Abstract
The North American Brain Injury Study: Hypothermia IIR (NABIS:H IIR) is a randomized clinical trial designed to enroll 240 patients with severe brain injury between the ages of 16 and 45 years. The primary outcome measure is the dichotomized Glasgow Outcome Scale (GOS) at 6 months after injury. The study has the power to detect a 17.5% absolute difference in the percentage of patients with a good outcome with a power of 80%. All patients are randomized by waiver of consent unless family is immediately available. Enrollment is within 2.5 h of injury. Patients may be enrolled in the field by emergency medical services personnel affiliated with the study or by study personnel when the patient arrives at the emergency department. Patients who do not follow commands and have no exclusion criteria and who are enrolled in the hypothermia arm of the study are cooled to 35°C as rapidly as possible by intravenous administration of up to 2 liters of chilled crystalloid. Those patients who meet the criteria for the second phase of the protocol (primarily a post-resuscitation GCS 3–8 without hypotension and without severe associated injuries) are cooled to 33°C. Patients enrolled in the normothermia arm receive standard management at normothermia. As of December 2007, 74 patients had been randomized into phase II of the protocol. Patients in the hypothermia arm reached 35°C in 2.7 ± 1.1 (SD) h after injury and reached 33°C at 4.4 ± 1.5 h after injury.
Key words: acute brain injury, clinical trials, hypothermia, normothermia
Introduction
A prior multicenter randomized study of surface-induced moderate hypothermia in 392 patients with acute traumatic brain injury (TBI) showed no benefit (Clifton et al., 2001). In that study, hypothermia was induced at 4.3 ± 1.1 h after injury and patients reached 33.0°C by 8.4 ± 3 h after injury. Hypothermia was maintained for 48 h, and then patients were slowly rewarmed. Thirty-eight percent of the patients were randomized with waiver of consent. Patients randomized to the hypothermia arm experienced an increased number of days with critical complications (78 ± 22%) as compared to normothermia patients (70 ± 29% percent of days, p = 0.005). Hypotension was the only complication that was significantly increased in the hypothermia group. Patients in the hypothermia group were hypotensive for at least 2 h with end-organ failure more often than normothermia patients (normothermia, 3%; hypothermia, 10%; p = 0.01). There was no overall treatment effect as measured by the dichotomized Glasgow Outcome Scale (GOS; good outcome =good recovery/moderate disability; poor outcome = severe disability/vegetative state/death) at 6 months after injury.
There were subgroup effects. Patients >45 years of age who were treated with hypothermia experienced a higher complication rate and more poor outcomes. Those patients who were <45 years of age, hypothermic upon admission (<35°C), and randomized to the hypothermia arm showed greater improvement at 6 months after injury as compared to patients <45 years of age who were hypothermic on admission, randomized to normothermia, and allowed to passively rewarm (hypothermia, 52% poor outcomes; normothermia, 76% poor outcomes; p = 0.02) (Clifton et al., 2001).
The protocol used in the National Acute Brain Injury Study: Hypothermia (NABIS:H) study was designed to test hypothermia as a neuroprotectant, presumably modifying the biochemical injury cascade, and was not designed to examine the effect of hypothermia upon elevated intracranial pressure (ICP). Induction of hypothermia and rewarming were instituted regardless of the patient's ICP. All patient groups (those aged >45 years, patients who were hypothermic on admission, and those who were normothermic on admission) experienced a decrease in elevated ICP during the period of hypothermia compared to those randomized to normothermic management (Clifton et al., 2001, 2002).
These findings could be interpreted in two ways. One possibility is that it could have been detrimental to become hypothermic after injury and then to be rewarmed. The other possibility is that patients who were hypothermic on admission experienced improved outcomes when hypothermia was maintained because their cooling began within a short treatment window. According to this interpretation of the data, those who were normothermic on admission and randomized to the hypothermia arm were cooled outside the treatment window. In experimental models of brain injury in rodents, hypothermia must be initiated within 90 min of injury to have any effect, so it is likely that there is some treatment window (Markgraf et al., 2001). Of course, there is no way of knowing what the human treatment window is or if there is a treatment window without conducting a clinical trial.
More recently, a multi-center randomized study of hypothermia in 225 pediatric patients (ages 1–17 years) with severe brain injury showed increased mortality rate with hypothermia treatment (p = 0.06). Cooling was initiated in this trial at 6.3 h after injury and 33°C was not reached until 10.2 h after injury, with a cooling duration of 24 h. Hypotension during rewarming was increased in the hypothermia group (p = 0.047), the only complication that was increased in the hypothermia group (Hutchison et al., 2008).
Based upon findings in NABIS:H and now verified by the more recent pediatric study, we designed a hypothermia trial with two priorities: (1) the earliest possible cooling; and (2) avoidance of hypothermia-induced hypotension via a rigid management protocol.
In NABIS:H IIR, we seek to test the hypothesis that induction of mild hypothermia (35°C) as soon as possible after injury followed by prolonged moderate hypothermia (33°C) reached within 4 h of injury and maintained for 48 h will improve the outcome of patients with severe brain injury. The 48-h duration of cooling was selected rather than a longer one because in NABIS:H patients treated with hypothermia had an increased number of hospital days with complications. Most of the complications from hypothermia are related to the depth and the duration of cooling.
The primary hypothesis of NABIS:H IIR is as follows: Induction of hypothermia after severe traumatic brain injury to reach 33.0°C within 4 h of injury and maintained for 48 h in patients aged 16–45 years will result in an increased number of patients with good outcomes at 6 months after injury compared to patients randomized to management at normothermia.
Study Design
NABIS:H IIR is a randomized clinical trial designed to enroll 240 patients with severe brain injury (Glasgow Coma Scale [GCS] 3–8), aged 16–45 years. Patient outcome is assessed at 3, 6, and 12 months after injury. The primary outcome measure is the dichotomized GOS (good outcome =good recovery/moderate disability; poor outcome = severe disability/vegetative/dead) at 6 months after injury using the admission age and the GCS score as co-variables. At 3, 6, and 12 months, the GOS, the Disability Rating Scale, and the Neurobehavioral Rating Scale–Revised (NRS-R) are measured, and at 6 and 12 months, a brief battery of neuropsychological tests is also administered. The neuropsychologist and neuropsychological technicians who perform outcome assessments are blinded to the patient's group assignment, and personnel involved in the acute management of the patients have no access to outcome data. An interim analysis is scheduled when 120 patients have 6-month outcome data.
The study has the power to detect a 17.5% absolute difference in the percentage of patients with a good outcome with a power of 80%. All patients are randomized by waiver of consent unless family is immediately available. Randomization is not delayed by efforts to reach family. An intent-to-treat analysis is used, and only those patients randomized to treatment in phase II of the protocol will be included in the intent-to-treat analysis of the primary outcome measure.
The use of waiver of consent for patients whose families are not immediately available was reviewed by six Institutional Review Boards (IRBs) and approved. Approval was based upon the criteria that the risk of the treatment is proportional to the severity of the injury, that the study could not practicably be performed without routine use of waiver, and that based upon animal and human studies, the treatment window was likely to be 2.5 h after injury or less.
Emergency medical services (EMS) personnel affiliated with the study may randomize and institute cooling if the providers reach the patient within 2.5 h of injury. Patients may also be enrolled by study personnel if the patient arrives by any mode of transport at a study hospital within 2.5 h of injury. Study personnel carry blinded envelopes that contain randomization assignments. Randomization is not stratified by GCS because the initial, randomizing GCS may change by the time of trauma triage or may not be accurate if determined in the field.
Whether randomized in transport or upon arrival at the emergency department (ED), the patient's rectal, esophageal, or bladder temperature is first measured. If randomized to the hypothermia arm and mild hypothermia (35°C) is not already present, it is induced or maintained by disrobing the patient and by the rapid intravenous administration of up to 2 liters of refrigerated crystalloid. If the patient is randomized to normothermia, then standard methods for that center are used to maintain or induce normothermia.
The criteria for randomization into the initial portion of the study (phase I of the protocol) are as follows:
Phase I inclusion criteria
Non-penetrating brain injury with GCS 3–8 on initial evaluation or deteriorates to that level during transport
Estimated or known age of ≥16 and ≤45 years
Injured ≤2.5 h prior to arrival at the ED for patients brought by non-affiliated EMS or reached in ≤2.5 h by study-affiliated EMS personnel
Phase I exclusion criteria
Following commands initially or after an initial period of coma
Penetrating mechanism of injury
Systolic blood pressure of <110 mm Hg
Diastolic blood pressure of <60 mm Hg
Sustained heart rate (pulse) of >120 beats per minute
Estimated or known age of >45 or <16 years
Suspected pregnancy
Injured >2.5 h prior to arrival of affiliated EMS personnel, or if randomized in ED, >2.5 h prior to arrival.
Overt evidence of major chest trauma (e.g., unilaterally absent breath sounds with tracheal deviation)
Patients who are randomized according to this initial set of inclusion/exclusion criteria then complete an evaluation in the ED. If these patients subsequently meet eligibility requirements for phase II of the protocol and the treatment assignment is to the hypothermia arm, the patient's temperature is rapidly reduced to 33.0°C. If their initial assignment is to the normothermia arm, then normothermia is maintained or induced. Patients stay in their initial treatment arm throughout the course of their treatment. Patients who are excluded from phase II of the protocol are subsequently managed by the preference of the treating physician and their complication data recorded until discharge.
The inclusion and exclusion criteria for phase II of the protocol are as follows:
Phase II inclusion criteria
Non-penetrating brain injury with post-resuscitation GCS of ≤8 (motor 1–5)
Estimated or known age of ≥16 and ≤45 years
Injured ≤2.5 h prior to arrival at the ED for patients brought by non-affiliated EMS or reached in ≤2.5 h by study-affiliated EMS personnel
Phase II exclusion criteria
GCS = 7 or 8 with a normal head computed tomography (CT) scan or showing only mild subarachnoid hemorrhage (SAH) or skull fracture or GCS of >8 after initial randomization
GCS = 3 and bilaterally non-reactive pupils
Abbreviated Injury Severity Score of ≥4 for any body area except head
Significantly positive abdominal ultrasound or CT scan
Persistent hypotension (systolic blood pressure of <110 mm Hg)
Persistent hypoxia (O2 saturation of <94%)
Estimated or known age of >45 or <16 years
Positive pregnancy test
Pre-existing medical conditions, if known
Phase II: Patient Management
Bladder temperature is measured hourly for 84 h (3.5 days) from the time of injury in all patients in both arms in phase II of the protocol. This period encompasses the 48 h of cooling plus the rewarming period for hypothermia patients. The temperature of patients randomized into the hypothermia arm in phase II is reduced to 33°C by use of the Arctic Sun Temperature Management System® (surface cooling), by use of cooled ventilated air, and by instillation of iced water or crystalloid into the stomach via nasogastric tube. Temperature is subsequently maintained by use of the Arctic Sun system. Forty-eight hours after the patient reaches 33°C, rewarming is instituted at a rate no faster than 0.25°C per hour. Fever in the normothermia group is defined as a temperature of ≥38°C and is treated by acetaminophen and surface cooling.
A detailed protocol delineates principles of intensive care unit (ICU) management, and all participating centers agree to its requirements. Compliance with the protocol for each individual case is independently monitored by a medical monitor who is unblinded to treatment group.
Brain temperature and brain PO2 are continuously measured by intraparenchymal Licox™ probe for 96 h. All patients undergo insertion of a pulmonary artery catheter for the purpose of calculating systemic vascular resistance, cardiac output, and the adequacy of fluid volume as judged by pulmonary capillary wedge pressure. Since one of the keys to the study is the avoidance of hypotension, a detailed protocol incorporating these variables is used to guide vasopressor and fluid administration throughout management and during rewarming in both patient groups.
The protocol's key components are maintenance of ICP at <20 mm Hg, cerebral perfusion pressure (CPP) = 60–70 mm Hg, and mean arterial pressure (MAP) of >70 mm Hg.
ICP is managed by a sequence of measures: ventricular drainage of cerebrospinal fluid, paralysis, analgesia, moderate hyperventilation, mannitol, and for ICP refractory to these measures, use of barbiturate coma according to published protocols (Eisenberg et al., 1988). Hyperventilation for ICP control is not to exceed PaCO2 = 30 mM Hg in order to avoid ischemia. Arterial blood gases are not corrected for temperature (alpha stat).
The protocol minimizes the occurrence of hypotension and decreased CPP by fluid replacement when mannitol is used, use of low doses of morphine (which acts as a vasodilator), and administration of vasopressors to maintain MAP. Dehydration is avoided by measurement of pulmonary artery capillary wedge pressure and daily measurement and recording of fluid intake and output.
Pancuronium 0.05–1 mg/kg/hr is given for resistance to ventilation, and to control ICP in normothermia patients and for the period of cooling for hypothermia patients. Morphine is administered at 0.05–0.1 mg/kg/hr titrated to the lowest effective dose with use of fentanyl, 1 μg/kg if needed for additional pain management. Phenytoin is given to both groups at 20 mg/kg loading dose with maintenance dose to maintain blood level of 10–20 μg/ml for 7 days after injury. Potassium and magnesium levels are reduced by hypothermia, and repletion of these electrolytes is guided by routine measurement of serum levels.
Safety Monitoring
The Data and Safety Monitoring Board (DSMB) examines complications in both treatment arms of the study at 3-month intervals. As an additional protection, a Complications Monitor independently examines complications and reports to the DSMB at the same interval.
Hypothermia is associated with poor outcomes in multitrauma patients, though only at temperatures of <35°C (Stinemann et al., 1990). Therefore, we examine whether a brief period of cooling to 35°C is associated with increased complications in patients rendered mildly hypothermic and then rewarmed by analyzing the following data in those randomized to phase I of the protocol and excluded from phase II: (1) volume of administration of blood products in the first 48 h, (2) coagulation profile and platelet count in the first 48 h, (3) mortality rate at discharge from hospital, (4) cause of death, and (5) all complications until discharge.
Preliminary Results
As of February 2008, five centers were participating, each with a principal investigator who is a neurosurgeon or an intensivist. Participating centers were the University of Alberta, Edmonton (Dr. Michael Jacka), University of Calgary (Dr. David Zygun), the University of Pittsburgh (Dr. David Okonkwo), St. Louis University (Dr. Ken Smith), and the University of Texas Health Science Center at Houston (Dr. Alex Valadka).
Enrollment began December 2005, with 107 patients randomized into phase I and 74 patients enrolled in phase II by December 2007. The reasons for exclusion of phase I patients from phase II are shown in Table 1. Patients may have more than one reason for exclusion. The most common reason for exclusion is an increase in the GCS from enrollment to the time of trauma triage or a negative CT scan in patients with GCS 7–8. The next most common reason was the presence of severe injuries other than the head injury.
Table 1.
Reasons for Exclusion from Phase II of NABIS:H IIR
| Reason for exclusion from phase II | Number of patients |
|---|---|
| GCS>8 or GCS = 7–8 with normal CT | 56 |
| Abbreviated Injury Severity Score ≥ 4, except head | 15 |
| GCS 3 with unreactive pupils | 12 |
| Persistent blood pressure < 110/60 | 12 |
| Significantly positive abdominal ultrasound or CT scan | 8 |
| Unable to obtain accurate GCS | 6 |
| Age of <16 or >45 years | 5 |
NABIS:H IIR, North American Brain Injury Study: Hypothermia IIR; GCS, Glasgow Coma Scale; CT, computed tomography.
Phase I: Patients
Hypothermia patients were excluded from phase II at a mean of 60 ± 31 min from the time of enrollment into phase I and received a mean of 685 cc of chilled crystalloid. Normothermia patients were excluded from phase II at a mean of 60 ± 41 min from randomization into phase I. From the time of randomization into phase I until exclusion from phase II, the temperature of hypothermia patients decreased only −0.13 ± 0.88°C and the temperature of normothermia patients increased 0.1 ± 1.13°C. Since the average admission temperature of phase I patients was 36 ± 1°C, the brief intervention in phase I served primarily to prevent hypothermia patients from warming until trauma triage could be completed, offering little possibility of producing hypothermia-related complications in multi-trauma patients.
Phase II: Patients
The mean age of patients enrolled into phase II was 29.23 ± 10.7 years, with a mean GCS of 4.2 ± 1.5. Ninety-five percent of patients were enrolled with waiver of consent. The mean temperature of phase II patients at randomization into phase I was the same as patients enrolled into phase I only, 36 ± 1°C. However, the time from randomization to inclusion into phase II was half that of the time from randomization to exclusion from phase II, 29 ± 31 min from randomization to inclusion into phase II for hypothermia patients and 33 ± 32 min for normothermia patients. Study personnel who were making the determination for eligibility for phase II had to wait longer for completion of studies and reversal of paralysis in patients who were ultimately excluded from phase II than they did for patients whose injuries were limited to their brain injury and were, therefore, more quickly evaluated.
Phase II hypothermia patients received a mean of 1644 cc of chilled crystalloid administered with pressure bags at the time of phase I randomization. The result for hypothermia patients was a decrease in temperature of −0.33 ± 0.57°C from the time of randomization into phase I to the time of inclusion into phase II (averaging about 30 min). The temperature of normothermia patients increased 0.38 ± 0.72°C in that half-hour period.
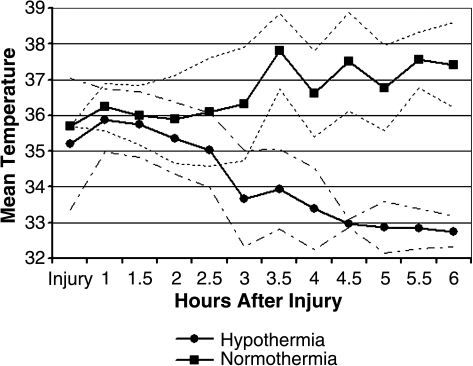
Patients reached 35°C in 2.7 ± 1.1 h after injury with 64.7% of patients reaching 35°C within 2.5 h of injury. The window for enrollment was also 2.5 h from injury. Patients reached 33°C at 4.4 ± 1.5 h after injury with 47.1% of patients reaching 33°C within 4 h of injury. Figure 1 illustrates the cooling rates of normothermia and hypothermia patients. Mean temperature at 4 h after injury was 33.5°C. Hypothermia patients rewarmed from 33°C to 37°C over 17.34 ± 3.1 h. The mortality rate of phase II patients was 21%.
FIG. 1.
Mean temperature from time of injury to 6 h post-injury for normothermia and hypothermia traumatic brain injury (TBI) patients with ranges.
ICU Management
There are significant improvements in ICU management in the present study as compared to the NABIS:H study for two reasons: (1) the ICU protocol is prescriptive; and (2) the medical monitor examines unblinded data to verify protocol compliance. Table 2 shows the percent of patients who had MAP of <70 mM Hg, CPP of <60 mM Hg, and ICP of >25 mM Hg at any time within the first 96 h in NABIS:H and the present study. Out of 74 enrolled, 62 patients with completed data are shown for the present study. The measures used to standardize ICU management dramatically decreased the percentage of patients with MAP of <70 mm Hg and CPP of <60 mm Hg.
Table 2.
Comparison of Percentage of Patients with MAP of <70 mm Hg, CPP of <60 mm Hg, and ICP of >25 mm Hg in First 96 Hours
| n | % with MAP <70 mm Hg | % with CPP <60 mm Hg | % with ICP >25 mm Hg | |
|---|---|---|---|---|
| Trial Ia | 392 | 52% | 71% | 66% |
| Hypothermia | 199 | 53% | 72% | 62% |
| Normothermia | 193 | 51% | 71% | 70% |
| Trial IIb | 62 | 32% | 57% | 66% |
National Acute Brain Injury Study: Hypothermia.
North American Brain Injury Study: Hypothermia IIR. Trial is in progress and cannot report blinded data regarding comparison of hypothermia and normothermia patients until conclusion of trial.
MAP, mean arterial pressure; CPP, cerebral perfusion pressure; ICP, intracranial pressure.
Fluid management also improved. Of 392 patients enrolled in NABIS:H, 26% were dehydrated with a negative cumulative fluid balance in the first 96 h. In the present study, the percentage of dehydrated patients is 10%.
Conclusion
Another 3 years will be necessary to complete enrollment, at a projected accrual rate of about 60 patients per year with six centers enrolling. It is unlikely that the cooling times will be any shorter than those recorded here. Centers do not start randomizing until they have successfully cooled run-in patients with the demonstrated ability to complete the necessary logistics.
It is unlikely that the use of intravascular catheters could achieve shorter cooling times. Some available large-bore catheters can cool more rapidly than surface methods but would require intravascular insertion during or immediately after trauma triage. We have found that delays in cooling are from lack of adequate paralysis or when study personnel cannot access the patient during the triage period or during emergency operative procedures. The limits of cooling are related more to the logistics of patient management than the limits of the technology we are employing.
The use of up to 2 liters of chilled crystalloid administered by pressure bags has been shown to reduce temperature by 1.5°C in neurological patients (Polderman et al., 2005). Our cooling times with use of chilled crystalloid administered with the same technique are longer, however. The temperature of normothermia patients increased by 0.38°C, and the temperature of hypothermia patients decreased by −0.33°C.
The treatment window for hypothermia in patients with severe brain injury is not known, and the goal of rapid cooling is complicated by the need to prevent multitrauma patients from cooling below 35°C. The present study probably offers the shortest cooling times to 33C° that is achievable in a multicenter study. The measures taken to improve the consistency of ICU management among centers have improved management over NABIS:H as judged by a lower incidence of hypotension and decreased cerebral perfusion pressure. Short cooling times and standardized ICU management to minimize hypotension offer the greatest opportunity of detecting a treatment effect of hypothermia as a neuroprotectant.
Acknowledgments
This work was funded by NIH/NINDS (grant 5U01NS043353-05).
References
- Clifton G.L. Miller ER. Choi S.C. Levin H.S. McCauley S. Smith K.R., Jr. Muizelaar J.P. Wagner F.C., Jr. Marion D.W. Luerssen T.G. Chesnut R.M. Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N. Engl. J. Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Miller E.R. Choi S.C. Levin H.S. McCauley S. Smith K.R., Jr. Muizelaar J.P. Marion D.W. Luerssen T.G. Hypothermia on admission in patients with severe brain injury. J. Neurotrauma. 2002;19:293–301. doi: 10.1089/089771502753594864. [DOI] [PubMed] [Google Scholar]
- Eisenberg H.M. Frankowski R.F. Contant C.F. Marshall L.F. Walker M.D. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J. Neurosurg. 1988;69:15–23. doi: 10.3171/jns.1988.69.1.0015. [DOI] [PubMed] [Google Scholar]
- Hutchison J.S. Ward R.E. Lacroix J. Hebert P.C. Barnes M.A. Bohn D.J. Dirks P.B. Doucette S. Fergusson D. Gottesman R. Joffe A.R. Kirpalani H.M. Meyer P.G. Morris K.P. Moher D. Singh R.N. Skippen P.W. Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- Markgraf C.G. Clifton G.L. Moody M.R. Treatment window for hypothermia in brain injury. J. Neurosurg. 2001;95:979–983. doi: 10.3171/jns.2001.95.6.0979. [DOI] [PubMed] [Google Scholar]
- Polderman K.H. Rijnsburger E.R. Peerdeman S.M. Girbes A.R. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit. Care Med. 2005;33:2744–2751. doi: 10.1097/01.ccm.0000190427.88735.19. [DOI] [PubMed] [Google Scholar]
- Steinemann S. Shackford S.R. Davis J.W. Implications of admission hypothermia in trauma patients. J. Trauma. 1990;30:200–202. doi: 10.1097/00005373-199002000-00011. [DOI] [PubMed] [Google Scholar]