Abstract
The Nlrp3 inflammasome is critical for the activation of caspase-1 in response to danger signals and particulate matter. However, its role in sterile inflammation remains unclear because pre-stimulation of phagocytic cells with microbial molecules is required for caspase-1 activation. We show here that exposure of macrophages and dendritic cells to TNF-α promotes ATP- or silica-mediated caspase-1 activation and IL-1β secretion in the absence of microbial stimulation. The effect of TNF-α was abolished in macrophages deficient in TNF receptor I and II, Nlrp3 or ASC whereas that induced by TLR ligands required MyD88/Trif. In addition to TNF-α, IL-1α and IL-1β promoted caspase-1 activation via Nlrp3 in response to ATP. Remarkably, macrophages tolerized to TNF-α, but not to LPS, retained full sensitivity to ATP stimulation via Nlrp3. These results provide a mechanism by which danger signals and particulate matter mediate inflammation via the Nlrp3 inflammasome in the absence of microbial infection.
Keywords: Monocytes/Macrophages, Inflammation, Cytokines
Introduction
The innate immune system provides the first line of defense against invading pathogens, but also is involved in the development of inflammatory responses that occurs in a sterile environment. DCs and macrophages contribute to the innate immune response by sensing microbial structures or danger signals which induces the production of pro-inflammatory molecules and the activation of adaptive immune responses (1). In infection, the induction of inflammatory responses relies on the activation of host pattern-recognition receptors including TLRs and Nod-like receptors (NLRs) that recognize conserved and unique microbial structures (2, 3). The mechanism by which inflammation is activated and maintained in a sterile environment is less understood (1, 4). Members of the NLR family of proteins have been recently linked to the recognition of microbial and danger signals and activation of inflammatory responses (5, 6). In response to microbial stimuli, certain NLR family members including Nlrc4 and Nlrp3 assemble a large multi-protein complex, called the ‘inflammasome’, that induces the processing of pro-caspase-1 into the enzymatically active enzyme composed of two p20 and p10 chains (6). Subsequently, active caspase-1 processes pro-IL-1β into the biologically active IL-1β molecule which acts synergistically with other cytokines in the orchestration of inflammatory responses (6). Nlrp3 (also called Cryopyrin and Nalp3) is critical for caspase-1 activation and secretion of IL-1β in macrophages stimulated with several microbial molecules, ATP, urate crystals, silica or asbestos particles (6). The importance of Nlrp3 in inflammatory homeostasis is underscored by the observation that mutations of this NLR are associated with the development of familial autoinflammatory syndromes (7). The activation of caspase-1 via Nlrp3 requires a signal provided by a microbial ligand such as LPS (6). In addition, a second signal that includes ATP or particulate matter, is required for caspase-1 activation induced via the Nlrp3 inflammation (6). Thus, it is unclear how the Nlrp3 inflammasome contributes to inflammation under sterile conditions. In the present study, we identified a mechanism involving TNF-α and IL-1 that promotes activation of the Nlrp3 inflammasome in the absence of microbial stimulation.
Experimental Procedures
Mice
Casp-1−/−, Nlrp3−/−, and Asc−/− in a C57BL6 background have been described (8). TLR4−/−and MyD88−/−/Trif−/− mice were provided by Dr. Shizuo Akira (Osaka University, Japan). TnfrI/II−/− and C57BL6 mice were purchased from Jackson Laboratories. All animal studies were approved by the University of Michigan Committee on Use and Care of Animals.
Reagents and Bacterial Infection
ATP was from Sigma. Ultrapure LPS from E. coli, Pam3CSK (BLP), poly I:C and CpG were from Invivogen. Recombinant TNF-α was purchased from R&D or Peprotech with identical results. Endotoxin contamination in TNF-α preparations was less than 0.1 ng/μg of protein. Recombinant sCD40L and IFN-γ were from Peprotech. Agonistic anti-Fas antibody (clone Jo2) was from BD Pharmingen. IL-1α, IL-1β and RANKL were from R&D. Salmonella enterica serovar Typhimurium strain SL1344 was a gift of D. Monack, Stanford University. Infection of macrophages with S. Typhimurium was performed as described (9).
Cell isolation
Bone marrow-derived macrophages (BMDM) were isolatedas previously described (9). For the differentiation of DCs, bone marrow cells were cultured with GM-CSF (10 ng/ml) with fresh GM-CSF added at day 3 and 5. After 6–7 days, greater than 90% of the floating cells were CD11c+ and were used as DCs. Human PBMC were purified from venous blood of healthy volunteers by density centrifugation over Ficoll-Paque (Pharmacia Biotech). Monocytes were then enrichedby adherence to plastic dishes.
Immunoblotting and cytokine measurements
Preparation of cell extracts and immunoblotting have been described (14). Membranes were probed with antibodies anti caspase-1, a gift from Dr. Vandanabeele (Ghent University, Ghent, Belgium). Antibodies against IκBα, p-IκBα, ERK and p-ERK were from Cell Signaling. Amounts of IL-1β, TNF-α and IL-6 were measured by ELISA (R & D Systems, Minneapolis, MN).
Statistical analysis
Statistical significance between groups was determined by two tailed Student’s t test. Differences were considered significant when p < 0.01.
Results and Discussion
TNF-α, IL-1α and IL-1β sensitize macrophages and dendritic cells to caspase-1 activation triggered by ATP
TNF-α induces IL-1β secretion (10). However, the mechanism involved remains largely unknown. To understand the link between TNF-α stimulation and IL-1β secretion, we initially assessed whether TNF-α can induce caspase-1 activation in mouse bone-marrow derived macrophages and DCs. Stimulation of macrophages with TNF-α, CD40L, agonistic anti-Fas antibody, IFN-γ or LPS alone did not induce caspase-1 activation as determined by immunoblotting with an antibody that recognizes the p20 subunit of mature caspase-1 (Fig. 1A). However, brief stimulation with ATP induced processing of pro-caspase-1 in macrophages pre-treated with TNF-α or LPS, but no or minimal caspase-1 activation was observed with CD40L, an agonistic anti-Fas antibody, or IFN-γ (Fig. 1). Incubation of macrophages with CD40L and Fas antibody induced IκBα phosphorylation and degradation indicating that they were stimulatory (supplemental Fig. 1). Likewise, stimulation with IL-1α or IL-1β, but not receptor activator of NF-κB-ligand (RANKL) or PMA, induced robust caspase-1 activation in the presence of ATP (Fig. 1B). Similar results were obtained with DCs (data not shown). Activation of caspase-1 by TNF-α was detected after incubation with ATP for 5 min and increased with longer ATP stimulation (supplemental Fig. 2). Furthermore, the levels of caspase-1 activation augmented with increasing concentrations of TNF-α (supplemental Fig. 2). TNF-α also promoted caspase-1 activation in response to silica (Fig. 1C), another stimulus that activates the Nlrp3 inflammasome. However, TNF-α did not enhance caspase-1 processing triggered by infection with S. Typhimurium (Fig. 1C), an intracellular bacterium that induces caspase-1 activation through Nlrc4 (9). These results indicate that cytokines can induce caspase-1 activation in macrophages stimulated with ATP or silica in the absence of microbial stimulation.
Figure 1.
TNF-α induces caspase-1 activation in response to ATP and silica in the absence of microbial stimulation. A, BMDM were stimulated for 6 hrs with TNF (100 ng/ml), CD40L (10μg/ml), agonistic anti-Fas antibody (10μg/ml), IFN-γ (100 U/ml) or LPS (10 ng/ml). When indicated ATP (5 mM) was added for the last 30 minutes B, BMDM were stimulated for 6 hrs with PMA (100 ng/ml), RANKL (1 μg/ml), IL-1α (10 ng/ml), IL-1β (10 ng/ml). When indicated ATP (5 mM) was added for the last 30 minutes. C, BMDM were stimulated with TNF-α and then stimulated with ATP, silica or infected with S. Typhimurium (Salm). (A–C) Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 (p45) and its processed p20 subunit. Results are representative of three separate experiments.
Induction of caspase-1 activation and IL-1β secretion by TNF-α requires TNFR-I/II and the Nlrp3 inflammasome
Next we determined the requirement of TNF-α for the induction of caspase-1 activation and IL-1β secretion in response to ATP. First, we compared the ability of TNF-α to induce IL-1β secretion in wild-type and mutant macrophages deficient in TNFR-I and TNFR-II, the surface receptors required for TNF-α signaling. Secretion of IL-1β induced by TNF-α and ATP was abolished in macrophages lacking TNFR-I and TNFR-II or Nlrp3 when compared to wild-type macrophages (Fig. 2A and B). Consistently, caspase-1 activation induced by TNF-α and ATP was abrogated in macrophages lacking TNFR-I and TNFR-II, Nlrp3 or ASC (Fig. 2C and supplemental Fig. 3). Similarly, caspase-1 activation triggered by IL-1α and ATP was abolished in macrophages deficient in Nlrp3 or Asc (supplemental Fig. 3). The requirement of TNF receptors was specific in that caspase-1 activation and IL-1β secretion triggered by LPS and ATP was abolished in macrophages lacking Nlrp3 but not TNFR-I and TNFR-II (supplemental Fig. 4). Stimulation of the purinergic P2X7 receptor, an ATP-gated ion channel, is required for activation of the Nlrp3 inflammasome in response to LPS and ATP (11). We found that the P2X7 receptor was also required for caspase-1 activation triggered by ATP in TNF-αstimulated macrophages (supplemental Fig. 5). Thus, activation of the inflammasome by TNF-α in response to ATP is mediated by Nlrp3 and requires the TNF and P2X7 receptors.
Figure 2.
TNF-α promotes IL-1β secretion and caspase-1 activation via TNFR-I/II and the Nlrp3 inflammasome. A–B, BMDC from wild-type (WT), TnfrI/II deficient (TNFRI/II-DKO) and Nlrp3 deficient (Nlrp3-KO) mice were stimulated with TNF-α for 6 hrs and then stimulated, or not, with ATP for 30 minutes. IL-1β was measured 4 hrs after stimulation in cell-free supernatants by ELISA. Values represent mean ± SD of triplicate cultures. * p < 0.01 between WT and mutant macrophages. C, BMDM were stimulated as in panels A and B. Extracts were immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 (p45) and its processed p20 subunit. (A–B) Results are representative of three separate experiments.
Induction of caspase-1 activation by TNF-α requires gene transcription
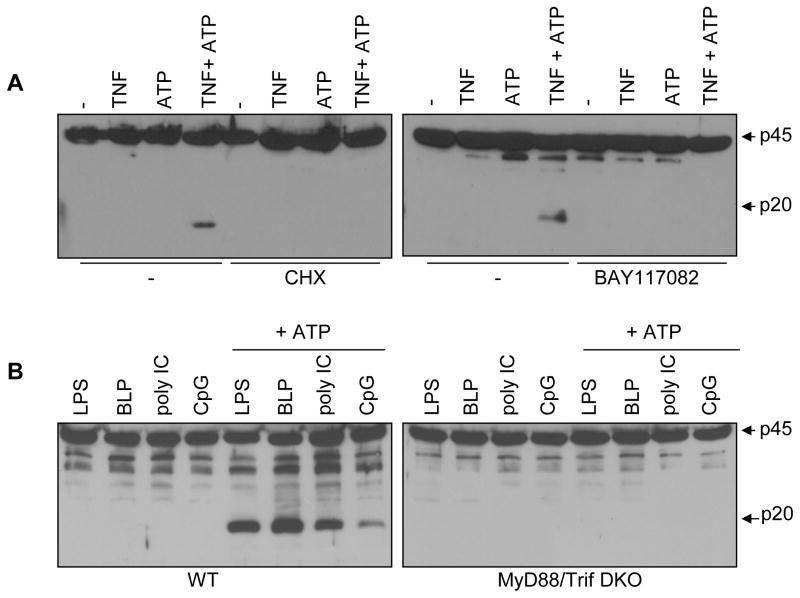
Next we sought to determine the mechanism by which TNF-α sensitizes macrophages to the danger signal ATP. Given that a long exposure to TNF-α was required for the induction of caspase-1 activation (see below), we tested whether sensitization to caspase-1 activation by TNF-α was affected by treatment with cyclohexamide (CHX), a general inhibitor of protein translation. Incubation of macrophages with CHX blocked caspase-1 activation induced by TNF-α and ATP (Fig. 3A). In addition, caspase-1 processing triggered by TNF-α and ATP was abrogated by treatment with BAY 11-7082 (Fig. 3A), a drug that inhibits NF-κB activation by targeting the Iκ-B kinase complex (12). Furthermore, the activation of NF-κB and mitogen-activated protein kinases (MAPKs) induced by TNF-α was comparable in wild-type and Nlrp3-deficient macrophages (supplemental Fig. 6), indicating that TNF-α does not act by regulating NF-κB or MAPKs activation through Nlrp3. CHX did not affect caspase-1 activation broadly in that it did not impair activation of caspase-1 induced by infection with S. Typhimurium which relies on the Nlrc4 inflammasome (supplemental Fig. 7). Initial experiments suggested that LPS triggers caspase-1 activation independently of TLR4 (13). However, recent studies showed that LPS can induce caspase-1 via a TLR4-Trif pathway (14). We reexamined this issue and found that, in agreement with the recent studies (14), LPS required TLR4 to promote caspase-1 activation in response to ATP (Supplemental Fig. 8). Similar to TNF-α, the induction of caspase-1 by LPS and ATP was inhibited by CHX and BAY 11-7082 (Supplemental Fig. 8). Consistently, activation of caspase-1 by LPS, synthetic lipopeptide (TLR2 ligand), poly I:C (TLR3 ligand) and CpG (TLR9 ligand) in response to ATP was abolished in macrophages deficient in MyD88 and Trif (Figure 3B). These results suggest that TNF-α and TLR ligands promote activation of the Nlrp3 inflammasome, at least in part, through gene transcription and NF-κB.
Figure 3.
TNF-mediated activation of caspase-1 requires gene transcription. A, BMDM were pretreated with 50 μM of cyclohexemide (CHX) or the NF-κB inhibitor BAY 11-7082 (20μM) for 1 hour, and then stimulated with TNF-α for 6 hrs. When indicated ATP was added for the last 30 minutes. B, BMDM from WT or MyD88−/−/Trif−/− mice (DKO) were stimulated for 6 hrs with LPS (1ug/ml), BLP (1ug/ml), poly IC (1ug/ml) or CpG (1ug/ml). When indicated ATP (5 mM) was added for the last 30 minutes. A and B, extracts were immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 (p45) and its processed p20 subunit Results are representative of three separate experiments.
TNF-α, but not LPS, promotes sustained ATP-induced caspase-1 activation and IL-1β secretion via the Nlrp3 inflammasome
Previous studies showed that prolonged stimulation with TNF-α rendered macrophages refractory to a successive stimulation with TNF-α or LPS (15). Therefore, we tested whether prolonged exposure of macrophages to TNF-α reduces the sensitivity to caspase-1 activation in response to ATP. In line with published results (15), the activation of NF-κB and MAPK in response to TNF-α was impaired in macrophages pre-stimulated with TNF-α for 24 hrs (Supplemental Fig. 9). Similarly, macrophages stimulated with TNF-α or LPS for 24 hrs become refractory to LPS, synthetic bacterial lipopeptide (TLR2 agonist) and CpG (TLR9 agonist) as determined by reduced secretion of TNF-α and IL-6 when compared to the response observed in naïve macrophages (Supplemental Fig. 10). We asked next whether macrophages tolerized to TNF-α or LPS could respond to a danger signal such as ATP. Remarkably, secretion of IL-1β and caspase-1 activation was induced in macrophages stimulated with TNF-α for 6 hrs and further enhanced with 24 hrs of stimulation, both of which required Nlrp3 (Fig. 4A and 4B). In contrast, stimulation of macrophages with LPS for 6 hrs induced IL-1β secretion and caspase-1 activation but the cells were refractory to ATP stimulation after LPS treatment for 24 hrs (Fig. 4C and 4D). We tested next whether these findings could be extended to human monocytes. In agreement with published results (16, 17), ATP potentiated the production of IL-1β when human monocytes were pre-treated with LPS for 4 hrs but not 24 hrs (Supplemental Fig. 11). Consistent with the mouse results, the secretion of IL-1β in response to ATP was enhanced ~ 40-fold in monocytes pre-stimulated for 24 hrs with TNF-α but not LPS (Supplemental Fig. 11). These results indicate that both human monocytes and mouse macrophages rendered insensitive to TNF-α by continuous stimulation with TNF-α remain responsive to ATP stimulation by producing IL-1β
Figure 4.
TNF-α, but not LPS, induces ATP-mediated IL-1β secretion and caspase-1 activation via Nlrp3 in tolerized macrophages. A–D, BMDM were left unstimulated or stimulated with TNF-α (100 ng/ml) (A–B) or LPS (C and D) for 6 or 24 hrs and then stimulated with ATP (5 mM) for 30 min. A–C, IL-1β was measured after stimulation in cell-free supernatants by ELISA. Values represent mean ± SD of triplicate cultures. n. d. refers to undetectable. * p < 0.01 between WT and mutant macrophages. B–D, Extracts were immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 (p45) and its processed p20 subunit. (A–D) Results are representative of three separate experiments.
We show that stimulation with TNF-α or IL-1 is sufficient to trigger caspase-1 activation and IL-1β secretion in response to ATP, providing a mechanism for activation of the Nlrp3 inflammasome in the absence of infection. TNF-α induces the synthesis of pro-IL-1β (18), but our results indicate that it cannot trigger alone the activation of caspase-1, a step that is required for IL-1β secretion, in mouse macrophages. TNF-α required the P2X7 receptor to induce caspase-1 in response to ATP which may act, at least in part, by inducing K+ efflux (6). Like LPS, the activation of caspase-1 induced by TNF-α was suppressed by CHX and by an inhibitor of NF-κB activation, suggesting that TNF-α and LPS stimulation induces a factor(s) which contributes to the activation of the Nrlp3 inflammasome. A potential candidate factor is Nlrp3 itself because its expression is induced by TNF-α and TLR ligands in human monocytes (19). Alternatively, TNF-α may promote the formation of endogenous ligand(s) that could act as an agonist of Nlrp3. Regardless of the mechanism, our results indicate that endogenous cytokines such as TNF-α and IL-1 can functionally substitute for microbial stimuli to activate the Nlrp3 inflammasome in response to ATP and silica.
Gain-of-function mutations in NLRP3 are associated with the development of autoinflammatory syndromes (7) which is associated with constitutive activation of NLRP3 and inappropriate production of IL-1β (20). Monocytes from patients with NLRP3 mutations produce elevated levels of TNF-α subsequently to IL-1β secretion (21). Thus, TNF-α and IL-1β could participate in a positive feedback loop to augment constitutive caspase-1 activation observed in patients with autoinflammatory syndromes. Similarly, the fibrillar peptide amyloid-beta which is associated to the pathogenesis of Alzheimer's disease, requires LPS stimulation to induce activation of the Nlrp3 inflammasome (22). Because amyloid-beta fibrils induce the release TNF-α (23), it is possible that this cytokine participates in the activation of Nlrp3 inflammasome in the brain of patients with Alzheimer's disease. Here we show that macrophages tolerized to TNF-α, but not to TLR ligands, retain full sensitivity to the danger signal ATP. Our results suggest that ATP is capable of breaking the tolerant state that results from long exposure to TNF-α through the activation of the Nlrp3 inflammasome. Thus, danger signals may play a role in maintaining inflammation in diseases characterized by chronic production of TNF-α and/or IL-1.
Supplementary Material
Acknowledgments
We thank Richard Flavell, Shizuo Akira and Millenium Pharmaceuticals for generous supply of mutant mice, Joel Whitfield for technical support, and Sherry Koonse for excellent animal husbandry.
Abbreviations used in this paper
- BMDM
bone marrow derived macrophages
- CHX
cyclohexamide
- NLR
Nod-like receptors
- Nlrp3
NLR family, pyrin domain containing 3
Footnotes
This work was supported by grants AI063331 and AI064748. L. Franchi was supported by a Fellowship from the Arthritis Foundation and T. Eigenbrod by a Fellowship from the Deutsche Forschungsgemeinschaft (DFG).
Disclosures
The authors declare that no competing interest exist
References
- 1.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 5.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 8.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 9.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA, Jr, O'Connor JV. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 12.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 13.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 15.Fraker DL, Stovroff MC, Merino MJ, Norton JA. Tolerance to tumor necrosis factor in rats and the relationship to endotoxin tolerance and toxicity. J Exp Med. 1988;168:95–105. doi: 10.1084/jem.168.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, Van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1{beta} in monocytes and macrophages. Blood. 2008 doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marucha PT, Zeff RA, Kreutzer DL. Cytokine regulation of IL-1 beta gene expression in the human polymorphonuclear leukocyte. J Immunol. 1990;145:2932–2937. [PubMed] [Google Scholar]
- 19.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol. 2003;171:6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 20.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren S, Mueller JL, Anderson JP, Niehaus BL, Misaghi A, Anderson S, Boyle DL, Hoffman HM. Monocytes from familial cold autoinflammatory syndrome patients are activated by mild hypothermia. J Allergy Clin Immunol. 2007;119:991–996. doi: 10.1016/j.jaci.2006.12.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casal C, Serratosa J, Tusell JM. Relationship between beta-AP peptide aggregation and microglial activation. Brain Res. 2002;928:76–84. doi: 10.1016/s0006-8993(01)03362-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.