Abstract
The purpose of the present study was to examine the effect of detraining on the glucose transport system after short-term swim training (5 days), long-term swim training (5 wk), and treadmill run training (5 wk). Skeletal muscles were isolated from female Wistar rats at 24 or 48 h posttraining. SST produces a 48% increase in GLUT-4 mRNA, a 30% increase in GLUT-4 protein, and a 60% increase in insulin-stimulated glucose transport activity at 24 h posttraining but not at 48 h posttraining. Similar to SST, long-term swim training produces a 60% increase in GLUT-4 mRNA and a 30% increase in GLUT-4 protein content at 24 h posttraining but not at 48 h posttraining. Finally, treadmill run training produces a transient 35% increase in GLUT-4 protein content that is completely reversed at 48 h after the last bout of exercise. These results demonstrate that the increase in GLUT-4 mRNA and GLUT-4 protein occurs during the first week of exercise training and is rapidly lost after training cessation. We believe that the transient enhancement in GLUT-4 protein after exercise training is due to a short GLUT-4 half-life, a process that is primarily regulated by pretranslational mechanisms.
Keywords: glucose transporters, detraining, glucose metabolism, training cessation, insulin action
The glucose transporter GLUT-4 is the primary isoform in skeletal muscle and is known to translocate to the cell surface in response to insulin (20, 21, 36), muscle contraction (2, 11, 27), and hypoxia (3). Because skeletal muscle is the predominant target tissue for glucose disposal and glucose transport is thought to be the rate-limiting step in glucose utilization, the importance of skeletal muscle GLUT-4 content is paramount. Several studies demonstrate that the glucose transport response to insulin in skeletal muscle is highly related to skeletal muscle GLUT-4 content (19, 33). Furthermore, recent evidence indicates that a larger total pool of GLUT-4 is associated with a greater amount of GLUT-4 translocated to the cell surface in response to insulin (5, 30).
It is well established that exercise training increases skeletal muscle GLUT-4 levels and insulin-stimulated glucose disposal (6, 15, 33). We and others have previously shown that exercise training enhances the glucose transport response to insulin by recruiting more GLUT-4 to the cell surface from a larger total muscle pool of GLUT-4 (5, 30). However, others have shown that the improvement in insulin action after exercise training appears to be lost within 48 h after the last bout of exercise (7, 17) despite total muscle GLUT-4 levels remaining elevated for several days (7, 14, 16). Therefore, it appears unlikely that the “short-lived” effect of exercise training on insulin-stimulated glucose uptake is due to a rapid reversal of the elevation in GLUT-4 content, although a recent report suggests otherwise (12).
Traditionally, several weeks of exercise training were thought to be necessary for skeletal muscle adaptations to occur. Recently, however, the adaptive response of skeletal muscle to exercise training has been shown to occur in the first week of exercise training if an adequate exercise stimulus is provided (9, 30, 34). Ren et al. (29) established that 2 days of exhaustive swimming results in a 100% increase in GLUT-4 content and a 40% increase in citrate synthase activity in rat epitrochlearis muscle. We have previously reported that 5 days of swim training produces an increase in both GLUT-4 levels and citrate synthase activity (30). In human subjects, skeletal muscle GLUT-4 levels are elevated after 7 days of exercise training (9, 13). Although it has been demonstrated that 2–7 days of exercise training induce a significant increase in total muscle GLUT-4 content in both rats (29, 30) and human subjects (9, 13), it is not clear how long this adaptation persists after short- and long-term exercise training. Furthermore, it is not known whether the adaptive increase in GLUT-4 content is different after swim training and treadmill run training (TRT).
In this context, the present study had three objectives: 1) to examine the effect of detraining on rat skeletal muscle GLUT-4 mRNA and protein levels and on insulin-stimulated glucose transport activity (GTA) after short-term swim training (SST); 2) to determine the effect of detraining on GLUT-4 mRNA and protein levels after long-term swim training (LST); and 3) to ascertain the effect of detraining on GLUT-4 protein content after TRT.
METHODS
Animals and housing
Specific pathogen-free female Wistar rats weighing 75–100 g were obtained from Charles River Breeding Laboratories. On arrival, rats were housed four per cage in a temperature-controlled animal room maintained on a 12:12-h light-dark cycle. The rats were fed National Institutes of Health (NIH) standard chow and water ad libitum. Two days after arrival, the rats were randomly assigned to an exercise training or sedentary control (Sed) group. All animal care and surgery were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (DHEW Publication No. 85-23). All experimental protocols were approved by the University of Michigan and NIH Committee for the Use and Care of Animals.
Swim training protocol
The rats assigned to the swim training group were acclimated to swimming for 10 min/day for 2 days and then exercise trained by a procedure similar to those previously described by Ploug et al. (28) and Ren et al. (29). Briefly, the animals performed two bouts of exercise in water maintained at 35°C, separated by a 1-h rest period. During the rest period animals were towel dried, kept warm, and given food and water. The rats swam in groups of six in 130-liter plastic barrels. On the first day of training, the rats performed a 1-h and a 2-h bout of swimming. On the second day of training, the swimming duration was increased so that the animals performed two 3-h bouts of exercise. This swimming regime was repeated for a total of 5 days (SST: 2 × 3 h/day, 5 days) or 5 wk (LST: 2 × 3 h/day, 5 days/wk). At the completion of either the SST or LST programs, the trained animals were randomly subdivided into two groups and studied at 20–24 h (T24) and 44–48 h (T48) after the last bout of swim training.
Treadmill exercise training protocol
The rats assigned to the TRT group were acclimated to running for 2 × 10 min/day for 3 days, and then the exercise intensity and duration were gradually increased so that by the end of the second week of training the rats were running for 60 min at 30 m/min on a 15% incline. This level of exercise was maintained for an additional 3 wk. After the training period, the animals were randomly subdivided into two groups and studied at T24 and T48 after the last bout of exercise training.
Muscle preparation and incubation
In the postprandial state (i.e., in the morning after having food during the night), rats were anesthetized with pentobarbital sodium (5 mg/100 g body wt). White gastrocnemius muscles were dissected out, rapidly frozen with tongs cooled to the temperature of liquid N2, and stored at −80°C until analyzed for GLUT-4 mRNA content. The epitrochlearis muscles were dissected out, blotted on gauze, and transferred to 25-ml Erlenmeyer flasks (1 muscle/flask) containing 2 ml of Krebs-Henseleit buffer (KHB) consisting of 0.1% BSA, 32 mM mannitol, and 8 mM glucose, with or without 13.3 nM (2.0 mU/ml) insulin. The flasks were incubated for 1 h at 35°C in a shaking water bath and were continually gassed with 95% O2−5% CO2. After the initial incubation, muscles were blotted and transferred to flasks containing 2 ml of KHB consisting of 0.1% BSA, 40 mM mannitol, 2 mM pyruvate, and 13.3 nM insulin if present in the previous incubation. These flasks were incubated for 10 min at 29°C in a shaking water bath to wash out the glucose; the gas phase in all the flasks was 95% O2−5% CO2.
Measurement of GTA
The measurement of GTA has been previously described (3). After the incubations described above, epitrochlearis muscles were blotted and transferred to 25-ml flasks (1 muscle/flask) containing 1.5 ml of KHB consisting of 1 mM 2-deoxy-[1,2-3H]glucose (1.5 µCi/mmol), and 39 mM [1-14C]mannitol (8 µCi/mmol) (New England Nuclear), and 13.3 nM insulin if present in the previous incubations. These flasks were incubated for 20 min at 29°C in a shaking water bath and were continually gassed with 95% O2−5% CO2. The uptake of 2-deoxyglucose in the presence of 8 mM 2-deoxyglucose and maximal concentrations of insulin has previously been shown to be linear for 120 min in epitrochlearis muscle, and therefore this glucose analog at a concentration of 1 mM is appropriate for the measurement of GTA (10). The muscles were then frozen between tongs cooled to the temperature of liquid N2 and stored at −70°C until processing. Frozen muscles were homogenized in 1 ml of ice-cold 0.6 N perchloric acid and centrifuged at 1,340 g, and aliquots of supernatant were counted for radioactivity in a liquid scintillation counter. After correcting for extracellular space, 2-deoxyglucose transport was calculated as micromole per milliter per 20 min.
Measurement of GLUT-4 mRNA
RNA from white gastrocnemius muscle was isolated via the method of Chomczynski and Sacchi (4) by using RNAzol (Molecular Research Center, Cincinnati, OH) as described in the manufacturer’s instructions. Ten micrograms of sample RNA were size fractionated on a 1.5% agarose gel containing 0.66 M formaldehyde, and the integrity of the RNA was verified by observation of the 16 and 18S ribosomal bands under ultraviolet light. The RNA was transferred to Nytran membranes using the Turboblotter system (Schleicher and Schuell, Keene, NH), following the manufacturer’s instructions. The blots were prehybridized for 5 h at 55°C in a solution consisting of 50% formamide, 5 × Denhardt’s solution, 5 × sodium chloride-sodium phosphate-EDTA (SSPE), 0.5% SDS, and 0.1 mg/ml sheared salmon sperm DNA. The blots were then hybridized overnight at 55°C in fresh hybridization solution containing 1 × 106 counts·min−1 · ml−1 of an [α-32P]UTP-labeled antisense full-length GLUT-4 riboprobe. The antisense riboprobe was generated from EcoR V-linearized plasmid PSM1-1 (gracious donation of Dr. Morris Birnbaum) by using T7 polymerase in an in vitro transcription reaction (Maxiscript kit, Ambion, Austin, TX). The filters were washed 3 × 10 min in 1 × SSPE, 0.5% SDS at 37°C and for 1 h in 0.1 × SSPE, 0.5% SDS at 65°C. Blots were visualized by phosphor imaging and quantified by using Imagequant software (Molecular Dynamics, Sunnyvale, CA). After quantification for GLUT-4 mRNA, blots were stripped and reprobed for the 18S ribosomal RNA subunit by use of an antisense 18S probe that was generated from the pT7 18S template (Ambion) by in vitro transcription (Maxiscript kit, Ambion). All GLUT-4 signals were normalized to the 18S signal to account for unequal loading of RNA.
Measurement of total muscle GLUT-4 protein
Freeze-clamped epitrochlearis muscles were weighed and homogenized on ice with a Brinkman polytron homogenizer at 1:40 (wt/vol) in HEPES-EDTA-sucrose buffer (20 mM HEPES, 1 mM EDTA, 250 mM sucrose, pH 7.4). The protein concentration was determined by the bicinchoninic acid method (Pierce, Rockford, IL) with crystalline BSA used as the standard. An aliquot of homogenate containing 40 µg of protein was solubilized in Laemmli sample buffer and subjected to 10% SDS-PAGE. The resolved proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes and incubated overnight at 4°C with anti-GLUT-4 antiserum. The PVDF membranes were then incubated in 0.25 µCi/ml of 125I-labeled protein A or goat anti-rabbit 125I-labeled IgG (New England Nuclear) for 1 h at 37°C and placed in a Phosphorimager cassette (Molecular Dynamics) for 72 h. After being corrected for background, the areas of each band were expressed as a percentage of a standard (40 µg of rat skeletal muscle intracellular membrane protein) run on each gel.
Measurement of citrate synthase activity and glycogen levels
Aliquots of the same epitrochlearis muscle homogenates used for the measurement of GLUT-4 were used for the determination of citrate synthase activity as described by Srere (35). For the measurement of glycogen, epitrochlearis muscles were homogenized in 0.3 M PCA on ice in Kontes (Vineland, NJ) glass-on-glass grinding tubes, and glycogen levels were determined by the amyloglucosidase method (26).
Statistical analysis
Statistically significant differences between T24, T48, and Sed groups for all dependent variables were detected by factorial ANOVA. Following a significant ANOVA F ratio, we performed selected mean comparisons to locate significant differences. Results are expressed as means ± SE, and differences are accepted as significant at the P ≤ 0.05 level.
RESULTS
Animal body weights and epitrochlearis muscle weights
The animals subjected to SST or LST weighed significantly less than Sed control animals (5 days: Sed, 144.3 ± 2.0 vs. T24, 127.7 ± 1.8 and T48, 135 ± 2; 5 wk: Sed, 242.8 ± 8.6 vs. T24, 208.9 ± 4.2 and T48, 214.8 ± 4.1 g). However, 5 wk of TRT did not produce significant changes in body weight in T24 or T48 animals (Sed, 226 ± 6.4 vs. T24, 227 ± 10.1 and T48, 236 ± 4.0 g). Epitrochlearis muscles from T24 animals after SST weighed significantly less than muscles from Sed animals (20.8 ± 0.6 vs. 18.7 ± 0.6 mg). Epitrochlearis muscle weights from T48 animals after SST were similar to those from Sed animals (20.8 ± 0.6 vs. 19.5 ± 0.8 mg). Epitrochlearis muscle weights from animals after LST also tended to be less than those of muscles from Sed animals, although this was not significant (Sed, 42.1 ± 4.0 vs. T24, 34.2 ± 1.0 and T48, 36.7 ± 2.0 mg). Epitrochlearis muscle weights from T24 animals after 5 wk of TRT were similar to those from Sed animals (35.1 ± 2.4 vs. 35.8 ± 1.4 mg), but epitrochlearis muscles from T48 animals after 5 wk of TRT weighed significantly less than muscles from Sed animals (35.1 ± 2.4 vs. 30.2 ± 2.6 mg).
GTA
Epitrochlearis muscle GTA after SST are illustrated in Table 1. Basal glucose transport did not change after 5 days of swim training. Insulin-stimulated GTA was enhanced by 62% in muscles from T24 animals compared with muscles from Sed animals. However, insulin-stimulated GTA was no longer elevated in muscles from T48 animals.
Table 1.
Effects of 5 days of swim training and detraining on basal and insulin-stimulated glucose transport activity in epitrochlearis muscles from swim-trained and sedentary control rats
| Glucose Transport Activity, µmol · ml−1 · 20 min−1 | ||
|---|---|---|
| Basal | Insulin (2.0 mU/ml) | |
| Control | 0.44 ± 0.03 (28) | 0.93 ± 0.02† (28) |
| 24 h Posttraining | 0.38 ± 0.02 (31) | 1.50 ± 0.08 †,* (27) |
| 48 h Posttraining | 0.33 ± 0.02 (30) | 1.13± 0.07† (14) |
Values are means ± SE; numbers of muscles are indicated within parentheses.
Significantly different from control muscles, P ≤ 0.05.
Significantly different from basal muscles, P ≤ 0.05.
GLUT-4 mRNA levels
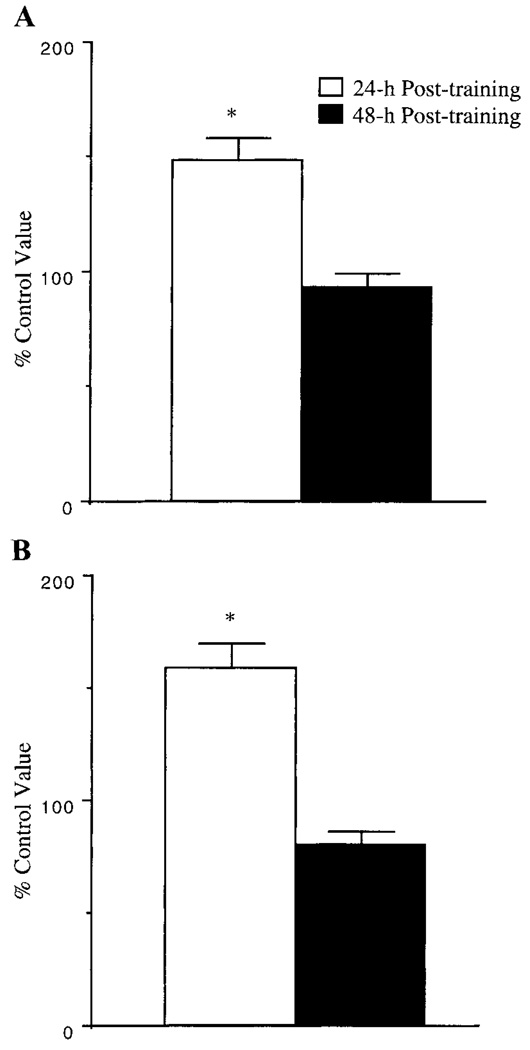
White gastrocnemius muscle GLUT-4 mRNAlevels were elevated by 48% and 60% in muscles from animals at 24 h posttraining after SST and LST, respectively (Fig. 1). However, these increases in GLUT-4 mRNA levels were reversed to control levels at 48 h posttraining. We utilized the white gastrocnemius muscle for GLUT-4 mRNA analysis because the epitrochlearis muscle homogenates were utilized for other assays. The white gastrocnemius muscle is similar to the epitrochlearis muscle in that it is a highly glycolytic muscle that contains predominantly type IIb fibers.
Fig. 1.
Effects of swim training on white gastrocnemius muscle GLUT-4 mRNA levels. After a 5-day (A) or a 5-wk (B) swim training program, white gastrocnemius muscles were isolated from fed rats at 24 h and 48 h after last bout of swim training, and GLUT-4 mRNA levels were assessed as described in methods. Results are means ± SE obtained from 6 muscles per group and are expressed as a percentage of control values. *Significantly different from control muscles, P ≤ 0.05.
Total GLUT-4 protein content
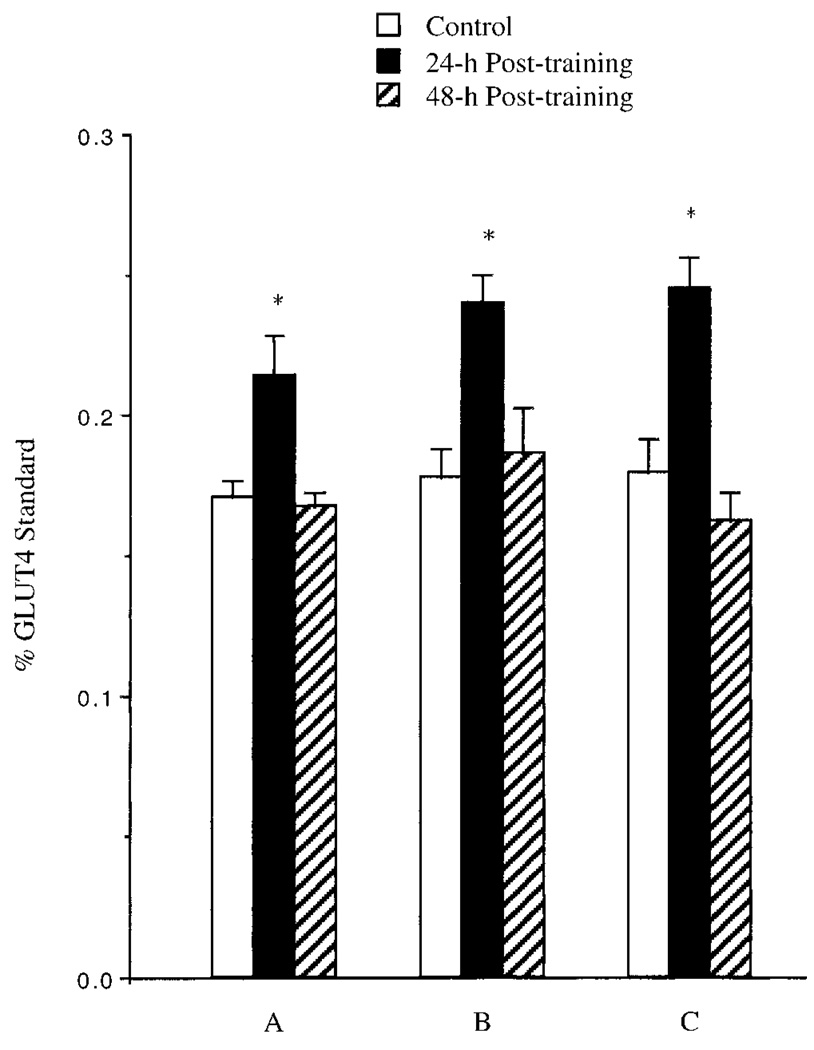
Epitrochlearis muscle GLUT-4 protein values from animals subjected to SST, LST, and TRT are illustrated in Fig. 2. All exercise training protocols produced a 25–35% increase in total muscle GLUT-4 content when assessed at 24 h posttraining. However, these increases in epitrochlearis muscle GLUT-4 were reversed to control levels when assessed at 48 h posttraining.
Fig. 2.
Effects of swim training on epitrochlearis muscle GLUT-4 levels after a 5-day (A) or 5-wk (B) swim training program or a 5-wk (C) treadmill exercise training program. Epitrochlearis muscles were isolated from fed rats at 24 h and 48 h after last bout of exercise training, and GLUT-4 levels were assessed as described in methods. Results are means ± SE obtained from 5–8 muscles. *Significantly different from control muscles, P ≤ 0.05.
Muscle glycogen levels
Muscle glycogen levels increased by 100% in epitrochlearis muscles from animals subjected to SST at 24 h posttraining compared with Sed controls but decreased to control levels at 48 h posttraining (Sed: 19.1 ± 1.0 vs. T24: 40.3 ± 2.7 vs. T48: 21.8 ± 2.2 µmol/g wetwt).
Citrate synthase activity
The SST and LST produced 35% and 30% increases, respectively, in epitrochlearis muscle citrate synthase activity when assessed at 24 h or 48 h posttraining (Table 2).
Table 2.
Citrate synthase activities in epitrochlearis muscles from swim-trained and sedentary control rats
| Citrate Synthase Activity, µmol · min−1 · g−1 | ||
|---|---|---|
| 5-day Swim-trained | 5-wk Swim-trained | |
| Control | 26.13 ± 0.94 (27) | 22.59 ± 1.6 (6) |
| 24 h Posttraining | 33.57 ± 0.29* (26) | 30.47 ± 1.6* (6) |
| 48 h Posttraining | 33.20 ± 0.27* (21) | 30.49 ± 1.5* (6) |
Values are means ± SE; numbers of muscles are indicated within parentheses.
Significantly different from control muscles, P ≤ 0.05.
DISCUSSION
The present study demonstrates that 5 days of swim training produced an increase in insulin-stimulated GTA, GLUT-4 mRNA, and GLUT-4 protein levels in rat skeletal muscle when assessed 24 h after the last bout of exercise. However, these increases were rapidly reversed to control levels by 48 h after the last bout of exercise. To ascertain whether the rapid reversal of the increase in GLUT-4 content was due to the short-term nature of the 5-day swim training program, we assessed the effect of detraining on GLUT-4 mRNA and protein levels after 5 wk of swim training. Similar to 5 days of swimming exercise, 5 wk produced an almost identical transient enhancement in skeletal muscle GLUT-4 mRNA and protein content. Furthermore, the effect of training cessation on skeletal muscle GLUT-4 protein content does not appear to be specific to swim training because 5 wk of TRT also produced a rapidly reversed increase in GLUT-4 protein levels.
The most interesting finding is the rapid reversal of GLUT-4 mRNA and GLUT-4 protein levels after 5 days of swim training. Although it is well established that exercise training produces a short-lived increase in insulin-stimulated glucose uptake, the mechanism responsible for this rapid reversal remains obscure. The effect of detraining on the exercise training-induced enhancement of skeletal muscle GLUT-4 protein levels has been inconsistent, varying from a transient increase after swim training (12, 18) to a persistent increase after treadmill and wheel-cage exercise training protocols (7, 8). Furthermore, previous exercise training studies in human subjects demonstrate that skeletal muscle GLUT-4 protein levels remain elevated several days after the last bout of exercise training (14, 16). Contrary to previous reports that examined longer periods of exercise training (7, 14, 16), the present SST regime produced a short-lived increase in GLUT-4 mRNA and protein levels, indicating pretranslational regulation (i.e., transcription rates or mRNA stability). To determine whether the transient enhancement of skeletal muscle GLUT-4 mRNA and protein content were due to the short-term nature of our 5-day swim training program, we subjected rats to 5 wk of swim training. Again, GLUT-4 mRNA and protein levels were elevated in muscles from swim-trained rats at 24 h after the last bout of exercise but not at 48 h postexercise. Other investigators (12, 18) also demonstrate that short-term (12, 18) and long-term (12) swim training induced a rapidly reversed increase in insulin-stimulated glucose transport capacity and skeletal muscle GLUT-4 protein content. Therefore, it appears that the transient enhancement in the glucose transport response to insulin after swim training is due primarily to the rapid reversal of the increased GLUT-4 protein content. This idea is further supported by the fact that, at 16–18 h posttraining, GLUT-4 levels are elevated by 85–90% (12, 18), but at 24 h posttraining the magnitude of the GLUT-4 response to exercise training appears to be considerably lower (30, 31). Perhaps the present 30–35% increase in GLUT-4 levels at 24 h posttraining would have been greater if assessed at 16–18 h posttraining.
The literature suggests that skeletal muscle GLUT-4 levels might be regulated differently after swim training compared with treadmill exercise training (7) or wheel-cage running (8). This idea is supported by the observation that one bout of treadmill running does not alter skeletal muscle GLUT-4 levels in rats (25, 33) and human subjects (15), but one bout of swimming exercise can induce a pronounced increase in GLUT-4 levels at 16 h postexercise (29). Therefore, we also examined the effect of detraining on skeletal muscle GLUT-4 protein content after 5 wk of TRT. Similar to both 5 days and 5 wk of swim training, TRT produced a short-lived increase in GLUT-4 protein content. In contrast, Etgen et al. (7) demonstrated that 12 wk of treadmill exercise training produced an increase in GLUT-4 protein content at both 24 and 48 h after the last bout of exercise. Perhaps the discrepancies between the present results and the Etgen et al. findings are due to the number of weeks of training. However, this seems unlikely because it appears that the adaptive increase in the GLUT-4 protein occurs during the first week of exercise training (12, 29).
Swimming and treadmill exercise are commonly employed to study the responses and adaptations to exercise, but little attention is paid to the stress associated with these activities. Therefore, another plausible explanation for the accelerated GLUT-4 protein turnover after exercise training might be related to the stress associated with forced exercise. Support for this idea stems from evidence that wheel-cage running, a voluntary activity, produced persistent long-lasting increases in skeletal muscle GLUT-4 content (8). In human subjects, the increase in GLUT-4 after exercise training appears to last several days (14, 16, 22). Houmard et al. (14) demonstrated that 14 days after the cessation of either endurance or resistance training, skeletal muscle GLUT-4 content was unaltered. Furthermore, McCoy et al. (22) reported that skeletal muscle GLUT-4 content was higher in detrained subjects (10 days of detraining) compared with their sedentary peers.
Another potential explanation for the rapid reversal of the increased GLUT-4 after exercise training is a short GLUT-4 half-life. Support for this idea is based on the present finding that swim training produced a persistent increase in skeletal muscle citrate synthase activity. At both 24 h and 48 h after the last bout of swim training, muscle citrate synthase activity was elevated, demonstrating an uncoupling of citrate synthase activity and total muscle GLUT-4 levels. Host et al. (12) suggested that the rapid reversal of the enhanced GLUT-4 levels was due to a short GLUT-4 half-life of ∼8–10 h, whereas citrate synthase activity remained elevated at 48 h posttraining because of its half-life of ∼7 days (1). The half-life of a protein is related to the time it takes to undergo a steady-state increase in response to a stimulus and the time it takes to reverse the increase once the stimulus is removed (32). Therefore, the exercise-induced rapid increase in GLUT-4 protein levels and the subsequent rapid return of GLUT-4 back to control levels indicate a short half-life (12).
In summary, the present findings indicate that as few as 5 days of exercise training produced a short-lived increase in insulin-stimulated GTA and in skeletal muscle GLUT-4 mRNA and protein levels. We also demonstrate that additional exercise training beyond what is necessary to cause a training effect did not alter the rapid reversal of the increased GLUT-4. These results provide evidence that the short-lived increase in the glucose transport response to insulin after exercise training is due primarily to a transient increase in GLUT-4 protein content. We believe that the transient enhancement in GLUT-4 protein levels is due to a short GLUT-4 half-life, a process that is regulated primarily by a pretranslational mechanism. In addition, the stress associated with the forced exercise of laboratory rats may contribute to the present results, and future studies examining the effect of detraining on skeletal muscle GLUT-4 content after voluntary exercise training are warranted.
Acknowledgments
T. H. Reynolds was supported in part by institutional National Research Service Award Grant T32-AG00114, and L. M. Larkin was supported by National Institute on Aging Grant AG-00710.
REFERENCES
- 1.Booth FW, Holloszy JO. Cytochrome c turnover in rat skeletal muscle. J Biol Chem. 1977;252:416–419. [PubMed] [Google Scholar]
- 2.Brozinick JT, Etgen GJ, Yaspelkis BB, Ivy JL. The effects of muscle contraction and insulin on glucose-transporter translocation in rat skeletal muscle. Biochem J. 1994;297:539–545. doi: 10.1042/bj2970539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartee GD, Douen AG, Ramal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol. 1991;70:1593–1600. doi: 10.1152/jappl.1991.70.4.1593. [DOI] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Dela F, Mikines KJ, Linstow M, Seccher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol Endocrinol Metab. 1992;263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- 6.Etgen GJ, Brozinick JT, Kang HY, Ivy JL. Effects of exercise training on skeletal muscle glucose uptake and transport. Am J Physiol Cell Physiol. 1993;264:C727–C733. doi: 10.1152/ajpcell.1993.264.3.C727. [DOI] [PubMed] [Google Scholar]
- 7.Etgen GJ, Jensen J, Wilson CM, Hunt DG, Cushman SW, Ivy JL. Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to cell surface. Am J Physiol Endocrinol Metab. 1997;272:E864–E869. doi: 10.1152/ajpendo.1997.272.5.E864. [DOI] [PubMed] [Google Scholar]
- 8.Goodyear LJ, Hirshman MF, Valyou PM, Horton ES. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes. 1992;41:1091–1099. doi: 10.2337/diab.41.9.1091. [DOI] [PubMed] [Google Scholar]
- 9.Gulve EA, Spina RJ. Effect of 7–10 days of cycle ergometer exercise on skeletal muscle GLUT-4 protein content. J Appl Physiol. 1995;79:1562–1566. doi: 10.1152/jappl.1995.79.5.1562. [DOI] [PubMed] [Google Scholar]
- 10.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- 11.Holloszy JO, Narahara HT. Studies of tissue permeability. X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J Biol Chem. 1965;240:3493–3500. [PubMed] [Google Scholar]
- 12.Host HH, Hansen PA, Nolte LA, Chen MA, Holloszy JO. Rapid reversal of adaptive increases in muscle GLUT-4 and glucose transport capacity after cessation of training. J Appl Physiol. 1998;84:798–802. doi: 10.1152/jappl.1998.84.3.798. [DOI] [PubMed] [Google Scholar]
- 13.Houmard JA, Hickey MS, Tyndall GL, Gavigan KE, Dohm GL. Seven days of exercise increase GLUT-4 protein contentin human skeletal muscle. J Appl Physiol. 1995;79:1936–1938. doi: 10.1152/jappl.1995.79.6.1936. [DOI] [PubMed] [Google Scholar]
- 14.Houmard JA, Hortobagyi T, Neufer PD, Johns RA, Frazier D, Israel RG, Dohm GL. Training cessation does not alter GLUT-4 protein levels in human skeletal muscle. J Appl Physiol. 1993;74:776–781. doi: 10.1152/jappl.1993.74.2.776. [DOI] [PubMed] [Google Scholar]
- 15.Houmard JA, Shinebarger MH, Neufer PD, Leggett-Frazier N, Bruner RK, McCammon MR, Israel RG, Dohm GL. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol Endocrinol Metab. 1993;264:E896–E901. doi: 10.1152/ajpendo.1993.264.6.E896. [DOI] [PubMed] [Google Scholar]
- 16.Hughes VA, Fiatarone MA, Fielding RA, Kahn BA, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 1993;264:E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- 17.Ivy JL, Young JC, McLane JA, Fell RD, Holloszy JO. Exercise training and glucose uptake by skeletal muscle in rats. J Appl Physiol. 1983;55:1393–1396. doi: 10.1152/jappl.1983.55.5.1393. [DOI] [PubMed] [Google Scholar]
- 18.Kawanaka K, Tabata I, Katsuta S, Higuchi M. Changes in insulin-stimulated glucose transport and GLUT-4 protein in rat skeletal muscle after training. J Appl Physiol. 1997;83:2043–2047. doi: 10.1152/jappl.1997.83.6.2043. [DOI] [PubMed] [Google Scholar]
- 19.Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, Tapscott EB, Pekala PH, Dohm GL. Insulin responsiveness in skeletal muscle is determined by glucose transporter (GLUT-4) protein level. Biochem J. 1990;270:397–400. doi: 10.1042/bj2700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klip A, Ramal T, Young DA, Holloszy JO. Insulinstimulated glucose transporters in rat hindlimb muscle. FEBS Lett. 1987;244:224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- 21.Lund S, Holman GD, Schmitz O, Pederson O. GLUT-4 content in the plasma membrane of rat skeletal muscle: comparative studies of the subcellular fractionation method and the exofacial photolabeling technique using ATB-BMPA. FEBS Lett. 1993;330:312–318. doi: 10.1016/0014-5793(93)80895-2. [DOI] [PubMed] [Google Scholar]
- 22.McCoy M, Proietto J, Hargreaves M. Effect of detraining on GLUT-4 protein in human skeletal muscle. J Appl Physiol. 1994;77:1532–1536. doi: 10.1152/jappl.1994.77.3.1532. [DOI] [PubMed] [Google Scholar]
- 25.Neufer PD, Shinebarger MD, Dohm GL. Effect of training and detraining on skeletal muscle glucose transporter (GLUT-4) content in rats. Can J Physiol Pharmacol. 1992;70:1286–1290. doi: 10.1139/y92-178. [DOI] [PubMed] [Google Scholar]
- 26.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurements in tissues. Anal Biochem. 1974;60:405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- 27.Ploug T, Galbo H, Richter EA. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol Endocrinol Metab. 1984;247:E726–E731. doi: 10.1152/ajpendo.1984.247.6.E726. [DOI] [PubMed] [Google Scholar]
- 28.Ploug T, Stallknecht MB, Pederson O, Kahn BB, Ohkuwa T, Vinten J, Galbo H. Effect of endurance training on glucose transport capacity and glucose transporter expression in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1990;259:E778–E786. doi: 10.1152/ajpendo.1990.259.6.E778. [DOI] [PubMed] [Google Scholar]
- 29.Ren JM, Semenkovich CF, Gulve EA, Gao J, Holloszy JO. Exercise induces rapid increases in GLUT-4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem. 1994;269:14396–14401. [PubMed] [Google Scholar]
- 30.Reynolds TH, Brozinick JT, Rogers MA, Cushman SW. Effects of exercise training on glucose transport and cell-surface GLUT-4 in isolated rat epitrochlearis muscle. Am J Physiol Endocrinol Metab. 1997;272:E320–E325. doi: 10.1152/ajpendo.1997.272.2.E320. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds TH, Brozinick JT, Rogers MA, Cushman SW. Mechanism of hypoxia-stimulated glucose transport in rat skeletal muscle: potential role of glycogen. Am J Physiol Endocrinol Metab. 1998;274:E773–E778. doi: 10.1152/ajpendo.1998.274.5.E773. [DOI] [PubMed] [Google Scholar]
- 32.Schimke RT. Regulation of protein degradation in mammalian tissues. In: Munro HN, editor. Mammalian Protein Metabolism. New York: Academic; 1970. pp. 177–228. [Google Scholar]
- 33.Slentz CA, Gulve EA, Rodnick KJ, Henriksen EJ, Youn JH, Holloszy JO. Glucose transporters and maximal transport are increased in endurance-trained rat soleus muscle. J Appl Physiol. 1992;73:486–492. doi: 10.1152/jappl.1992.73.2.486. [DOI] [PubMed] [Google Scholar]
- 34.Spina RJ, Chi MM, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol. 1996;80:2250–2254. doi: 10.1152/jappl.1996.80.6.2250. [DOI] [PubMed] [Google Scholar]
- 35.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- 36.Wilson CM, Cushman SW. Insulin stimulation of glucose transport activity in rat skeletal muscle: increase in cell-surface GLUT-4 as assessed by photolabeling. Biochem J. 1994;299:755–759. doi: 10.1042/bj2990755. [DOI] [PMC free article] [PubMed] [Google Scholar]