Abstract
Background
Roughex (Rux) is a cell-cycle regulator that contributes to the establishment and maintenance of the G1 state in the fruit fly Drosophila. Genetic data show that Rux inhibits the S-phase function of the cyclin A (CycA)–cyclin-dependent kinase 1 (Cdk1) complex; in addition, it can prevent the mitotic functions of CycA and CycB when overexpressed. Rux has no homology to known Cdk inhibitors (CKIs), and the molecular mechanism of Rux function is not known.
Results
Rux interacted with CycA and CycB in coprecipitation experiments. Expression of Rux caused nuclear translocation of CycA and CycB, and inhibited Cdk1 but not Cdk2 kinase activity. Cdk1 inhibition by Rux did not rely on inhibitory phosphorylation, disruption of cyclin–Cdk complex formation or changes in subcellular localization. Rux inhibited Cdk1 kinase in two ways: Rux prevented the activating phosphorylation on Cdk1 and also inhibited activated Cdk1 complexes. Surprisingly, Rux had a stimulating effect on CycA–Cdk1 activity when present in low concentrations.
Conclusions
Rux fulfils all the criteria for a CKI. This is the first description in a multicellular organism of a CKI that specifically inhibits mitotic cyclin–Cdk complexes. This function of Rux is required for the G1 state and male meiosis and could also be involved in mitotic regulation, while the stimulating effect of Rux might assist in any S-phase function of CycA–Cdk1.
Background
Cell-cycle progression is controlled by cyclin-dependent kinases (Cdks) [1]. Downregulation of Cdk activity can occur by inhibitory phosphorylation, cyclin proteolysis or through the action of Cdk inhibitors (CKIs) [2,3]. CKIs mediate cell-cycle arrest at specific stages by acting on Cdks, cyclins or cyclin (Cyc)–Cdk complexes. In higher eukaryotes, there are two distinct families of CKIs, the p21Kip/p27Cip family and the INK4 family of inhibitors [3]. Both inhibit S-phase-promoting Cdk activity, thereby safeguarding the stability of G1. CKI targets are complexes consisting of G1 cyclins with Cdk2, Cdk4 or Cdk6 [4–7]. No inhibitors of Cyc–Cdk1 complexes have been described so far in higher eukaryotes.
In Drosophila, a single CKI, Dacapo, has been identified that belongs to the p21/p27 class of CKIs [8,9]. Dacapo is required for the establishment of the first G1 state of embryonic development during cycle 17. It binds CycE–Cdk2 and inhibits kinase activity in vivo and in vitro [8]. Dacapo is also required at later stages, for example during eye development, for the establishment and maintenance of G1 [9].
Although CycE–Cdk2 is the main S-phase inducer in Drosophila, CycA–Cdk1 is also able to trigger a G1–S transition [10]. For the establishment and maintenance of the G1 state, CycA–Cdk1 activity must therefore be downregulated as well. During Drosophila embryogenesis, this S-phase function of CycA is normally suppressed in G1 by at least three different mechanisms: inhibitory phosphorylation of Ckd1, CycA instability during G1 and the activity of Roughex (Rux) [10].
The rux gene was initially identified in a screen for mutations affecting development of the Drosophila eye [11]. During eye development, cells are synchronized in G1 in the morphogenetic furrow where neuronal differentiation is initiated. In rux mutants, this critical G1 phase is not established and cells undergo a premature S phase. Mutations in rux also cause reduced viability and male sterility [11,12]. During spermatogenesis, rux germ cells go through meiosis I and II but then attempt an additional aberrant division, resulting in aneuploid nuclei. The rux phenotypes are dominantly suppressed by mutations in cycA and genes encoding the Drosophila CDC25 homologs, string and twine [11,12]. A genetic interaction between cycA and rux can also be demonstrated when both genes are overexpressed: the expression of CycA can induce ectopic S phases but the simultaneous expression of Rux inhibits these S phases [10,13]. By contrast, S phases that are triggered by CycE–Cdk2 activity are not prevented when Rux is overexpressed [13]. This indicates that Rux is not a general S-phase suppressor, but that it specifically prevents CycA-associated S-phase activity. In addition, when Rux is expressed during S or G2, Cdk1-dependent mitoses are inhibited [13]. At the cellular level, both effects of Rux are associated with a change in the subcellular distribution of the mitotic cyclins CycA and CycB. Normally, both cyclins are present in the cytoplasm during interphase; during prophase they accumulate in the nucleus and are degraded during mitosis [14,15]. In G1, turnover of mitotic cyclins is high and only very low levels can be detected. When Rux is overexpressed in G1 together with CycA, a transient nuclear localization can be observed, followed by degradation of CycA [13]. When Rux is expressed during S and G2 or if the rux gene dose is increased during spermatogenesis, CycA is again translocated to the nucleus [11,12].
All of the above data suggest that Rux acts by inhibiting mitotic Cyc–Cdk activity. Rux shares no homology to any other CKI or other proteins in the sequence databases, however, nor have any molecular interactions between CycA and Rux been demonstrated. Rux does interact with CycE in vitro and in a yeast two-hybrid system and is phosphorylated by CycE–Cdk2 in vitro. Overexpression of CycE results in degradation of Rux, suggesting that phosphorylation of Rux by CycE–Cdk2 activity at the G1–S transition targets Rux for degradation [13]. The failure to detect any direct association between Rux and CycA in vitro and the lack of homology to known CKIs led to the suggestion that Rux itself is not a CKI but, rather, promotes the activities of an unknown CKI [10,13].
Here, we provide evidence that Rux is a CKI. We found that CycA and CycB associate with Rux in vivo. The expression of Rux in embryos resulted in a strong reduction of Cdk1 kinase activity. This inhibition of kinase activity was independent of inhibitory phosphorylation of Cdk1. In vitro analysis demonstrated that Rux prevents the activating phosphorylation of Cdk1 on Thr161 and that Rux also inhibits Cdk1 activity by other means. Interestingly, Rux had a dual effect on CycA-associated kinase activity, resulting in activation of kinase activity at lower concentrations and inhibition at higher concentrations. These data indicate that Rux is a novel CKI that is present in a higher eukaryote and acts specifically on mitotic cyclins.
Results
Rux subcellular localization, its effect on cyclin localization and mitotic progression
As a tool to study protein interaction, we developed a Rux antibody (see Materials and methods). On western blots, it specifically recognized in vitro translated Rux protein and detected a specific band in 3–6 hour old embryos after Rux expression from a heat-inducible promoter (data not shown). In extracts from wild-type embryos of this stage, no Rux band was detected, consistent with the low abundance of Rux mRNA. To determine the subcellular localization of Rux, we performed indirect immunofluorescent labeling of embryos after heat-induced expression of Rux (Figure 1c,e) and after injection of mRNA coding for a haemagglutinin (HA)-tagged Rux (HA–Rux, Figure 1i,k). In both cases, a nuclear staining was observed, consistent with previous findings [13]. We identified a bipartite nuclear localization sequence (NLS; Figure 1h) at the carboxyl terminus of Rux, and created a deletion of HA–Rux lacking the last 13 amino acids (HA–RuxΔNLS), including the second basic cluster of the putative NLS. Immunofluorescent labeling of embryos after injection of HA–RuxΔNLS mRNA now showed a predominantly cytoplasmic staining of Rux (Figure 1p,r), indicating that the identified NLS is required for the normal nuclear localization of Rux.
Figure 1.
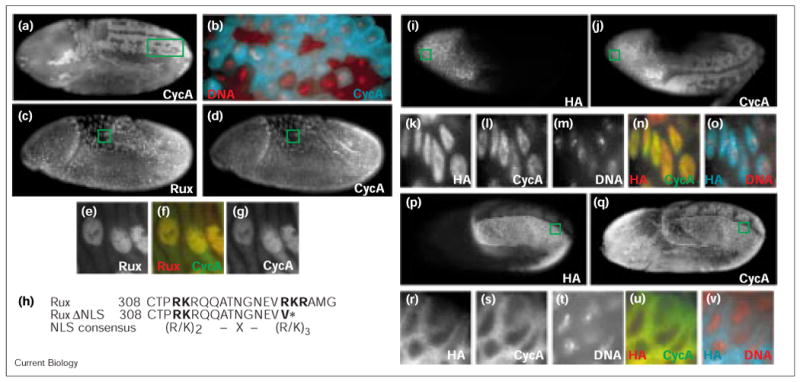
Rux is a nuclear protein that causes CycA accumulation in the nucleus and prevents mitosis. (a,b) CycA staining pattern in a wild-type embryo undergoing the 14th division cycle, revealing the normal mitotic pattern. (b) Magnified view of the boxed region in (a), stained for DNA (red) and CycA (cyan). Note the predominantly cytoplasmic staining in interphase cells (upper right corner) and the mitotic degradation of CycA starting in metaphase. (c–g) Staining of (c) Rux and (d) CycA in a 14th cell-cycle embryo after expression of Rux from a heat-shock transgene (hs–rux). The mitotic pattern of CycA turnover is absent. (e–g) High magnification views of amnioserosa cells (boxed in panels c and d). (f,g) CycA and (e,f) Rux colocalize in the nucleus, while CycA is also present in the cytoplasm. (h) Comparison of the Rux sequence commencing at amino-acid position 308 with the consensus sequence for bipartite NLSs [43] and the sequence of the RuxΔNLS construct (a stop codon was introduced at the position marked with an asterisk). (i–o) Expression and subcellular localization of (i) HA–Rux and (j) CycA in embryos injected at the anterior end with HA–Rux mRNA. High levels of (i) HA staining can be observed in the head region where mitotic degradation of (j) CycA is blocked. Normal turnover of CycA can be seen in the remainder of the embryo where HA–Rux is absent. (k–o) High magnification views of cells marked in (i,j), showing nuclear localization of (k) Rux, (l) CycA and (m) DNA, and (n) merged Rux and CycA staining and (o) merged Rux and DNA staining. (p,q) Embryo injected with mRNA encoding HA–RuxΔNLS. (p) High levels of HA–RuxΔNLS can be seen in the posterior region on one side of the embryo. In this region, mitotic progression is blocked, as visualized by (q) the lack of CycA turnover. (r–v) High magnification views of cells marked in (p,q) showing cytoplasmic localization of (r) RuxΔNLS, (s) CycA and (t) nuclear DNA, as well as (u) merged Rux and CycA staining and (v) merged Rux and DNA staining.
Rux expression causes nuclear accumulation of mitotic cyclins [10,13]. Using our Rux antibodies, we could now determine whether Rux and mitotic cyclins occupy identical compartments within the cell. We found that Rux overexpression caused CycA accumulation in the nucleus (Figure 1d). Rux and CycA both occupied most of the nuclear space but were excluded from the nucleolus (Figure 1e–g). Although most of the Rux staining was restricted to the nucleus, we found that a proportion of CycA remained in the cytoplasm. On the other hand, when we expressed HA–RuxΔNLS, both Rux and CycA remained cytoplasmic (Figure 1p–v), showing that nuclear localization of Rux is required for the induced nuclear accumulation of CycA.
In cycles 14–16 of Drosophila embryogenesis, Cdk1 is held in an inactive state throughout S phase and G2 by inhibitory phosphorylations [16]. Controlled expression of Cdc25String phosphatase relieves these inhibitory constraints and induces mitosis in a complex spatial and temporal pattern. Mitotic progression can be monitored by the turnover of CycA, which is degraded in each mitosis in a stereotypical pattern (Figure 1a,b). After expression of Rux, no CycA turnover could be observed (Figure 1d), indicating that cells are prevented from undergoing mitosis. The absence of other mitotic markers (tubulin, condensed DNA or phosphorylated histone H3; data not shown) indicated that cells are blocked at a pre-mitotic step, most likely in a G2-like state. In embryos in which Rux was locally expressed by mRNA injection, we also observed a lack of mitotic progression in regions with high Rux levels, but normal mitotic pattern of CycA degradation in regions that have little or no Rux levels (Figure 1j). A lack of a normal mitotic pattern was also observed when HA–RuxΔNLS mRNA was injected into embryos. In the embryo shown in Figure 1q, the normal bilaterally occurring mitoses are blocked on the side of the embryos with high levels of HA–RuxΔNLS, whereas CycA degradation and, thus, mitotic progression occurs on the opposing side.
In summary, Rux, a nuclear protein, causes CycA localization to the nucleus and prevents entry into mitosis. The nuclear localization of Rux and CycA requires a functional NLS at the carboxyl terminus of Rux. In its absence, Rux remains predominantly cytoplasmic and no nuclear accumulation of CycA is observed. Nevertheless, Rux lacking the NLS is still able to inhibit mitosis, indicating that this effect of Rux does not require a nuclear localization.
Rux interacts with mitotic cyclins and causes inhibition of Cdk1 activity
The overlapping localization of CycA with Rux and RuxΔNLS raises the possibility that both proteins physically associate. To test whether Rux associates with cyclins in vivo, we performed immunoprecipitations from embryonic extracts, using Rux antiserum and looked for coprecipitation of cyclins and Cdk1. Using extracts from 3–5 hour hs–rux embryos, we immunoprecipitated Rux and could detect coprecipitation of CycA, CycB and, to a lesser extent, Cdk1 (Figure 2a,b). Rux did not precipitate control proteins like Even-skipped or β-tubulin (Figure 2a and data not shown).
Figure 2.
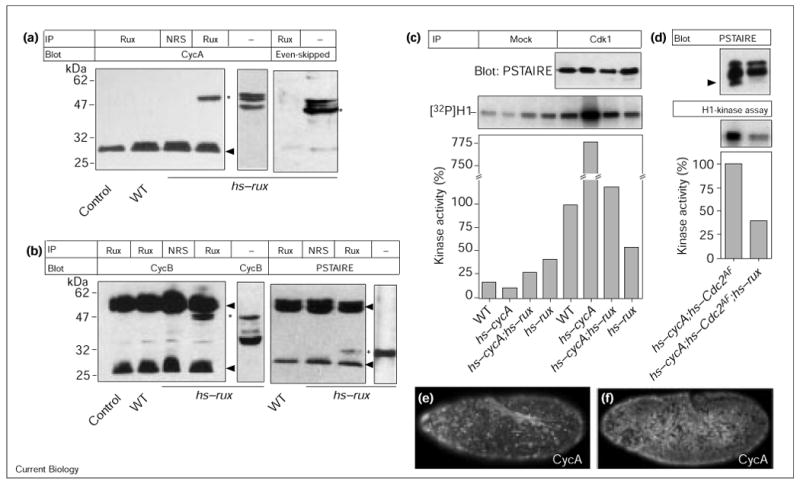
Rux binds CycA and CycB and inhibits Cdk1 kinase activity. (a,b) Rux coimmunoprecipitates mitotic cyclins and Cdk1. Wild-type (WT) and hs–rux embryonic extracts were immunoprecipitated (IP) with normal rat serum (NRS) or Rux antibodies and analyzed on western blots using antibodies against CycA, CycB, Even-skipped and the PSTAIRE motif. As a control, the extract was blotted with the respective antibodies to indicate the position of the relevant antigens (asterisks). In extracts, CycA migrates as a doublet, the band below the doublet is a cross-reacting band. Arrowheads, position of the immunoglobulin heavy and light chains. The secondary antibody used to detect the CycA primary antibody only recognizes light chain from the rat serum. (c) Cdk1 kinase activity is reduced after Rux expression. Extracts were made from 4–6 h old wild-type (lanes 1,5), hs–cycA (lanes 2,6), hs–cycA;hs–rux (lanes 3,7), and hs–rux (lanes 4,8) embryos. Precipitation was performed using normal rabbit serum (mock, lanes 1–4) or with Cdk1 antiserum (lanes 5–8). Kinase activity of wild-type embryos was set to 100%. Expression of cycA (lane 6) resulted in a sevenfold stimulation of kinase activity towards histone H1. Expression of rux (lane 8) caused reduction of kinase levels to half that found in the wild type; in embryos expressing cycA and rux (lane 7), levels were reduced to a seventh of those for embryos expressing CycA alone (lane 6). (d) Rux can suppress kinase activity of Cdc2AF, a mutant form of Cdk1 that lacks inhibitory phosphorylation sites. Extracts from heat-shocked hs–cycA;hs–Cdc2AF and hs–cycA;hs–Cdc2AF;hs–rux embryos were precipitated with Cdk1 antiserum and either blotted for the PSTAIRE western blot or used for the kinase assay with histone H1 as a substrate. The expression of Rux reduced total kinase levels to 40%. In the PSTAIRE western blot, several isoforms of Cdk1 could be detected, which derive from both endogenous, wild-type Cdk1 and Cdc2AF. The latter only comes in two forms, unphosphorylated and Thr161-phosphorylated. The majority of the wild-type Cdk1 is Thr14 and Tyr15 phosphorylated at this stage of embryogenesis and migrates with slower electrophoretic mobility. The bulk of the fastest migrating form is the Thr161-phosphorylated form of Cdc2AF (arrowhead). The coexpression of Rux led to a marked reduction in the levels of this phosphoisoform. (e,f) CycA staining in (e) hs–cycA;hs–Cdc2AF and (f) hs–cycA;hs–Cdc2AF;hs–rux embryos. Rux inhibited progression through mitosis, independent of inhibitory phosphorylations. Coexpression of CycA and Cdc2AF overran the normal mitotic pattern of cell division 14 in the embryos and induced simultaneous mitoses throughout the embryo. This resulted in a mitotic degradation of CycA in most of the cells in (e). Simultaneous expression of Rux prevented the execution of mitosis, visualized in (f) by the persistence of CycA staining in most cells.
To determine what consequence the interaction of Rux with mitotic cyclins has on Cdk1 kinase activity, we precipitated Cdk1 from embryonic extracts and determined its kinase activity using histone H1 as a substrate. High kinase activity levels were found in wild-type embryos (Figure 2c), which increased several fold upon CycA induction (hs-cycA; Figure 2c). The expression of Rux in either of these two situations led to a decrease in Cdk1 kinase activity (Figure 2c). Similar amounts of Cdk1 were precipitated in all four cases (Figure 2c, PSTAIRE immunoblot).
Inhibition of Cdk1 kinase activity can occur at multiple levels, including inhibitory phosphorylation of Cdk1 on Thr14 and Tyr15 [1]. To test whether Rux inhibits Cdk1 activity by modulating Thr14 and Tyr15 phosphorylation, we expressed a mutant form of Cdk1 in which the Thr14 and Tyr15 residues were substituted with Ala and Phe and, thus, cannot be phosphorylated on these residues (Cdc2AF). A simultaneous overexpression of Cdc2AF and CycA (hs–Cdc2AF;hs-cycA) was sufficient to induce mitosis throughout the entire embryo (Figure 2e). The coexpression of Rux prevented mitosis in most cells of the embryo (Figure 2f), however, demonstrating that the effect Rux does not rely on the Thr14 and Tyr15 phosphorylation state of Cdk1. In the absence of Thr14 and Tyr15 phosphorylation of Cdc2AF, the only phosphoisoform is the Thr161-phosphorylated form. This isoform shows a higher electrophoretic mobility than unphosphorylated Cdk1 on SDS–PAGE [17] and can readily be detected when Cdc2AF and CycA are coexpressed. The simultaneous expression of Rux led to a drastic reduction in the levels of this phosphoisoform and a corresponding decrease in kinase activity towards histone H1 (Figure 2d).
In conclusion, we observe an interaction of Rux with mitotic cyclins and a downregulation of Cdk1 activity. This inhibition is not mediated by inhibitory phosphorylation on Thr14 and Tyr15. We observe a significant reduction in the level of Thr161 phosphorylation on Cdk1, however, suggesting that Rux can modulate the Thr161 phosphorylation state of Cdk1.
Rux inhibits mitotic cyclins in vitro
To analyze Rux-dependent Cdk regulation in greater detail, we used an in vitro system that enabled us to analyze Cyc–Cdk complexes, permitted the addition of Rux at different steps of the Cdk activation process and allowed analysis of Cdk activation and activity. The proteins used were translated individually in reticulocyte lysate in the presence of [35S]Met (Figure 3a). We used HA-tagged Cdks to facilitate immunoprecipitation and amino-terminally truncated forms of mitotic cyclins (CycAΔ170 and CycBΔ46), which are more stable in our system. Cdk activation requires phosphorylation of Thr161 (Thr163 in Cdk2) by a Cdk-activating kinase (CAK) [18]. We used an embryonic extract from 0–1 hour old embryos as a source of CAK (‘extract’). This extract contains few free cyclins, which would interfere with our assay, and does not phosphorylate Cdk1 on Thr14 or Tyr15 [19]. The CAK in this extract is likely to be of the CycH–Cdk7 type, as extracts from cdk7 mutants fail to provide CAK activity (data not shown and [20]). After immunoprecipitation of HA–Cdks, kinase activity was measured using histone H1 as a substrate. In addition, a fraction of the immunoprecipitate was used to monitor Thr161 phosphorylation of Cdk1 by high-resolution SDS–PAGE (Figure 3c).
Figure 3.
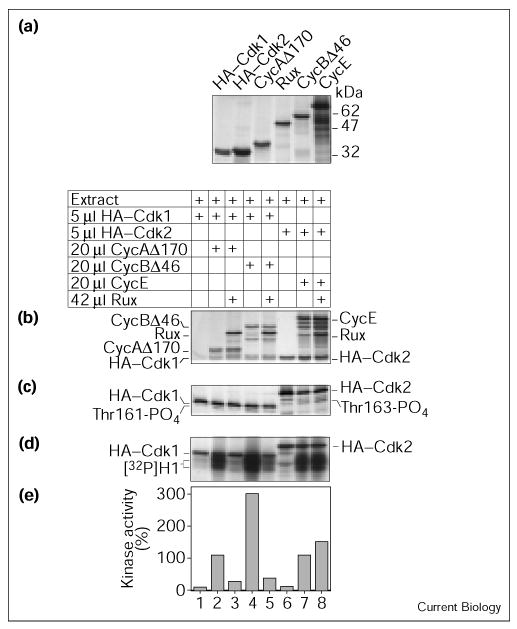
Rux is a specific inhibitor of mitotic cyclins. (a) Autoradiogram of 35S-labeled reticulocyte-translated proteins. (b–e) Immune-complex kinase assay of various cyclin–Cdk complexes in the absence or presence of Rux using histone H1 as substrate. Drosophila extract was used as a source of CAK. Immunoprecipitation was done using antibodies against the HA tag on Cdk1 and Cdk2. The presence of Rux reduced kinase activity of CycA–Cdk1 and CycB–Cdk1 but not that of CycE–Cdk2. (b) ‘Input gel’ showing presence and abundance of the different translation products in the individual experimental samples. (c) Phosphorylation state of the immunoprecipitated Cdks. Autoradiogram of a high-resolution SDS–PAGE to visualize the level of Thr161 and Thr163 phosphorylation of Cdk1 and Cdk2, respectively. Only the molecular weight range between 28 and 38 kDa is shown. (d) Autoradiogram after histone H1 phosphorylation and high-resolution SDS–PAGE. The 32P-labelled histone H1 signal appears as two blocks. The sharp bands above the histone H1 signal correspond to the 35S-labeled Cdks. (e) Phosphorimaging results from counting the 32P signal. The level of phosphorylation obtained with CycA–Cdk1 (lane 2) was set to 100%. We do not consider the minor increase in kinase activity between lanes 7 and 8 significant as it is not reproducible.
Incubation of reticulocyte-translated HA–Cdk1 or HA–Cdk2 with Drosophila extract alone did not result in significant Cdk activity (Figure 3d,e; lanes 1,6). Cdk activation was achieved by adding cyclin (lanes 2,4,7). The extent of activation varied for the different cyclins used, probably reflecting their different affinities for their Cdk partners. The presence of Rux in this system had a strong inhibitory effect on Cdk1 kinase activity, reducing the level of histone H1 phosphorylation to almost background levels (lanes 3,5). Both cases of inhibition displayed a corresponding decrease in the extent of Thr161 phosphorylation on Cdk1 (Figure 3c; lanes 3,5). In contrast, no significant change in the kinase activity of Cdk2 was observed (lane 8). This experiment was repeated three times, with similar results obtained each time. These data demonstrate that Rux specifically inhibits mitotic Cyc–Cdk1 activity, but does not inhibit CycE–Cdk2 activity. Using a Rux fusion protein tagged at the amino terminus with glutathione-S-transferase (GST), we did not observe any effects on Cdk1 activity. The addition of GST to the amino terminus might abrogate Rux function and could explain the failure of previous attempts to show inhibition of CycA-dependent kinase activity by Rux in vitro [13].
Rux modulation of CycA- and CycB-dependent kinase activity in vitro
To better analyze Rux regulation of Cdk1 kinase activity, we monitored the effects of different doses of Rux on CycA- and CycB-dependent kinase activity. When CycB was used as a cyclin partner for Cdk1, we observed an inhibition of kinase activity by Rux that increased with increasing amounts of Rux (Figure 4d,e). Looking at the Thr161-phosphorylated form of Cdk1, we found this isoform to some extent even in the absence of added CycB, which could result from the presence of free cyclins in the extract used (Figure 4c). Addition of CycB stimulated Thr161 phosphorylation but addition of Rux resulted in a marked decrease in the abundance of this phosphoisoform. The amount of CycB that coprecipitated with HA–Cdk1, on the other hand, remained constant throughout the experiment (Figure 4b).
Figure 4.
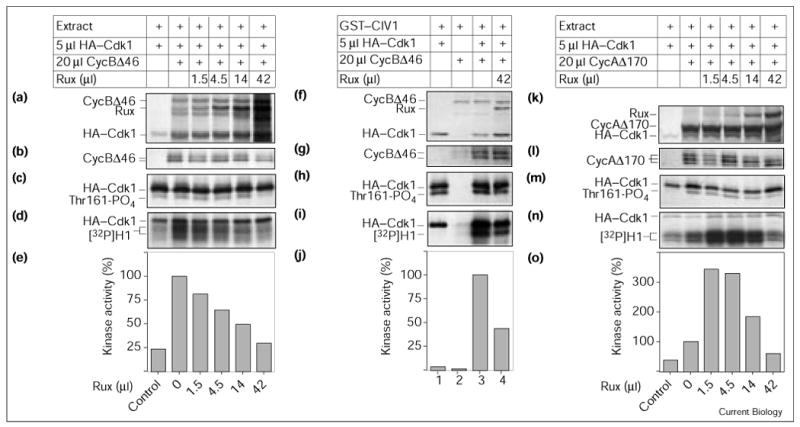
Rux modulation of CycA- and CycB-dependent kinase activity in vitro. (a,f,k) Levels of in vitro translated proteins used in this assay. (b,g,l) Levels of associated cyclins and (c,h,m) level of Thr161-phosphorylated HA–Cdk1 were visualized after SDS–PAGE and autoradiography of the immune complexes. (d,e,i,j,n,o) Kinase assays using histone H1 as substrate were performed after immunoprecipitation of HA–Cdk1. (a–e) Rux inhibits CycB-dependent Cdk1 kinase activity in a progressive fashion. Drosophila extract was used as a source of CAK. Incubation of HA–Cdk1 with extract resulted in some kinase activity, which was stimulated fourfold by the addition of CycB. Increasing amounts of Rux led to a reduction of kinase activity to one-third of the positive control. (b,c) The presence of larger amounts of Rux consistently led to a reduction in the Thr161-phosphorylated form of Cdk1 while no significant change in the level of CycB coprecipitation was seen. (f–j) Rux inhibition does not require additional Drosophila proteins and is independent of the source of CAK. Bacterially expressed GST–CIV1 was used instead of the crude Drosophila extract as a source of CAK. (h) GST–CIV1 was able to efficiently phosphorylate monomeric Cdk1 (lane 1) but kinase activity still depended on adding cyclin (lanes 1,3). Rux inhibited CycB–Cdk1 kinase activity to 43% of the positive control (lanes 3,4). No change in (g) cyclin coprecipitation or (h) Thr161 phosphorylation was seen. (k–o) Rux is a bimodal regulator of CycA–Cdk1 kinase activity. (n,o) CycA-induced kinase activity was stimulated threefold by lower concentrations of Rux (0 μl Rux versus 1.5 μl Rux). Any further addition of Rux had an inhibitory effect, reducing kinase levels close to background levels. (l) Levels of CycA coprecipitating remained fairly constant. Nevertheless, a reduction in the level of the slowest migrating phosphoisoform of CycA could be seen. (m) The level of Thr161 phosphorylation was reduced when higher amounts of Rux were present.
In the above experiments, we used a crude embryonic extract to provide CAK activity. This extract may contain accessory Drosophila molecules that mediate the effects of Rux. To exclude this possibility, we replaced the embryonic extract with a bacterially expressed GST fusion protein containing the CAK activity from the Saccharomyces cerevisiae CIV1 gene [21–23]. While this monomeric enzyme shares no homology with the CycH–Cdk7 complex, it can phosphorylate the Thr161 (or equivalent) position of several Cdks from higher eukaryotes [22]. Using GST–CIV1, we could activate Cdk1 in a cyclin-dependent manner. The level of Thr161 phosphorylation on Cdk1 remained constant in this experiment. CIV1 was able to phosphorylate monomeric Cdk1 (Figure 4h, lane 1) [22] and we did not find a significant increase in the Thr161-phosphorylated form after the addition of CycB. An effect of Rux on Thr161 phosphorylation of CycB–Cdk1 complexes could therefore not be detected. Addition of Rux resulted in an inhibition of kinase activity to about 40% of the positive control. This indicates that no additional Drosophila proteins are required for Rux inhibition of Cdk1 kinase activity. Furthermore, because Rux can inhibit CycB–Cdk1 activated by either extract or CIV1, we believe that Rux does not inhibit by acting on CAK.
In contrast to the effects of Rux on CycB–Cdk1 kinase activity, Rux had a more complex effect on CycA–Cdk1 activity. Figure 4k–o shows a typical example, which demonstrates that Rux has a dual effect on CycA–Cdk1 activity. In this assay, the addition of CycAΔ170 led to an approximate threefold stimulation of Cdk1 kinase activity in comparison to Cdk1 alone (Figure 4o, 0 μl Rux versus control). Unexpectedly, the presence of small amounts of Rux (1.5–4.5 μl) resulted in a further threefold increase in Cdk1 kinase activity. Any further addition of Rux resulted in a decline in Cdk1 kinase activity. The addition of 42 μl Rux reduced Cdk1 kinase activity to less than 50% of that for the positive control (42 μl Rux versus 0 μl Rux). This experiment was repeated seven times and similar results were recorded each time. Small amounts of Rux (1.5–4.5 μl) enhanced CycA–Cdk1 activity by about 300% (± 100%), whereas large amounts exerted a visible inhibitory effect, reducing kinase activity to 40% (± 8%) of the positive control. This value was similar to basal levels of Cdk1 activity in this assay. Large amounts of Rux led to a decline in the extent of Thr161 phosphorylation, whereas the stimulatory effect of Rux on Cdk1 did not have a corresponding effect on Thr161 phosphorylation (Figure 4m). No significant change in the amount of CycA coprecipitation was observed (Figure 4l). A similar bimodal effect of Rux on CycA–Cdk1 was also observed when GST–CIV1 was used instead of the Drosophila extract to activate Cdk1 (data not shown), indicating that these effects of Rux do not require additional Drosophila proteins.
Rux can inhibit active Cyc–Cdk1 complexes
The inhibition of Cyc–Cdk1 complexes observed in the previous experiments was to a certain extent associated with a decrease in Thr161 phosphorylation of Cdk1. To determine whether Rux can also inhibit fully activated Cyc–Cdk1 complexes, we pre-activated Cyc–Cdk1 and added Rux in a second incubation step. In the first set, we used CycA–Cdk1 activated by CIV1 in reticulocyte lysates (Figure 5a–c). Kinase activity in this experimental setup was measured directly by taking an aliquot of the reaction mixture (Figure 5b,c). High levels of CycA-dependent kinase activity were seen (Figure 5b, lane 3). The addition of Rux resulted in a strong inhibition of kinase activity, however, while Thr161 phosphorylation of Cdk1 was not affected (Figure 5b and 5a, respectively; lane 6). In the second setup, we assayed the kinase activity of embryonic extract from 0–1 hour old embryos. These extracts contained high levels of CycB–Cdk1-dependent kinase activity (Figure 5f, lane 7; F.S., unpublished data). This activity was efficiently inhibited by HA–Rux (Figure 5e; compare lanes 7,8). We did not observe phosphorylation of HA–Rux under these conditions (Figure 5e), demonstrating that HA–Rux does not compete with the added histone H1 as a substrate for Cdk1 phosphorylation. We also did not observe a diminishment of Thr161 phosphorylation on Cdk1 (Figure 5d) after the addition of Rux, suggesting that the inhibition being observed in this case is a direct inhibition of Cdk1 activity. The fact that Rux can directly inhibit Cdk1 under both of these conditions further supports the idea that Rux does not solely function by inhibiting CAK activity.
Figure 5.
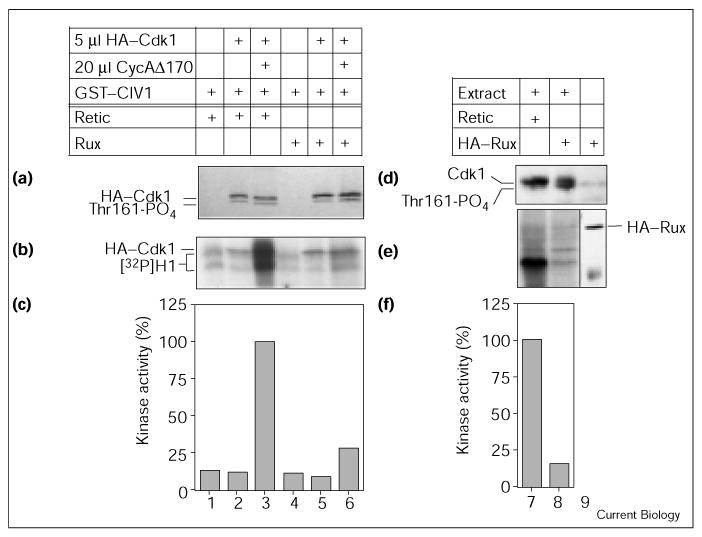
Rux can inhibit pre-activated CycA–Cdk1 complexes. (a–c) HA–Cdk1 was activated with GST–CIV1 and CycA for 30 min before addition of Rux or control reticulocyte lysate (retic). (b,c) An aliquot of the protein mixture was analyzed in a direct kinase assay using histone H1 as substrate. Reticulocyte extract contains low kinase levels, and neither Cdk1 nor GST–CIV1 alone stimulated kinase activity (lanes 1,2). The combined presence of Cdk1, CycA and GST–CIV1 resulted in high kinase activity (lane 3). The addition of Rux after the activation step resulted in strong inhibition of kinase levels (lane 6). (a) After immunoprecipitation of HA–Cdk1, Thr161 phosphorylation was analyzed by SDS–PAGE. No change in the level of Thr161 phosphorylation was observed. (d–f) Rux inhibits Cdk1 activity present in embryonic extracts. Extracts from 0–1 h old embryos were incubated with control reticulocyte lysate (lane 7) or HA–Rux (reticulocyte lysate translated in the absence of [35S]Met, lane 8), and (e,f) an aliquot of each sample was analyzed in a direct kinase assay using histone H1 as substrate. (e) A control containing reticulocyte-lysate-translated HA–Rux was loaded next to the kinase reaction and immunoblotted with HA antibodies to mark the position of the HA–Rux protein (lane 9). (d) A second aliquot of the three reactions was analyzed on a western blot using PSTAIRE antibodies. High levels of kinase activity towards histone H1 are present in embryonic extracts (lane 7). The addition of HA–Rux caused a reduction in kinase levels to 14% (lane 8). We did not observe incorporation of radiolabeled phosphate into HA–Rux during the kinase reaction, indicating that HA–Rux does not act as a competitive substrate in this reaction. (d) The analysis of Cdk1 using the PSTAIRE antibody revealed that Thr161 phosphorylation is not reduced by the addition of Rux.
Discussion
Several mechanisms contribute toward maintenance of the G1 state, including the activity of CKIs. These proteins have the following characteristics (as defined in [2]): they physically associate with a cyclin, a Cdk or both but do not covalently modify either. CKIs downregulate Cdk activity by inhibiting Cdk activation, its activity or both. Here, we have presented evidence that Rux acts as a CKI specific for Cdk1.
A molecular interaction of Rux with mitotic cyclins was seen in immunoprecipitation experiments. Both CycA and CycB and, to a lesser extent, Cdk1 were coprecipitated in Rux precipitations, indicating that Rux can form complexes with cyclins and possibly with Cyc–Cdk complexes. The interaction of a variety of proteins, including CKIs, with cyclins is mediated by RXL motifs [24]. Rux contains three RXL motifs, starting at positions 30, 197 and 249, that could mediate the observed interaction of Rux with cyclins. An association of Rux with mitotic cyclins is supported by the observed changes in subcellular localization of cyclins upon expression of Rux. We found that a large proportion of CycA, which is normally cytoplasmic during interphase, moves into the nucleus and overlaps with Rux. The Rux protein itself is nuclear and requires a functional bipartite NLS sequence at its carboxyl terminus for its localization. RuxΔNLS failed to localize into the nucleus and CycA remained in the cytoplasm. The observed nuclear accumulation of CycA after Rux expression could thus be explained by a nuclear transport of CycA–Rux complexes mediated by the NLS of Rux. Alternatively, Rux could interfere with a putative nuclear export of CycA, leading to a nuclear accumulation of CycA.
Rux can inhibit Cdk1-dependent mitosis and CycA–Cdk1-dependent S phases [10,13]. Here, we have presented evidence that the molecular basis of these effects is inhibition of CycA- and CycB-dependent Cdk1 kinase activity. We found that Rux expression led to a marked decrease in Cdk1 kinase activity from embryos, and we demonstrated an inhibition of kinase activity using in vitro assembled and activated Cyc–Cdk1 complexes. In the latter assays, both CycA- and CycB-dependent kinase activities were suppressed. Although we cannot strictly rule out the interference of reticulocyte proteins in some of our in vitro assays, we consider this unlikely, as the effects observed using in vitro translated proteins were identical to those seen using embryonic extracts. Genetic data have already indicated the importance of Rux in downregulation of CycA–Cdk1 activity during G1. The importance of inhibiting CycB–Cdk1 kinase activity is less clear, as CycB is unable to induce S phase in Drosophila [10]. Nevertheless, the effects of Rux on mitotic Cyc–Cdk1 complexes opens up the possibility that it may also contribute to regulating entry into or exit from mitosis. It is interesting to note that Sic1, a CKI from S. cerevisiae that inhibits S-phase-inducing activity during G1 can also contribute to exit from mitosis under certain circumstances [25]. Rux has no effect on CycE–Cdk2 kinase activity in vitro and cannot inhibit CycE/Cdk2-dependent S phases in vivo [10,13]. Thus, inhibition by Rux is specific for mitotic cyclins and, like the Sic1 inhibitor of S. cerevisiae, would help to enforce a requirement for G1 cyclins to promote S phase.
How does Rux inhibit Cdk1 activity? Activation of Cdk1 requires cyclin association, phosphorylation of Thr161 in the T-loop and dephosphorylation of inhibitory Thr14 and Tyr15 phosphorylation sites. Rux inhibition does not require modulation of the inhibitory phosphorylations. Firstly, Rux was able to inhibit kinase activity and induction of mitosis by Cdc2AF, a mutant form of Cdk1 which lacks the inhibitory phosphorylation sites. Secondly, phosphorylation on Thr14 and Tyr15 was not observed in the in vitro assays in which Rux was able to inhibit kinase activity. The mechanism of Cdk1 inhibition by Rux also does not rely on preventing Cyc–Cdk1 complex formation. We found no significant change in the level of cyclins coprecipitating with Cdk1 in the presence of Rux. We did, however, find markedly reduced levels of Thr161 phosphorylation both after expression in vivo and in the in vitro experiments. Phosphorylation of Thr161 in the T-loop is carried out by a CAK [26]. Rux could influence the level of Thr161 phosphorylation in several ways. First, Rux could have a Thr161-dephosphorylating activity. This is unlikely as Rux is not able to change the state of Thr161 phosphorylation when added after the initial Thr161-phosphorylation event. The second possibility is that Rux inhibits CAK activity directly. Rux prevents Thr161 phosphorylation by two very different CAKs, however. In one case, we used a monomeric kinase, CIV1, the in vivo CAK in S. cerevisiae [21,23,27]. The other source of CAK was a crude Drosophila extract that contained CycH–Cdk7. Embryos lacking Cdk7 activity did not provide CAK activity (data not shown), indicating that the CAK activity in our extracts depends on CycH–Cdk7 activity. CIV1 and CycH–Cdk7 are very different in nature; therefore, it is very unlikely that Rux can inhibit both kinase activities. Finally, should Rux function by inhibiting CAK we would expect to see an inhibition of Cdk2–CycE by Rux, which is not the case in our in vitro assays. Instead, Rux might prevent CAK access to the T-loop or recognition of Cyc–Cdk complexes by CAK. Rux does not act solely by preventing Thr161 phosphorylation, however, as it also is able to inhibit activated, Thr161-phosphorylated Cdk1 kinase activity. The molecular nature of this inhibition is at present not known. In summary, Rux can inhibit kinase activity by at least two mechanisms: prevention of Thr161 phosphorylation and inhibition of active Cyc–Cdk complexes. Such dual effects have previously been described for a number of CKIs [28–30].
The inhibition of kinase activity by Rux in vitro occurred in a progressive fashion when using CycB–Cdk1, but a more complex effect on CycA–Cdk1 was observed. The addition of small amounts of Rux resulted in a stimulation of kinase activity and only larger amounts resulted in an inhibition. The increase in activity was not associated with an increase in Cyc–Cdk1 association or Thr161 phosphorylation. The seemingly contradictory ability of CKIs to enhance the activity of Cyc–Cdk complexes has previously been described for members of the CIP/KIP family [7,31,32]. How Rux stimulates activity in this situation remains to be resolved. Several explanations are possible. Rux could have a chaperone-type function for CycA, or different stoichiometric configurations of Rux and cyclins might exist that can be either stimulatory or inhibitory. Finally, Rux might contain several binding sites with different affinities whose effect on CycA might be qualitatively different.
It has been suggested previously that Rux acts by targeting mitotic cyclins for destruction [13]. CycA destruction is not a necessary component of Rux function, however. Rux prevents the S-phase-inducing activity of a non-destructible CycA (CycAΔ170) in vivo [10] and it can inhibit kinase activity stimulated by CycAΔ170 in vitro. Cyclin degradation in G1 is caused by fizzy-related/HCT1-dependent anaphase-promoting complex (APC) activity [33]. This function in turn is downregulated by Cyc–Cdk activity [34]. Thus, by inhibiting Cdk1 kinase activity, Rux may contribute towards maintaining a G1 by keeping APC activity high and causing cyclin degradation. Disappearance of mitotic cyclins was also described when Rux was expressed during S and G2 phases [13]. We repeated these experiments by expressing Rux in paired stripes in the embryo and also followed CycA disappearance after heat-shock expression of Rux. In both cases, CycA disappearance was only observed after a considerable time (3 hours after Rux expression). Embryos of this age are older than 7 hours and would normally prepare to enter G1 of cycle 17, a stage when CycE is downregulated and fizzy-related is upregulated in the epidermis. These changes, and not the presence of Rux, most likely lead to the ‘eventual disappearance’ [13] of CycA.
Inhibition by Rux also does not rely on changes in the subcellular distribution of cyclins. Although both CycA and CycB moved to the nucleus upon Rux expression, mitosis could still be suppressed when a mutant form of Rux lacking the NLS was expressed and no CycA accumulation in the nucleus was observed. The presence of Rux in the nucleus would, however, be advantageous in protecting the nucleus from S-phase-inducing CycA–Cdk1 activity during G1.
Conclusions
To our knowledge, Rux is the first CKI to be reported in a multicellular organism that is specific for mitotic cyclins. As similar CKIs have been identified in unicellular eukaryotes, such as SIC1 from S. cerevisiae [35] and rum1 from Schizosaccharomyces pombe [36], there may be an evolutionarily conserved requirement for an activity which keeps mitotic cyclins in check during G1 [37].
During the G1 state, cyclin turnover is high, resulting in low mitotic cyclin levels. At this stage, even low levels of Rux are high relative to cyclins and Rux can prevent Cyc–Cdk1 kinase activity by interfering with Thr161 phosphorylation and inhibiting Cyc–Cdk1 kinase activity. As such, Rux is a typical CKI involved in control of the G1 state. As the cell progresses through G1 CycE levels rise. Rux is a substrate for CycE–Cdk2, and CycE has been shown to promote Rux turnover [13]. Thus, while CycE levels rise, Rux levels decrease, and switching off APC activity at the G1–S transition allows CycA levels to rise. At this stage, the ability of small amounts of Rux to enhance CycA–Cdk1 kinase activity may have a physiological relevance. It is conceivable that low levels of Rux enhance any S-phase and/or mitotic functions of CycA by increasing CycA–Cdk1 kinase activity and promoting their transport to the nucleus.
Materials and methods
Drosophila stocks, heat-shock procedure and embryonic extracts
The hs–rux, hs–cycA, hs–Cdc2AF and hs–stg flies have been described previously [10,13,16,38]. The wild-type strain used was OregonR. The heat-shock procedure and RNA injections were performed as described [10,39]. Embryonic extracts were prepared as described [19] and typically have a protein concentration around 35 mg/ml.
DNA constructs and in vitro translation
All constructs were cloned in an SP64-based vector containing the Xenopus β-globin 5′ leader sequence. HA–Cdk1 (pSF191), CycBΔ46 (pSF283) and CycAΔ170 (pSF281) have been described [10,17]. Rux (pSF821) contains the coding region from the rux cDNA [11] flanked by the Xenopus β-globin 3′ and 5′ untranslated regions. HA–Rux (EF018) was constructed by cloning the coding region of pSF821 into the vector pSF398, which contains the HA tag. CycE (pSF828) was obtained by cloning the CycE type I cDNA [40] into the vector pSF036 containing the 5′ Xenopus β-globin region. HA–Cdk2 (pSF905) was cloned by PCR amplification using the Dmcdc2c cDNA [41] and oligonucleotides that contained the coding sequence of the HA tag. Transcription in vitro and translation in reticulocyte lysate (Promega) were performed as described [39] using Expre35S35S-protein labeling mix from NEN.
Antibodies, immunofluorescence, immunoprecipitations and immunoblotting
Rux antibody was made by immunizing rats with bacterially produced GST–Rux, which had been purified with glutathione–Sepharose beads (Pharmacia). Antibodies against CycA, CycB and Cdk1 have been described [10,38]. The antibodies against β-tubulin, HA and PSTAIRE were obtained from Amersham, Boehringer Mannheim and Sigma, respectively. The anti-Even-skipped antibody was provided by Manfred Frasch. The PSTAIRE antibody recognizes Drosophila Cdk1 100 times better than Drosophila Cdk2 [42]. Secondary antibodies were obtained from Dianova. For immunoprecipitations, embryonic extract was incubated with 10 μl protein G–Sepharose beads (Pharmacia) and 15 μl Rux antibody for 2 h at 4°C. The immunoprecipitate was then washed twice in immunoprecipitation buffer (IP buffer: 10 mM Tris pH 7.5, 80 mM K-β-glycerophosphate pH 7.3, 20 mM EGTA pH 8.0, 15 mM MgCl2, 0.5 mM DTT, 2 mM Na3VO4, 10% glycerol). The supernatant was removed, the beads resuspended in 20 μl loading buffer, boiled for 5 min and proteins separated by SDS–PAGE. The gel was blotted onto nitrocellulose, western blots performed and developed using the ECL system (Amersham).
Histone H1 kinase assays
Proteins were translated individually, mixed and each sample brought to an equal volume using rabbit reticulocyte lysate. The mixture was incubated to allow complex formation and Cdk1 phosphorylation on Thr161 by the addition of CAK, 10 mM MgCl2 and 2 mM ATP for 30 min at room temperature. As a source of CAK, approximately 35 μg bacterially produced GST–CIV1p (kindly provided by M. Mann) [21], which had been purified with glutathione–Sepharose beads (Pharmacia), or 8 μl extract prepared from 0–1 h old wild-type embryos was used. After mixing of all components, an aliquot was removed and analyzed by SDS–PAGE and autoradiography to visualize the presence and abundance of the different translation products (input gel). For immunocomplex histone H1 kinase assays, HA–Cdks were immunoprecipitated for 2 h at 4°C in 300 μl IP buffer, containing 20 mM EDTA, 10 μl protein G–Sepharose beads (Pharmacia) and 1.5 μg anti-HA antibody. The beads were washed twice in 300 μl IP buffer and then split into two equal fractions. One half was analyzed by high-resolution (25 cm) SDS–PAGE and autoradiography to visualize the levels of coprecipitated cyclins and to allow distinction of Cdk phosphoisoforms. The second half was washed twice in 300 μl pre-kinase buffer (25 mM HEPES pH 7.4, 10 mM MgCl2, 1 mM DTT and 25 μM ATP). The supernatant was removed and the beads gently resuspended in histone H1 kinase assay buffer (25 mM HEPES pH 7.4, 10 mM MgCl2, 10 μM ATP, 250 μg/ml histone H1 and 3 mCi/ml [γ-32P]ATP). Kinase reactions were incubated for 30 min at room temperature, stopped by adding an equal volume of sample buffer and analyzed by SDS–PAGE followed by autoradiography and phosphorimaging; 32P intensities are displayed as percentage of kinase activity normalized to a positive control. Kinase assays from embryo extract were performed as above with the exception that the embryos were first homogenized in HB buffer and Cdk1 was immunoprecipitated with a polyclonal anti-Cdk1 antiserum for 2 h at 4°C in IP buffer. Direct kinase assays in Figure 5 were performed by incubating reticulocyte-translated proteins directly with 250 μg/ml histone H1 and 3 mCi/ml [γ-32P]ATP.
Acknowledgments
The authors acknowledge the technical assistance of Hayati Özden. We are grateful to Barbara Thomas for rux cDNA and hs–rux flies, and Nikita Yakubovitch for discussions and communication of results. The plasmid encoding the GST–CIV1 was a gift of Matthias Mann and the CycE clone used was a gift of Helena Richardson. We are also grateful to Axel Dienemann, Bob Duronio, Ruth Grosskortenhaus, Uwe Irion, Thomas Klein, Maria Leptin and Rene Medena for critical reading of the manuscript. E.F. thanks Maria Leptin for her encouragement and comments throughout the course of this project. E.F. is supported by a fellowship from the Boehringer Ingelheim Fonds. This work was supported by the DFG through SFB 243 and by NIH grant GM37193 to P.O.F.
Footnotes
Because Current Biology operates a ‘Continuous Publication System’ for Research Papers, this paper has been published on the internet before being printed. The paper can be accessed from http://biomednet.com/cbiology/cub – for further information, see the explanation on the contents page.
References
- 1.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 2.Peter M, Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell. 1994;79:181–184. doi: 10.1016/0092-8674(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 4.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 5.Guan KL, Jenkins CW, Li Y, Nichols MA, Wu X, O'Keefe CL, et al. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 6.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 7.Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- 8.Lane ME, Sauer K, Wallace K, Jan YN, Lehner CF, Vaessin H. Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell. 1996;87:1225–1235. doi: 10.1016/s0092-8674(00)81818-8. [DOI] [PubMed] [Google Scholar]
- 9.de Nooij JC, Letendre MA, Hariharan IK. A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell. 1996;87:1237–1247. doi: 10.1016/s0092-8674(00)81819-x. [DOI] [PubMed] [Google Scholar]
- 10.Sprenger F, Yakubovich N, O'Farrell PH. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr Biol. 1997;7:488–499. doi: 10.1016/s0960-9822(06)00220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas BJ, Gunning DA, Cho J, Zipursky L. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994;77:1003–1014. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 12.Gönczy P, Thomas BJ, DiNardo S. roughex is a dose-dependent regulator of the second meiotic division during Drosophila spermatogenesis. Cell. 1994;77:1015–1025. doi: 10.1016/0092-8674(94)90441-3. [DOI] [PubMed] [Google Scholar]
- 13.Thomas BJ, Zavitz KH, Dong X, Lane ME, Weigmann K, Finley RL, Jr, et al. roughex down-regulates G2 cyclins in G1. Genes Dev. 1997;11:1289–1298. doi: 10.1101/gad.11.10.1289. [DOI] [PubMed] [Google Scholar]
- 14.Lehner CF, O'Farrell PH. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Raff JW. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 1999;18:2184–2195. doi: 10.1093/emboj/18.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgar BA, O'Farrell PH. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell. 1990;62:469–480. doi: 10.1016/0092-8674(90)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar BA, Sprenger F, Duronio RJ, Leopold P, O'Farrell PH. Distinct molecular mechanisms regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994;8:440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draetta GF. Cell cycle: will the real Cdk-activating kinase please stand up. Curr Biol. 1997;7:R50–R52. doi: 10.1016/s0960-9822(06)00020-0. [DOI] [PubMed] [Google Scholar]
- 19.Campbell SD, Sprenger F, Edgar BA, O'Farrell PH. Drosophila Wee1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila Cdc2 in vitro. Mol Biol Cell. 1995;6:1333–1347. doi: 10.1091/mbc.6.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larochelle S, Pandur J, Fisher RP, Salz HK, Suter B. Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev. 1998;12:370–381. doi: 10.1101/gad.12.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thuret JY, Valay JG, Faye G, Mann C. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell. 1996;86:565–576. doi: 10.1016/s0092-8674(00)80130-0. [DOI] [PubMed] [Google Scholar]
- 22.Kaldis P, Russo AA, Chou HS, Pavletich NP, Solomon MJ. Human and yeast Cdk-activating kinases (CAKs) display distinct substrate specificities. Mol Biol Cell. 1998;9:2545–2560. doi: 10.1091/mbc.9.9.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinoza FH, Farrell A, Erdjument-Bromage H, Tempst P, Morgan DO. A cyclin-dependent kinase-activating kinase (CAK) in budding yeast unrelated to vertebrate CAK. Science. 1996;273:1714–1717. doi: 10.1126/science.273.5282.1714. [DOI] [PubMed] [Google Scholar]
- 24.Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WG., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 26.Solomon MJ, Lee T, Kirschner MW. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992;3:13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaldis P, Sutton A, Solomon MJ. The Cdk-activating kinase (CAK) from budding yeast. Cell. 1996;86:553–564. doi: 10.1016/s0092-8674(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 28.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 29.Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor p27Kip1 of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 30.Aprelikova O, Xiong Y, Liu ET. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995;270:18195–18197. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- 31.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Hannon GJ, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 33.Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- 34.Jaspersen SL, Charles JF, Morgan DO. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- 35.Schwob E, Bohm T, Mendenhall MD, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 36.Correa-Bordes J, Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Diaz A, Gonzalez I, Arellano M, Moreno S. The cdk inhibitors p25rum1 and p40SIC1 are functional homologues that play similar roles in the regulation of the cell cycle in fission and budding yeast. J Cell Sci. 1998;111:843–851. doi: 10.1242/jcs.111.6.843. [DOI] [PubMed] [Google Scholar]
- 38.Knoblich JA, Lehner CF. Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO J. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprenger F, Nüsslein-Volhard C. Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell. 1992;71:987–1001. doi: 10.1016/0092-8674(92)90394-r. [DOI] [PubMed] [Google Scholar]
- 40.Richardson H, O'Keefe LV, Marty T, Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121:3371–3379. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- 41.Lehner CF, O'Farrell PH. Drosophila cdc2 homologs: a functional homolog is coexpressed with a cognate variant. EMBO J. 1990;9:3573–3581. doi: 10.1002/j.1460-2075.1990.tb07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 43.Görlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]


