Abstract
Catheter-associated urinary tract infection, a common and potentially preventable complication of hospitalization, is one of the hospital-acquired complications chosen by the Centers for Medicare and Medicaid Services (CMS) for which hospitals no longer receive additional payment. To help understand the potential consequences of the recent CMS rule changes we examine the preventability of catheter-associated infection, review the CMS rules changes regarding catheter-associated urinary tract infection, offer our assessment of the possible consequences of these changes, and provide guidance for hospital-based administrators and clinicians. Though controversial, we conclude that the CMS rule changes related to catheter-associated urinary tract infection may do more good than harm since hospitals are likely to re-double their efforts in preventing catheter-associated urinary tract infection, which may minimize unnecessary placement and facilitate prompt removal of indwelling catheters. While we applaud CMS for forcing hospitals to increase efforts to prevent complications stemming from hospital-acquired infection, the opportunity costs and potential for unintended consequences cannot be overlooked. Consequently, how hospitals and physicians respond to the CMS rule changes must be monitored closely.
“By failing to prepare, you are preparing to fail.”
Benjamin Franklin (the inventor of the flexible urinary catheter)
Introduction
Catheter-associated urinary tract infection is the most frequent healthcare-associated infection in the United States (1, 2). Urinary catheter use is common, with approximately one in every five patients admitted to an acute care hospital receiving an indwelling catheter (1, 3); the rate of catheter use is even higher among Medicare patients (4). Infection frequently occurs after placement of urinary catheters; each day of catheter use is associated with an approximately 5% increase in bacteriuria (5), which is asymptomatic most of the time (3, 6) and usually requires no treatment. Since clinicians must distinguish asymptomatic bacteriuria from symptomatic urinary tract infection in order to avoid unnecessary administration of antimicrobial therapy, we provide a clinical definition of asymptomatic bacteriuria in Table 1 (7). Each episode of catheter-associated urinary tract infection costs at least $600 (3, 8, 9); each episode of urinary tract-related bacteremia costs at least $2800 (10). Because catheter-associated urinary tract infection is common, costly, and believed to be “reasonably preventable,” it was chosen by the Centers for Medicare and Medicaid Services (CMS) as one of the complications for which hospitals no longer receive additional payment to compensate for the extra cost of treatment as of October 1, 2008. Thus, from a hospital's perspective, catheter-associated urinary tract infection may become an even more costly complication (11-13).
Table 1.
Clinical Definition of Asymptomatic Bacteriuria (7)
| Asymptomatic bacteriuria can be defined as: |
| Isolation of a specified quantitative count of bacteria in an appropriately collected urine specimen obtained in a manner that minimizes contamination: |
| • Women: 2 consecutive voided urine specimens with isolation of the same bacterial strain in quantitative counts ≥105 colony forming units (CFU)/mL or a single catheterized urine specimen with 1 bacterial species isolated in a quantitative count ≥102 CFU/mL |
| • Men: A single, clean-catch voided urine specimen with 1 bacterial species isolated in a quantitative count ≥105 CFU/mL or a single catheterized urine specimen with 1 bacterial species isolated in a quantitative count ≥102 CFU/mL |
| AND |
| The absence of signs and symptoms that may suggest a urinary infection, such as: |
| • Fever |
| • Urgency |
| • Frequency |
| • Dysuria |
| • Suprapubic tenderness |
| • Costovertebral angle pain or tenderness |
Given the possible far-reaching consequences of the CMS rule changes and the high frequency of catheter-associated infection, our aim in this narrative review is to provide practical and timely information and guidance for hospital-based administrators, policymakers, epidemiologists, and clinicians. We first address the preventability of catheter-associated urinary tract infection, then discuss the CMS rules changes regarding payment for treatment of catheter-associated urinary tract infection, and offer our assessment of the possible consequences of the rule changes, as well as guidance for hospital administrators and clinicians.
How Preventable is Catheter-Associated Urinary Tract Infection?
CMS was asked to select hospital-acquired complications that could reasonably be prevented through the application of evidence-based guidelines. Does catheter-associated urinary tract infection fit this criterion? Perhaps. The Centers for Disease Control and Prevention proposed recommended practices for preventing catheter-associated urinary tract infection over two decades ago that appropriately emphasize the benefits of hand hygiene, aseptic catheter insertion, and proper maintenance using a closed urinary drainage system (14). More recently, the Healthcare-Associated Infection Taskforce from the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America provided an evidence-based compendium of the various practices available (15, 16). With regard to catheter-associated urinary tract infection, the compendium focused on the importance of maintaining an appropriate infrastructure for infection surveillance and prevention, education and training of healthcare personnel about catheter-associated urinary tract infection, appropriate insertion and maintenance of the indwelling catheter, consideration of alternatives to indwelling catheter use (e.g., condom and intermittent catheterization), and early removal of the indwelling catheter using reminders or stop-orders (15, 16).
Importantly, practices can be bundled together, just as most Michigan intensive care units did to reduce the incidence of vascular catheter-related infection (17). Several multi-modal intervention studies provide opportunities to examine the preventability of catheter-associated urinary tract infection, including interventions such as:
Educational programs directed at nurses, physicians, or both (included nearly universally in the studies reviewed below);
Restricting the initial placement of indwelling urinary catheters in various settings (e.g., emergency department, intensive care unit/inpatient floor, pre-operative area/operative room);
Systems to remind physicians and/or nurses of urinary catheter presence, with recommendation for removal;
Methods to facilitate prompt removal when a urinary catheter is unnecessary, such as nurse-initiated catheter removal protocols that do not require a physician order; and
Surveillance and feedback of catheter-associated urinary tract infection rates.
Implementing multi-modal interventions to prevent hospital-acquired catheter-associated urinary tract infection is not a new idea (18, 19). For example, approximately a decade ago Dumigan and colleagues employed a multidisciplinary team approach to produce guidelines for appropriate catheter placement in addition to a protocol enabling nurses to remove unnecessary catheters without a physician order; these interventions implemented in three intensive care units decreased catheter-associated urinary tract infection rates by 17 to 45%, with post-intervention catheter-associated urinary tract infection rates of 8.3 to 11.2 per 1000 catheter-days (19). Daily reminders from nurses to physicians after specific durations of catheter placement (such as 3 to 5 days) have been included as part of several multi-modal interventions (20-22); these before-and-after (uncontrolled) studies demonstrate significantly reduced incidence of catheter-associated urinary tract infection. Reminders to physicians have also been evaluated in several forms, including reminders to physicians that a urinary catheter was placed in the emergency department (23) and by placing restrictions on catheter placement to appropriate indications (usually by prompting physicians to designate an appropriate indication as part of the catheter placement order) (24-26). Expiring urinary catheter orders (e.g., “stop orders”) serve to remind and prompt removal of catheters after pre-specified time periods and can be directed to physicians (24) or nurses, and can authorize nurses to remove unnecessary catheters (based on criteria provided) without requiring an additional order from the physician (23, 25, 27). Multi-modal studies including “stop orders” have had mixed results, ranging from no significant change in the only randomized controlled trial performed to evaluate this intervention (27) to reduced catheter-associated urinary tract infection rates in before-and-after studies, including two studies demonstrating more than a 50% reduction in catheter-associated urinary tract infection rates (23, 25)
The most impressive reductions have come from interventions that use a reminder system to facilitate early removal of unnecessary catheters, often in combination with urinary catheter placement restrictions. Most of these studies, however, excluded patients requiring chronic catheterization and the risk of catheter-associated urinary tract infection was not completely eliminated. The bulk of the evidence is consistent with the view that between 25% and 75% of catheter-associated urinary tract infections could be prevented if multi-modal strategies were used. Given these findings, we conclude that reduction (not elimination) of catheter-associated urinary tract infection is possible.
Inaction, however, is common. In a national study of approximately 600 U.S. hospitals conducted in 2005, 56% reported not having a system for monitoring which patients had urinary catheters placed and 74% reported not monitoring catheter duration (28). In terms of preventive practices being used, only 9% used some type of catheter removal reminder or stop-order (28).
Overview of the CMS Rule Changes
“Value-based purchasing” is a quality improvement strategy explicitly linking payment with healthcare outcomes by paying more for better healthcare, and less for inferior care. Value-based purchasing could improve the quality of hospital care while also lowering health care costs. In many ways, the current hospital payment system is the antithesis of value-based purchasing because hospitals can receive additional payments when patients develop complications during their stay, including hospital-acquired infection. One approach is to hold hospitals financially accountable for not preventing complications. This strategy underlies the hospital payment rule change, implemented by CMS as the “Hospital-Acquired Conditions Initiative,” in which CMS will no longer pay hospitals extra when patients develop specific complications after admission (see Table 2) (4, 25, 29-31).
Table 2.
| Effective October 1, 2008: | Considered for Future Implementation: |
|---|---|
| Catheter-Associated Urinary Tract Infection | Ventilator-Associated Pneumonia |
| Decubitus Ulcer (Pressure Ulcers) | Staphylococcus aureus Septicemia |
| Vascular-Catheter Associated Infection | Clostridium difficile-Associated Disease |
| Serious preventable “Never Events”: | Iatrogenic Pneumothorax |
| Foreign Object retained after surgery, | |
| Air Embolism, | Legionnaires’ disease |
| Blood Incompatibility | |
| Delirium | |
| Falls and Trauma | |
| Manifestations of Poor Glycemic Control: | |
| Diabetic Ketoacidosis, | |
| Nonketotic Hyperosmolar coma, | |
| Hypoglycemic Coma, | |
| Secondary Diabetes with Ketoacidosis or Hyperosmolarity | |
| Deep Vein Thrombosis or Pulmonary Embolism following certain orthopedic surgeries | |
| Surgical Site Infections after certain surgical procedures: | |
| Mediastinitis after coronary artery bypass | |
| Certain orthopedic surgical site infections | |
| Certain bariatric surgical site infections |
The Deficit Reduction Act of 2005 (Section 5001c) mandated CMS to choose at least 2 hospital-acquired complications that meet 3 criteria:
high cost or high volume or both;
result in the assignment of the case to a diagnosis-related group that has a higher payment when present as a secondary diagnosis; and
could reasonably have been prevented through the application of evidence-based guidelines.
For discharges occurring on or after October 1, 2008, hospitals paid by the Inpatient Prospective Payment System will not receive additional payment for the following conditions when acquired during hospitalization: catheter-associated urinary tract infection, decubitus ulcer, vascular-catheter associated infection, serious preventable events (such as blood incompatibility), injury due fall or trauma, serious glycemic control states, and specific post-operative infections and venous thromboembolic conditions (detailed in Table 2).
This initiative has two main components: 1) mandated use of a code called a “present-on-admission indicator” that indicates whether each diagnosis occurred prior to or after hospital admission, and 2) a payment change, mandating that specific hospital-acquired conditions no longer warrant increased hospital payment (Table 2). The expected consequence of this policy is simple: with no potential for extra payment to compensate for care of hospital-acquired complications, hospitals will vigorously pursue strategies to prevent such complications. However, the details required for policy implementation are complex, and there is potential for the policy's impact on hospital payment to be negligible if the data used to identify complications are inaccurate. With this in mind, we review the details necessary to understand the CMS policy implementation with regard to catheter-associated urinary tract infection.
The present-on-admission indicator reporting requirement necessitates that for all diagnoses documented for payment generation (via the established diagnosis-related group-based Prospective Payment System) (32), hospitals must indicate whether the diagnosis was present-on-admission, or if it occurred after admission by using the new code called the present-on-admission indicator. The present-on-admission indicator can be coded as Y (condition was present-at-admission), N (hospital-acquired), W (provider has determined by data and clinical judgment that it is not possible to document when the onset of the condition occurred), or U (insufficient documentation to determine if condition was present-on-admission); CMS will be monitoring and analyzing whether these codes are being used appropriately. Present-on-admission is defined as “present at the time the order for inpatient admission occurs.” As such, “conditions that develop during an outpatient encounter, including emergency department, observation, or outpatient surgery,” are considered present-on-admission. The present-on-admission status of a diagnosis is assigned by hospital coders, who have been given general instructions in the Appendix of the ICD-9-CM Official Guidelines for Coding and reporting (33). Coders are instructed to assign a condition as “present-on-admission” if it is (a) explicitly documented by the provider as present-on-admission, (b) a chronic condition diagnosed prior to admission (such as asthma), or (c) a condition “clearly present-on-admission” but not diagnosed until after admission occurred, such as a diagnosis suspected at admission due to symptoms but confirmed sometime later.
Although instructions provided to hospital coders include a few general examples for how to apply the present-on-admission indicator, specific instructions or examples have not been provided regarding catheter-associated urinary tract infection. Coders are dependent upon accurate and complete documentation from physicians and physician extenders (e.g., physician assistants) regarding whether a urinary tract infection was catheter-associated or not. Coders are instructed only to use physician and physician extender documentation to determine diagnoses for requesting payment for care provided. If physician documentation is unclear whether a condition was present-on-admission, the coder is advised to query the physician for clarification, and the physician is expected to update the medical record. This is likely to be burdensome for both the coder and the clinician.
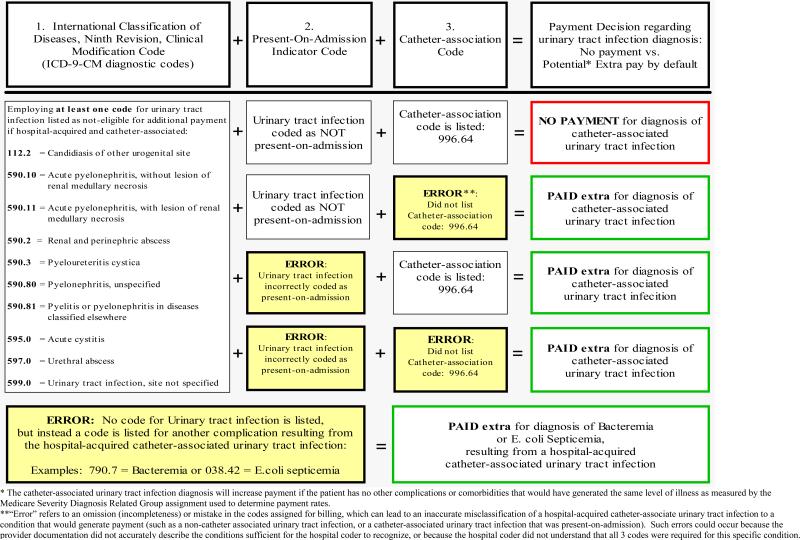
The new CMS policy contains very detailed criteria for how specific hospital-acquired complications are to be identified by ICD-9-CM codes. Some conditions (including catheter-associated urinary tract infection) will require coders to choose multiple codes to identify a complication for which additional payment should not be provided. As detailed in Figure 1, requesting payment for a urinary tract infection will occur unless three pieces of information are correctly listed to identify the diagnosis:
a urinary tract infection code (any one of 10 codes, outlined in Figure 1);
a present-on-admission indicator coded as “N” indicating the infection was not present-on-admission or “U” (hospital coder could not determine status at admission due to insufficient documentation), and
the catheter-association code (996.64).
If any one of these three codes is omitted or coded inaccurately, the hospital could receive additional payment for the urinary tract infection as a co-morbidity by default.
Figure 1.
Coding for a hospital-acquired catheter-associated urinary tract infection, resulting in outcomes of no extra payment (if corrected correctly) or potential extra payment by default (if coded incorrectly).*
Recent analyses suggest that not providing additional payment for hospital-acquired conditions could impact a significant portion of Medicare payments to hospitals ($800 million nationwide) (34). The additional payment at stake can be substantial, as it is a proportion of the base payment the hospital would receive for an otherwise uncomplicated admission. Payment to a hospital is determined by multiplying the hospital base payment by the diagnosis's “relative weight,” which increases if the patient has complications or comorbidities. For example, a patient admitted to the University of Michigan with pneumonia will yield a payment of $6072 if uncomplicated, $8346 if complicated by a minor condition such as a simple catheter-associated urinary tract infection, and $11,891 if complicated by a major complication such as a renal abscess associated with a urinary catheter. Under the new rules, however, the hospital will receive payment of $6072 for the pneumonia admission without additional payment for treatment of hospital-acquired catheter-associated urinary tract infection. Thus, the amount of payment potentially lost per admission can be substantial.
Non-payment for hospital-acquired conditions in Medicare patients could decrease or increase healthcare costs. Ideally, healthcare costs overall should decrease if hospitals are able to reduce the number of hospital-acquired complications, decreasing costs incurred by hospitals and payers. Yet, healthcare costs could also increase. First, “defensive testing” in order to document bacteriuria as “present-on-admission” could lead to increased laboratory testing costs. Then, increased documentation of bacteriuria (even if asymptomatic) could result in more antibiotic use and antibiotic-related complications such as infection due to Clostridium difficile (which may also prolong the hospital stay and qualify for outlier payments). Second, there is potential for cost-shifting, if hospitals shift the uncompensated cost of Medicare patient care to other hospital chargers or non-Medicare payers.
Likely Consequences: The Good and Not-So-Good
With any intervention, there are likely to be expected and unexpected consequences of the CMS rule changes. We review them below.
The Good
Increased focus on catheter-associated urinary tract infection
Recent research suggests preventing catheter-associated urinary tract infection has been a low priority compared with other types of hospital-acquired infections (35), and is viewed by many as the “Rodney Dangerfield of nosocomial infections” given the lack of respect it receives. While such prioritization may be appropriate, it also means that many hospitals have yet to employ even basic strategies for preventing catheter-associated urinary tract infection (35). Increased attention on catheter-associated urinary tract infection and its prevention will therefore likely improve patient care.
Specific education for healthcare workers focusing on appropriate and inappropriate indications for urinary catheterization
In addition to removing catheters in a timely fashion, it is extremely important to ensure that only patients who truly need them receive them. Therefore, healthcare workers involved in urinary catheter placement, including staff in the emergency department, intensive care units, extended care units, and even general medical wards, need to be aware of the appropriate indications for catheter use. In some particularly challenging settings, such as the emergency department, education alone may not be sufficient and more innovative strategies to facilitate appropriate catheter use may be needed (35). However, increased adherence to the use of appropriate indications for catheter use is certainly a positive step.
Increased focus on early catheter removal
Although catheter-associated urinary tract infection is often a low priority for healthcare providers, there is general agreement that catheters should be removed as soon as possible, both to prevent catheter-associated urinary tract infection and to reduce patient discomfort, activity restrictions, and discharge delays (35, 36). For some patients urinary catheters operate as a physical restraint, tantamount to binding them to the bed. We have even argued that the urinary catheter often acts as a “one-point restraint” (37) by restricting patient activity and promoting other nosocomial complications, such as venous thromboembolism. Consequently, early catheter removal will likely benefit patients.
More focus on the use of alternatives to indwelling catheterization
Condom catheters and intermittent catheterization are alternatives to indwelling catheters that are less likely to cause bacteriuria in certain patient groups (38-44). Unfortunately, no ideal alternative to the indwelling urinary catheter is available, as drawbacks exist for both options; condom catheters, for example, are only useful in men without bladder outlet obstruction.
The Not-So-Good
More urinalyses and urine cultures performed, leading to more antibiotic treatment for asymptomatic bacteriuria
Given the importance of the present-on-admission indicator there may be an increase in urinalyses and urine cultures performed at the time of hospital admission to document the presence of infection. This increase in testing alone may result in an increase in overall healthcare costs. Of more concern, however, is that performing additional cultures also increases the likelihood of more positive urine cultures, which in turn leads to an increased use of antibiotics for treating patients with asymptomatic bacteriuria. Increased antibiotic resistance and cases of C. difficile infection may, therefore, result.
Increased chance/opportunity for fraud
Coding is complicated, and at the present time code 996.64 may not be commonly used. However, if this code is not used and the patient has a catheter-associated urinary tract infection, then hospitals will still be reimbursed even though the condition is hospital-acquired. Some facilities may thus unwittingly commit fraud because of coding errors.
The loss of important information for research and surveillance
Given the complexity of coding for complications, some diagnoses may simply disappear from the administrative discharge record. Currently, unless a hospital is requesting payment there is no requirement to use the complication-related diagnoses codes. On one hand, this means hospitals would not receive payment for catheter-associated urinary tract infection. This loss of information, however, may negatively impact epidemiological studies and tracking of these conditions.
Reduced access for some high-risk patients
Certain patient populations (e.g., the elderly) are more likely to experience infectious complications while hospitalized (45-47). The CMS rule change may disproportionately affect hospital payments for these patients and ultimately could lead to reduced healthcare access for patient populations already among the most vulnerable and who often face other barriers to care.
Opportunity costs
The resources used to address and/or enforce the rule change, including implementing new preventive and coding practices, and monitoring for fraud, will no longer be available to support other activities. We must therefore be mindful of other opportunities that might be forgone by hospitals, by CMS, and by other payers in order to respond to this change, and whether the potential benefits are likely to outweigh these more broadly defined costs.
Practical Implications & Next Steps for Hospitals
There are several steps hospitals should be undertaking given the CMS rule changes; these are outlined in Table 3. As important, there is one action hospitals should not take: obtain a urine culture from asymptomatic patients at admission. First, this would add substantially to hospital costs without additional benefit. Second, this would increase the workload for the microbiology laboratory, perhaps hindering their ability to perform indicated laboratory tests in a timely fashion. Third, this process is likely to identify a relatively large number of patients with asymptomatic bacteriuria, a condition for which treatment is not indicated in most circumstances (48).
Table 3.
Recommendations for hospitals to address the Center for Medicare and Medicaid rule changes regarding catheter-related urinary tract infection
| 1) Develop or adopt existing protocols (19, 23, 25) to ensure that indwelling urinary catheters are used only when medically indicated and that they are inserted and maintained using proper technique. Using, for example, the following specific strategies: |
| • Develop a list of indications for the use of indwelling urinary catheters and make sure that catheter use is limited to persons with an accepted indication. Indications for catheter insertion may include: urinary retention, close monitoring of urinary output in critically ill patients, urinary incontinence that poses a risk to the patient (e.g., associated with major skin breakdown or at risk of contaminating a surgical site), and some surgical procedures. |
| • Develop training standards for those who insert catheters and manipulate urinary catheters and drainage bags in daily patient care. |
| • Provide all necessary supplies for proper catheter insertion and maintenance. |
| 2) Develop systems to promote removal of urinary catheters when they are no longer indicated. These systems may include: |
| • Daily review of catheter necessity during medical, nursing, or multidisciplinary rounds. |
| • Automated nurse or physician reminders of catheter presence. |
| • A protocol that authorizes nurses to discontinue catheters without a physician order when patients meet established criteria. |
| • Catheter stop-orders that are entered automatically with each order for catheter insertion so that discontinuation of the catheter becomes the default after a pre-designated period of time, rather than catheter continuation (which would require an additional physician order). |
| 3) Educate clinicians about the appropriate use and interpretation of urinalysis and urine culture. |
| • Bacteriuria and pyuria are relatively common among patients with indwelling urinary catheters. These findings do not necessarily indicate the presence of infection or the need for treatment in the absence of symptoms. |
| • Adequate knowledge of these issues may result in improved accuracy of documentation and more appropriate use of antimicrobial therapy. |
Conclusions
Though controversial (49), we conclude that the CMS rule changes may end up doing more good than harm since hospitals are likely to re-double their efforts to prevent catheter-associated urinary tract infection, which will likely minimize catheter placement and facilitate removal of unnecessary indwelling catheters. While this simple approach should reduce infection rates, there are usually additional benefits to early catheter removal in improving patient comfort and function. We also suspect collaborative efforts to combat catheter-associated urinary tract infection will increase. For example, the state of Michigan has developed a novel statewide initiative (the “Bladder Bundle”) that focuses on continual assessment and early removal of urinary catheters and considering alternatives to indwelling catheterization. Furthermore, we agree with Pronovost and colleagues that the CMS rule changes should be monitored closely in order for unintended consequences to be identified and ameliorated (13). While we applaud CMS for encouraging hospitals to increase efforts to prevent complications stemming from hospital-acquired infections, the opportunity costs and unintended complications are real and cannot be overlooked. These need to be followed and, if they are present, changes to the rules will become necessary.
Acknowledgements
Dr. Saint was supported by an Advanced Career Development Award from the Health Services Research & Development Program of the Department of Veterans Affairs during a portion of the time this manuscript was written. Dr. Saint is currently supported by award R21-DK078717 from the National Institute of Diabetes and Digestive and Kidney Diseases and Drs. Saint and Krein are currently supported by award R01-NR010700 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Role of Funding Sources
This work was supported with funding from the National Institute of Diabetes and Digestive and Kidney Diseases (R21-DK078717) and the National Institute of Nursing Research (R01-NR010700). Salary support for one of the authors was also provided through an Advanced Career Development Award from the Health Services Research & Development Program of the Department of Veterans Affairs. The funding sources were not involved in the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: “This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
References
- 1.Haley RW, Hooton TM, Culver DH, et al. Nosocomial infections in U.S. hospitals, 1975-1976: estimated frequency by selected characteristics of patients. Am J Med. 1981;70(4):947–59. doi: 10.1016/0002-9343(81)90561-1. [DOI] [PubMed] [Google Scholar]
- 2.Haley RW, Culver DH, White JW, Morgan WM, Emori TG. The nationwide nosocomial infection rate. A new need for vital statistics. Am J Epidemiol. 1985;121(2):159–67. doi: 10.1093/oxfordjournals.aje.a113988. [DOI] [PubMed] [Google Scholar]
- 3.Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68–75. doi: 10.1016/s0196-6553(00)90015-4. [DOI] [PubMed] [Google Scholar]
- 4.Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Fed Regist. 2007;72(162):47129–8175. [PubMed] [Google Scholar]
- 5.Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerging Infectious Diseases. 2001;7(2):342–7. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med. 2000;160(5):678–82. doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]
- 7.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–54. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17(8):552–7. doi: 10.1086/647371. [DOI] [PubMed] [Google Scholar]
- 9.Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27–31. doi: 10.1086/501964. [DOI] [PubMed] [Google Scholar]
- 10.Saint S, Veentra DL, Lipsky BA. The clinical and economic consequences of nosocomial central venous catheter-related infection: are antimicrobial catheters useful? Infect Control Hosp Epidemiol. 2000;21:375–380. doi: 10.1086/501776. [DOI] [PubMed] [Google Scholar]
- 11.Pear R. Medicare Says It Won't Cover Hospital Errors. The New York Times. 2007 August 19; 2007. [Google Scholar]
- 12.Wald HL, Kramer AM. Nonpayment for harms resulting from medical care: catheter-associated urinary tract infections. JAMA. 2007;298(23):2782–4. doi: 10.1001/jama.298.23.2782. [DOI] [PubMed] [Google Scholar]
- 13.Pronovost PJ, Goeschel CA, Wachter RM. The wisdom and justice of not paying for “preventable complications”. JAMA. 2008;299(18):2197–9. doi: 10.1001/jama.299.18.2197. [DOI] [PubMed] [Google Scholar]
- 14.Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983;11(1):28–36. doi: 10.1016/s0196-6553(83)80012-1. [DOI] [PubMed] [Google Scholar]
- 15.Yokoe DS, Mermel LA, Anderson DJ, et al. Executive Summary: A Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute Care Hospitals. Infect Control Hosp Epidemiol. 2008;29(s1):S12–S21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 16.Lo E, Nicolle L, Classen D, et al. Strategies to Prevent Catheter-Associated Urinary Tract Infections in Acute Care Hospitals. Infect Control Hosp Epidemiol. 2008;29(s1):S41–S50. doi: 10.1086/591066. [DOI] [PubMed] [Google Scholar]
- 17.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. New England Journal of Medicine. 2006;355(26):2725–32. doi: 10.1056/NEJMoa061115. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Goetz AM, Kedzuf S, Wagener M, Muder RR. Feedback to nursing staff as an intervention to reduce catheter-associated urinary tract infections. Am J Infect Control. 1999;27(5):402–4. doi: 10.1016/s0196-6553(99)70005-2. [DOI] [PubMed] [Google Scholar]
- 19.Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172–8. [PubMed] [Google Scholar]
- 20.Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974–8. doi: 10.1086/502329. [DOI] [PubMed] [Google Scholar]
- 21.Crouzet J, Bertrand X, Venier AG, Badoz M, Husson C, Talon D. Control of the duration of urinary catheterization: impact on catheter-associated urinary tract infection. J Hosp Infect. 2007;67(3):253–7. doi: 10.1016/j.jhin.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791–8. doi: 10.1086/518453. [DOI] [PubMed] [Google Scholar]
- 23.Topal J, Conklin S, Camp K, Morris V, Balcezak T, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121–6. doi: 10.1177/1062860605276074. [DOI] [PubMed] [Google Scholar]
- 24.Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114(5):404–7. doi: 10.1016/s0002-9343(02)01568-1. [DOI] [PubMed] [Google Scholar]
- 25.Stephan F, Sax H, Wachsmuth M, Hoffmeyer P, Clergue F, Pittet D. Reduction of urinary tract infection and antibiotic use after surgery: a controlled, prospective, before-after intervention study. Clin Infect Dis. 2006;42(11):1544–51. doi: 10.1086/503837. [DOI] [PubMed] [Google Scholar]
- 26.Doyle B, Mawji Z, Horgan M, et al. Decreasing nosocomial urinary tract infection in a large academic community hospital. Lippincotts Case Manag. 2001;6(3):127–36. doi: 10.1097/00129234-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Loeb M, Hunt D, O'Halloran K, Carusone SC, Dafoe N, Walter SD. Stop orders to reduce inappropriate urinary catheterization in hospitalized patients: a randomized controlled trial. J Gen Intern Med. 2008;23(6):816–20. doi: 10.1007/s11606-008-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: a national study. Clin Infect Dis. 2008;46(2):243–50. doi: 10.1086/524662. [DOI] [PubMed] [Google Scholar]
- 29.Medicare Program; Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2009 Rates. Fed Regist. 2008;73(161):48471–491. [PubMed] [Google Scholar]
- 30.Medicare Program; Proposed Changes to the Hospital Inpatient Prospective Payments Systems and Fiscal Year 2008 Rates; Proposed Rule. Fed Regist. 2007;72(85):24984–25063. [PubMed] [Google Scholar]
- 31.Medicare Program; Proposed Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2009 Rates; Proposed Rules. Fed Regist. 2008;73(84):23547–62. [Google Scholar]
- 32.Platt R, Polk BF, Murdock B, Rosner B. Prevention of catheter-associated urinary tract infection: a cost-benefit analysis. Infect Control Hosp Epidemiol. 1989;10(2):60–4. doi: 10.1086/645962. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare and Medicaid Services (CMS) the National Center for Health Statistics (NCHS) ICD-9-CM Official Guidelines for Coding and Reporting. 2007. Effective October 1.
- 34.Zhan C, Elixhauser A, Friedman B, Houchens R, Chiang YP. Modifying DRG-PPS to include only diagnoses present on admission: financial implications and challenges. Med Care. 2007;45(4):288–91. doi: 10.1097/01.mlr.0000256969.34461.cf. [DOI] [PubMed] [Google Scholar]
- 35.Saint S, Kowalski CP, Forman J, et al. A multicenter qualitative study on preventing hospital-acquired urinary tract infection in US hospitals. Infect Control Hosp Epidemiol. 2008;29(4):333–41. doi: 10.1086/529589. [DOI] [PubMed] [Google Scholar]
- 36.Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: What type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453–1457. doi: 10.1111/j.1532-5415.1999.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 37.Saint S, Lipsky BA, Goold SD. Indwelling urinary catheters: A one-point restraint? Ann Intern Med. 2002;137:125–127. doi: 10.7326/0003-4819-137-2-200207160-00012. [DOI] [PubMed] [Google Scholar]
- 38.Hirsh DD, Fainstein V, Musher DM. Do condom catheter collecting systems cause urinary tract infection? JAMA. 1979;242(4):340–1. [PubMed] [Google Scholar]
- 39.Ouslander JG, Greengold B, Chen S. Complications of chronic indwelling urinary catheters among male nursing home patients: a prospective study. J Urol. 1987;138(5):1191–5. doi: 10.1016/s0022-5347(17)43546-4. [DOI] [PubMed] [Google Scholar]
- 40.Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc. 1987;35(12):1063–70. doi: 10.1111/j.1532-5415.1987.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 41.Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055–61. doi: 10.1111/j.1532-5415.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn W, Rist M, Zaech GA. Intermittent urethral self-catheterisation: long term results (bacteriological evolution, continence, acceptance, complications). Paraplegia. 1991;29(4):222–32. doi: 10.1038/sc.1991.33. [DOI] [PubMed] [Google Scholar]
- 43.Lapides J, Diokno AC, Silber SJ, Lowe BS. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107(3):458–61. doi: 10.1016/s0022-5347(17)61055-3. [DOI] [PubMed] [Google Scholar]
- 44.Diokno AC, Sonda LP, Hollander JB, Lapides J. Fate of patients started on clean intermittent self-catheterization therapy 10 years ago. J Urol. 1983;129(6):1120–2. doi: 10.1016/s0022-5347(17)52599-9. [DOI] [PubMed] [Google Scholar]
- 45.Wagenlehner FM, Naber KG. Hospital-acquired urinary tract infections. J Hosp Infect. 2000;46(3):171–81. doi: 10.1053/jhin.2000.0821. [DOI] [PubMed] [Google Scholar]
- 46.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 47.Forster AJ, Asmis TR, Clark HD, et al. Ottawa Hospital Patient Safety Study: incidence and timing of adverse events in patients admitted to a Canadian teaching hospital. CMAJ. 2004;170(8):1235–40. doi: 10.1503/cmaj.1030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin K, Fajardo K. Screening for asymptomatic bacteriuria in adults: evidence for the U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2008;149(1):W20–4. doi: 10.7326/0003-4819-149-1-200807010-00009-w1. [DOI] [PubMed] [Google Scholar]
- 49.Graves N, McGowan JE., Jr Nosocomial Infection, the Deficit Reduction Act, and Incentives for Hospitals. JAMA. 2008;300(13):1577–1579. doi: 10.1001/jama.300.13.1577. [DOI] [PubMed] [Google Scholar]