Abstract
Purpose
To conduct a Phase I trial of recombinant immunotoxin SS1P given by continuous infusion in chemoresistant solid tumors expressing mesothelin.
Experimental Design
Eligible patients had mesothelioma, ovarian, or pancreatic cancer which was recurrent or unresectable despite standard therapy, and were mesothelin positive by immunohistochemistry. SS1P was administered by continuous infusion for 10 days and cycles could be repeated at 4 week intervals in the absence of neutralizing antibodies or progressive disease.
Results
Twenty-four patients, 5 with peritoneal mesothelioma, 9 with pleural mesothelioma, 2 with pleural-peritoneal mesothelioma, 7 with ovarian carcinoma, and 1 with pancreatic carcinoma, received 4, 8, 12, 18 and 25 ug/Kg/day x10. The maximum tolerated dose was 25 ug/Kg/day ×10, where one of 6 patients had dose limiting toxicity due to reversible vascular leak syndrome. Immunogenicity was observed in 18 (75%) of 24 patients and 5 (21%) received a 2nd cycle. Constant plasma levels of SS1P were maintained for most of the 10-day infusion time, with median peak levels up to 153 ng/ml. One patient had a partial response. Non-major responses included cessation of ascites and independence from paracentesis, resolution of masses by PET, and improved pain and range of motion.
Conclusions
As a single agent by continuous infusion, recombinant immunotoxin SS1P was well-tolerated up to 25 ug/Kg/day x10, and showed evidence of modest clinical activity. Continuous infusion showed no significant advantage over bolus dosing, and further clinical development of SS1P is proceeding by bolus dosing in combination with chemotherapy.
Introduction
Mesothelin is a 40 kDa glycosylphosphatidylinositol-anchored membrane glycoprotein (1, 2). Mesothelin was originally identified by the monoclonal antibody (Mab) K1 produced by immunization of mice with the OVCAR3 cell line. Mesothelin is made as a 69 kDa precursor protein and then processed into the 30 kDa megakaryocyte potentiating factor (MPF) and the 40 kDa mesothelin (3). Mesothelin has also been shown to be important for binding CA-125 and probably has a role in malignant invasion (4). Mesothelin is expressed by a variety of solid tumors including non-mucinous ovarian cancer (5), epithelial and mixed but not sarcomatous mesothelioma (5), squamous-cell cancers arising in lung, head and neck, cervix, or esophagus (6), adenocarcinoma of the lung (7) and pancreatic adenocarcinoma (8, 9). Although normal mesothelial tissues express mesothelin, no reactivity is detectable on liver, heart, brain, kidney, bone marrow, cervix, prostate, stomach, esophagus, or skin (10).
mAb K1 was shown to target human mesothelin-positive tumors in mice (11) and had antitumor activity when chemically conjugated to truncated Pseudomonas exotoxin (PE) (12). To target mesothelin with a recombinant immunotoxin, mice were DNA-immunized with mesothelin and an Fv expression library screened by phage display to yield SS(Fv)-PE38 (13). PE38 is a truncated form of PE which is missing its binding domain, and can be directed by a ligand to bind, internalize into, and kill target cells by ADP-ribosylation and inactivation of elongation factor 2 and apoptosis (14). To improve its affinity for mesothelin, somatic mutational ′hot spots′ in the hypervariable regions were randomized and selected by phage display to result in the high affinity recombinant immunotoxin SS1(Fv)-PE38 (15–17). This immunotoxin was stabilized by conversion of the Fv to a disulfide-stabilized form called SS1(dsFv)-PE38, or SS1P (18, 19). SS1P is cytotoxic toward primary cultures of human ovarian and cervical cancer cells, mesothelin-expressing cell lines (20–22), and toward human mesothelin-expressing tumors grown as xenografts in mice (3).
To determine its clinical activity, SS1P was administered in a Phase I trial to 34 patients with mesothelin-expressing solid tumors as a 30 minute infusion every other day (QOD) (23). The maximum tolerated dose (MTD) in 17 patients treated QOD ×6 was 18 ug/Kg ×6, and in 17 patients treated QOD ×3 was 45 ug/Kg QOD ×3. There were 4 minor responses in 33 evaluable patients, and in addition, resolution of malignant ascites was documented. Immunogenicity by day 29 of cycle 1 was observed in 88% of patients and plasma levels demonstrated a mean half-life of 466 min at the MTD.
Despite excellent antitumor activity achievable in mice with recombinant immunotoxins delivered by continuous infusion (24, 25), this method of administration of recombinant immunotoxins has not been reported in patients. Several clinical trials of larger (∼200 kDa) immunotoxin chemical conjugates have been reported (26–28). Although obvious benefit was not observed relative to bolus dosing, these large mAb-containing chemical conjugates already had prolonged half-life in the plasma. Because solid tumors are closely packed together making tumor penetration a limiting factor for efficacy (29–31), and because the smaller immunotoxins may lack sufficient time in the plasma to achieve significant penetration, we reasoned that maintenance of constant drug levels in the plasma might improve therapeutic efficacy. We therefore assessed the safety and clinical activity of SS1P given by continuous infusion over a 10 day period. A wide variety of tumor types were included to optimally explore its biological activity in mesothelin-expressing malignancies (5–9).
Patients and Methods
Eligibility
Diagnoses included mesothelioma, ovarian cancer, squamous cell cancer of the head and neck, lung or cervix, or pancreatic cancer. Disease had to be unresectable after standard therapy and mesothelin positive by immunohistochemistry. Patients could not have had treatment for ≥ 4 weeks before SS1P. Age ≥ 18, life expectancy ≥ 12 weeks, and performance status ECOG 0–2 were required. Labs required included ANC ≥ 1000, platelets ≥ 75,000, creatinine < 2, normal bilirubin, and AST and ALT < grade 2. Albumin needed to be at least 3 and oxygen saturation ≥ 92%. CNS tumor was disqualifying. Eligibility for retreatment required absence of high levels of neutralizing antibodies, defined as > 75% neutralization by patient serum of 200 ng/ml of the cytotoxic activity of SS1P toward target A431-K5 cells, and also absence of progressive disease.
Study design
SS1P was administered by continuous infusion for 10 days. Doses of 4–25 ug/Kg/day were diluted to 250 ml using 0.9% NaCl containing 0.2% albumin, and infused by portable pump at a rate of 10 ml/hr. The beginning dose of 4 ug/Kg/day × 10 was chosen because the total dose of 40 ug/Kg/cycle was similar to the total of dose/cycle of other recombinant immunotoxins which was associated with some efficacy and without significant toxicity (32–34). Also, continuous infusion doses up to 400 ug/Kg/day ×7 days were non-lethal in Balb/c mice. Retreatment of patients was allowed in the absence of progressive disease or high levels of neutralizing antibodies. Retreatment cycles were 4–6 weeks apart. To prevent allergic reactions and fever, patients received oral hydroxyzine 25 mg and ranitidine 150 mg 1 hour before and 8 hours after each dose, and acetaminophen 650 mg every 6 hours ×4 beginning 1 hour before each dose. The dose of treatment in new patients was escalated if 0 of 3 or 1 of 6 patients at the previous dose level had dose-limiting toxicity (DLT). The MTD was defined as the highest dose level which caused DLT in 0–1 of 6 patients. No intrapatient dose escalation was allowed. DLT was defined as grade ≥ 3 toxicity, and exceptions were made for grade 3 fever, nausea, vomiting, transaminase elevations, grade 4 hematologic toxicity lasting < 5 days, and grade 3 proteinuria of 3.5–10 g/day without creatinine elevation or lasting < 2 weeks. Excessive interruption or failure to complete a cycle of treatment was also considered DLT. Standard response criteria were used as defined previously (23). Neutralizing antibodies were measured by incubating serum with purified SS1P in a 90:10 mixture with a final concentration of 200 ng/ml of SS1P, and determining the percent neutralization of cytotoxicity on A431-K5 cells as described (23). Plasma levels were determined before (day 1) and after beginning the infusion on days 3, 5, and 8, and then before and after stopping the infusion on day 11. Levels were quantified by measuring cytotoxic activity of dilutions of plasma compared to a standard cytotoxicity curve using purified SS1P, as described (23).
Results
Patients and dose escalation
A total of 24 patients were treated (Table 1), including 5 with peritoneal mesothelioma, 9 with pleural mesothelioma, 2 with pleural-peritoneal mesothelioma, 7 with ovarian carcinoma, and 1 with pancreatic carcinoma. Compared with ovarian cancer, patients with mesothelioma had fewer prior therapies (median 2 vs 6, p=0.004, Wilcoxon), probably due to mesothelioma being more aggressive and less treatable. Groups of 3 patients received 4 and 8 ug/Kg/day ×10, and 6 patients each received 12, 18 and 25 ug/Kg/day ×10.
Table 1.
Patient characteristics
| Diagnosis | No. Cases | Prior Tx |
|---|---|---|
| Peritoneal mesothelioma | 5 | 1–3 (2) |
| Pleural mesothelioma | 9 | 0–4 (2) |
| Pleural-peritoneal mesothelioma | 2 | 1–3 (2) |
| Ovarian cancer | 7 | 2–9 (6) |
| Pancreatic carcinoma | 1 | 1 |
| Total | 24 | 0–9 (2) |
| Dose levels of SS1P | No. Enrolled | No. Patients with DLT |
| 4 µg/Kg/day ×10 | 3 | 0 |
| 8 µg/Kg/day ×10 (3) | 3 | 0 |
| 12 µg/Kg/day ×10 (3) | 6 | 1 |
| 18 µg/Kg/day ×10 (3) | 6 | 0 |
| 25 µg/Kg/day ×10 (3) | 6 | 1 |
| Ages: 31–69, median 60 | ||
| Males: 13, Females: 11 |
DLT, dose-limiting toxicity, Prior Tx, number of prior chemotherapy regimens per patient, range (median).
SS1P safety and dose-escalation
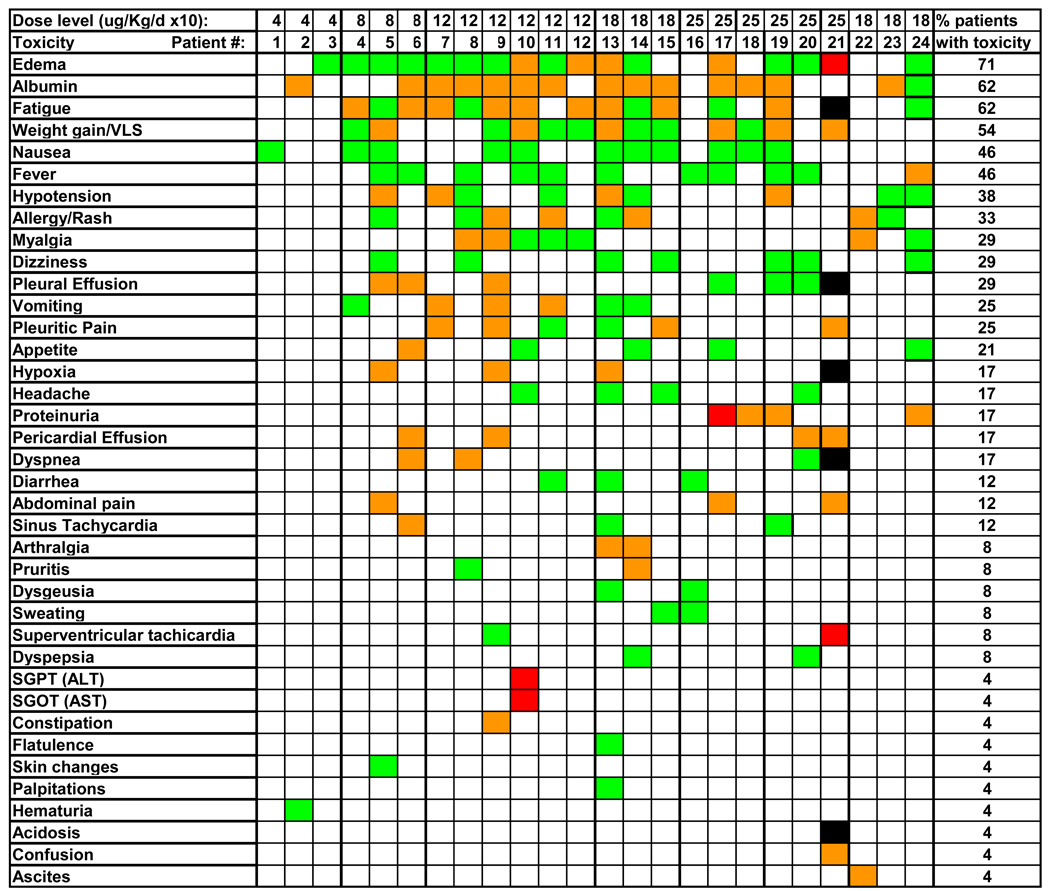
As shown in Fig. 1, toxicities were usually grade 1–2 and the most common adverse events were edema (71%), hypoalbuminemia (62%), fatigue (62%), weight gain/vascular leak syndrome (VLS) (54%), nausea (46%), fever (46%), hypotension (38%), and allergy/rash (33%). Grade 3 transaminase elevations and proteinuria were observed in 1 patient each and were not considered dose-limiting by protocol. The 4 and 8 ug/Kg/day ×10 dose levels were completed with 3 patients each. The 12 ug/Kg/day ×10 dose level was expanded to 6 patients because the 1st patient stopped treatment after 5 days due to pleuritic chest pain without evidence of cardiopulmonary origin, and did not resume the infusion. Groups of 3 patients each then received 18 and 25 ug/Kg/day ×10. Pleuritic chest pain not due to a cardiopulmonary cause was observed in 3/6 patients at 12 ug/Kg/day ×10 and 2 patients at 18 ug/Kg/day ×10, and typically involved the normal lung in patients with pleurodesis on the contralateral side. One patient at the 25 ug/Kg/day ×10 dose level had grade III proteinuria not associated with symptoms or with a creatinine elevation, resolving several days later with the next 24 hour urine. Although this event was not considered DLT, 3 additional patients were enrolled at the 25 ug/Kg/day ×10 dose level. The last patient had baseline pulmonary hypertension and diastolic dysfunction, and with SS1P plus exogenous fluid developed large pleural effusions and respiratory failure, resolving with aggressive diuresis. An additional 3 patients were treated at the 18 ug/Kg/day dose level without DLT. Dose escalation beyond 25 ug/Kg/day ×10 was not attempted. Thus 0 of 6 at 18 and 1 of 6 at 25 ug/Kg QOD ×3 had DLT, defining the higher dose level as the MTD.
Fig. 1. Toxicity of SS1P by continuous infusion.
Adverse events shown were grade 1 (green), 2 (orange), 3 (red) or 4 (black).
Immunogenicity
High levels of neutralizing antibodies, defined as > 75% neutralization of 200 ng/ml of SS1P, were observed in 3 of 3 patients at 4 and 8 ug/Kg/day ×10, 3 of 6 at 12 and 18 ug/Kg/day ×10, and 6 of 6 at 25 ug/Kg/day ×10. Five patients received 2 cycles, with the 2nd cycle of SS1P the same dose level as cycle 1. Thus, the immunogenicity rate on cycle 1 was 18 (75%) of 24 patients.
Pharmacokinetics
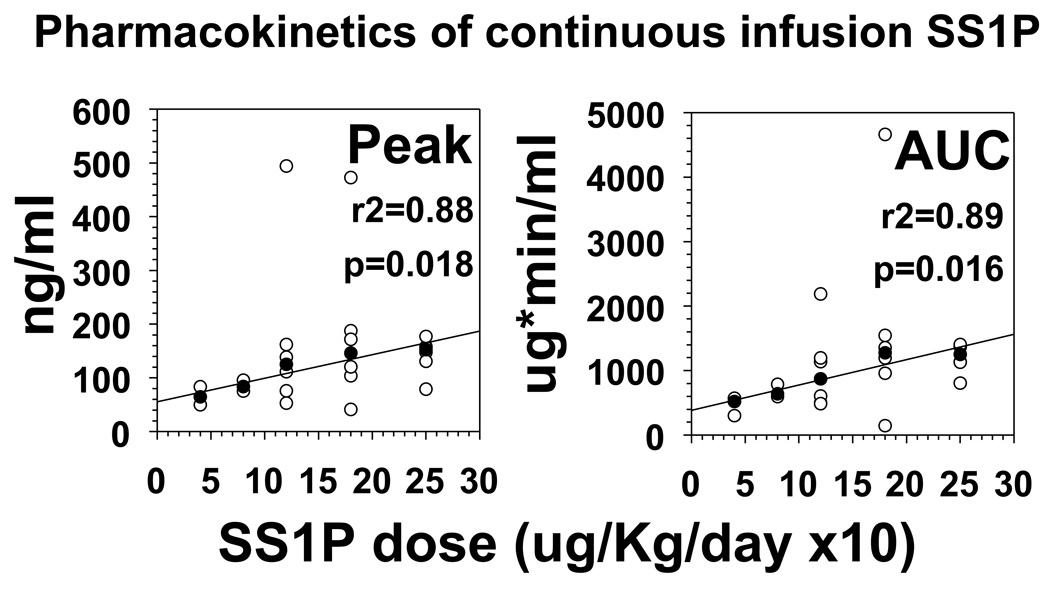
Plasma levels of SS1P were determined by incubating dilutions of plasma from treated patients and comparing cytotoxicity on A431-K5 cells to a standard curve of cytotoxicity generated simultaneously by known concentrations of purified SS1P. Plasma levels were measured prior to infusion, and on days 3, 5, 7, 9, and 11 before and after the infusion ended. Plasma levels typically reached a peak at day 5 and declined prior to day 11 when patients made neutralizing antibodies early. As shown in Fig. 2, median peak levels of SS1P were 64, 95, 119, 146, and 153 ng/ml at 4, 8, 12, 18, and 25 ug/Kg/day ×10, respectively. The medians of both peak concentrations and median AUCs (the latter determined by the trapezoid method on the cytotoxicity curves) were directly proportional to dose (r2 = 0.88 – 0.89, p=0.016 – 0.018).
Fig. 2. Pharmacokinetics of SS1P by continuous infusion.
The peak levels reached during infusion (A) and the total calculated AUCs are shown for each patient in open circles, and the medians of each dose level are shown by closed circles.
Efficacy
All 24 patients were considered evaluable for response. One patient had a partial response (PR), 12 had stable disease (SD), and 11 had progressive disease (PD). The patient with PR had ovarian cancer and received 25 ug/Kg/day ×10. She had a 15 × 26 mm hepatic lesion which decreased in size to 10 × 19 mm by C1D29, and to 7 × 16 mm by C1D64. Her CA-125 decreased from 384–392 pretreatment to 309 by C1D90 and 243 by C1D119. Another patient at this dose level with peritoneal mesothelioma had severe ascites prior to SS1P requiring frequent paracentesis and had hypoalbuminemia due to removal of albumin in the fluid. After finishing the SS1P infusion, the patient did not require paracentesis for several months and was able to return to jogging. One patient with thoracic mesothelioma had severe chest pain requiring high dose narcotics and was unable to move his arms without pain. By the end of the infusion of 18 ug/Kg/day ×10, the patient′s pain had resolved, he regained full range of motion of his arms without pain, and was taken off of narcotics. His CTs showed a slight decrease in the size of the chest mass. Another patient at 18 ug/Kg/day ×10 with mesothelioma had complete disappearance of a supraclavicular lymph node by PET scan, which appeared slightly smaller but present on CT. Finally, one patient at 25 ug/Kg/day ×10 had a significant decrease in uptake of an abdominal ovarian cancer lesion by PET. Overall, one of 24 patients had a PR based on improvement of the CT, which was required by protocol, although other patients not making CT response criteria responded by PET.
Discussion
We found that SS1P given by continuous infusion was well tolerated at doses up to 25 ug/Kg/day ×10. The major toxicities included pleuritic pain and 3rd spacing, both reversible. Immunogenicity after 1 cycle was observed in 75% of patients. The median peak plasma level and AUC correlated with dose level, with significant variability between patients. One major response was documented and several patients had less than protocol-defined PR but evidence of antitumor activity.
Mesothelial targeting of SS1P
While the adverse events related to VLS, including edema, hypoalbuminemia, weight gain, and hypotension are common to other recombinant immunotoxins and suggest nonspecific toxicity (32, 34), the pleuritic pain observed with SS1P is unique to this recombinant immunotoxin (23) and thus suggests direct targeting of normal mesothelial cells. Since pleuritic chest pain typically involved normal lung, rather than the side which had undergone pleurodesis, the cause of pain may be inflammation of normal mesothelium lining in the thoracic cavity. Typically the pain subsided spontaneously by day 7–9 of infusion even when significant plasma levels were present at that time. This suggests that the process of mesothelial inflammation or toxicity was self-limited. Although this toxicity was not a major dose-limiting event in this trial, it is potentially a problem and will be addressed in further SS1P development possibly through anti-inflammatory therapy.
Comparison of continuous infusion and bolus dosing
As shown in Table 2, the total dose of SS1P delivered at the MTD, 250 ug/Kg, is slightly higher than that achieved by bolus dosing of 3 doses of SS1P (23). Although immunogenicity of SS1P when given by continuous infusion was slightly lower than that observed by bolus dosing, it represented a major potential limitation in response. At the MTD, the median AUC over the 10 days of continuous infusion, 1800 ug*min/ml, was about 3-fold higher than the median estimated AUC by bolus dosing, 590 ug*min/ml. The total AUC by bolus dosing was obtained by multiplying by 3 the AUC determined on the 1st of 3 bolus doses.
Table 2.
Comparison of continuous infusion and bolus dosing of SS1P
| Continuous Infusion | Bolus (30 min) Infusion | |
|---|---|---|
| MTD | 25 µg/Kg/day ×10 | 45 µg/Kg QOD ×3 |
| MTD (total dose) | 250 µg/Kg | 135 µg/Kg |
| Immunogenicity | 18 (75%) of 24 | 30 (88%) of 34 |
| AUC (median at MTD) | 1800 µg*min/ml | 590 µg*min/ml |
MTD, maximum tolerated dose
QOD, every other day
Clinical development of SS1P
Response was not dramatically different on this trial compared to the Phase I trial of bolus SS1P (23). Anti-tumor activity was modest despite median plasma levels up to 153 ng/ml, even though concentrations of SS1P at 1–10 ng/ml were sufficient to kill mesothelin-expressing cells lines (20, 22) and tumors in organotypic culture (21). It is possible that high levels of soluble mesothelin, within the tumors of patients, interfered with delivery of SS1P to the tumor cells (35), and that chemotherapy along with SS1P would decrease the soluble receptor within tumors, facilitating effective targeting to all tumor cells (35–37). To test this hypothesis, a phase II trial is now underway pretreating mesothelioma patients with pemetrexed-cisplatinum prior to SS1P, beginning with an SS1P bolus dosage of 25 ug/Kg QOD ×3. If chemotherapy can allow even distribution of SS1P to tumor cells, then bolus dosing, which can achieve peak SS1P levels of nearly 500 ng/ml, with mean half-lives of nearly 8 hours, should be adequate to result in major response in several types of mesothelin-expressing malignancies.
STATEMENT OF TRANSLATIONAL RELEVANCE.
This manuscript describes clinical results of a phase I trial in which the recombinant anti-mesothelin immunotoxin SS1P was administered by continuous infusion to patients with mesothelin-positive solid tumors, most commonly mesothelioma. A phase I trial of this agent administered by bolus infusion has recently been reported. Recombinant immunotoxins contain an Fv fragment of a monoclonal antibody genetically fused to a truncated bacterial toxin. These agents (∼63 kDa) are much smaller than chemical conjugates of whole antibody and toxin, and their plasma lifetimes are much shorter. This manuscript is the first to document that significant plasma levels of recombinant immunotoxin can be maintained in patients by continuous infusion. The results will be useful for understanding and predicting the pharmacokinetics of other proteins of similar type or size. Moreover, this manuscript is an important part of the clinical development of SS1P, which is now undergoing phase II testing.
Acknowledgments
The authors thank research nurses Karen Bergeron, Rita Mincemoyer, Diana O’Hagan, Kelly Cahill, and Michelle Zancan, and Dr. David Squires for their work with patients on this trial. We recognize Dr. Lee Pai-Scherf for writing a portion of the protocol, and Dr. Mark Willingham for performing immunohistochemistry. We recognize the efforts of NCI Medical Oncology Branch fellows and nurses. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo C, Bei R, Procopio A, Modesti A. Molecular targets and targeted therapies for malignant mesothelioma. Curr Med Chem. 2008;15:855–867. doi: 10.2174/092986708783955446. [DOI] [PubMed] [Google Scholar]
- 3.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 4.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang K, Pai LH, Pass H, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol. 1992;16:259–268. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chang K, Pastan I, Willingham MC. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer. 1992;51:548–554. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- 7.Kushitani K, Takeshima Y, Amatya VJ, Furonaka O, Sakatani A, Inai K. Immunohistochemical marker panels for distinguishing between epithelioid mesothelioma and lung adenocarcinoma. Pathol Int. 2007;57:190–199. doi: 10.1111/j.1440-1827.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- 8.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 9.Ryu B, Jones J, Blades NJ, et al. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819–826. [PubMed] [Google Scholar]
- 10.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 11.Hassan R, Wu C, Brechbiel MW, Margulies I, Kreitman RJ, Pastan I. 111Indium-labeled Monoclonal antibody K1: biodistribution study in nude mice bearing a human carcinoma xenograft expressing mesothelin. Int J Cancer. 1999;80:559–563. doi: 10.1002/(sici)1097-0215(19990209)80:4<559::aid-ijc13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Hassan R, Viner J, Wang QC, Kreitman RJ, Pastan I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer and malignant mesothelioma. J Immunotherapy. 2000;23:473–479. doi: 10.1097/00002371-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury PS, Chang K, Pastan I. Isolation of anti-mesothelin antibodies from a phage display library. Mol Immunol. 1997;34:9–20. doi: 10.1016/s0161-5890(97)00011-4. [DOI] [PubMed] [Google Scholar]
- 14.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin Treatment of Cancer. Annu Rev Med. 2007;58:221. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotechnol. 1999;17:568–572. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci U S A. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury PS, Vasmatzis G, Lee B, Pastan I. Improved stability and yield of a Fv-toxin fusion protein by computer design and protein engineering of the Fv. Journal of Molecular Biology. 1998;281:917–928. doi: 10.1006/jmbi.1998.1980. [DOI] [PubMed] [Google Scholar]
- 18.Reiter Y, Brinkmann U, Kreitman RJ, Jung S-H, Lee B, Pastan I. Stabilization of the Fv fragments in recombinant immunotoxins by disulfide bonds engineered into conserved framework regions. Biochemistry. 1994;33:5451–5459. doi: 10.1021/bi00184a014. [DOI] [PubMed] [Google Scholar]
- 19.Pastan I, Hassan R, FitzGerald DJP, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 20.Onda M, Nagata S, Tsutsumi Y, et al. Lowering the isoelectric point of the Fv portion of recombinant immunotoxins leads to decreased nonspecific animal toxicity without affecting antitumor activity. Caner Res. 2001;61:5070–5077. [PubMed] [Google Scholar]
- 21.Hassan R, Lerner MR, Benbrook D, et al. Antitumor Activity of SS(dsFv)PE38 and SS1(dsFv)PE38, Recombinant Antimesothelin Immunotoxins against Human Gynecologic Cancers Grown in Organotypic Culture in Vitro. Clin Cancer Res. 2002;8:3520–3526. [PubMed] [Google Scholar]
- 22.Li Q, Verschraegen CF, Mendoza J, Hassan R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004;24:1327–1335. [PubMed] [Google Scholar]
- 23.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 24.Kreitman RJ, Wang QC, FitzGerald DJP, Pastan I. Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by Cynomolgus monkeys. Int J Cancer. 1999;81:148–155. doi: 10.1002/(sici)1097-0215(19990331)81:1<148::aid-ijc24>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Benhar I, Reiter Y, Pai LH, Pastan I. Administration of disulfide-stabilized Fv-immunotoxins B1(dsFv)-PE38 and B3(dsFv)-PE38 by continuous infusion increases their efficacy in curing large tumor xenografts in nude mice. Int J Cancer. 1995;62:351–355. doi: 10.1002/ijc.2910620320. [DOI] [PubMed] [Google Scholar]
- 26.Grossbard ML, Lambert JM, Goldmacher VS, et al. Anti-B4-blocked ricin: a Phase I trial of 7-day continuous infusion in patients with B-cell neoplasms. J Clin Oncol. 1993;11:726–737. doi: 10.1200/JCO.1993.11.4.726. [DOI] [PubMed] [Google Scholar]
- 27.Stone MJ, Sausville EA, Fay JW, et al. A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood. 1996;88:1188–1197. [PubMed] [Google Scholar]
- 28.Grossbard ML, Fidias P, Kinsella J, et al. Anti-B4-blocked ricin: a phase II trial of 7 day continuous infusion in patients with multiple myeloma. Br J Haematol. 1998;102:509–515. doi: 10.1046/j.1365-2141.1998.00799.x. [DOI] [PubMed] [Google Scholar]
- 29.Sung C, Dedrick RL, Hall WA, Johnson PA, Youle RJ. The spatial distribution of immunotoxins in solid tumors: assessment by quantitative autoradiography. Cancer Res. 1993;53:2092–2099. [PubMed] [Google Scholar]
- 30.Sung C, Youle RJ, Dedrick RL. Pharmacokinetic analysis of immunotoxin uptake in solid tumors: role of plasma kinetics, capillary permeability, and binding. Cancer Res. 1990;50:7382–7392. [PubMed] [Google Scholar]
- 31.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab')2, and Fab in tumors. Cancer Res. 1989;49:5656–5663. [PubMed] [Google Scholar]
- 32.Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin Anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1614–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 33.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the Anti-CD22 Recombinant Immunotoxin BL22 in Chemotherapy-Resistant Hairy-Cell Leukemia. New Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 34.Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Xiang L, Hassan R, Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc Natl Acad Sci U S A. 2007;104:17099–17104. doi: 10.1073/pnas.0708101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan R, Broaddus VC, Wilson S, Liewehr DJ, Zhang J. Anti-mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin-expressing tumor xenografts. Clin Cancer Res. 2007;13:7166–7171. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Xiang L, Hassan R, et al. Synergistic anti-tumor activity of taxol and immunotoxin SS1P in tumor bearing mice. Clin Cancer Res. 2006;12:4695–4701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]