Abstract
We know very little about aging (senescence) in natural populations, and even less about plant aging. Demographic aging is identified by an increasing rate of mortality following reproductive maturity. In natural populations, quantifying aging is often confounded because changes in mortality may be influenced by both short- and long-term environmental fluctuations as well as age-dependent changes in performance. Plants can be easily marked and monitored longitudinally in natural populations yet the age-dependent dynamics of mortality are not known. This study was designed to determine whether a plant species, Plantago lanceolata, shows demographic aging in its natural environment. A large, multiple-cohort design was used to separate age-independent and age-dependent processes. Seven years of results show environmental influences on mortality as evidenced by synchronous changes in mortality across four cohorts over time. Age-dependent mortality was found through an age-by-environment interaction when the oldest cohorts had significantly higher mortality relative to the younger cohorts during times of stress. Neither size nor quantity of reproduction could explain this variation in mortality across cohorts. These results demonstrate demographic senescence in a natural population of plants.
Keywords: age-by-environment interaction, aging, cohorts, mortality, natural population, Plantago lanceolata, senescence
Introduction
Senescence, or aging, is quantified either demographically as an increase in mortality, or physiologically as a decline in function with increasing age. This study was designed to study aging in a natural population. Most of our current information about demographic aging is derived from relatively short-lived species that have been removed from their natural environment. In most experimental, laboratory, studies of senescence, a single cohort is followed over the course of its life span and environmental influences on mortality are deliberately minimized to focus on intrinsic aspects of aging. In natural populations, mortality is influenced not only by age-dependent intrinsic processes but also by environmental processes that fluctuate seasonally or even daily (cf. Roach 2003, Menges and Quintana-Ascencio 2004, Pico and Retana 2008). These age-independent environmental factors can have major impacts on mortality that may obscure age-dependent processes and complicate the interpretation of changes in mortality (see also Roach and Gampe 2004). In particular, a longitudinal demographic study that shows an increase in mortality with age does not verify the existence of aging in a population because these mortality changes may be strictly due to a deteriorating environment and may not reflect the aging status of individuals.
The interpretation of mortality changes over time may be further complicated if there are interactions between age-dependent intrinsic and age-independent environmental processes (Williams and Day 2003). These interactions have been found in field studies of large herbivores (cf. Gaillard et al. 1998, 2000, Coulson et al. 2001) and are manifest when individuals of different ages show dissimilar susceptibility to extrinsic sources of mortality.
One open question is whether all species eventually show aging or whether there are species that may be able to escape this deleterious process. Demographic aging has been shown in many species of animals (reviewed in Finch 1990), and has also been demonstrated in bacteria that reproduce by symmetric division (Stewart et al. 2005). In these species, the increasing rate of mortality following reproductive maturity is evolutionarily caused by a decline in the intensity of selection on late-life traits. Species such as plants, and a few animals, may be able to escape aging if indeterminate growth allows individuals to continue to increase in size and fertility following the age of first reproduction (Vaupel et al. 2004). Aging would not be expected with these patterns of growth and reproduction if the intensity of selection remains high throughout the life span of an individual.
There have been two distinct definitions of “plant senescence” that have developed within the literature. “Physiological senescence” occurs within an individual and as a part of an internally controlled program of development, whereas “demographic senescence” is concerned with variation in mortality patterns among individuals within populations and between species. It is this latter approach that we will focus on here. Plant species offer a unique opportunity to study aging in natural populations because individuals of known age can be easily marked and followed longitudinally over time (Roach 2004). Yet, we currently have very little longitudinal demographic data to test the hypothesis that plants can escape aging.
This study describes a seven-year longitudinal experiment in a natural population of a perennial species Plantago lanceolata. In a previous study with this species, a covariate model was used to describe the mortality dynamics of an experimental population in North Carolina (Roach and Gampe 2004, see also Roach 2003). The results of that study showed no evidence for an increase in mortality with advancing age. Unfortunately, the previous study had complete data on only one cohort and thus age-dependent and -independent processes could not be clearly separated. The question addressed here is whether a longitudinal analysis of multiple cohorts will reveal aging in a natural population of a perennial plant species.
Materials and Methods
Plantago lanceolata (ribwort plantain) is a short-lived perennial species that germinates in fall and spring (D. A. Roach, unpublished data) and flowers in midsummer. It retains a green rosette all year, thus individuals can be easily identified and an accurate assessment of mortality can be made in every month. The “natural” habitat for this species is roadsides and mown fields. In our field site, located at the Shadwell Preserve of the Jefferson Monticello Foundation in Virginia, USA, a large population of P. lanceolata exists that has been subjected to one or two mowings per year for many years. To create seed for this study, we isolated random parental genotypes from throughout the 70×35 m research area. Chosen plants were mapped, dug up, brought to the greenhouse, and potted into standard high-quality conditions. Each genotype was cloned, by whole-plant division, and clones were grown to be large enough to support sustained reproduction. All genotypes survived transplantation into the greenhouse and subsequent cloning, therefore, this process imposed no selection on our pool of available genotypes.
Parental genotypes were crossed using a modified North Carolina II design (originally developed by Comstock and Robinson [1948]) in which distinct sets of sires and dams were used to produce 50–200 seedlings for 80 sire–dam combinations. The crosses included five sets of “large crosses.” A large cross consisted of four sires crossed to each of two dams resulting in eight sire–dam combinations and 200 offspring from each. This was repeated for five unique sets of sires and dams, for a total of 20 sire genotypes, 10 dam genotypes, 40 sire–dam combinations, and 8000 individual offspring. A second set of “small crosses” were made consisting of two sires crossed to each of two dams with 50 offspring produced from each of those four resulting crosses. Ten unique sets of sires and dams were used in the small crosses, adding 20 sire genotypes, 20 dam genotypes, 40 sire–dam pairs, and 2000 individuals to the experiment. This complete design was used for cohorts 1 and 2 and one-half of the total number of individuals per cross was used for cohorts 3 and 4. No parental genotypes were used in more than one set of crosses and the same parental genotypes were used for all cohorts. Seeds were stored dry in envelopes until planting. We found no change in seed mass or germination percentage over time using this method (D. A. Roach, unpublished data). When necessary, the parental genotypes maintained in the greenhouse were crossed again one year later to attain the required seeds/cross.
A previously published protocol (Roach 2003) for raising seedlings, planting, and marking individuals was used for this study. Multiple cohorts were planted at different times to facilitate separation of age- and environmental-effects. The cohort number, date, and number of individual seedlings per cohort planted into the field were as follows: cohort 1, October 2000, 10 000; cohort 2, October 2001, 10 000; cohort 3, April 2002, 5000; cohort 4, October 2002, 5000. These 30 000 individuals from the four cohorts were then monitored for reproduction, size, and mortality for seven years from November 2000–November 2007.
Individual seedlings were planted and marked with minimal disturbance to the surrounding community. Plants were located 15 cm apart in rows each 20 cm apart. This spacing was sufficient to avoid competition between individuals and is within the natural density of the field. Seedlings were planted in a randomized block design with replication of genotypes and cohorts within 1 × 14 m “sections.” Sections were separated by 1 m to allow researchers to measure plants without disturbing them. There were 72 total sections, and four adjacent sections constituted one of 18 blocks. Mortality was quantified as q(x), the number of deaths between ages x and x + 1, divided by the number of individuals alive at age x. In this paper, we report mortality over the six-month intervals November to April and May to October. In a longitudinal demographic analysis, all individuals are revisited at each census but the calculation of mortality is independent for each census interval (Carey 1999), thus a chi-square test was used to determine significant differences in mortality across cohorts. Mature inflorescences were collected continuously during the reproductive period to quantify reproduction. Age-specific fecundity is the total number of inflorescence spikes produced by an individual in one year. The mean number of inflorescences reported here is the mean for a cohort of individuals for all reproductive individuals within a year (zeros excluded). The percentage of nonreproductive individuals was quantified for a cohort as the proportion of individuals alive in May that did not reproduce in a particular year. Size of the experimental individuals was quantified as the total number of leaves every May and November for all cohorts. A previous study showed that leaf number is a good estimate of aboveground biomass in this species (D. A. Roach, unpublished data; see also Roach and Gampe 2004). In order to determine the significant differences in size and reproduction across cohorts within years, PROC MIXED (SAS 2003) was used, with independent contrasts and block as a random factor. A log-transformation was used for these two traits to improve normality of the residuals. In order to determine whether mortality was influenced by reproductive effort, an index of reproductive effort was calculated for each individual as the number of inflorescences produced in a reproductive year divided by its size at the beginning of the reproductive season (May). The percentage mortality (May to October) was calculated for each year for reproductive and size quartiles.
Meteorological data, including minimum, maximum, and average temperatures, and precipitation were obtained from the State Climate Office of North Carolina (available online).4 The weather station is located at Monticello, Virginia, USA approximately 1 mile (1.67 km) from the field site.
Results
The six-month mortality rates show synchronous changes across cohorts (Fig. 1). Mortality rates were initially relatively low (0.05 ± 0.01 [mean ± SE] for November 2000–November 2002) until a major increase between May and October 2003. Mortality rate was then consistently high for three years (0.29 ± 0.01). In May–October 2006, it finally dropped below 0.2 for all cohorts (0.13 ± 0.01 for May 2006–November 2007). Within this overall pattern of synchronous mortality changes, several census intervals showed significant differences in mortality among cohorts. This cohort differentiation was generally apparent only during the period when mortality was high (Fig. 1). Most interestingly, when there are differences among cohorts, the oldest cohorts consistently show higher mortality. The only mortality interval that did not fit this general pattern was May–October 2002 when cohort 2 was in its first reproductive season and had higher mortality than cohort 1 (Fig. 1).
Fig. 1.
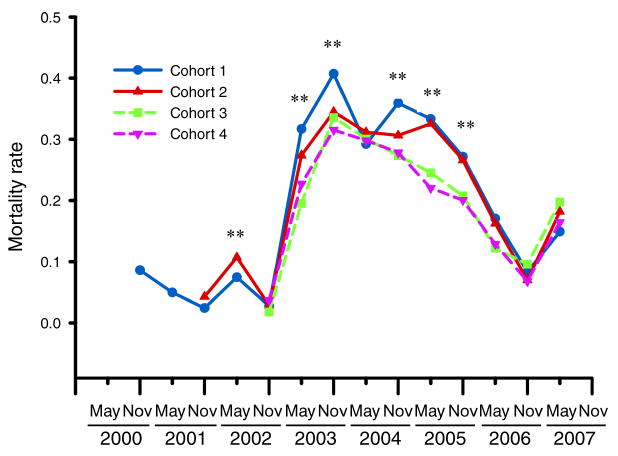
Six-month mortality rate q(x) by cohort. Major ticks represent mortality from November to April, and minor ticks represent mortality from May to October from November 2000–2007. Differences among cohorts were tested with a contingency analysis, and significant differences between cohorts are indicated by asterisks.
** P < 0.01.
The coordinated shifts in mortality across all cohorts suggest that major environmental factors influenced these patterns. However, there were no consistent differences in mean monthly maximum or minimum temperatures across years that could explain the relatively high mortality from May–October 2003–2005 and the lower mortality across cohorts in the years before and after this interval (Fig. 2A, B). The only major deviation in weather across this study period was in 2003 when there was a significantly higher level of accumulated precipitation (Fig. 2C). The initial increase in mortality across all cohorts occurred at this same time.
Fig. 2.
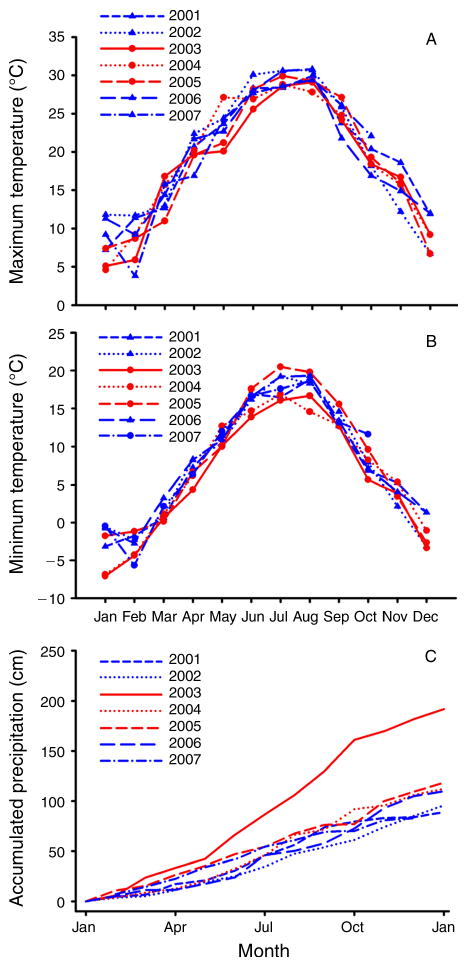
Changes in weather in Monticello, Virginia, USA, during the study period November 2001–November 2007: (A) monthly mean maximum temperature (°C); (B) monthly mean minimum temperature (°C); and (C) cumulative monthly precipitation (cm). Note that the years with the highest mortality are represented with red lines and the other years with blue lines.
Given that cohorts all experienced the same environment, the variation in mortality among cohorts may have been due to differences in size, reproduction, reproduction by size, or age. To determine whether size or reproduction influenced the observed mortality patterns, the mean size in May, and number of inflorescences was compared for cohorts across years (Table 1). A population-level analysis showed that across years, individuals in the largest size quartiles had the lowest risk of mortality (Table 2), as had been found in a previous study (Roach 2003). However there were no consistent differences in size across cohorts that could explain the higher mortality of the older cohorts from 2003–2005 (Table 1).
Table 1.
Size (mean number of leaves ± SE) in May, number of inflorescences (mean ± SE), and percentage of nonreproductive individuals for Plantago lanceolata by cohort and year in the experimental population.
| Year and cohort | Size | Inflorescences | Nonreproductive individuals | |||
|---|---|---|---|---|---|---|
| Number of leaves | n | Number | n | % | Number | |
| 2002 | ||||||
| 1 | 16.00 ± 0.21 | 3761 | 11.18 ± 0.17 | 3957 | 19.80 | 977/4934 |
| 2† | 3.98 ± 0.03 | 6285 | 4.08 ± 0.07 | 1963 | 75.57 | 6073/8036 |
| 2003 | ||||||
| 1 | 21.17a ± 0.33 | 2669 | 6.18a ± 0.13 | 2397 | 47.02 | 2127/4524 |
| 2 | 12.74b ± 0.23 | 1879 | 4.95b ± 0.07 | 4157 | 42.73 | 3102/7259 |
| 3 | 13.13c ± 0.30 | 1097 | 5.85a,b ± 0.10 | 2524 | 32.33 | 1206/3730 |
| 4† | 4.38 ± 0.08 | 993 | 2.86 ± 0.05 | 1601 | 61.12 | 2517/4118 |
| 2004 | ||||||
| 1 | 14.68a ± 0.53 | 1363 | 5.77a ± 0.29 | 573 | 68.36 | 1238/1811 |
| 2 | 10.32b ± 0.28 | 1713 | 4.48b ± 0.15 | 1175 | 65.88 | 2269/3444 |
| 3 | 15.40c ± 0.64 | 552 | 6.20a ± 0.23 | 871 | 56.23 | 1119/1990 |
| 4 | 10.64b ± 0.47 | 492 | 4.62b ± 0.14 | 929 | 57.33 | 1248/2177 |
| 2005 | ||||||
| 1 | 13.04a ± 0.61 | 619 | 2.94a ± 0.23 | 158 | 80.64 | 658/816 |
| 2 | 10.89b ± 0.41 | 853 | 2.82a ± 0.15 | 357 | 77.89 | 1258/1615 |
| 3 | 15.03c ± 0.64 | 496 | 3.13a ± 0.16 | 280 | 71.89 | 716/996 |
| 4 | 13.45a,c ± 0.62 | 473 | 2.97a ± 0.16 | 348 | 67.75 | 731/1079 |
| 2006 | ||||||
| 1 | 9.67a ± 0.62 | 315 | 3.86a ± 0.38 | 111 | 72.32 | 290/401 |
| 2 | 9.27a ± 0.49 | 468 | 4.04a ± 0.27 | 229 | 71.69 | 580/809 |
| 3 | 13.28a ± 0.78 | 366 | 4.31a ± 0.28 | 207 | 64.97 | 384/591 |
| 4 | 12.59a ± 0.76 | 380 | 4.11a ± 0.25 | 239 | 64.96 | 443/682 |
| 2007 | ||||||
| 1 | 8.88a ± 0.49 | 253 | 4.18a ± 0.38 | 88 | 71.15 | 217/305 |
| 2 | 9.88a ± 0.44 | 552 | 4.09a ± 0.25 | 206 | 67.76 | 433/639 |
| 3 | 11.73a ± 0.64 | 401 | 4.31a ± 0.32 | 167 | 65.06 | 311/478 |
| 4 | 10.43a ± 0.46 | 441 | 4.50a ± 0.33 | 177 | 68.39 | 383/560 |
Notes: The number of inflorescences does not include zeros. Different superscript letters within years denote significant differences among cohorts based on independent contrasts (P < 0.05). For percentage nonreproductive, there were significant differences among cohorts for all years (P < 0.001) except 2007. Cohorts that were less than six months old at the time of measurement, denoted by
were not included in the statistical analysis.
Table 2.
The percentage of mortality six months after reproduction by May size and reproductive quartile across years for all individuals in the population (cohorts pooled).
| Year | Size | Reproduction | ||||
|---|---|---|---|---|---|---|
| Quartile | Number of leaves | Mortality (%) | Quartile | Number of inflorescences | Mortality (%) | |
| 2002 | 1 | 0.5–6.5 | 9.13*** | 1 | 1–3 | 3.98 ns |
| 2 | 7–12.5 | 3.66 | 2 | 4–7 | 2.86 | |
| 3 | 13–21.5 | 1.76 | 6 | 8–14 | 2.57 | |
| 4 | >21.5 | 3.20 | 4 | >15 | 3.02 | |
| 2003 | 1 | 0.5–6.5 | 41.23*** | 1 | 1 | 20.25*** |
| 2 | 7–12.5 | 25.96 | 2 | 2–3 | 17.65 | |
| 3 | 13–24.0 | 24.37 | 3 | 4–6 | 13.98 | |
| 4 | >24 | 18.60 | 4 | >6 | 10.17 | |
| 2004 | 1 | 0.5–4.0 | 50.17*** | 1 | 1 | 9.20*** |
| 2 | 4.5–7.5 | 19.57 | 2 | 2–3 | 8.80 | |
| 3 | 8.0–15.0 | 12.70 | 3 | 4–6 | 5.19 | |
| 4 | >15.0 | 5.87 | 4 | >6 | 3.05 | |
| 2005 | 1 | 0.5–4.0 | 42.10*** | 1 | 1 | 7.18* |
| 2 | 4.5–8.0 | 24.59 | 2 | 2 | 6.95 | |
| 3 | 8.5–15.5 | 18.87 | 3 | 3 | 7.91 | |
| 4 | >15.5 | 8.42 | 4 | >3 | 2.94 | |
| 2006 | 1 | 0.5–3.0 | 41.22*** | 1 | 1 | 1.62 ns |
| 2 | 3.5–6.0 | 8.63 | 2 | 2–3 | 2.34 | |
| 3 | 6.5–13.0 | 5.81 | 3 | 4–5 | 0.56 | |
| 4 | >13.0 | 2.33 | 4 | >5 | 0 | |
| 2007 | 1 | 0.5–3.5 | 48.83*** | 1 | 1 | 2.72 ns |
| 2 | 4.0–7.0 | 11.36 | 2 | 2–3 | 1.95 | |
| 3 | 7.5–14.0 | 6.62 | 3 | 4–5 | 0.78 | |
| 4 | >14.0 | 1.27 | 4 | >5 | 0.63 | |
Notes: The quartiles for size were defined by the number of leaves and the quartiles for reproduction were defined by the number of inflorescences. These respective quartiles changed each year. Cohorts that were less than six months old at the time of measurement were not included in this analysis (see Table 1). Significant differences in mortality across all four quartiles within a year are indicated.
P < 0.05;
P < 0.001; ns, not significant.
During the years of high mortality 2003–2005, individuals with the lowest reproductive output had a significantly higher risk of mortality relative to individuals who reproduced more (Table 2), yet the reproductive output of the oldest cohorts was not significantly different from the younger cohorts (Table 1). For 2003, the index of reproductive effort showed that individuals in the lowest quartiles, in other words those that reproduced less per leaf number, had higher mortality than those in the highest quartiles (P < 0.001). However, there were no differences in mortality by reproductive effort for the other two years of high mortality (2004–2005) thus the cohort variation in this trait was not evaluated. Across all years, the percent mortality for nonreproductive individuals was very high and ranged from 19.34% in 2002 to 46.94% in 2004. Mortality of these nonreproductive individuals was significantly higher than the other reproductive classes across all years (P < 0.001 for all years). There was also a significant trend from 2003–2005 that the older cohorts had a significantly higher percentage of nonreproductive individuals (Table 1). In other words, the percentage of nonreproductive individuals reflected the observed mortality patterns. The only exceptions to these patterns were when a cohort was in its first year of reproduction (e.g., cohort 2 in 2002 and cohort 4 in 2003).
Discussion
This long-term demographic study of a plant species in its natural environment shows two significant phenomena. First, different-aged cohorts can have coordinated changes in mortality over time suggesting important age-independent influences on mortality. Second, when mortality is high, older cohorts have the highest levels of mortality, suggesting age-dependent influences on mortality.
Extrinsic mortality can influence all individuals in a population in a similar manner, regardless of their age. In 2003, the mortality of all cohorts increased and remained high for three years before declining, which suggests that there were changing levels of ecological stress over time. One hypothesis is that this stress-induced mortality may have been caused by changes in environmental parameters. Previous work with this species showed that temperature and precipitation could explain a large percentage of the variance in mortality in an experimental population in North Carolina (Roach 2003), and similar environmental influences on mortality have been found with other species (cf. Menges and Quintana-Ascencio 2004, Pfeifer et al. 2006, Pico and Retana 2008). However, no covariates could explain the sustained level of ecological stress observed in this study in Virginia.
An alternative hypothesis is that the abnormally high cumulative precipitation in 2003 induced a long-term shift in the surrounding biotic community. Analysis of spatial variation across the study site showed that the areas of highest mortality had wetter soils and taller vegetation in the surrounding community than areas of low mortality (D. A. Roach, unpublished data). Plantago lanceolata is a poor competitor, particularly under low light conditions created by taller surrounding vegetation (Van Tienderen and Van der Toorn 1991), and it does not perform well in winter-waterlogged soil (Sagar and Harper 1964). In another long-term plant field study, Silvertown et al. (1994) showed that rainfall could have indirect effects on community composition that can then have lag effects of competitive suppression of study species.
The second major result from this study is that during the period of high ecological stress, when all cohorts showed relatively high mortality, the oldest cohorts performed significantly worse. This differential susceptibility of older individuals to extrinsic mortality is indicative of aging, regardless of whether the mortality is caused directly by an environmental change or mediated by a shift in the surrounding community. Similar to the results from previous work with this species (Roach 2003), smaller individuals had a higher probability of dying. However, there was no evidence that size variation across cohorts could explain the higher mortality in the older cohorts. There was also no evidence for an age-dependent cost to reproduction. In fact, individuals with high reproductive output had a lower risk of mortality across all cohorts and years. This suggests that for this species there is no evidence for an increase in reproductive effort as the prospects for future survival decline as has been recently found in a long-lived bird (Velando et al. 2006). However, whether an individual is reproducing may be an indicator of health and physiological aging in this species because mortality risk was higher for non-reproductive individuals and was highest for the oldest cohorts.
Regardless of whether the exact ecological causes of the mortality increases and decreases can be identified, these results show that long-term mortality patterns are very dynamic and can mask age-dependent demography. In a previous study with this species, a covariate model was used to describe the mortality dynamics of an experimental population in North Carolina (Roach and Gampe 2004, see also Roach 2003). The results of that study showed that when extrinsic weather covariates and intrinsic covariates such as size, reproduction, and genetics, are accounted for, this species shows no evidence for age-dependent mortality. Unfortunately, this covariate model could only be applied to one cohort of the North Carolina population and it could not be applied to the study reported here, with more cohorts, because there were no extrinsic weather covariates that could be found to explain the observed periods of low and high mortality (D. A. Roach and J. Gampe, unpublished data). Although the North Carolina population experienced one year of very high mortality, mortality was relatively low (Roach 2003) compared to the sustained level of 20–50% annual mortality reported here. Under these more stressful conditions, it is clear that older individuals perform relatively poorly. While further analysis is needed to determine whether the cohort differences seen here were due to variation in the selective history experienced by these cohorts, the most likely explanation is that these patterns of differential morality were due to higher rates of aging of the oldest individuals.
The importance of interactions between organismal condition and environmental stress has been underappreciated in most studies of senescence evolution (Williams and Day 2003). In the lab, condition-dependent aging has been demonstrated in Drosophila mutants where aging is only found under stress conditions of caloric restriction (Marden et al. 2003). The results from this field study demonstrate that, in contrast to controlled laboratory studies, demographic senescence may not be manifest as a smooth increasing rate of mortality with age. As has been shown with guppies (Reznick et al. 2004), a thorough understanding of possibly changing environmental conditions is critical to our ability to discern age-dependent influences on mortality over time.
This study shows that when multiple cohorts experience relatively high levels of age-independent mortality, older individuals disproportionately decline. In other words, interactions between age-independent and age-dependent processes demonstrate a demographic pattern of senescence in this plant species in its natural environment. In future analyses of this longitudinal study, aging will be explored at the level of the individual plants to determine how reproductive patterns change with age and whether there is genotypic variation in these patterns of mortality and reproduction.
Acknowledgments
We thank all of the technicians and undergraduate students at the University of Virginia who worked in the hot sun and the cold winter for many many hours gathering this data over the past seven years. We also thank Jutta Gampe for her consultation throughout this project and the members of the Roach lab group for comments on an earlier draft of this manuscript. This work was supported by NIH Grant PO1-AG08761 to D. A. Roach.
Footnotes
Literature Cited
- Carey JR. Population study of mortality and longevity with Gompertzian analysis. In: Yu BP, editor. Methods in aging research. CRC Press; Boca Raton, Florida, USA: 1999. pp. 3–24. [Google Scholar]
- Comstock RE, Robinson HF. The components of genetic variance in populations of biparental progenies and their use in estimating the average degree of dominance. Biometrics. 1948;4:254–266. [PubMed] [Google Scholar]
- Coulson T, Catchpole EA, Albon SD, Morgan BJT, Pemberton JM, Clutton-Brock TH, Crawley MJ, Grenfell BT. Age, sex, density, winter weather, and population crashes in Soay sheep. Science. 2001;292:1528–1531. doi: 10.1126/science.292.5521.1528. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, senescence, and the genome. University of Chicago Press; Chicago, Illinois, USA: 1990. [Google Scholar]
- Gaillard JM, Festa-Bianchet M, Yoccoz NG. Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends in Ecology and Evolution. 1998;13:58–63. doi: 10.1016/s0169-5347(97)01237-8. [DOI] [PubMed] [Google Scholar]
- Gaillard JM, Festa-Bianchet M, Yoccoz NG, Loison A, Toigo C. Temporal variation in fitness components and population dynamics of large herbivores. Annual Review of Ecology and Systematics. 2000;31:367–393. [Google Scholar]
- Marden JH, Rogina B, Montooth KL, Helfand SL. Conditional trade-offs between aging and organismal performance of Indy long-lived mutant flies. Proceedings of the National Academy of Sciences (USA) 2003;100:3369–3373. doi: 10.1073/pnas.0634985100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges ES, Quintana-Ascencio PF. Population viability with fire in Eryngium cuneifolium: deciphering a decade of demographic data. Ecological Monographs. 2004;74:79–99. [Google Scholar]
- Pfeifer M, Wiegand K, Heinrich W, Jetschke G. Long-term demographic fluctuations in an orchid species driven by weather: implications for conservation planning. Journal of Applied Ecology. 2006;43:313–324. [Google Scholar]
- Pico FX, Retana J. Age-specific, density-dependent and environment-based mortality of a short-lived perennial herb. Plant Biology. 2008;10:374–381. doi: 10.1111/j.1438-8677.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Bryant MJ, Roff D, Ghalambor CK, Ghalambor DE. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature. 2004;431:1095–1099. doi: 10.1038/nature02936. [DOI] [PubMed] [Google Scholar]
- Roach DA. Age-specific demography in Plantago: variation among cohorts in a natural plant population. Ecology. 2003;84:749–756. [Google Scholar]
- Roach DA. Evolutionary approaches to the study of whole plant senescence. In: Nooden LD, editor. Plant cell death processes. Academic Press; San Diego, California, USA: 2004. pp. 331–348. [Google Scholar]
- Roach DA, Gampe J. Age-specific demography in Plantago: uncovering age-dependent mortality in a natural population. American Naturalist. 2004;164(1):60–69. doi: 10.1086/421301. [DOI] [PubMed] [Google Scholar]
- Sagar GR, Harper JL. Biological flora of the British Isles: Plantago major L., P. media L., and P. lanceolata L. Journal of Ecology. 1964;52:189–221. [Google Scholar]
- SAS Institute. SAS 9.1. SAS Institute, Inc.; Cary, North Carolina, USA: 2003. [Google Scholar]
- Silvertown J, Dodd ME, McConway K, Potts J, Crawley M. Rainfall, biomass variation, and community composition in the Park Grass experiment. Ecology. 1994;75:2430–2437. [Google Scholar]
- Stewart EJ, Madden R, Paul G, Taddei F. Aging and death in an organism that reproduces by morphologically symmetric division. PLOS Biology. 2005;3:295–300. doi: 10.1371/journal.pbio.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tienderen PH, Van der Toorn J. Genetic differentiation between populations of Plantago lanceolata. I. Local adaptation in three contrasting habitats. Journal of Ecology. 1991;79:27–42. [Google Scholar]
- Vaupel JW, Baudisch A, Dolling M, Roach DA, Gampe J. The case for negative senescence. Theoretical Population Biology. 2004;65:339–351. doi: 10.1016/j.tpb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Velando A, Drummond H, Torres R. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proceedings of the Royal Society B. 2006;273:1443–1448. doi: 10.1098/rspb.2006.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PD, Day T. Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution. 2003;57:1478–1488. doi: 10.1111/j.0014-3820.2003.tb00356.x. [DOI] [PubMed] [Google Scholar]


