Abstract
There is controversy about the abuse liability of methylphenidate (MPH) in humans, and MPH has yet to be established fully as a reinforcer in rats. The present experiment examined if intravenous MPH served as a reinforcer in rats, and how individual differences in impulsivity impacted MPH self-administration. Rats were exposed to a delay discounting procedure, and then were implanted with an intravenous catheter to assess self-administration of 0.56 mg/kg/infusion MPH at different fixed ratio (FR) values. Self-administration rates of different MPH doses (0.03–1.0 mg/kg/infusion) were also examined. Both high and low impulsive rats acquired MPH self-administration at the same rate. All rats pressed more on the active lever than the inactive lever regardless of MPH dose, and pressed more for MPH than for saline. High impulsive rats self-administered more MPH than low impulsive rats at a low unit dose (0.1 mg/kg/infusion), indicating that individual differences in impulsive choice influence the dose-dependent reinforcing effects of MPH.
Keywords: methylphenidate, self-administration, rat, impulsivity, delay discounting
Introduction
Impulsivity, generally defined as behavior without foresight (Winstanley et al., 2006) can refer to a variety of behaviors (Evenden, 1999a, 1999b). Delay discounting is defined by the choice for a small, immediate reinforcer over a larger, delayed reinforcer (Ainslie, 1975). While there are many ways to measure impulsivity, this delay discounting procedure is used widely in the operant conditioning literature to define and measure impulsive choice, and has been found to be predictive of drug use initiation (Perry et al., 2005; Perry et al., 2008a). Individual differences in impulsive choice in humans have been implicated in the use of a variety of drugs, including alcohol (Vuchinich and Simpson, 1998; Petry, 2001), cocaine (Coffey et al., 2003), nicotine (Bickel et al., 1999; Mitchell 1999; Baker et al., 2003), and opiates (Kirby et al., 1999; Madden et al., 1997; Madden et al.,1999). Although impulsivity and drug use are correlated, studies in humans have not established firmly if impulsivity leads to drug use, or if drug use leads to impulsivity, or if there is a reciprocal relationship between these two variables.
While impulsive choice is viewed as one type of impulsivity, another form of impulsivity is impulsive action, defined as the inability to withhold a response (Winstanley et al., 2006). This type of impulsivity refers to physical action, whereas impulsive choice refers to decision making. Impulsive action is measured with the go/no go, stop-signal reaction time task (SSRT), or five-choice serial reaction time task (5CSRT; Winstanley et al., 2006). These procedures require animals to respond or stop responding depending on a signal (Winstanley et al., 2006).
The effects of stimulants on impulsivity depend on the particular measure of impulsivity used (impulsive choice vs. impulsive action). Exposure to drugs of abuse, including nicotine (Dallery and Locey, 2005) increases impulsive choice; however, research on d-amphetamine has been mixed. Amphetamine has been reported to increase (Perry et al., 2008b) or decrease (Winstanley et al., 2003) impulsive choice in animals. Interestingly, however, methylphenidate (MPH) has been reported to either not affect impulsive choice (Perry et al., 2008b) or decrease impulsive choice in rats (Bizot et al., 2007; Pitts and McKinney, 2005) and humans (Pietras et al., 2003). In contrast, MPH increases impulsivity on a 5CSRT task (Milstein et al., 2008); however, this increase in impulsivity may be limited to high doses of MPH (Navarra et al., 2008). On a stop-signal reaction time task (SSRT), MPH has been found to decrease SSRT for slow responders and increase SSRT for fast responders (Eagle et al., 2007). On a fixed consecutive number (FCN) task of shock avoidance, MPH has been found to decrease impulsivity (Evenden and Ko, 2005). Therefore, the particular effects of MPH on impulsivity depend on the particular measure of impulsivity and dose of MPH used.
Preclinical evidence indicates that impulsivity is related to drug addiction and the maintenance of drug use (Jentsch and Taylor, 1999). In rats, individual differences in impulsivity predict the initiation and maintenance of drug use, including nicotine self-administration (Diergaarde et al., 2008) and cocaine self-administration (Perry et al., 2005; Perry et al., 2008a). Rats high in impulsivity also consume more ethanol than rats low in impulsivity (Poulos et al., 1995). Rats high in impulsivity have also been found to be more persistent and compulsive in cocaine self-administration (Belin et al., 2008).
Results from preclinical studies on MPH are interesting because MPH is one of the most commonly prescribed treatments for attention deficit hyperactivity disorder (ADHD; Challman and Lipsky, 2000; Swanson and Volkow, 2002), a disorder characterized by impulsive behaviors (Sonuga-Barke, 2005). MPH has abuse potential in both human and nonhuman animals (see Kollins et al., 2001 for a review) and up to 20% of children prescribed MPH for ADHD have been asked to sell, trade, or give their medication to other children (Kollins et al., 2001). Approximately 5–15% of the U.S. population under the age of 18 is treated with MPH (Barbaresi et al., 2002), and because ADHD may continue beyond adolescence, many of these individuals continue to take MPH during adulthood (Himelstein and Halperin, 2000). Because of the wide spread availability of MPH, nonclinical use and abuse of MPH is a health concern for children, adolescents, and adults.
Although much preclinical research has examined self-administration of psychomotor stimulants, little research has examined MPH self-administration in laboratory animals, regardless of individual differences in impulsivity. In one study, the reinforcing effect of a variety of drugs self-administered by rats was evaluated using a rating scale. Investigators found that MPH was as reinforcing as cocaine at similar doses (Collins et al., 1984). In another study, rats were trained to self-administer amphetamine (0.06 mg/kg/infusion), then switched to MPH (0.2 or 0.4 mg/kg/infusion). The investigators showed that rats continued to self-administer MPH at the same rate as amphetamine (Nielsen et al., 1984). Similarly, nonhuman primates trained to lever press for cocaine will subsequently lever press for MPH (Bergman et al., 1989; Gasior et al., 2005; Lile et al., 2003; Johanson and Schuster, 1975). In the most extensive study on MPH self-administration in rats, it was found that drug naive rats would self-administer MPH doses of 0.06125–0.5 mg/infusion (Botly et al., 2008). These investigators, however, did not give MPH doses based on body weight, did not compare infusions of MPH earned relative to saline infusions, and did not report inactive (non-reinforced) lever presses during the session. Therefore, the reinforcing effect of MPH in rats remains to be established fully.
The purpose of the present study in rats was two-fold. The first purpose was to determine if individual differences in impulsive choice predict acquisition and dose-dependent maintenance of MPH self-administration. Regardless of individual differences in impulsive choice, the second purpose was to determine if MPH serves as a reinforcer across varying doses when drug-reinforced responses are compared to responses on an inactive lever and responses for saline.
Method
Subjects
Subjects were twelve 65-day-old male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN). All subjects were experimentally and drug naive at the beginning of the experiment. To provide sufficient motivation to lever press for food in the delay discounting task, subjects were restricted to 15 g of food/day, delivered immediately after each session. Access to water was continuous in the home cage. Subjects were housed individually in plastic home cages and were maintained on a 16/8 hr light/dark cycle (lights on at 6:00 am). Subjects remained in their home cage except during their 1–2 hr daily experimental sessions. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky, and followed APA ethical standards of laboratory animal care (American Psychological Association, 2002).
Apparatus
Experimental sessions were conducted in operant conditioning chambers for rats (28 cm × 24 cm × 25 cm; ENV-001; MED Associates, St. Albans, VT). The front and back walls were Plexiglas, side walls were aluminum, and floors were made of metal rods. Operant chambers were housed inside sound-attenuating chambers (ENV-018M; MED Associates, St. Albans, VT). During delay discounting sessions, a 28-V house light centered 2.5 cm from the ceiling on the left side wall illuminated the chamber. Sucrose pellets (45 mg, Bio-Serv, Frenchtown, NJ) were dispensed individually from a pellet dispenser (ENV-203M-45; MED Associates, St. Albans, VT) into a recessed food cup (5 cm × 5 cm × 3 cm) located 2 cm above the floor and centered on the right side wall. Retractable levers (4.5 cm) were located 6 cm above the floor on each side of the food tray. White stimulus lights (28 V; 3 cm in diameter) were located 3 cm above each lever. A fan in the sound-attenuating chamber produced noise to mask extraneous sounds. Experimental events were arranged and recorded by MED-PC software (Med-Associates, St. Albans, VT) on a computer located in the experimental room.
Procedures
Delay Discounting
Subjects were exposed to an adjusted delay procedure (1 pellet available immediately vs. 3 pellets available after an adjusting delay) for 28 sessions using previously published methods (Perry et al., 2005, Perry et al., 2008a). Sessions contained 15 blocks of 4 trials. The first two trials in each block were forced-choice trials, one for the immediate option and one for the delayed option. The order in which the forced-choices were presented alternated randomly within the session. During forced-choice trials, the active lever was extended into the chamber and the stimulus light above the active lever was illuminated. The lever retracted following one lever press on forced-choice trials. The delay to the delayed option did not change during forced choice trials. Lever presses during on all trials were followed by an inter-trial interval (ITI) of an adjusted duration such that the total trial, including the ITI, was 60 s. The third and fourth trials in each block were free-choice trials. During free-choice trials, both levers were extended and both stimulus lights were illuminated. A response on the immediate option produced one pellet immediately, and a response on the delayed option produced three pellets after an adjusting delay. Levers associated with the immediate and delayed options alternated sides of the chamber across days. The only stimulus that signaled which side of the chamber was associated with each option was the number of pellets that followed a lever press. All lights were extinguished and lever presses had no programmed consequences during ITIs. Levers were retracted following the completion of all trials in a block.
For the first session, the duration to the delayed option started at 0 s, and the delay changed subsequently based on choices during free-choice trials. This allowed subjects to titrate the delay depending on their choices rather than starting subjects with an arbitrarily chosen delay. Responses during the free-choice trials on the immediate option produced a 1-s decrease in the delay and responses on the delayed option produced a 1-s increase in the delay. The delay had a lower limit of 0 s and upper limit of 45 s. During the delay, the house light was extinguished, but stimulus lights remained on until food was delivered. The delay at the end of each session was used as the starting delay for the following session. Mean adjusted delays (MADs) were calculated at the end of each session by calculating the average adjusting delay over the free-choice trials. MADs were used as a measure of impulsive choice, with lower MADs indicating higher levels of impulsivity. Sessions ended after 60 trials, or 2 hr, whichever occurred first. Subjects completed all trials during each session, except for some subjects during the first two sessions. This phase continued for 28 consecutive daily sessions. Because some individual rat’s behavior did not stabilize on the adjusting delay procedure (cf. Cardinal et al., 2002), stability was based on the group mean of all 12 subjects as described previously (Perry et al., 2005; Perry et al., 2008a). Following 28 sessions, the MAD for the group mean varied by less than 5 s across the last 5 days.
Extinction
Subjects were then exposed to lever press extinction (EXT) for seven sessions. During EXT sessions, both levers were extended and both stimulus lights and the house light were illuminated. Lever presses had no programmed consequence. The purpose of this phase was to minimize the carryover of lever pressing for food to lever pressing for MPH, which was implemented in the next phase.
Methylphenidate self-administration
Following two days of free feeding, rats were surgically implanted with a chronic indwelling jugular catheter (0.2 mm in diameter). Rats were anesthetized with 100 mg/kg ketamine (i.p.) and 5 mg/kg diazepam (i.p.). One end of the catheter was inserted into the jugular vein, and the other end was attached to a metal cannula that exited the skin and was secured in a dental acrylic head mount. The head mount was adhered to the skull with dental acrylic and metal jeweler screws. Catheter patency was maintained by daily 0.2 ml infusions of a mixture containing 20 ml saline, 0.6 ml heparin, and 0.2 ml gentamicin. Rats were given five days of recovery and then food restriction was re-introduced. Rats were maintained on 20 g of food/day during the self-administration phase, given immediately after each session. During experimental sessions, cannulas were attached to tubing within a flexible, spring covered leash (PHM-120; MED Associates, St. Albans, VT). The leash was connected to a swivel (PHM-115; MED Associates, St. Albans, VT) that was outside the operant chamber. The tubing exited the operant chamber and was connected to an infusion pump (PHM-100; MED Associates, St. Albans, VT).
Following recovery from surgery, subjects were exposed to three more sessions of lever press EXT. Subjects were then given access to 0.56 mg/kg/infusion MPH (0.1 ml volume), available on a fixed ratio 1 (FR 1) schedule of reinforcement for 60-min daily sessions. The 0.56 mg/kg/infusion dose of MPH was chosen because it was estimated to engender reliable responding based on previous literature (Botly et al., 2008). During self-administration sessions, both levers were extended into the chamber and no lights were illuminated. Presses on one lever produced drug infusions (active lever) and presses on the other lever had no programmed consequence (inactive lever). The side of the chamber containing the active lever was counterbalanced across subjects. Following each active lever press, MPH was infused over 5.9 s, followed by a 20-s time out signaled by the illumination of both stimulus lights. Presses on either lever during the time out had no programmed consequence. Following seven consecutive FR 1 sessions, the FR value was incremented by one response every three sessions, up to an FR 5.
Since one rat failed to make any responses on the first two FR 1 sessions, it was given additional training using an autoshaping procedure (Carroll and Lac, 1993) before moving to the FR 2 schedule. Once subjects reached FR 5, they remained on this ratio for nine consecutive sessions.
Dose-effect determinations
During the next phase of the experiment, subjects were given access to 0.1, 0.3 and 1.0 mg/kg/infusion MPH on an FR 5 schedule of reinforcement. Subjects were given access to each MPH dose for three consecutive sessions. Half the subjects in each impulsivity group proceeded through the doses in ascending order (0.1, 0.3, 1.0), and the other half proceeded in descending order (1.0, 0.3, 0.1). All subjects were then given access to 0.03 mg/kg/infusion MPH for three consecutive sessions, followed by access to saline for seven consecutive sessions.
Drug
Methylphenidate HCl (Mallinckrodt, St Louis, MO) was prepared in sterile 0.9% NaCl (saline).
Data Analysis
The average MAD was calculated for all subjects during the last 5 days of delay discounting. Subjects with MADs above the median were designated the Low Impulsivity (Lo I) group, and subjects with MADs below the median were designated as the High Impulsivity (Hi I) group. Mixed model analyses of variance (ANOVAs) were used to compare group means with impulsivity group (Hi I vs. Lo I) as a between subjects factor using a median-split approach (Piazza et al., 2000). This test was conducted with factors “FR schedule” (FR 1-FR 5) and “lever type” (active and inactive) for each group to compare active and inactive lever presses across FR schedules using the individual subject mean of the last three sessions of exposure to each FR schedule. This test was also conducted with factors “session” (sessions 1–7) and “impulsivity group” (Hi I and Lo I) to compare the mean number of lever presses for the two impulsivity groups during EXT. These tests were also conducted with factors “FR schedule” (FR 1-FR 5), “session of exposure to FR schedule” (1–3), and “impulsivity group” (Hi I and Lo I) to compare active lever presses and number of infusions earned during acquisition of MPH self-administration across the last three sessions of exposure to each FR schedule. Only the last two sessions of exposure to each dose were included in the data analyses during the dose-effect determination phase. Three subjects (two from the Hi I group, and one from the Lo I group) were removed from the dose-effect phase due to catheter malfunction. A mixed model ANOVA was also used to examine the number of infusions earned and MPH intake during dose-effect determinations with factors “MPH dose” (0.0–1.0 mg/kg/infusion) and “impulsivity group” (Hi I and Lo I). Pair wise comparisons (t-tests with Bonferroni correction) were used to compare MPH infusions earned between groups for individual doses during dose-effect determination. All statistical tests were considered significant at p < .05.
Results
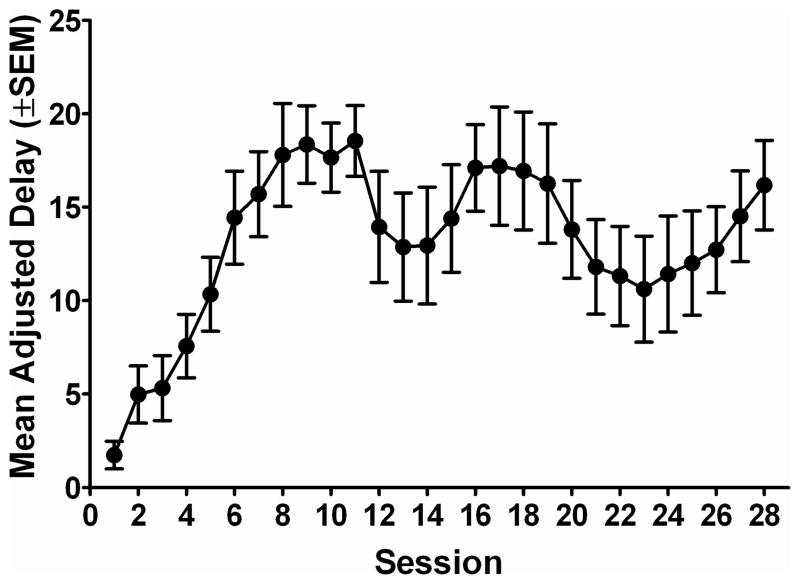
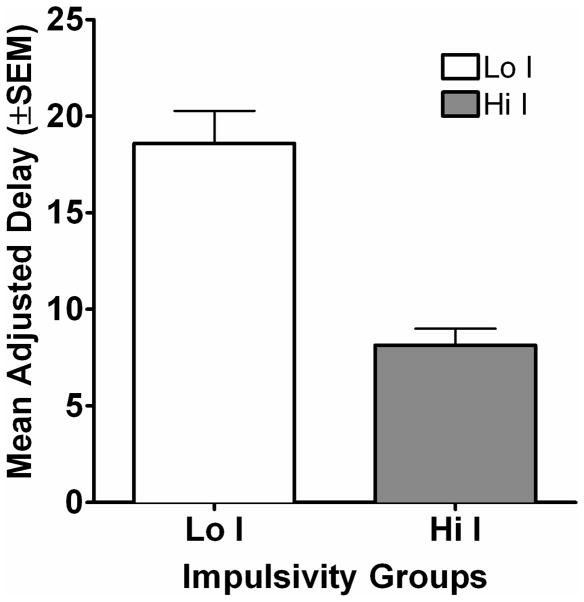
Average MADs increased across the first eight sessions, and then showed some variability during the rest of the phase. Figure 1a shows the MAD for all rats across the 28 sessions of delay discounting. Subjects were divided into two groups based on each subject’s average MAD derived from sessions 24–28 of the delay discounting task. Figure 1b shows the MADs for the two impulsivity groups collapsed across the last five sessions of the delay discounting task. The average (mean ± S.E.M.) MADs during the last five days of the delay discounting task in Hi I subjects and Lo I subjects were 8.15 s (± 0.85) and 18.60 s (± 1.68) respectively (see Table 1 for individual subject MADs).
Figure 1.
Figure 1a. Mean adjusted delay (MAD) is plotted as a function of session for the group average. Error bars show the standard error of the mean.
Figure 1b. Average MADs across the last five days of exposure to the delay discounting task are plotted for each group. The open bar shows data from the Lo I group and the filled bar shows data for the Hi I group.
Table 1.
Mean adjusted delays (MADs) for each subject during the last five sessions of the delay discounting task. Values in parentheses show the standard error of the mean.
| Subject | MAD |
|---|---|
| 801 | 30.90 |
| 807 | 26.29 |
| 808 | 14.70 |
| 809 | 10.86 |
| 810 | 14.21 |
| 811 | 14.63 |
| Lo I Group Mean | 18.60 (1.68) |
| 802 | 9.87 |
| 803 | 8.20 |
| 804 | 8.09 |
| 805 | 8.20 |
| 806 | 9.85 |
| 812 | 4.67 |
| Hi I Group Mean | 8.15 (0.85) |
During the lever press EXT phase (results not shown), subjects in both groups decreased the number of lever presses across EXT sessions. A 2 × 7 mixed model ANOVA (impulsivity group × session) showed that the groups’ lever pressing was not significantly different across the seven pre-surgery sessions of EXT [F (1, 10) = 2.71; p > .05]. Following i.v. catheter placement, subjects showed a slight increase in lever pressing that decreased across the three post-surgery EXT sessions (results not shown). During the final EXT session, subjects averaged only 11 responses during the 60-min session.
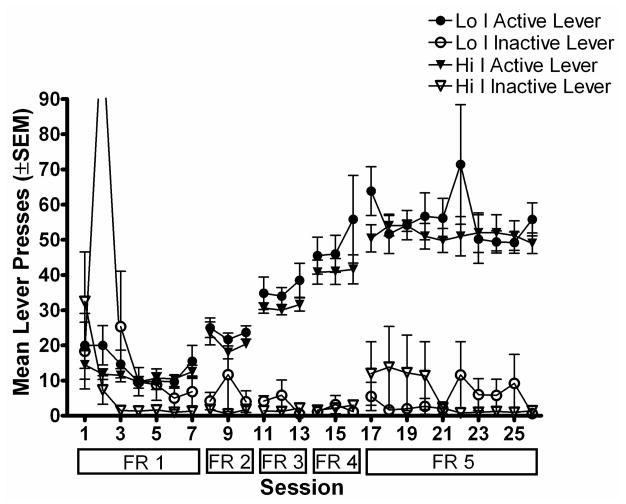
Figure 2a shows the number of lever presses for Lo I and Hi I groups during acquisition of MPH self-administration using a unit dose of 0.56 mg/kg/infusion. During the first few sessions of self-administration, subjects pressed both the active and inactive levers; however, during the last few days on FR 1, subjects were primarily pressing the active lever. As the FR requirement increased, subjects showed an increase in active lever presses, while inactive lever presses remained at a low rate. A within group 5 × 2 (FR schedule × lever type) factorial ANOVA indicated that subjects in both groups pressed the active lever significantly more than the inactive lever during the last three days of exposure to each FR schedule [Lo I group: F (4, 1) = 84.21; p < .05; Hi I group: F (4, 1) = 152.31; p < .05]. Following nine days on FR 5, all subjects except one had less than 20% variability in the number of infusions earned across three consecutive sessions, and all subjects had greater than a 2:1 ratio of active to inactive lever presses. There were no significant differences in self-administration based on impulsivity groups across the different FR values. This was confirmed by a mixed model 5 × 2 × 3 (FR schedule × group × session of exposure to schedule) factorial ANOVA [F (1, 7) = 1.99; p > .05], indicating that differences in impulsivity did not affect acquisition of MPH self-administration.
Figure 2.
Figure 2a. Mean total number of lever presses during the session is plotted as a function of session during acquisition of MPH self-administration. Filled symbols show lever presses on the active lever and open symbols show lever presses on the inactive lever. Circles show data from the Lo I group and triangles show data from the Hi I group. Error bars show the standard error of the mean. Boxes below the x-axis show the FR value in effect during each session. Note that the off-scale point for inactive lever presses for the Lo I group during the second session was due to one subject pressing the inactive lever 652 times.
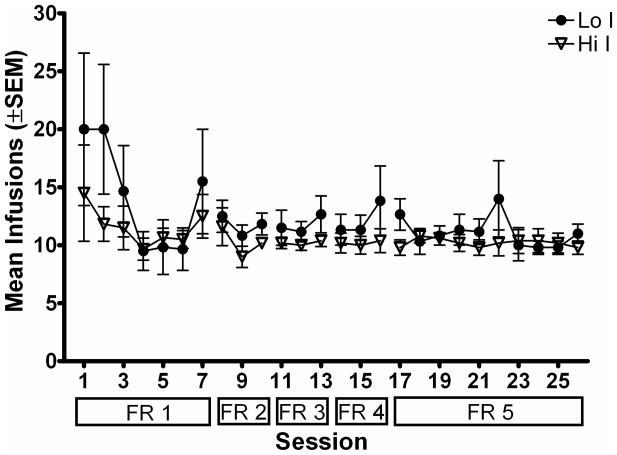
Figure 2b. Mean total number of infusions earned during the session is plotted as a function of session during acquisition of MPH self-administration. Filled circles show data from the Lo I group and open triangles show data from the Hi I group. Other details are as in Figure 2a.
Figure 2b shows the corresponding number of infusions of MPH earned during acquisition of MPH self-administration. Again, there were no significant differences between Lo I and Hi I groups across any FR schedule. This was confirmed by a mixed model 5 × 2 × 3 (FR schedule × group × session of exposure to schedule) factorial ANOVA [F (1, 7) = 0.66; p > .05]. Subjects in both groups earned approximately the same number of infusions regardless of the FR requirement. The consistency in number of MPH infusions earned despite the increase in response requirement, and the low number of inactive lever presses compared to active lever presses, indicate that MPH served as a reinforcer for subjects in both groups.
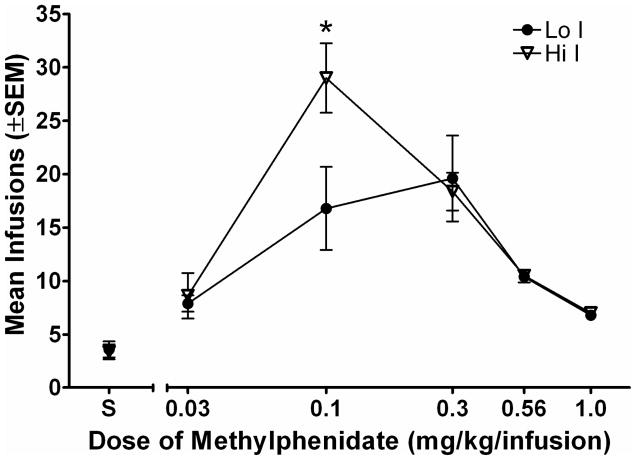
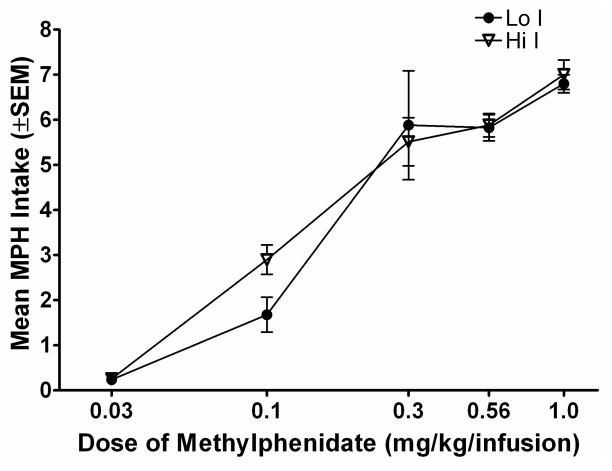
Figure 3a shows the number of infusions earned for subjects in each group during the dose-effect assessment phase. Subjects earned more of each unit dose of MPH compared to saline, indicating that each dose of MPH examined served as a reinforcer. Subjects earned the highest number of infusions at the 0.1 and 0.3 mg/kg/infusion MPH doses. A mixed model 6 × 2 (dose × group) factorial ANOVA showed a significant dose × group interaction [F (5, 35) = 2.56; p < .05] in the number of infusions earned. There were no significant differences in the number of infusions earned between groups at any dose except 0.1 mg/kg/infusion. A Bonferroni-corrected t-test revealed that subjects in the Hi I group earned more infusions of the 0.1 mg/kg/infusion MPH than subjects in the Lo I group [t (7) = 4.16; p < .05]. Inactive lever presses remained low regardless of dose (results not shown). Figure 3b shows total drug intake for each group at each dose of MPH. Lo I and Hi I rats took approximately the same amount of drug at the three highest doses (0.3, 0.56, 1.0 mg/kg/infusion), and took less of the lower doses. A 5 × 2 (dose × group) ANOVA revealed only a significant main effect of dose [F (4, 28) = 48.27; p < .05]. There were no significant differences in intake of MPH between the two impulsivity groups.
Figure 3.
Figure 3a. Mean total number of infusions earned during the session is plotted as a function of dose of methylphenidate (log scale). Filled circles show data from the Lo I group and open triangles show data from the Hi I group. Error bars show the standard error of the mean. The asterisk indicates a significant difference between groups at the MPH unit dose of 0.1 mg/kg/infusion, p < .05.
Figure 3b. Mean MPH intake is plotted as a function of methylphenidate dose (log scale). Filled circles show data from the Lo I group and open triangles show data from the Hi I group. Other details are as in Figure 3a.
Discussion
The present experiment found that rats readily acquired MPH self-administration at a unit dose of 0.56 mg/kg/infusion. Across a wide range of doses (0.03–1.0 mg/kg/infusion), MPH was found to be a reinforcer when compared to responding on an inactive lever or responding during saline substitution. Differences in impulsivity on a delay discounting task predicted self-administration of 0.1 mg/kg/infusion, with Hi I rats responding more for this dose than Lo I rats; animals responded similarly for other doses of MPH regardless of their levels of impulsivity. Based on the overall dose-effect curve, Hi I rats were more sensitive than Lo I rats to adjustments in the unit dose of MPH.
Results of the present experiment also suggest that rats can regulate their intake of MPH. Subjects earned approximately the same total amount of drug across the three highest doses tested (0.3–1.0 mg/kg/infusion; see Figure 3b). Subjects were also able to regulate their intake of the training dose regardless of the FR value used across a range of FR values (FR 1-FR 5). These results are consistent with prior research on self-administration of MPH by rhesus monkeys (Wilson et al., 1971).
The present experiment extends past research on MPH self-administration in rats. Previous investigators have shown that rats respond for MPH across a wide range of doses (Botly et al., 2008; Collins et al., 1984; Nielsen et al., 1984). The results of the present experiment also found that inactive lever presses remained at a low rate regardless of the dose of MPH available for self-administration and that rats respond more for MPH than saline. These results establish more fully that MPH is a reinforcer in rats. Because drug self-administration in non-human animals, as used in the present experiment, is a valid model of human drug use (O’Dell and Khroyan, 2009; Thomsen and Caine, 2005), results of the present experiment suggest that MPH has significant abuse liability. These results, combined with those suggesting that humans also abuse MPH (Kollins et al., 2001), indicate that caution may be needed when prescribing MPH as a treatment for ADHD among individuals prone to drug abuse.
The results of the present experiment contrast with other research showing that impulsivity predicts acquisition of self-administration for cocaine (Perry et al., 2005; Perry et al., 2008a), nicotine (Diergaarde et al., 2008), and alcohol (Poulos et al., 1995). The present experiment did not find any differences in acquisition of MPH self-administration based on impulsive choice; however, previous studies examining individual differences in impulsivity in self-administration of cocaine and nicotine tested only a single unit dose of the drug (Diergaarde et al., 2008; Perry et al., 2005; Perry et al., 2008a). Other work has shown that the unit dose of drug tested is important in finding individual differences in response to novelty. For example, in one study, it was found that high novelty responders self-administered more amphetamine at unit doses of 0.03 and 0.01 mg/kg/infusion, but not at higher or lower doses (Cain et al., 2007). In similar studies, it was found that high novelty responders self-administered more amphetamine than low responders at a dose of 0.03 mg/kg/infusion, but not at higher doses (Klebaur et al., 2001), and that individual differences in self-administration of amphetamine based on rearing conditions are observed when animals self-administer low but not high doses (Green et al., 2002). Since individual differences tend to be more pronounced at low unit doses, it is possible that Lo I and Hi I rats may have differed in the acquisition of MPH self-administration if they were trained initially with a unit dose lower than the one used in the present experiment (0.56 mg/kg/infusion). Alternatively, it may be that the ability of impulsive choice to predict acquisition of cocaine self-administration (Perry et al., 2005; Perry et al., 2008a) does not generalize to acquisition of MPH self-administration.
It is not clear why Hi I rats self-administered more MPH than Lo I rats when a low dose (0.1 mg/kg/infusion) was tested, but not when other doses were tested. One possibility is that individual differences may be masked when rats self-administer high unit doses because the direct effects of MPH overcome individual differences in impulsive choice. Another possibility is that individual differences in impulsive choice may only predict self-administration of drug doses that engender a high rate of lever pressing. Unit doses that generate more responding overall may allow individual differences in impulsive choice to be expressed more readily.
Results of the present experiment are generally consistent with the literature on human drug abuse. Among humans, individuals with ADHD are impulsive than individuals without ADHD (Sonuga-Barke, 2005), and they are more likely to initiate drug use and are more likely to have problems with substance abuse than individuals without ADHD (Biederman et al., 1998; Bukstein, 2000; Ernst et al., 2006; Faraone et al., 2007; Mannuzza et al., 1998; Milberger et al., 1997; Molina and Pelham, 2003). The present experiment also found that Hi I rats self-administered more MPH (0.1 mg/kg/infusion) than Lo I rats. Thus, these results corroborate the evidence that impulsive behavior increases drug abuse vulnerability.
Acknowledgments
Research supported by NIH Grants P50 DA05312 and T32 DA007304. Reprints may be obtained from the first author at University of Kentucky, 741 S. Limestone, BBSRB Room 448B, Lexington, KY 40536, or jmarusich@uky.edu.
The authors thank Joshua Beckmann, Emily Denehy, Cassandra Gipson, A. Chip Meyer, and Lindsay Pilgram for assistance.
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Ethical principles of psychologists and code of conduct. Am Psychol. 2002;57:1060–1073. [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and neverbefore cigarette smokers: Similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Oankratz VZ, Weber AL, Mrazek KJ, Jacobsen SJ. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002;156:217–224. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1353–1354. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–5. [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44:269–273. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- Bizot J, Chenault N, Houze B, Herpin A, David S, Pothion S, Trovero F. Methylphenidate reduces impulsive behaviour in juvenile Wistar rats, but not in adult Wistar, SHR and WKY rats. Psychopharmacology. 2007;193:215–223. doi: 10.1007/s00213-007-0781-4. [DOI] [PubMed] [Google Scholar]
- Botly LCP, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology. 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Bukstein OG. Disruptive behavior disorders and substance use disorders in adolescents. J Psychoactive Drugs. 2000;32:67–79. doi: 10.1080/02791072.2000.10400213. [DOI] [PubMed] [Google Scholar]
- Cain ME, Denehy ED, Bardo MT. Individual differences in amphetamine self-administration: The role of the central nucleus of the amygdala. Neuropsychopharmacology. 2007;33:1149–1161. doi: 10.1038/sj.npp.1301478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Daw N, Robbins TW, Everitt BJ. Local analysis of behaviour in the adjusting-delay task for assessing choice of delayed reinforcement. Neural Netw. 2002;15:617–634. doi: 10.1016/s0893-6080(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: Effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Collins RJ, Weeks RJ, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology. 1984;82:6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MRA, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eshela N, London ED, Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999a;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacology. 1999b;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Evenden J, Ko T. The psychopharmacology of impulsive behaviour in rats VIII: Effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology. 2005;180:294–305. doi: 10.1007/s00213-005-2163-0. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Wilens TE, Petty C, Antshel K, Spencer T, Biederman J. Substance use among ADHD adults: Implications of late onset and subthreshold diagnoses. Am J Addict. 2007;16:24–34. doi: 10.1080/10550490601082767. [DOI] [PubMed] [Google Scholar]
- Gasior M, Bergman J, Kallman MJ, Paronis CA. Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a concurrent schedule of food and i.v. drug delivery in rhesus monkeys. Neuropsychopharmacology. 2005;30:758–64. doi: 10.1038/sj.npp.1300593. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Himelstein J, Halperin JM. Identification of AD/HD subtypes using laboratory-based measures: A cluster analysis. CNS Spectrums. 2000;5:58–64. [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: Cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–88. [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: A review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, Nader MA. The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307:356–66. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using controls participants: Drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, Jacobs EA. Discounting of delayed rewards in opioid-dependent outpatients: Exponential or hyperbolic discounting functions. Exp Clin Psychopharmacol. 1999;7:284–293. doi: 10.1037//1064-1297.7.3.284. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Milstein JA, Dalley JW, Robbins TW. Methylphenidate-induced impulsivity: Pharmacological antagonism by β-adrenoreceptor blockade. J Psychopharmacol. 2008 doi: 10.1177/0269881108098146. Online First. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Duda NJ, Mokler DJ, Moore KE. Self-administration of central stimulants by rats: A comparison of the effects of d-amphetamine, methylphenidate and McNeil 4612. Pharmacol Biochem Behav. 1984;20:227–232. doi: 10.1016/0091-3057(84)90247-8. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent Models of Nicotine Reward: What do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of iv cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008a;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008b;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rogue-Pont F, Moal ML. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology. 2003;170:390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP. Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: Implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 2005;Chapter 9(Unit 920) doi: 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self-administration as a function of dosage per injection in the rhesus monkey. Psychopharmacologia. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]