Abstract
Background
Activation of the insulin (IN)/IRS-1/MAPK and the Wnt/β-catenin signaling cascades occurs frequently in hepatocellular carcinoma (HCC) associated with persistent viral infection. The aims of this study were to provide a chronic proliferative stimulus via IRS-1 in the context of hepatitis Bx (HBx) protein expression in transgenic mice and determine if constitutive expression of these genes is sufficient to cause hepatocyte dysplasia and cellular transformation.
Methods
We generated transgenic mice in which the HBx (ATX), IRS-1 or both (ATX+/IRS-1) genes were expressed under a liver specific promoter. We also assessed histology and oxidative damage as well as upregulation of molecules related to these signal transduction cascades in the liver by qRT-PCR.
Results
Whereas mice with a single transgene (ATX or IRS-1) did not develop tumors, ATX+/IRS-1+ double transgenic livers had increased frequency of hepatocellular dysplasia and developed HCC. All three transgenic lines had significantly increased IGF-1, Wnt 1 and Wnt 3 mRNA levels, and evidence of DNA damage and oxidative stress. The ATX+/IRS+ double transgenic mice were distinguished by having the highest level of activation of Wnt 3 and Frizzled 7 and selectively increased expression of IGF-II, PCNA, and aspartyl (-asparaginyl)-β-hydroxylase (AAH) a gene associated with increased cell migration.
Conclusions
These results suggest that continued expression of the ATX or IRS-1 transgenes can contribute to hepatocyte transformation but are not sufficient to trigger neoplastic changes in the liver. However, dual expression that activates both the IN/IRS-1/MAPK and Wnt/β-catenin cascades is sufficient to cause dysplasia and HCC in a previously normal liver.
Keywords: Hepatocellular carcinoma, signal transduction, Wnt, β-catenin, insulin receptor substrate, Hepatitis B virus, DNA damage, transgenic mice, liver disease
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 5th leading cause of cancer and third leading cause of cancer death worldwide (1). The major etiologic agents include chronic infection with hepatitis B or hepatitis C virus (2). In addition, co-factors that contribute to HCC pathogenesis include chronic alcohol abuse, aflatoxin exposure, and metabolic liver disease such as hemachromatosis (3). Although significant progress has been made in unraveling sequential cellular changes that precede HCC development, the molecular pathogenesis of HCC is still under intense investigation. In this regard, the aggregate results stemming from experiments and studies conducted by multiple independent groups have brought into focus that there are two main signal transduction pathways which are consistently up-regulated in HCC: 1) the insulin/IGF/MAPK cascade; and 2) the Wnt/Frizzled/β-catenin pathway.
In human HCC, the insulin/IGF/MAPK pathway has been shown to be dysregulated due to increased expression of growth factors, such as IGF2 (4–6), or downstream signaling molecules, such as the insulin receptor substrate-1 (IRS-1) (7, 8), which promotes mitogenesis and growth by activating both Erk MAPK and PI3 kinase-Akt/Protein kinase B pathways (9). IRS-1 is over-expressed in nearly 90% of human HCCs, and the degree to which IRS-1 is over-expressed in liver correlates with tumor size and tumor progression (9–13). Yet, over-expression of IRS-1 alone was found to be insufficient to cause hepatocellular transformation, since transgenic mice that constitutively over-expressed IRS-1 in liver, never developed HCC, even after more than 2 years of observation (9). This suggests that, in addition to persistent growth and increased hepatocellular turnover, another factor besides IRS-1 over-expression is required for HCC to develop in the otherwise normal liver.
The canonical Wnt/Frizzled/β-Catenin signaling pathway, which has a crucial role in regulating cell fate and tissue patterning during development, is also frequently dysregulated in both malignant and pre-neoplastic states (8, 14, 15). Ordinarily, glycogen synthase kinase 3β (GSK-3β) constitutively phosphorylates and thereby targets β-Catenin for degradation through the ubiquitin-mediated proteosomal pathway. However, up-regulation of Wnt signaling by interaction of Wnt ligand with Frizzled (FZD) receptors results in phosphorylation and inactivation of GSK-3β, and attendant destabilization of the CK1-β-catenin-axin-APC (adenomatous polyposis coli)-GSK-3β pentameric regulatory complex, leading to β-catenin accumulation and subsequent gene activation (16) through nuclear translocation and binding of β-catenin to TCF/LEF-1 family of transcription factors (17). In HCC, Wnt signaling is increased due to: 1) mutations that stabilize β-catenin and render it resistant to proteasomal degradation (6, 18); 2) up-regulation of stimulatory components, such as FZD-7 (19) and Wnt3 (20) or factors such as PIN-1 that reduce β-catenin interaction with APC (21); or 3) mutations in structural components of the complex, e.g. axins (22).
Since the majority of HCCs are associated with HBV or HCV chronic infection (2), it would be of interest to evaluate the potential effects of viral gene expression and protein-protein interactions on HCC-associated signaling pathways. The X gene (HBx), the smallest open reading frame (ORF) in the HBV genome, encodes a 154 amino acid protein that is essential for productive HBV infection and replication (23–25). HBx has pleiotropic functions including its broad transactivation of transcription, activation of signal transduction cascades; and interference with proteasomal, mitochondrial and DNA repair functions (26). HBx is often integrated into the cellular genome of HCC (27). In addition, HBx was shown have transforming capabilities in some but not all (28, 29) HBx transgenic mice, and to have a role in activating both the Wnt (30, 31) and Ras/MAPK (32, 33) pathways. Nonetheless, by itself, HBx is generally regarded as weakly oncogenic (24, 26).
Despite the independent evidence that both insulin/IGF/Ras/MAPK and Wnt/Frizzled/β-catenin pathways are up-regulated in human HCCs, definitive causal evidence with respect to the molecular pathogenesis hepatocellular dysplasia, transformation and/or HCC development in the previously normal liver is still lacking. Since over-activation of either pathway alone has proven to be insufficient to cause HCC in vivo, we hypothesized that more than one hit was required for malignant transformation of the liver. The observations that HBx can activate both signaling mechanisms and is expressed during active HBV replication and integrated in human HCCs (27), led us to design experiments to determine if the combined effects of HBx expression and constitutive activation of the insulin/IGF/Ras/MAPK pathway would cause hepatocellular dysplasia and HCC. Therefore, we generated a new transgenic mouse model to investigate potential synergistic effects of HBx and IRS-1 in relation to insulin/IGF/Ras/MAPK and Wnt/Frizzled/β-catenin signaling and hepatocellular transformation in vivo.
METHODS
Transgenic Mouse Models
Mice expressing the human IRS-1 gene (9) were mated with ATX transgenic mice, which express the Hepatitis B virus X gene derived from the adw2 strain. Transgene expression was driven by a liver-specific promoter as previously described (9, 29). In brief, heterozygous ATX mice were crossed with heterozygous IRS-1 and the offspring (WT, ATX, IRS-1, and ATX/IRS-1) were analyzed. Non-transgenic (WT) littermates were used as controls. For the ATX mice, we used the human alpha-1-anti-trypsin regulatory region (29); the IRS-1 mice used the albumin promoter (9). The IRS-1 mice were derived from the inbred FVB genetic background; the ATX mice from the outbred ICR background. Four groups of male mice were studied: wild-type (Wt), ATX+, IRS-1+ and IRS-1+/ATX+. F1 progeny were sacrificed at 15 and 18 months of age and pooled for subsequent analysis since there was no difference in the frequency of dysplasia and HCC between these groups, respectively. Liver tissue was harvested for quantitative RT-PCR analysis of gene expression or histopathological studies of immersion fixed (Histochoice, Amresco Corp, Solon, OH) liver tissue. All genotypes were confirmed by qPCR using transgene-specific primer pairs (available upon request).
Histopathological Studies
Paraffin-embedded sections (5 µM thick) were stained with Hematoxylin and Eosin (H&E), and subjected to blind analysis with a semi-quantitative scale of 0 to 3 for lobular inflammation, portal inflammation, nuclear pleomorphism, microsteatosis, and macrosteatosis. In addition, we assessed the presence of dysplasia as defined by hepatocyte pleomorphism, highly atypical nuclei, multiple nucleoi and nuclear hyperchromatism and HCC. Liver samples were derived from individual mice within the following groups: ATX (N=16); IRS-1 (N=15); ATX+IRS-1 (N=23); and wild type litter mate controls (N=12). We simplified the data presentation by graphing the frequency distributions of the combined non-trivial grade lesions, i.e. 2+ plus 3+ severity, as the percent of the animals exhibiting such changes in the liver compared to the total according to genotype. We simplified the data presentation by graphing the frequency distributions of lesions according to genotype. Adjacent histological sections were immunostained with polyclonal antibodies to 4-hydroxy-2,3-nonenal (HNE), 8-hydroxy-deoxyguanosine (8-OHdG) (Chemicon International., Temecula, CA), proliferating cell nuclear antigen (PCNA), or β-catenin to detect lipid peroxidation, DNA damage, DNA synthesis, or activation of Wnt signaling, respectively as previously described, except that immunoreactivity was revealed using horseradish peroxidase-conjugated polymer-linked secondary antibody, and diaminobenzidine or NovaRed as the chromogen (Vector Laboratories, Burlingame, CA). The sections were lightly counterstained with hematoxylin and examined by light microscopy. The percentages of PCNA-positive nuclei in 4 representative 200x magnification fields were determined using Image-Pro Plus software. The sections were examined and scored under code by pathologists (AR and SMdIM).
Quantitative (Q) RT-PCR Assays
Total liver RNA isolated from liver with TRIzol reagent (Invitrogen, Carlsbad, CA), was reverse transcribed using random oligodeoxynucleotide primers, and the resulting cDNA templates were used to measure IGF-1, IGF-2, insulin, and their corresponding receptors, IRS-1(primers detect the endogenous form and not the human IRS-1 driven from the transgene), IRS-2, and IRS-4, Wnt-1, Wnt-3, Frizzled-3 (FZD-3), FZD-7, PCNA, aspartyl-(asparaginyl)-β-hydroxylase (AAH), hepatocyte growth factor (HGF), transformation-related protein 53 (TP53), and β-catenin by qRT-PCR with gene specific primers (available upon request). Ribosomal 18S measured in parallel reactions was used to calculate relative mRNA abundance. PCR amplifications were performed with QuantiTect SYBR Green PCR Mix (Qiagen Inc, Valencia, CA). The amplified signals were detected continuously with the Mastercycler ep realplex instrument and software (Eppendorf AG, Hamburg, Germany) as previously described.
Source of Reagents
QuantiTect SYBR Green PCR Mix was obtained from Qiagen Inc (Valencia, CA). Polyclonal antibodies to 8-OHdG and HNE were purchased from Chemicon (Tecumsula, CA). All other reagents used for immunohistochemical staining were purchased from Abcam (Cambridge, MA) or Vector Laboratories (Burlingame, CA). All other fine chemicals were purchased from CalBiochem (Carlsbad, CA) or Sigma-Aldrich (St. Louis, MO).
Statistical Analysis
Data depicted in the graphs represent the means ± S.E.M.’s for each group. Inter-group comparisons of gene expression levels were made using repeated measures Analysis of Variance with the Tukey-Kramer or Dunn’s post-hoc significance test. Inter-group analysis of frequency distributions of lesions was performed with Chi-square tests. Statistical analyses were performed using the Number Cruncher Statistical System (Dr. Jerry L. Hintze, Kaysville, UT). The computer software generated significant P-values are indicated either over the graphs or in the figure legends.
RESULTS
Co-expression of IRS-1 and HBx causes pre-neoplastic changes in live
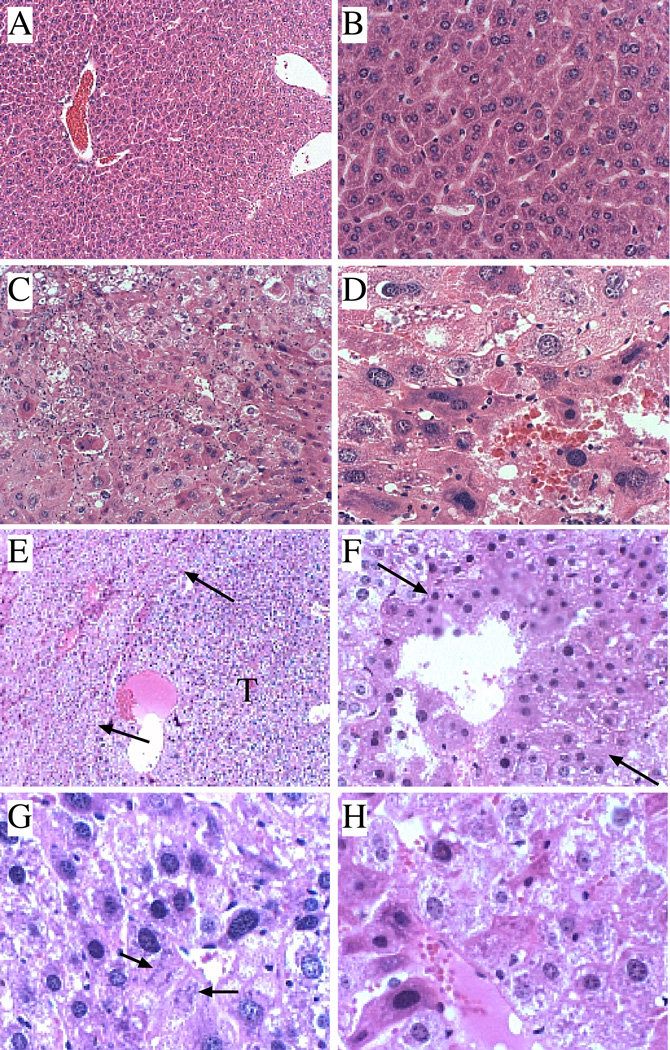
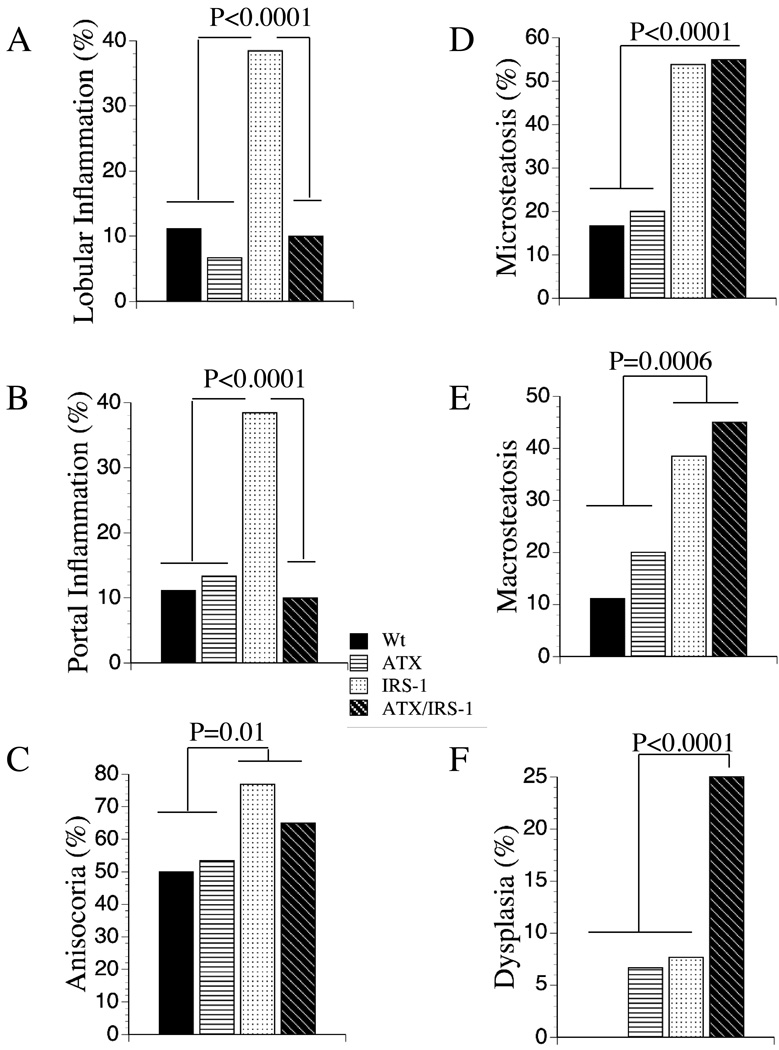
Histological sections of wildtype liver showed the expected ordered and cord-like architecture with well delineated portal and centrilobular areas, and only small foci of mild chronic inflammation, occasional hepatocytes with micro- or macrosteatosis, but no evidence of apoptosis, necrosis, or dysplasia (Figure 1A, 1B, Figure 2). Livers of ATX+ mice, which expressed HBx in liver at levels similar to that found in chronic hepadnaviral infection, had minimal histopathological change relative to wildtype controls as previously reported (29, 34, 35). Constitutive over-expression of the IRS-1 gene significantly increased the frequencies of prominent lobular and portal inflammation, anisocoria (nuclear pleomorphism), micro- and macrosteatosis, and focal dysplasia (8%) (Figure 2). In addition, foci of apoptosis or necrosis with Councilman bodies were readily detected, as previously reported (36). Livers from double-transgenic ATX+/IRS-1+ mice exhibited increased nuclear pleomorphism and hyperchromasia, micro- and macrosteatosis, foci of necrosis and apoptosis, and dysplasia (25%) (Figure 2). Like the IRS-1+ mice, apoptotic bodies and marked lobular disorganization were evident in the ATX+/IRS-1+ livers. Dysplastic foci in ATX+/IRS-1+ livers were often nodular, and in 3 cases, the lesions were histologically indistinguishable from HCC based on hypercellularity, nuclear pleomorphism, mitoses, necrosis, architectural disarray, and infiltrative pattern of growth (Figures 1C–1H). All 3 HCCs exhibited non-trabecular growth with embedded micro-acini, irregularly increased density of nuclei, high nuclear-cytoplasmic ratios, and scattered mitoses. Nodularity with compression of adjacent uninvolved liver parenchyma imparted an encapsulated appearance to the neoplasms (Figure 1E).
Figure 1.
Histopathological features of transgenic mouse livers. The histopathological features of Wt, ATX+, IRS-1+, and ATX+/IRS-1+ livers from mice 15–18 months old were examined. (A, B) Wt control livers exhibited well-organized lobular architecture (A) with minimal inflammation and relatively uniform hepatocellular morphology (B). (C, D) Livers from ATX+/IRS-1+ mice exhibited (C) focal areas of hepatocellular dysplasia juxtaposed to regions with microsteatosis. (D) Higher magnification images demonstrate hepatocellular pleomorphism, disarray, and nuclear hyperchromasia. (E–H) Dysplastic and HCC foci in ATX+/IRS-1+ livers. (E) Nodular, well-delineated dysplastic focus (right side) compressing adjacent liver tissue (arrows point to boundaries with liver tissue on left side). Note bluer hue of cells within the nodule due to increased cellularity and nuclear hyperchromasia. (F) Infiltrating HCC with nodular growth pattern. (G, H) Infiltrating HCC cells with disorganized architecture, nuclear hyperchromasia and atypical mitoses (G-arrows). Original magnifications: A-160x; B, C, F-320x; D, G, H-640x; E-100x.
Figure 2.
Frequency distribution of histopathological lesions in transgenic mouse livers. The histopathological features of Wt, ATX+, IRS-1+, and ATX+/IRS-1+ livers. The slides were analyzed under code for the presence or absence of (A) lobular inflammation, (B) portal inflammation, (C) anisocoria (nuclear pleomorphism), (D) microsteatosis, (E) macrosteatosis, and (F) dysplasia. Each group consisted of 24 mice. Graphs depict the percentage of cases/specimens exhibiting the specific lesion indicated on the ordinates. Significant P-values relative to other groups are indicated above the bars.
DNA damage, lipid peroxidation, DNA synthesis, and β-catenin accumulation in hepatocytes of ATX+/IRS-1+ mice
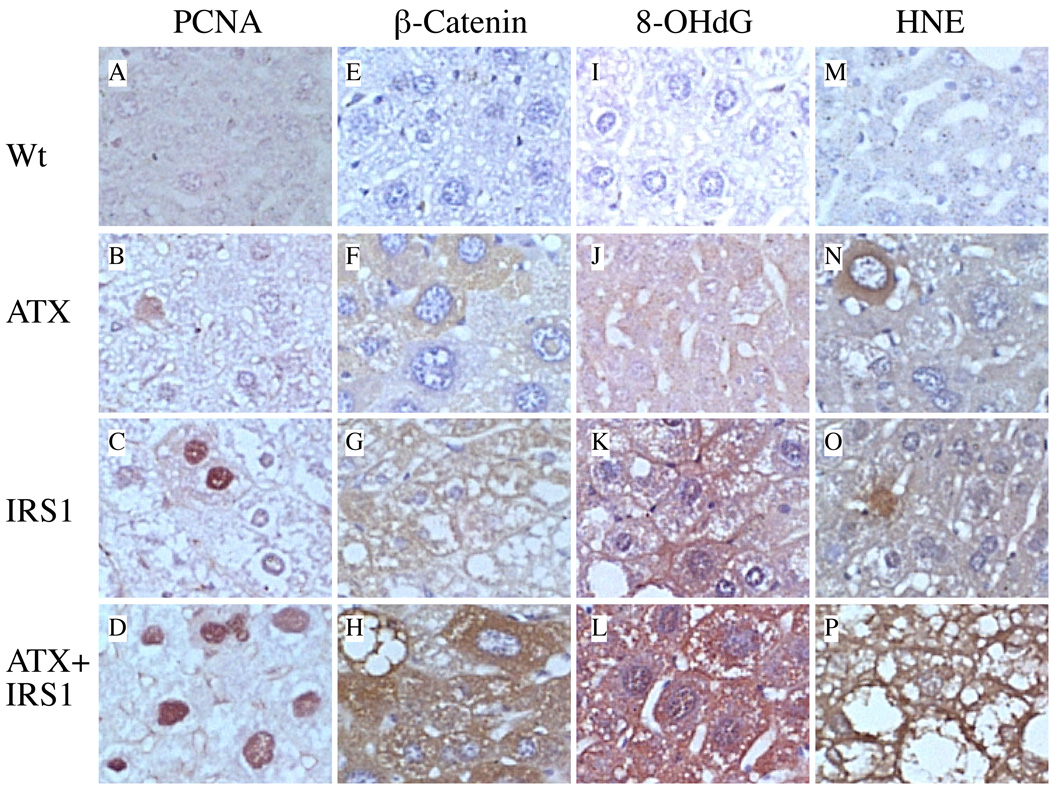
Given the increased frequencies and prominence of pleomorphism, steatosis, and dysplasia in the transgenic livers, it was important to determine if these abnormalities were associated with increased DNA damage, lipid peroxidation, PCNA, and/or β-catentin accumulation. 8-OHdG immunoreactivity was used as an index of DNA damage. HNE immunostaining was used to detect lipid peroxidation. PCNA was used as a measure of DNA synthesis and cell proliferation. β-catenin immunoreactivity was used to assess activation of the canonical Wnt-Frizzled pathway (14) (Figure 3). The wildtype livers exhibited minimal or no immunoreactivity for PCNA, β-catenin, 8-OHdG, or HNE (Figures 3A, 3E, 3I). ATX+ livers had occasional focal or low-level diffusely increased staining for PCNA, HNE, 8-OHdG, and β-catenin (Figures 3B, 3F, 3J). Livers from IRS-1+ mice had more conspicuously increased labeling for PCNA, β-catenin, and 8-OHdG relative to control and ATZ+ livers (Figures 3C, 3G, 3K). The double transgenic ATX+/IRS-1+ livers displayed the highest and most diffusely increased levels of PCNA, β-catenin, 8-OHdG, and HNE, and immunoreactivity in hepatocytes relative to all other groups (Figures 3D, 3H, 3L). Image analysis demonstrated that the ATX+/IRS-1+ livers had significantly higher mean percentages of PCNA+ nuclei (9.06 ± 1.08) relative to Wt controls (0.13 ± 0.03) (P<0.0001), whereas the percentages of PCNA+ nuclei in ATX+ (0.10 ± 0.07) and IRS-1+ (0.79 ± 0.32) were not significantly different from control by one-way ANOVA.
Figure 3.
Effects of ATX, IRS-1, and ATX+IRS-1 chronic over-expression on cell proliferation, β-catenin expression, DNA damage, and lipid peroxidation in the liver. (A, E, I) Wt control livers exhibited minimal or no immunoreactivity to PCNA, β-catenin, 8-OHdG, or HNE in hepatocytes. (B, F, J) ATX+ transgenic mouse livers had focally increased immunoreactivity to PCNA, β-catenin, 8-OHdG, or HNE in hepatocytes. (C, G, K) IRS-1+ transgenic mouse livers had more conspicuously increased immunoreactivity to PCNA, β-catenin, 8-OHdG, and HNE relative to ATX+ livers. (D, H, L) ATX+/IRS-1+ transgenic mouse livers had prominently increased immunoreactivity to PCNA, β-catenin, 8-OHdG, and HNE relative to all other groups. All imaged originally photographed at 400x magnification.
Effects of chronic expression of the IRS-1 and HBx transgenes on insulin and IGF signaling mechanisms
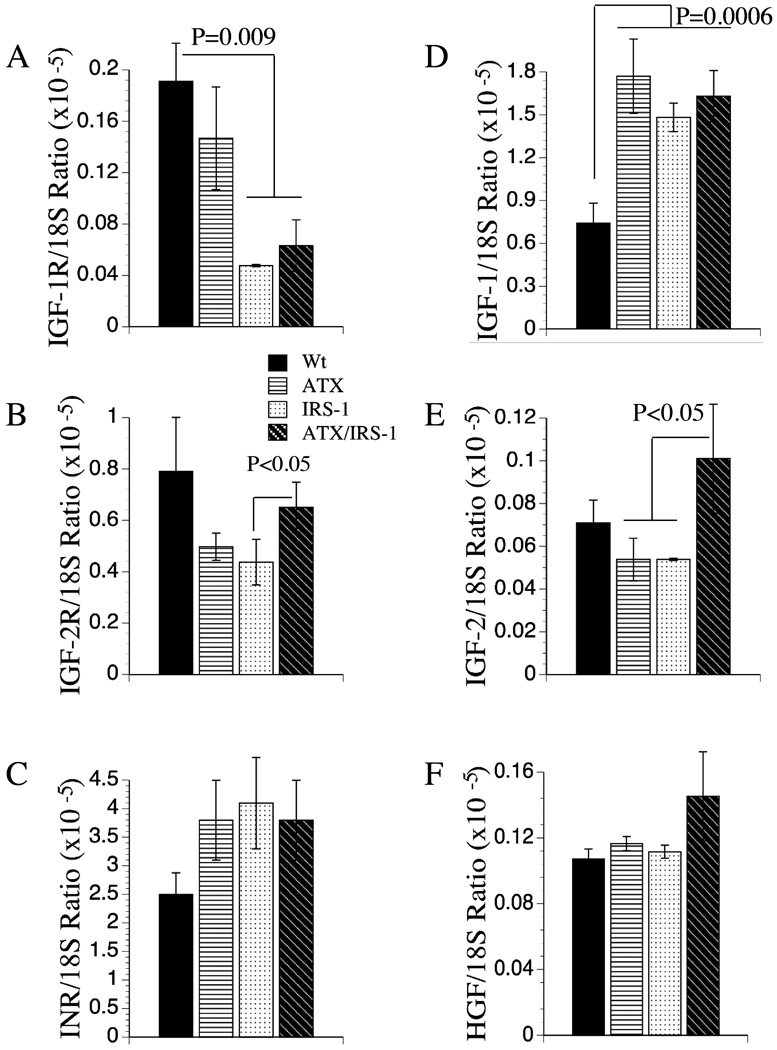
We characterized the effects of HBx and IRS-1 -expression in relation to insulin and IGF signaling mechanisms in liver because the majority of HCCs are associated with dysregulation of these pathways (7, 8, 12, 37). Therefore, we measured gene expression corresponding to insulin, IGF-I, and IGF-2 receptors (R) and polypeptide genes, and endogenous (murine) IRS-1 and IRS-2 by qRT-PCR. In addition, we measured hepatocyte growth factor (HGF) expression because HGF is a major growth factor in liver and it has been implicated in HCC development and/or progression (8). These studies demonstrated significant reductions in IGF-1R in IRS-1 and ATX+/IGS-1+ livers, and reduced expression of IGF-2R in IRS-1+ transgenic livers (Figures 4A–4B). In contrast, IGF-1 polypeptide gene expression was significantly increased in all 3 transgenic lines relative to control, and IGF-2 expression was selectively increased in ATX+/IGS-1+ double transgenic livers (Figures 4D, 4E). No significant inter-group differences were observed with respect to the insulin receptor or HGF expression in liver (Figures 4C, 4F).
Figure 4.
Effects of ATX, IRS-1, and ATX+IRS-1 expression in liver on mRNA levels of (A) IGF-1 receptor (R), (B) IGF-2R, (C) InsulinR (INR), (D) IGF-1, (E) IGF-2, and (F) hepatocyte growth factor (HGF). Graphs depict the mean ± S.E.M. levels of gene expression in 8–10 mice per group. Significant inter-group differences are indicated with P-values above the bars.
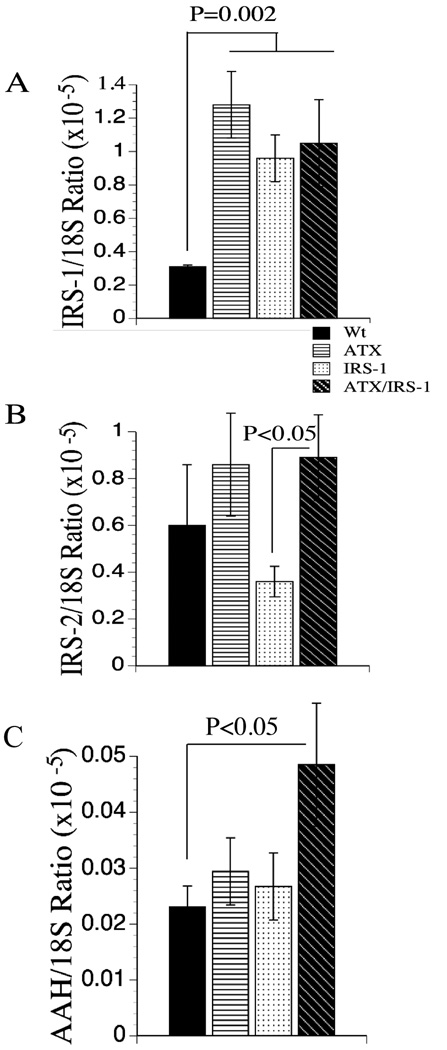
Endogenous (murine) IRS-1 mRNA levels were significantly increased in all transgenic relative to control livers (Figure 5A). Moreover, the levels were similarly increased, irrespective of hepatic expression of the IRS-1 transgene. Since the transgene was derived from the human cDNA (9), the murine and human mRNA transcripts were readily distinguished by qRT-PCR using gene-specific primers. IRS-2 expression levels were highly varied within each group. Nonetheless, the mean levels of IRS-2 mRNA were significantly reduced in IRS-1+ transgenic livers relative to all other groups (Figure 5B).
Figure 5.
Effects of ATX, IRS-1, and ATX+IRS-1 chronic over-expression on mRNA levels of IRS-1, IRS-2 and AAH. Gene expression corresponding to (A) IRS-1, (B) IRS-2, and (C) aspartyl-asparaginyl-β-hydroxylase (AAH) by qRT-PCR. Graphs depict the mean ± S.E.M. levels of gene expression in 8–10 mice per group. Significant P-values relative to other groups are indicated above the bars.
Effects of IRS-1 and HBx -expression on downstream target gene expression
Proliferating cell nuclear antigen (PCNA) was used as a marker of increased DNA synthesis. Aspartyl asparaginyl-β-hydroxylase (AAH) has a demonstrated role in cell motility and invasion (10, 38–40), is over-expressed in the majority of HCCs (10, 37). In addition, AAH is stimulated by insulin/IGF signaling through Erk MAPK (10, 37), and up-regulated with IRS-1 over-expression in liver. Therefore, we measured AAH mRNA levels to provide an index of premalignant phenotype associated with increased signaling through the insulin/IGF/IRS-1/MAPK cascade. The results demonstrated selectively increased AAH expression in only the ATX+/IRS-1+ double transgenic livers (Figure 5C). AAH is a downstream IN/IGF responsive gene that mediates hydroxylation of EGF-like domains of molecules such as Notch and its ligand Jagged, which have roles in cell motility and differentiation (37).
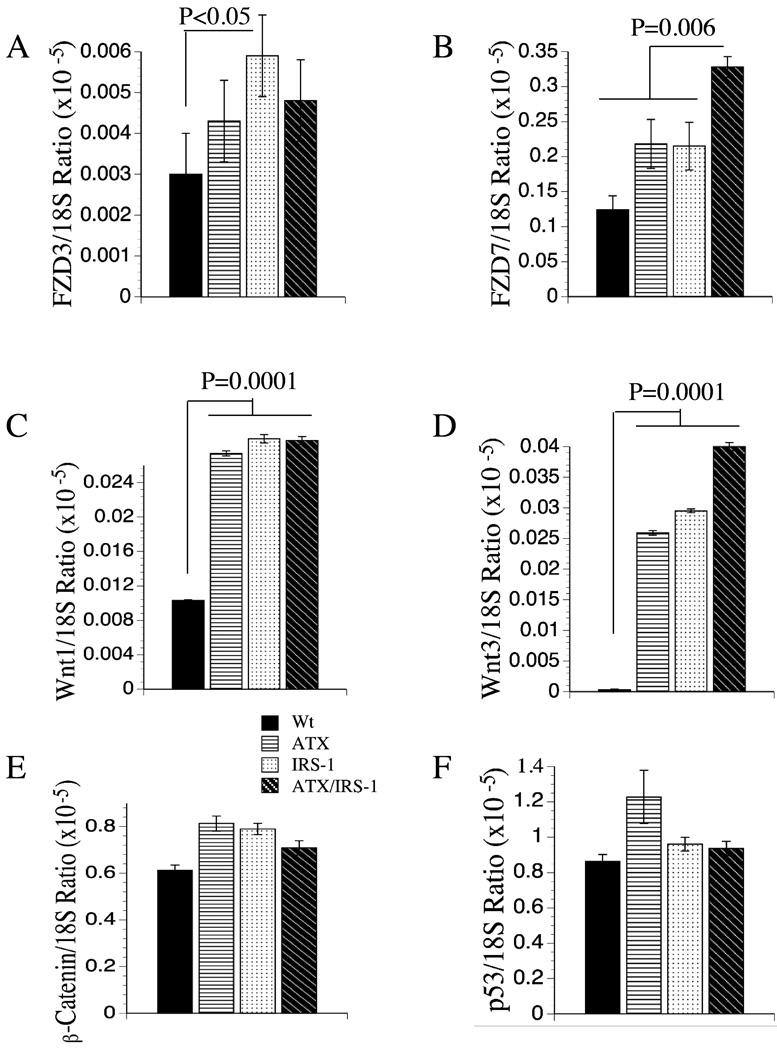
Constitutive expression of IRS-1 or HBx enhances Wnt-Frizzled signaling
We were interested in Wnt-1, Wnt-3, FZD3 and FZD7 since they have been shown to be overexpressed in human HCC with low or negligible expression in normal liver (19, 20, 41). Both Wnt-1 and Wnt-3 mRNA levels were significantly increased in livers of all three transgenic lines relative to wildtype controls (Figures 6C, 6D). In addition, FZD-3 was significantly increased in the IRS-1+ livers (Figure 6A), whereas FZD-7 was selectively increased in the ATX+/IRS-1+ double transgenic livers (Figure 6B). The significantly increased expression of Wnt ligands and/or FZD receptors in the transgenic mouse livers correlates with the increased levels/accumulations of β-catenin detected by immunohistochemical staining (Figure 3). However, the qRT-PCR studies demonstrated that the increased β-catenin immunoreactivity observed in transgenic livers was not mediated at the mRNA level since β-catenin mRNA expression was not significantly increased in the IRS-1+, ATX+, or ATX+/IRS-1+ livers (Figure 6E). Finally, we measured p53 mRNA levels to determine if its expression was altered in these transgenic lines, given its probable role in destabilizing the genome. Those studies demonstrated no significant inter-group differences with respect to p53 mRNA levels (Figure 6F).
Figure 6.
Effects of ATX, IRS-1, and ATX+IRS-1 constituative expression on mRNA levels of (A) Frizzled 3 (FZD3), (B) FZD7, (C) Wnt-1, (D) Wnt-3, (E) β-catenin, and (F) p53. Graphs depict the mean ± S.E.M. levels of gene expression in 8–10 mice per group. Significant inter-group differences are indicated with P-values above the bars.
DISCUSSION
This study demonstrates that while continued expression of HBx causes minimal histopathology and IRS-1 causes hepatocellular unrest with inflammation, apoptosis, and increased steatosis, dual chronic over-expression of these genes had a synergistic effect on the histopathology of the liver. Of particular note is that hepatic dysplasia was observed in 25% of the ATX+/IRS-1+ mice, while HCC occurred in 3 cases, whereas no examples of HCC have been observed in 400 and 250 livers from the single IRS-1 and HBx transgenic lines, respectively [(9, 25, 41) and Wands et al unpublished]. These findings confirm that consituative expression of HBx or IRS-1 alone is not sufficient to promote hepatocarcinogenesis, unless other factors, i.e. a second hit is present (25, 34, 42). These results correspond with epidemiological data showing that only a fraction of individuals with chronic HBV infection ever develop HCC. A potential gain from this research is that by utilizing molecular approaches, we may be able to identify distinct abnormalities that mark propensity toward HCC in the context of chronic HBV infection.
Increased propensity toward dysplasia in general was associated with hepatic steatosis, and evidence of increased DNA damage and lipid peroxidation, as demonstrated by immunohistochemical staining (Fig. 2 and Fig 3). It is well established that a key component in carcinogenesis is persistent DNA damage. In the context of these transgenic models, it appears that the micro- and macrosteatosis, reflecting injury and impaired energy metabolism, may serve as the template for lipid peroxidation, oxidative stress, and ultimately DNA damage. While increased DNA damage would most likely lead to further tissue injury, hepatocellular drop-out, inflammation, and fibrogenesis, the same process is responsible for the mutations resulting in activation of proto-oncogenes or down-regulation or silencing of onco-suppressor genes, which are key steps in HCC development.
Another common feature of HCC is constitutive activation of pro-growth signaling with particular involvement of two major signal transduction cascades: 1) insulin-IGF/IRS-1/Ras/Raf/Erk MAPK and 2) the Wnt/FZD/β-catenin pathway. Although previous studies established that constitutive over-expression of IRS-1 leads to increased pro-growth and pro-survival signaling via activation of the insulin/IGF/IRS-1/Erk MAPK pathway in liver (9, 12, 13) little was known about the effects of constitutive expression of HBx in this regard. The studies herein demonstrated expression of IRS-1, HBx, or IRS-1+HBx resulted in significantly increased levels of IGF-1 gene expression in liver; however, increased levels of PCNA reflecting hepatocyte proliferation was found only in the ATX+/IRS-1 mice. In addition, these animals were further distinguished by its selective increase in IGF-II mRNA as well as FZD7 and Wnt3 levels. This observation carries significance because IGF-II is the main growth factor that is dys-regulated in HCC, in both humans and experimental animal models (7, 8) and FZD7 and WNT3 are highly upregulated in these tumors as well (19, 20, 43).
IRS-1 and IRS-2 are over-expressed in the great majority, i.e. 90% or more of HCCs and HCC cell lines (37, 44), and constitutive over-expression of IRS-1 promotes growth (45) and transformation of NIH3T3 cells (13). However, in vivo, over-expression of IRS-1 alone does not cause HCC as demonstrated previously (9, 10) and in the present study. Consequences of increased IRS-1 and/or IRS-2 expression include increased signaling through growth and survival pathways such as Erk MAPK and PI3 kinase/Akt. In HCC, corresponding with the up-regulation of IRS-1 and IRS-2, both of these downstream signaling pathways are highly activated (9). It was of particular interest to find increased levels of murine IRS-1 in the IRS-1+, ATX+, and ATX+/IRS-1+ livers, as the mechanism is not entirely clear. Previous studies have established that the IRS-1 transgenic mice exhibit increased hepatocyte proliferation compared to wild type litter mates (9) but this phenomenon is confined to young mice (46). More important, the stimulatory effects on pathways that mediate growth was found exclusively in the ATX+/IRS-1+ transgenic line as measured by PCNA.
A downstream target gene of insulin-IGF-1/IRS-1/Erk MAPK signaling is AAH (37). AAH is an α-ketoglutarate dependent dioxygenase that hydroxylates aspartyl and asparaginyl residues in epidermal growth factor (EGF)-like domains of proteins such as Notch and Jagged, which have known roles in cell motility. AAH expression is increased in the majority of HCCs and HCC cell lines (10, 37, 47), and over-expression of AAH is sufficient to cause malignant transformation (40). The selectively increased levels of AAH mRNA in ATX+/IRS-1+ livers correlate with the significantly increased frequencies of hepatocyte proliferation, dysplasia and HCC development in that transgenic line.
In addition to increased signaling through the insulin/IGF/IRS-1 pathways a common feature of hepatocellular carcinoma is dysregulation and over-activation of the Wnt-Frizzled/β-catenin pathway. These responses have been associated with mutation of various components in the pathway, such as its effectors, β-catenin and axin, or gene over-expression (8, 14, 19). The Wnt-FZD pathway mediates cell growth and motility, thereby enabling expansion and spread of malignant neoplasms (17). Our analyses demonstrated significant up-regulation of Wnt-1 and Wnt-3 ligands in both the single and double transgenic lines relative to wildtype controls. However, the double transgenic mice were distinguished from the ATX+ and IRS-1+ mice by their selectively increased levels of FZD-7 expression, and higher levels of Wnt3, which is a common feature of HCCs and associated with pre-neoplastic change in liver (8, 19, 20, 43).
We also detected increased levels of β-catenin immunoreactivity principally in the cytoplasem (19, 43) of transgenic livers and much higher levels in the double transgenic compared with single transgenic lines by immunohistochemical staining. With respect to the potential molecular mechanisms involved, it is noteworthy that HBx and IRS-1 over-expression may both contribute to β-catenin accumulation in hepatocytes. In this context, the IRS-1 pathway cross-talks with the Wnt mediated cascade through GSK-3β (48). Since GSK-3β is a component of the β-catenin destruction complex, inhibition of its activity promotes β-catenin accumulation and translocation to the nucleus where it acts in concert with Tcf/Lef transcription factors to up-regulate Wnt responsive genes. Similarly, HBx expression has recently been shown to inhibit GSK-3β activity as well, which allows β-catenin to accumulate in the cytoplasm of HCC cells (30). Also participating in β-catenin accumulation in hepatocytes is the over-expression of Wnt3 and FZD7, which activates the canonical signaling cascade in HCC (20). Thus, there are at least three major mechanisms that may activate and amplify WNT/β-catenin signaling in the ATX+/IRS-1 mice.
Taken together, constitutive expression of IRS-1 and HBx promotes hepatocyte dysplasia and HCC. Activation of IN/IGF/IRS-1/MAPK and Wnt/β-catenin signaling cascades is necessary and sufficient to transform mammalian hepatocytes. Therefore, the double HBx/IRS-1 transgenic mouse model replicates many of the cellular and molecular abnormalities found in human HCC.
Acknowledgments
Supported by AA-02666, AA-11431, AA-12908, CA035711, CA095388 (BLS), and CA123544 from the National Institutes of Health
Footnotes
Presented in part at the AASLD Annual Meeting November 2–6, 2007.
There is no conflict of interest to disclose.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093–5107. doi: 10.1038/sj.onc.1206557. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Breuhahn K, Vreden S, Haddad R, Beckebaum S, Stippel D, Flemming P, Nussbaum T, et al. Molecular profiling of human hepatocellular carcinoma defines mutually exclusive interferon regulation and insulin-like growth factor II overexpression. Cancer Res. 2004;64:6058–6064. doi: 10.1158/0008-5472.CAN-04-0292. [DOI] [PubMed] [Google Scholar]
- 5.Sohda T, Yun K, Iwata K, Soejima H, Okumura M. Increased expression of insulin-like growth factor 2 in hepatocellular carcinoma is primarily regulated at the transcriptional level. Lab Invest. 1996;75:307–311. [PubMed] [Google Scholar]
- 6.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem Pharmacol. 2004;68:1003–1015. doi: 10.1016/j.bcp.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787–3800. doi: 10.1038/sj.onc.1209556. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Mohr L, Schmidt EV, Sugimachi K, Wands JR. Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26:598–604. doi: 10.1002/hep.510260310. [DOI] [PubMed] [Google Scholar]
- 10.de la Monte SM, Tamaki S, Cantarini MC, Ince N, Wiedmann M, Carter JJ, Lahousse SA, et al. Aspartyl-(asparaginyl)-beta-hydroxylase regulates hepatocellular carcinoma invasiveness. J Hepatol. 2006;44:971–983. doi: 10.1016/j.jhep.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka S, Wands JR. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Invest. 1996;98:2100–2108. doi: 10.1172/JCI119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka S, Wands JR. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta1-induced apoptosis. Cancer Res. 1996;56:3391–3394. [PubMed] [Google Scholar]
- 13.Ito T, Sasaki Y, Wands JR. Overexpression of human insulin receptor substrate 1 induces cellular transformation with activation of mitogen-activated protein kinases. Mol cell Biol. 1996;16:943–951. doi: 10.1128/mcb.16.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901–1915. doi: 10.2741/1933. [DOI] [PubMed] [Google Scholar]
- 15.Buendia MA. Genetics of hepatocellular carcinoma. Semin Cancer Biol. 2000;10:185–200. doi: 10.1006/scbi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 16.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 17.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 18.Prange W, Breuhahn K, Fischer F, Zilkens C, Pietsch T, Petmecky K, Eilers R, et al. Beta-catenin accumulation in the progression of human hepatocarcinogenesis correlates with loss of E-cadherin and accumulation of p53, but not with expression of conventional WNT-1 target genes. J Pathol. 2003;201:250–259. doi: 10.1002/path.1448. [DOI] [PubMed] [Google Scholar]
- 19.Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, et al. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang R, Yuen J, Yuen MF, Lai CL, Lee TK, Man K, Poon RT, et al. PIN1 overexpression and beta-catenin gene mutations are distinct oncogenic events in human hepatocellular carcinoma. Oncogene. 2004;23:4182–4186. doi: 10.1038/sj.onc.1207493. [DOI] [PubMed] [Google Scholar]
- 22.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 23.Cougot D, Neuveut C, Buendia MA. HBV induced carcinogenesis. J Clin Virol. 2005;34 Suppl 1:S75–S78. doi: 10.1016/s1386-6532(05)80014-9. [DOI] [PubMed] [Google Scholar]
- 24.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keasler VV, Lerat H, Madden CR, Finegold MJ, McGarvey MJ, Mohammed EM, Forbes SJ, et al. Increased liver pathology in hepatitis C virus transgenic mice expressing the hepatitis B virus X protein. Virology. 2006;347:466–475. doi: 10.1016/j.virol.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58–66. doi: 10.1016/j.lab.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Feitelson MA, Lee J. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007;252:157–170. doi: 10.1016/j.canlet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 29.Lee TH, Finegold MJ, Shen RF, DeMayo JL, Woo SL, Butel JS. Hepatitis B virus transactivator X protein is not tumorigenic in transgenic mice. J Virol. 1990;64:5939–5947. doi: 10.1128/jvi.64.12.5939-5947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology. 2004;39:1683–1693. doi: 10.1002/hep.20245. [DOI] [PubMed] [Google Scholar]
- 32.Klein NP, Schneider RJ. Activation of Src family kinases by hepatitis B virus HBx protein and coupled signaling to Ras. Mol Cell Biol. 1997;17:6427–6436. doi: 10.1128/mcb.17.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benn J, Schneider RJ. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci U S A. 1994;91:10350–10354. doi: 10.1073/pnas.91.22.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden CR, Finegold MJ, Slagle BL. Hepatitis B virus X protein acts as a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. J Virol. 2001;75:3851–3858. doi: 10.1128/JVI.75.8.3851-3858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madden CR, Finegold MJ, Slagle BL. Expression of hepatitis B virus X protein does not alter the accumulation of spontaneous mutations in transgenic mice. J Virol. 2000;74:5266–5272. doi: 10.1128/jvi.74.11.5266-5272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedmann M, Tamaki S, Silberman R, de la Monte SM, Cousens L, Wands JR. Constitutive over-expression of the insulin receptor substrate-1 causes functional up-regulation of Fas receptor. J Hepatol. 2003;38:803–810. doi: 10.1016/s0168-8278(03)00117-x. [DOI] [PubMed] [Google Scholar]
- 37.Cantarini MC, de la Monte SM, Pang M, Tong M, D'Errico A, Trevisani F, Wands JR. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–457. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- 38.Lavaissiere L, Jia S, Nishiyama M, de la Monte S, Stern AM, Wands JR, Friedman PA. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J Clin Invest. 1996;98:1313–1323. doi: 10.1172/JCI118918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda T, Sepe P, Lahousse S, Tamaki S, Enjoji M, Wands JR, de la Monte SM. Antisense oligodeoxynucleotides directed against aspartyl (asparaginyl) beta-hydroxylase suppress migration of cholangiocarcinoma cells. J Hepatol. 2003;38:615–622. doi: 10.1016/s0168-8278(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 40.Ince N, de la Monte SM, Wands JR. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase is associated with malignant transformation. Cancer Res. 2000;60:1261–1266. [PubMed] [Google Scholar]
- 41.Bengochea A, de Souza MM, Lefrancois L, Le Roux E, Galy O, Chemin I, Kim M, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slagle BL, Lee TH, Medina D, Finegold MJ, Butel JS. Increased sensitivity to the hepatocarcinogen diethylnitrosamine in transgenic mice carrying the hepatitis B virus X gene. Mol Carcinog. 1996;15:261–269. doi: 10.1002/(SICI)1098-2744(199604)15:4<261::AID-MC3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Merle P, Kim M, Herrmann M, Gupte A, Lefrancois L, Califano S, Trepo C, et al. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43:854–862. doi: 10.1016/j.jhep.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama M, Wands JR. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1992;183:280–285. doi: 10.1016/0006-291x(92)91640-c. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Ito T, Wands JR. Neoplastic transformation induced by insulin receptor substrate-1 overexpression requires an interaction with both Grb2 and Syp signaling molecules. J Biol Chem. 1996;271:14610–14616. doi: 10.1074/jbc.271.24.14610. [DOI] [PubMed] [Google Scholar]
- 46.Mohr L, Banerjee K, Kleinschmidt M, Bartolome Rodriguez MM, Wands JR. Transgenic overexpression of insulin receptor substrate 1 in hepatocytes enhances hepatocellular proliferation in young mice only. Hepatol Res. 2008 doi: 10.1111/j.1872-034X.2008.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda T, Taguchi K, Aishima S, Shimada M, Hintz D, Larusso N, Gores G, et al. Clinicopathological correlates of aspartyl (asparaginyl) beta-hydroxylase overexpression in cholangiocarcinoma. Cancer Detect Prev. 2004;28:313–318. doi: 10.1016/j.cdp.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Branda M, Wands JR. Signal transduction cascades and hepatitis B and C related hepatocellular carcinoma. Hepatology. 2006;43:891–902. doi: 10.1002/hep.21196. [DOI] [PubMed] [Google Scholar]