J, Clementi E. Nitric oxide effects on cell growth: GMP-dependent stimulation of the AP-1 transcription complex and cyclic GMP-independent slowing of cell cycling. Br J Pharmacol. 1997;122:687–697. doi: 10.1038/sj.bjp.0701413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374:811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB. Foraging strategies of Drosophila melanogaster: a chromosomal analysis. Behav Genet. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld M, Ward M, Nystrom G, Mosher J, Stahl S, Crews S. The Drosophila tango gene encodes a bHLH-PAS protein that is orthologous to mammalian Arnt and controls CNS midline and tracheal development. Development. 1997;124:4571–4582. doi: 10.1242/dev.124.22.4571. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Truman JW, De Vente J, Ball EE. Nitric oxide sensitive guanylate cyclase activity is associated with the maturational phase of neuronal development in insects. Development. 1996;122:3949–3958. doi: 10.1242/dev.122.12.3949. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268:21513–21528. [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton AR, Simonds DB, Piantadosi CA. Regulation of nitric oxide synthesis by oxygen in vascular endothelial cells. Am J Physiol. 1997;272:1161–1166. doi: 10.1152/ajplung.1997.272.6.L1161. [DOI] [PubMed] [Google Scholar]
- Wildemann B, Bicker G. Developmental expression of nitric oxide/cyclic GMP synthesizing cells in the nervous system of Drosophila melanogaster. J Neurobiol. 1999;38:1–15. doi: 10.1002/(sici)1097-4695(199901)38:1<1::aid-neu1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Yao KS, Clayton M, O’Dwyer PJ. Apoptosis in human adenocarcinoma HT29 cells induced by exposure to hypoxia. J Natl Cancer Inst. 1995;87:117–122. doi: 10.1093/jnci/87.2.117. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Naughton D, Winyard PG, Benjamin N, Blake DR, Symons MC. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 1998;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]