Abstract
We previously found that female mice exhibited Fos responses in the accessory olfactory bulb (AOB) after exposure to volatile opposite-, but not same-sex, urinary odours. This effect was eliminated by lesioning the main olfactory epithelium, raising the possibility that the AOB receives information about gender via centrifugal inputs originating in the main olfactory system instead of from the vomeronasal organ. We asked which main olfactory forebrain targets send axonal projections to the AOB, and whether these input neurons express Fos in response to opposite-sex urinary volatiles. Female mice received bilateral injections of the retrograde tracer, cholera toxin B (CTB), into the AOB, and were exposed to either same- or opposite-sex volatile urinary odours one week later. We found CTB- labeled cell bodies in several forebrain sites including the bed nucleus of the accessory olfactory tract, the rostral portion of the medial amygdala (MeA), and the posteromedial cortical nucleus of the amygdala. A significant increase in the percentage of CTB/Fos co-labeled cells was seen only in the MeA of female subjects exposed to male but not to female urinary volatiles. In Experiment 2, CTB-injected females were later exposed to volatile odours from male mouse urine, food, or cat urine. Again, a significant increase in the percentage of CTB/Fos co-labeled cells was seen in the MeA of females exposed to male mouse urinary volatiles but not to food or predator odours. Main olfactory - MeA -AOB signaling may motivate approach behaviour to opposite-sex pheromonal signals that ensure successful reproduction.
Keywords: pheromone, ovariectomy, retrograde, volatile, Fos
Introduction
The murine main olfactory system (MOS) detects general volatile odourants, while the accessory olfactory system (AOS) is thought to respond to non-volatile pheromones. Volatile odourants bind to olfactory sensory neurons (OSNs) in the main olfactory epithelium (MOE), which in turn project to the same 1-2 glomeruli in the main olfactory bulb (MOB) (Buck & Axel, 1991, Chess et al., 1992). Mitral cells in the MOB project to the ipsilateral olfactory cortex as well as cortical amygdaloid nuclei. Detection of non-volatile pheromones occurs in the vomeronasal organ (VNO), which contains receptor neurons (VSNs) that are unrelated to those found in the MOE (Dulac & Axel, 1995). VSNs project to multiple glomeruli in the accessory olfactory bulb (AOB) (Wagner et al., 2006). Mitral cells of the AOB project to the medial amygdala (MeA), which subsequently conveys inputs to the bed nucleus of the stria terminalis as well as a number of downstream hypothalamic targets (Halpern & Martinez-Marcos, 2003; Kevetter & Winans 1981).
Recent studies have shown that the functions of the main and accessory olfactory systems may complement each other (Brennan & Keverne, 2004; Brennan & Zufall, 2006; Shepherd, 2006). The MOB may be activated by non-volatile major histocompatibility complex (MHC) class I peptides (Spehr et al., 2006). Additionally, cells in both the MOB and AOB express Fos in response to volatile urinary odours (Martel & Baum, 2007; Muroi et al., 2006), although it seems likely that access to the AOS by urinary volatiles is limited to opposite-sex odours and is mediated via centrifugal inputs (projections from central forebrain sites to the periphery) as opposed to direct inputs to the AOB that originate in the VNO (Martel & Baum, 2007). Previous anatomical studies showed that forebrain sites including the posteromedial cortical nucleus of the amygdala (PMCo), bed nucleus of the accessory olfactory tract (BnAOT), and medial amygdala (MeA) (Barber, 1982), as well as the locus coeruleus (LC) (Shipley et al., 1985; McLean et al., 1989) send centrifugal projections to the granule cell layer (PMCo and LC), internal plexiform layer (BnAOT and MeA), and the external plexiform and mitral cell layers (LC) of the AOB.
To further explore the possibility that sex-specific AOB Fos responses to volatile urinary odours are induced via centrifugal inputs, we sought to confirm that neurons in each of the above-mentioned forebrain regions send projections to the AOB and asked whether AOB-projecting neurons in any of these regions selectively express Fos in response to opposite-sex urinary odours. In a pilot experiment, CTB-IR cell bodies were found in several forebrain regions of female mice including the anterior MeA, BnAOT, and the PMCo 7 days after injection of CTB into the ipsilateral AOB. Fewer than 1% of CTB labeled cells in the PMCo were co-labeled with Fos following odour exposure. Thus, we chose to focus on odour-induced Fos expression in AOB-projecting neurons in the MeA and BnAOT. In Experiment 1, we compared percentages of AOB-projecting cells in these two regions that expressed Fos in response to same-versus opposite-sex urinary odours. A subset of AOB-projecting neurons in the females’ MeA selectively responded to opposite-sex urinary odours. To determine whether this circuit responds selectively to volatile reproductive odours, in Experiment 2 we compared Fos expression in AOB-projecting BnAOT and MeA neurons of female mice in response to food odours, or to cat or male mouse urinary odours.
Materials and Methods
Subjects
Seventy-five sexually naïve female Swiss Webster mice (Charles River Laboratories, Wilmington, MA, USA) were purchased at 6-8 weeks of age and group housed under a 12h light-dark photoperiod (lights on at 09:00h). Food and water were provided ad libitum. All procedures were approved by the Boston University Animal Care and Use Committee and were in accordance with NIH guidelines. Subjects underwent bilateral ovariectomy under 2% isoflurane anesthesia, and were given 1 week to recover. In our previous study (Martel & Baum, 2007), a robust sex difference was seen in AOB Fos responses to male or female urinary volatiles in gonadectomized male and female mice given no replacement sex hormone treatments. Since our aim in the present study was to illuminate the possible contribution of MOS inputs to this apparently hard-wired sex difference in AOB Fos responses, we chose not to administer sex hormones to our ovariectomized subjects. We cannot rule out the possibility that a different profile of results might have been obtained in estradiol-treated subjects. However, if anything, we would expect that estradiol treatment would have amplified any neural Fos responses seen in response to pheromonal stimuli. Ovariectomized mice were again deeply anesthetized using a ketamine (120mg/kg) and xylazine (20mg/kg) cocktail whereupon their heads were fixed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA); animals received continuous 1% isoflurane anesthesia throughout the entire procedure. A midline incision was made to expose the skull, and two small holes were drilled above the left and right olfactory bulbs. The dura was then penetrated by passing 200μA of current through a tungsten microelectrode (500μm diameter, FHC, Bowdoin, ME, USA) attached to a Grass Lesion Maker, with the ground electrode attached to the subject’s tail. Bilateral iontophoretic injections of 0.5% CTB (List Biological Laboratories, Campbell, CA, USA) in 0.1M phosphate buffer (pH=6.0) were then made into the AOBs using a glass capillary tube (OD=1.5mm, ID=0.75mm, Stoelting, Wood Dale, IL, USA) with a pulled tip diameter of 20-25μm. The capillary tube was lowered at a 40° angle at a point 1.0mm anterior and 0.8mm lateral to the intersection of the midline and the inferior cerebral vein. A silver wire was placed in the open end of the capillary tube and attached to the positive electrode, while the ground electrode was again attached to the subject’s tail. A 2mA positive alternating current was then applied for 15 min, followed by a 5-min rest period prior to retraction of the capillary tip to avoid leakage of the tracer into the entry tract. The skull holes were then filled with gel foam, and the incision was closed with absorbable suture.
Odour exposure
Stimuli
One week after bilateral CTB injection into the AOB, subjects were exposed to one of three volatile odour conditions in Experiment 1 including clean air, male, or female mouse urine, and to one of four volatile odour conditions in Experiment 2 including clean air, food, cat urine, or male mouse urine. Urine donors included 5 gonadally intact adult male Swiss Webster mice and 5 ovariectomized Swiss Webster female mice given a series of subcutaneous injections of estradiol benzoate (20μg, 24 and 48 hours prior to collection) and progesterone (500μg, 4 hours prior to collection) to induce oestrus. Urine was obtained by scruffing the animal over a funnel and gently applying pressure to the abdomen. Urine was subsequently pooled according to sex and stored at −80°C. Female cat urine was obtained from 3 domestic female cats. Regular cat litter was replaced with Nosorb® urine collecting litter (Catco Vet Products, Cape Coral, FL, USA) overnight. Urine was collected into clean containers by tilting the litter tray, pooled, and stored at −80°C. All urinary stimuli were presented in 5ml solutions of 20% urine in distilled water. Nutter Butter® cookies were used as the food odour stimulus by crushing a single cookie and dissolving it in 10ml of mineral oil. In Experiment 2, all subjects were given experience eating Nutter Butter® cookies on day 5 following CTB injection.
Odour delivery
All subjects were singly housed 48h prior to odour exposure. Subjects were food deprived for 24h prior to odour exposure to minimize exposure to the subject’s own fecal odours. Subjects were placed in a clean Plexiglas chamber (5×10×25cm) on top of a perforated metal plate raised 2.5cm above the bottom of the chamber. These perforations allowed for any urine and fecal excrement to fall below the subject, once again reducing access to background odour stimuli. All odour exposures were performed in an odour-free fume hood during the light phase of subjects’ light/dark cycle. Subjects were first exposed for 40 minutes to clean air at a flow rate of 1500ml/min. Air was passed to the test chamber via odour-free tubing through activated charcoal for purification followed by fresh distilled water for humidification. Subjects were then exposed to a particular volatile stimulus odour (Experiment 1: clean air (n=8), female mouse urine (n=6), male mouse urine (n=10); Experiment 2: clean air (n=6), food (n=6), cat urine (n=6), male mouse urine (n=7)), in which clean, humidified air was passed through a 250ml flask containing one of the previously described mixtures, or just distilled water in the case of clean air controls. Volatile stimuli were delivered through an odour port 0.75cm in diameter) located 4cm above the perforated metal plate on which the subject stood. Stimulus odours were pulsed at 3:2 min on:off intervals for 30 min to avoid habituation to the odour (Schaefer et al., 2001). To ensure that possible group differences in odour-induced neural Fos activation were not the result of subjects being exposed to different concentrations of the odours presented, in Experiment 2 we recorded the amount of time that a subset of subjects in the different groups spent sniffing at the odour port across each consecutive 3-min odour on interval. Subjects were deeply anesthetized with Sleepaway and sacrificed 90 min after the onset of odour exposure via transcardiac perfusion with 0.1M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in 0.1M PBS. Brains were removed and post-fixed for 4 hours, and cryoprotected in 30% sucrose:PBS solution for 48 hours. Forebrains and olfactory bulbs were then blocked and stored at −80°C.
Histology
Brain hemispheres were divided by a midline cut, and ipsilateral sagittal olfactory bulb and coronal forebrain sections were cut (30μm thickness) on a freezing sledge microtome (Leica Microsystems Inc., Bannockburn, IL, USA). Every other section was immersed free floating in 0.1M PBS, and remaining sections were stored in antifreeze at −20°C. CTB/Fos double label ICC was subsequently performed. To first visualize c-Fos positive cells, tissue was rinsed 3 times in 0.1M PBS, and then placed in a solution of 40% methanol and 3% hydrogen peroxide in 0.1M PBS for 10 minutes. Following 4 rinses in 0.1M PBS, the tissue was placed in a blocking solution containing 5% normal donkey serum in 0.1% Triton-X 100 PBS (PBST) for 1 hour. Sections were then incubated overnight in anti-cFos rabbit IgG (1:1000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and 5% normal donkey serum in PBST. The following day, sections were rinsed 6 times with PBST and then placed in biotynilated donkey anti-rabbit secondary antibody (1:600) (Jackson Immunoresearch, West Grove, PA, USA) in PBST. After another series of rinses in PBST, the tissue was incubated with ABC (1:200) (ABC Kit; Vector Laboratories Elite Kit), rinsed, and developed with DAB with nickel enhancement. For CTB labeling in the same tissue, sections were first re-fixed in 4% paraformaldehyde. The same protocol was used for CTB staining as for c-Fos with the exception of the blocking serum (normal rabbit serum), and primary (anti-CTB goat IgG; 1:40,000) (List Biological Laboratories, Campbell, CA, USA) and secondary (biotynilated rabbit anti-goat; 1:600) (Vector Laboratories, Burlington, CA, USA) antibodies. CTB positive cells were developed using a DAB kit without nickel enhancement. After staining was complete, sections were mounted sequentially on gelatin-coated slides, rostral-caudal for forebrains, and lateral-medial for olfactory bulbs. Slides were air-dried over night, rinsed with deionized water, and coverslipped with Permount.
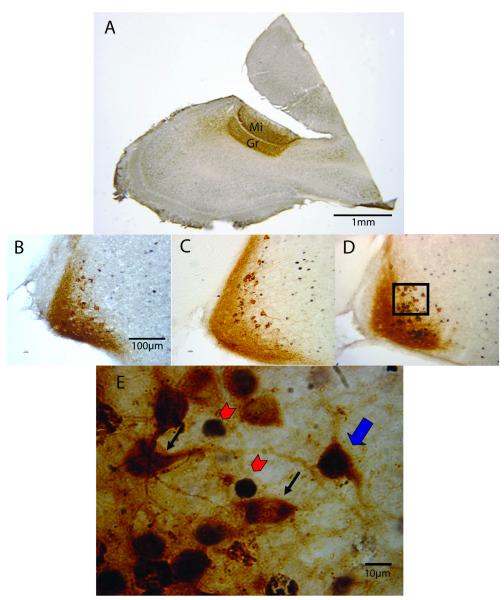
In order for individual forebrain hemispheres of each animal to be included in the study, CTB injections had to be confined to and fill at least 2/3 of the granule and mitral cell layers of the AOB (Figure 1A). Subjects with insufficient injection sizes or extensive CTB labeling in the surrounding MOB tissue were discarded (14/38 animals in Experiment 1, and 12/37 animals in Experiment 2). Presumably, data collected from either forebrain hemisphere of a single subject would not significantly differ, thus only one hemisphere was used even in subjects that received accurate injections bilaterally. All slides were coded to conceal the identity of individual subjects. Individually labeled CTB-immunoreactive (IR) (histology, Figure 1; counting regions, Figure 2D and E) and Fos-IR cells (histology, Figure 1; counting regions, Figure 2 all panels) were quantified in forebrain regions by tracing cells in three anatomically matched sections (60μm apart to avoid double counting of IR cells) onto paper using a camera lucida attachment to an Olympus microscope, as previously described (Halem et al., 2001; Pankevich et al., 2006). Mean numbers of CTB-IR or Fos-IR cells per circular standard counting area (0.1mm2) were calculated for each animal. Double-labeled cells were quantified on a separate occasion in the BnAOT and MeA using the same circular field as was used for single-labeled cells under oil immersion at 100x magnification, and were characterized by nuclear Fos staining surrounded by cytoplasmic CTB staining (Figure 1E). These data were expressed as the percentage of CTB cells that were co-labeled with Fos-IR. One-way ANOVAs followed by Student-Newman-Keuls post hoc comparisons of pairs of means were performed to determine significant differences among odour exposure groups in Experiments 1 and 2.
Figure 1.
Identification of retrogradely labeled cholera toxin B immunoreactive (CTB-IR) cells in the rostral medial amygdala (MeA) of female mice that were co-labeled with Fos after exposure to volatile male or female mouse urinary odours. (A) Successful CTB injections into the accessory olfactory bulb (AOB) were defined as having filled at least 2/3 of the mitral (Mi) and granule (Gr)-cell layers without significant expansion of CTB-IR into the main olfactory bulb (MOB). Panel A shows a representative sagittal section with a successful CTB injection. (Panels B-D): CTB-IR and Fos-IR cells can be seen in the MeA (coronal sections) under low power magnification (20x) in ovariectomized female subjects exposed to (B) clean air, (C) female or (D) male mouse urinary volatiles. Panel E shows the area enclosed by the rectangle in panel D under high magnification (100x, oil immersion, rectangle does not depict entire counting region), which allows for identification of CTB/Fos co-labeled cells. Co-labeled cells exhibit black nuclear Fos staining and brown cytoplasmic staining (thick blue arrow). Single labeled Fos-IR (red arrowheads) and CTB-IR (thin black arrows) cells are also pointed out.
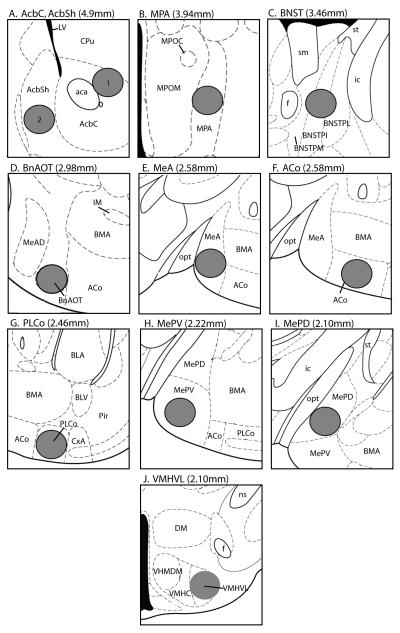
Figure 2.
Drawings adapted and modified from the mouse brain atlas of Paxinos and Franklin (2001) showing circular standard counting areas (0.1mm2, gray circles) for single-labeled forebrain Fos-IR cells (all brain regions) and CTB-IR cells (BnAOT and MeA), as well as for CTB/Fos double labeled cells (BnAOT and MeA). The distance rostral to the interaural line is given in parentheses. (A1) Nucleus accumbens core (AcbC); (A2) Nucleus accumbens shell (AcbSh); (B) Medial preoptic area (MPA); (C) Bed nucleus of the stria terminalis (BNST); (D) Bed nucleus of the accessory olfactory tract (BnAOT); (E) Medial amygdala (MeA); (F) Anterior cortical nucleus of the amygdala (ACo); (G) Posterolateral cortical nucleus of the amygdala (PLCo); (H) Posteroventral medial amygdala (MePV); (I) Posterodorsal medial amygdala (MePD); (J) Ventrolateral nucleus of the ventromedial hypothalamus (VMHVL). Reprinted from The Mouse Brain in Stereotaxic Coordinates (2nd ed) G. Paxinos and K. B. J. Franklin, Copyright (2001), with permission from Elsevier.
Results
Experiment 1: Neuronal CTB labeling and Fos activation in ovariectomized females exposed to male versus female urinary volatiles
CTB/Fos co-labeling in the forebrain
Since CTB is both an anterograde and retrograde tracer, labeled cell bodies and axonal fibers can be seen in both of these regions due to their respective afferent and efferent connections with the AOB (Figure 1). Surprisingly, we did not observe a significant number of CTB-IR cell bodies in the locus coeruleus (LC) as suggested from earlier anterograde tracing studies (Shipley et al., 1985; McLean et al., 1989) in which the LC was shown to send a robust projection to the AOB.
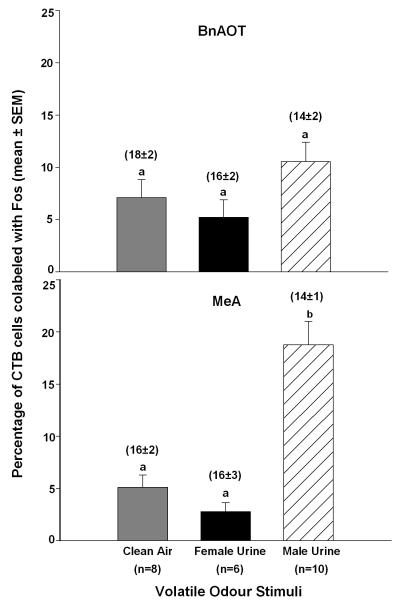
Ovariectomized female subjects exposed to male urinary volatile odours showed a significantly higher percentage of CTB-IR cells in the MeA (F2,23=24.3, p<0.001), but not the BnAOT, that co-expressed Fos-IR compared to subjects exposed to female urinary volatiles or clean air (Figure 3). To be sure that the percentages of co-labeled CTB cells in each group were not skewed by different degrees of retrograde labeling, we averaged the total number of CTB-IR cells in each forebrain region per section and found that there were no differences across odour exposure groups (see mean ± SEM values located above each bar in Figure 3). Sample MeA sections from each of the three odour exposure groups are shown in Figure 1 (panels B-D).
Figure 3.
Percentage of cholera toxin B immunoreactive (CTB-IR) cells in the bed nucleus of the accessory olfactory tract (BnAOT) and the rostral medial amygdala (MeA) of female mice that were co-labeled with Fos-IR following exposure to male or female mouse urinary volatiles. All female subjects received bilateral accessory olfactory bulb (AOB) injections of CTB 7 days earlier. There were no differences among groups of female subjects in the BnAOT (top panel). Means with different superscript letters were significantly different from each other based on Student-Newman-Keuls post hoc tests (bottom panel). The average number of retrogradely labeled CTB-IR cells (shown in parentheses above each bar) did not differ across groups in either the BnAOT or the MeA.
Fos labeling in the forebrain
Several forebrain regions in the vomeronasal projection pathway and mesolimbic dopamine system showed significantly augmented Fos-IR responses to male urinary volatiles, but not to female urinary volatiles (Table 1). These respective brain regions include the MeA (F2,23=18.5, p<0.001), bed nucleus of the stria terminalis (BNST) (F2,23=9.6, p=0.001), medial pre-optic area (MPA) (F2,23=10.9, p<0.001), ventrolateral nucleus of the ventromedial hypothalamus (VMHVL) (F2,23=14.8, p<0.001), and the nucleus accumbens core (AcbC) (F2,23=18.4, p<0.001) and shell (AcbSh) (F2,23=27.8, p<0.001). The posterodorsal nucleus of the medial amygdala (MePD) showed significantly increased Fos-IR in subjects exposed to both male and female urinary volatiles compared to clean air (F2,23=11.3, p<0.001). Additionally, amygdaloid targets of main olfactory input including the anterior cortical nucleus (ACo) and the posterolateral cortical nucleus (PLCo) showed a significant increase in Fos-IR cells in response to male urine compared to clean air, and the response to female urine was intermediate between that of clean air and male urine (PLCo, F2,23=5.7, p=0.01; ACo, F2,23=6.2, p=0.008). These results indicate that neurons of downstream targets of both the main and accessory olfactory systems expressed Fos in response to opposite-sex urinary volatiles, while only the MePD, ACo, and PLCo showed a significant Fos response to same-sex urinary volatiles.
Table 1.
Forebrain Fos responses to male or female mouse volatile urinary odours in ovariectomized female subjects in Experiment 1.
| Volatile Odour Stimuli | |||
|---|---|---|---|
| Brain Region | Clean Air (n=8) |
Female Urine (n=6) |
Male Urine (n=10) |
|
Vomeronasal Projection Pathway MeA |
10 ± 1a | 16 ± 3a | 31 ± 3b |
| MePD | 4 ± 1a | 11 ± 2b | 16 ± 2b |
| MePV | 6 ± 1a | 7 ± 2a | 10 ± 2a |
| BNST | 6 ± 1a | 7 ± 1a | 16 ± 2b |
| MPA | 21 ± 3a | 29 ± 3a | 45 ± 2b |
| VMHVL | 2 ± 1a | 4 ± 1a | 7 ± 1b |
|
Amygdaloid targets of main olfactory input Aco |
15 ± 2a | 21 ± 3ab | 30 ± 4b |
| PLCo | 8 ± 1a | 10 ± 2ab | 16 ± 2b |
|
Mesolimbic Dopamine System AcbC |
2 ± 1a | 2 ± 1a | 10 ± 1b |
| AcbSh | 15 ± 2a | 16 ± 2a | 40 ± 3b |
Data are expressed as the number of Fos-IR cells (mean ± SEM) per standard counting area averaged across three anatomically matched sections. The number of subjects in each group is given in parentheses. In each row, means with different superscript letters are significantly different (p<.05) based on Student-Newman-Keuls post hoc tests.
Experiment 2: CTB labeling and Fos activation in ovariectomized females exposed to volatile reproductive versus food and predator odours
CTB/Fos co-labeling in the forebrain
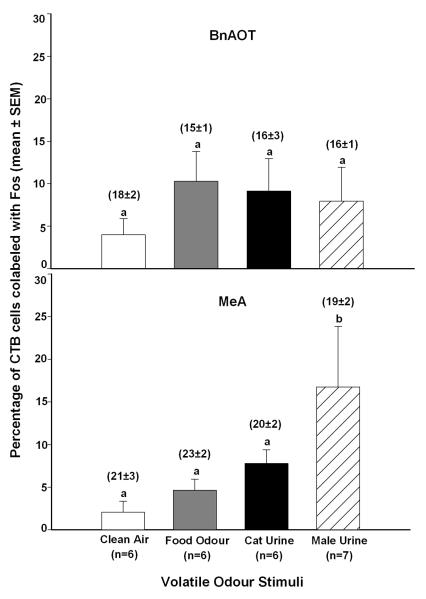
Ovariectomized female subjects exposed to male mouse urinary volatile odours showed a significantly higher percentage of CTB-IR cells in the MeA (F3,24=8.3, p<0.001), but not the BnAOT, that were co-labeled with Fos-IR compared to subjects exposed to cat urinary volatiles, food odour, or clean air (Figure 4). The total number of CTB labeled cells in each forebrain region per section was averaged for each group, and we again found no significant differences across odour exposure groups (see mean ± SEM values given above each bar in Figure 4).
Figure 4.
Percentage of cholera toxin B immunoreactive (CTB-IR) cells in the bed nucleus of the accessory olfactory tract (BnAOT) and the rostral medial amygdala (MeA) of female mice that were co-labeled with Fos-IR following exposure to food odours, male mouse urinary volatiles, or cat urinary volatiles. All female subjects received bilateral accessory olfactory bulb (AOB) injections of CTB 7 days earlier. There were no differences among groups of female subjects in the BnAOT (top panel). Means with different superscript letters were significantly different from each other based on Student-Newman-Keuls post hoc tests (bottom panel). The average number of retrogradely labeled CTB-IR cells (shown in parentheses above each bar) did not differ across groups in either the BnAOT or the MeA.
Fos labeling in the forebrain
Several forebrain regions showed a significant increase in the number of Fos-IR cells in response to male mouse urinary volatiles but not food odour, or cat urine compared with clean air (Table 2). These regions included the VHMVL (F3,24=6.1, p=0.004) in the vomeronasal projection pathway, and the AcbC (F3,24=5, p=0.009) and AcbSh (F3,24=7.7, p=0.001) in the mesolimbic dopamine system. The MeA showed augmented Fos-IR in response to male and cat urine compared to clean air (F3,24=3.3, p=0.038), and intermediate responses to food odour. Subjects exposed to male urine showed significantly more Fos-IR cells in the BNST compared to clean air (F3,23=3.9, p=0.023), while subjects exposed to cat urine and food odour both showed intermediate levels of Fos activation in response to these stimuli. Non-significant trends for increased Fos in groups exposed to male urine, cat urine, and food odour versus clean air were observed in forebrain targets of the vomeronasal projection pathway including the MePD and MPA, as well as amygdaloid targets of the main olfactory system including the ACo and PLCo. Additionally, a non-significant trend for increased Fos-IR in the MePV in response to cat urine versus other odour stimuli was noted.
Table 2.
Forebrain Fos responses to food odour, male mouse urinary volatiles, or cat urinary volatiles in ovariectomized female subjects in Experiment 2.
| Volatile Odour Stimuli | ||||
|---|---|---|---|---|
| Brain Region | Clean Air (n=6) |
Food (n=6) |
Cat Urine (n=6) |
Male Urine (n=7) |
|
Vomeronasal Projection Pathway MeA |
9 ± 2a | 22 ± 6 ab | 30 ± 6 b | 28 ± 5 b |
| MePD | 5 ± 1a | 12 ± 4a | 15 ± 3a | 17 ± 5a |
| MePV | 6 ± 1a | 11 ± 3a | 22 ± 7a | 12 ± 3a |
| BNST | 6 ± 1a | 8 ± 1ab | 11 ± 1ab | 14 ± 3b |
| MPA | 20 ± 4a | 29 ± 6a | 34 ± 6a | 37 ± 6a |
| VMHVL | 2 ± 1a | 5 ± 1a | 3 ± 1a | 10 ± 2b |
|
Amygdaloid targets of main olfactory input Aco |
11 ± 2a | 25 ± 7a | 32 ± 4a | 27 ± 6a |
| PLCo | 6 ± 2a | 15 ± 3a | 16 ± 2a | 16 ± 4a |
|
Mesolimbic Dopamine System AcbC |
2 ± 1a | 4 ± 1a | 4 ± 1a | 9 ± 2b |
| AcbSh | 10 ± 3a | 19 ± 4a | 22 ± 5a | 37 ± 4b |
Data are expressed as the number of Fos-IR cells (mean ± SEM) per standard counting area averaged across three anatomically matched sections. The number of subjects in each group is given in parentheses. In each row, means with different superscript letters are significantly different (p<.05) based on Student-Newman-Keuls post hoc tests.
Odour port investigation times
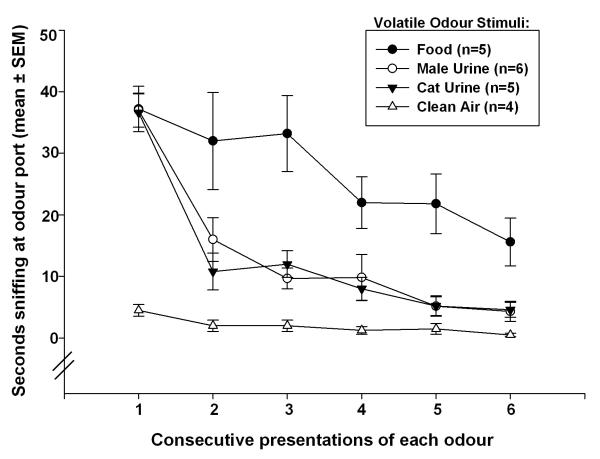
Subjects exposed to male urine, cat urine, and food odours spent significantly more time sniffing at the odour port during the first 3-min presentation of these odours than subjects exposed to clean air (Figure 5). Investigation times were significantly reduced during the second 3-min presentation for groups exposed to male urine and cat urine indicating that the subjects habituated to these odours. Habituation to food odour occurred more slowly, perhaps because all subjects had been food deprived for 24h prior to odour exposure. An overall 2-way Repeated Measures ANOVA showed significant effects of odour groups (F3,119=18.1, p<0.001), and odour presentation sequence (F5,119=36.3, p<0.001), as well as a significant groups × presentation sequence interaction (F15,119=4.5, p<0.001). Additional post-hoc comparisons between pairs of groups using 2-way Repeated Measures ANOVAs revealed significant differences between groups exposed to clean air versus food odour (F1,53=35, p<0.001), cat urine (F1,53=26.9, p=0.001), and male mouse urine (F1,59=33.2, p<0.001). Additionally, mice spent significantly more time investigating food odour than either cat urine (F1,59=11.7, p=0.009) or male mouse urine (F1,65=12.4, p=0.007). There was no significant difference in investigation times between groups exposed to cat urine versus male mouse urine. These behavioral results suggest that observed group differences in neural Fos responses were unlikely to have resulted from subjects having been exposed to different concentrations of these various odours due to intrinsic differences in subjects’ motivation to approach the port from which these odours were emitted.
Figure 5.
Time that female mice in different groups spent investigating the odour port in Experiment 2.
Discussion
The present results expand on our previous observation (Martel & Baum, 2007) that the murine AOB expressed Fos in response to opposite-, but not same-sex urinary volatiles, likely via a centrifugal feedback circuit that originates in the MOS. Our new results identify the MeA as the source of this centrifugal input which selectively conveys information to the AOB about opposite-sex conspecifics’ pheromones. While the MeA is part of the “vomeronasal amygdala” and has traditionally been thought to receive direct inputs only from the AOB, a recent study (Kang et al., 2008) has shown that a subset of mitral cells in the MOB send direct projections to the MeA. Many of these particular mitral cells which are located in the ventral/medial portion of the MOB expressed Fos specifically in response to opposite-sex as opposed to same-sex mouse urinary volatiles or cat urinary odours. While no definitive link has been made between activation of MOB mitral cells by opposite-sex odours and centrifugal activation of the AOB via the MeA, these data collectively suggest a possible anatomical pathway by which volatile odours detected by the MOE, which are specific to reproduction, gain access to the AOS. Alternatively, recent anatomical evidence from the rat (Larriva-Sahd, 2008) raises the possibility that the AOB receives axon collaterals directly from the mitral cells of the MOB. More research is needed to determine whether these particular MOB mitral cells exist in the mouse and respond selectively to opposite-sex urinary volatiles. Finally, fMRI imaging of the main and accessory olfactory bulbs of female mice during presentation of male urinary volatiles showed robust activation in both the MOB and AOB; however, the maximum response observed in the AOB was delayed until after the peak BOLD signal occurred in the MOB (Xu et al., 2005). This sequential time course may reflect AOB processing of these odour cues after their initial detection by the main olfactory system.
MOB responses to volatile urinary odours
Numerous studies have shown the MOS to be both necessary and sufficient for discrimination of urinary odours according to sex and endocrine status in male and female mice. Home cage habituation/dishabituation tests were used to show that MOE lesions disrupted the ability of subjects to make sex discriminations (Keller et al., 2006a; Ma et al., 2002), while MOE-intact subjects with (Baum and Keverne, 2002; Pierman et al., 2004) or without a functional VNO (Pankevich et al., 2004; K.L. Martel & M.J. Baum, unpublished data) successfully discriminated between same and opposite-sex volatile urinary odours. While the MOS clearly detects and distinguishes between male and female urinary odours, MOS-mediated responses specific to opposite-sex odours have recently been noted in the AOB as well. Muroi et al. (2006) reported an augmented Fos response in the granule cell layer of the AOB in sexually naïve male mice when exposed to air drawn over an oestrous female as opposed to an intact male, an ovariectomized female, or soiled bedding from an oestrous female mouse. In addition, detection of alien male odours by the MOS of female mice following mating and the implantation of embryos has been shown to disrupt pregnancy (Serguera et al., 2008). It is still unclear, however, what chemical components of opposite-sex body odours stimulate these sex-specific responses and potentially induce communication between the main and accessory olfactory systems. Lin et al. (2005) showed that mitral cells in the ventrolateral portion of the MOB in female mice responded robustly to (methylthio)methanethiol (MTMT), a pheromone only present in male urine. Several other volatile pheromonal constituents of male urine have also been shown to affect the neuroendocrine responses and behaviour of female mice (Novotny, 2003). These compounds bind to major urinary proteins (MUPs) and individually or in combination induce synchronization of the oestrous cycle (Jemiolo et al., 1986) or accelerate puberty in female mice (Novotny et al., 1999). Further investigation will be necessary to determine whether any of these compounds play a role in MOS-mediated stimulation of Fos expression in the AOB in response to opposite-sex urinary volatiles in female mice.
Forebrain responses to volatile urinary odours
In Experiment 1, ovariectomized female subjects showed augmented Fos responses to opposite, but not same-sex urinary odours in several forebrain targets of the vomeronasal projection pathway including the MeA, BNST, MPA, and VMHVL, as well as the AcbC and AcbSh in the mesolimbic dopamine pathway. Amygdaloid targets of main olfactory input also showed significant increases in Fos-IR in response to male urinary odours compared to clean air, while female urinary odours elicited intermediate levels of cellular Fos activation. While these results parallel our previous results (Martel & Baum, 2007), the current findings show more robust differences in Fos activation in response to opposite versus same-sex urinary volatiles. In addition, this sex-specific Fos response in forebrain targets of the main and accessory olfactory systems can be further augmented by inducing oestrus in ovariectomized females (Kang et al., 2008), indicating that these responses are hardwired but may be enhanced by activational sex hormones. The results of Experiment 2 showed a significantly higher cellular Fos response to male mouse urinary odours in the VMHVL, AcbC, and AcbSh compared with food or cat odours; however, these odours induced similar levels of Fos in the other vomeronasal and main olfactory target regions examined. Our data indicate that biologically relevant cues including reproductive, predator, and food odours all induce Fos in amygdaloid and hypothalamic nuclei that receive inputs from the murine MOB and AOB.
We have shown that an anatomical pathway exists allowing for communication between the MOS and the AOS via the MeA in female mice, and that male urinary volatiles selectively induced Fos expression in a significant percentage of MeA neurons that project to the AOB. However, the functional role of these centrifugal inputs to the AOB is unclear. Why would this circuitous pathway persist in parallel with the more direct VNO-AOB signaling pathway? While there is some behavioural (Trinh & Storm, 2003) and in vitro imaging evidence (Sam et al., 2001) that the VNO is able to detect volatile odours, in vivo electrophysiological evidence suggests that activation of putative AOB mitral cells required direct nasal contact with non-volatile pheromonal stimuli (Luo et al., 2003). Additionally, our previous study (Martel & Baum, 2007) using ovariectomized female mice found that in the absence of functional OSNs due to ZnSO4 ablation, the AOB no longer expressed Fos in response to opposite-sex volatile urinary odours whereas AOB Fos was stimulated by direct nasal contact with opposite-sex urine and soiled bedding. This AOB Fos response was further augmented in ovariectomized females treated with estradiol benzoate (Martel & Baum, 2007). An alternative explanation for the AOB Fos response to opposite-sex urinary volatiles is that detection of the stimulus by the MOS enhanced the pumping of these volatile pheromones to the VNO (Meredith et al., 1980), thereby upregulating Fos expression in the mitral and granule cell layers of the AOB. Our current results cannot rule out this possibility. Preliminary data (K.L. Martel & M.J. Baum, unpublished data), however, indicate that female mice that received either VNO removal surgery or a sham operation showed equivalent AOB Fos responses to opposite-sex urinary volatiles in the mitral and granule cell layers of the AOB. These results are consistent with our conclusion that the ability of opposite-sex urinary volatiles to stimulate AOB Fos responses may rely on centrifugal MOS inputs to the AOB rather than on VNO inputs.
Much evidence suggests that VNO inputs play a prominent role in enhancing the motivation of mice to seek out non-volatile opposite-sex odours. VNO lesions eliminated the preference to establish direct nasal contact with opposite sex urine in male mice (Pankevich et al., 2004, 2006), female mice (Keller et al., 2006b), female hamsters (Petrulis et al., 1999), female ferrets (Woodley & Baum, 2004), and male guinea pigs (Beauchamp et al., 1982). We propose that while the VNO is likely not activated by opposite-sex urinary volatiles, centrifugal inputs from the main olfactory system to the AOB may increase the salience of these cues and motivate the animal to maintain proximity to these stimuli. Interestingly, Pankevich et al. (2004) found that in home cage habituation/dishabituation tests in which male subjects did not have direct nasal access to the stimulus, VNO lesioned mice did not show any deficits in detection or investigation times when presented with intact male versus oestrous female urinary volatiles. Using the same testing paradigm; however, Jakupovic et al. (2008) found that male mice that received bilateral AOB lesions were able to distinguish between intact male and oestrous female urine, but showed a significant reduction in the time spent investigating female (but not same-sex male) volatile urinary odours compared to sham-operated subjects. These behavioural results suggest that inputs to the AOB, other than those originating in the VNO, may play an important role in the motivating approach behaviour to opposite-sex pheromonal signals that ensure successful reproduction.
Acknowledgements
This work was supported by NIH grant HD 044897. We thank Sarah Elmiligy for technical assistance.
Abbreviations
- ABC
avidin-biotin-peroxidase complex
- AcbC
nucleus accumbens core
- AcbSh
nucleus accumbens shell
- ACo
anterior cortical nucleus of the amygdala
- AOB
accessory olfactory bulb
- AOS
accessory olfactory system
- BnAOT
bed nucleus of the accessory olfactory tract
- BNST
bed nucleus of the stria terminalis
- CTB
cholera toxin B
- DAB
diaminobenzidine
- ICC
immunocytochemistry
- IR
immunoreactivity
- LC
locus coeruleus
- MeA
medial amygdala
- MePD
posterodorsal medial amygdala
- MePV
posteroventral medial amygdala
- MOB
main olfactory bulb
- MOE
main olfactory epithelium
- MOS
main olfactory system
- MPA
medial pre-optic area
- OSN
olfactory sensory neuron
- PBS
phosphate buffered saline
- PBST
Triton X-100 phosphate buffered saline
- PLCo
posterolateral cortical nucleus of the amygdala
- PMCo
posteromedial cortical nucleus of the amygdala
- VMHVL
ventrolateral nucleus of the ventromedial hypothalamus
- VNO
vomeronasal organ
- VSN
vomeronasal sensory neuron
References
- Barber PC. Adjacent laminar terminations of two centrifugal afferent pathways to the accessory olfactory bulb in the mouse. Brain Res. 1982;245:215–21. doi: 10.1016/0006-8993(82)90803-4. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41:213–9. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Martin IG, Wysocki CJ, Wellington JL. Chemoinvestigatory and sexual behavior of male guinea pigs following vomeronasal organ removal. Physiol Behav. 1982;29:329–36. doi: 10.1016/0031-9384(82)90022-1. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14:R81–9. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–15. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–87. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Chess A, Buck L, Dowling MM, Axel R, Ngai J. Molecular biology of smell: expression of the multigene family encoding putative odorant receptors. Cold Spring Harb Symp Quant Biol. 1992;57:505–16. doi: 10.1101/sqb.1992.057.01.056. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Central forebrain Fos responses to familiar male odours are attenuated in recently mated female mice. Eur J Neurosci. 2001;13:389–99. [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Jakupovic J, Kang N, Baum MJ. Effect of bilateral olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in mice. Physiol Behav. 2008;93:467–73. doi: 10.1016/j.physbeh.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo B, Harvey S, Novotny M. Promotion of the Whitten effect in female mice by synthetic analogs of male urinary constituents. Proc Natl Acad Sci. 1986;83:4576–9. doi: 10.1073/pnas.83.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. Opposite-sex volatile pheromones activate a novel main olfactory bulb-medial amygdala projection and downstream forebrain targets in mice. Society for Neuroscience Annual Meeting; Washington DC. 2008. p. 866.24. [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006a;31:315–23. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006b;23:521–30. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. The accessory olfactory bulb in the adult rat: a cytological study of its cell types, neuropil, neuronal modules, and interactions with the main olfactory system. J Comp Neurol. 2008;510:309–50. doi: 10.1002/cne.21790. [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–7. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, Brennan PA. Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP-ntr transgenic mice. Eur J Neurosci. 2002;16:2317–23. doi: 10.1046/j.1460-9568.2002.02303.x. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26:463–75. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CK. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol. 1989;285:339–49. doi: 10.1002/cne.902850305. [DOI] [PubMed] [Google Scholar]
- Meredith M, Marques DM, O’Connell RO, Stern FL. Vomeronasal pump: significance for male hamster sexual behavior. Science. 1980;207:1224–6. doi: 10.1126/science.7355286. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Ishii T, Komori S, Kitamura N, Nishimura M. Volatile female odors activate the accessory olfactory system of male mice without physical contact. Neurosci. 2006;141:551–8. doi: 10.1016/j.neuroscience.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Weidong M, Wiesler D, Zidek L. Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc R Soc Lond B Biol Sci. 1999;266:2017–22. doi: 10.1098/rspb.1999.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny MV. Pheromones, binding proteins and receptor responses in rodents. Biochem Soc Trans. 2003;31:117–22. doi: 10.1042/bst0310117. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–7. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci. 2006;120:925–36. doi: 10.1037/0735-7044.120.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed Academic Press; San Diego, CA: 2001. [Google Scholar]
- Petrulis A, Peng M, Johnston RE. Effects of vomeronasal organ removal on individual odor discrimination, sex-odor preference, and scent marking by female hamsters. Physiol Behav. 1999;66:73–83. doi: 10.1016/s0031-9384(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Pierman S, Douhard Q, Balthazart J, Baum MJ, Bakker J. Attraction thresholds and sex discrimination of urinary odorants in male and female aromatase knockout (ArKO) mice. Horm Behav. 2005;49:96–104. doi: 10.1016/j.yhbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Neuropharmacology. Odorants may arouse instinctive behaviours. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–7. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serguera C, Triaca V, Kelly-Barrett J, Banchaabouchi MA, Minichiello L. Increased dopamine after mating impairs olfaction and prevents odor interference with pregnancy. Nat Neurosci. 2008;11:949–56. doi: 10.1038/nn.2154. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Behaviour: smells, brains, and hormones. Nature. 2006;439:149–151. doi: 10.1038/439149a. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res. 1985;329:294–9. doi: 10.1016/0006-8993(85)90537-2. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–70. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–25. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Wagner S, Gresser AL, Torello AT, Dulac C. A multireceptor genetic approach uncovers an ordered integration of VNO sensory inputs in the accessory olfactory bulb. Neuron. 2006;50:697–709. doi: 10.1016/j.neuron.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex-discrimination and odor preferences in female ferrets. Chem Senses. 2004;29:659–69. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–50. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]