Abstract
Objective
To examine the effect of selective fetoscopic laser photocoagulation (SFLP) versus serial amnioreduction (AR) on perinatal mortality in severe twin-twin transfusion syndrome (TTTS).
Study Design
5-year multicenter prospective randomized controlled trial. The primary outcome variable was 30-day postnatal survival of donors and recipients.
Results
There is no statistically significant difference in 30-day postnatal survival between SFLP or AR treatment for donors at 55% (11/20) vs 55% (11/20) (p=1, OR=1, 95%CI=0.242 to 4.14) or recipients at 30% (6/20) vs 45% (9/20) (p=0.51, OR=1.88, 95%CI=0.44 to 8.64). There is no difference in 30-day survival of one or both twins on a per pregnancy basis between AR at 75% (15/20) and SFLP at 65% (13/20) (p=0.73, OR=1.62, 95%CI=0.34 to 8.09). Overall survival (newborns divided by the number of fetuses treated) is not statistically significant for AR at 60% (24/40) vs SFLP 45% (18/40) (p=0.18, OR=2.01, 95%CI=0.76 to 5.44). There is a statistically significant increase in fetal recipient mortality in the SFLP arm at 70% (14/20) versus the AR arm at 35% (7/20) (p=0.25, OR=5.31, 95%CI=1.19 to 27.6). This is offset by increased recipient neonatal mortality of 30% (6/20) in the AR arm. Echocardiographic abnormality in recipient twin Cardiovascular Profile Score is the most significant predictor of recipient mortality (p=0.055, OR=3.025/point) by logistic regression analysis.
Conclusions
The outcome of the trial does not conclusively determine whether AR or SFLP is a superior treatment modality. TTTS cardiomyopathy appears to be an important factor in recipient survival in TTTS.
Keywords: twin-twin transfusion syndrome, amnioreduction, selective fetoscopic laser photocoagulation
Introduction
Twin-twin transfusion syndrome (TTTS) is the single most common serious complication of monochorionic twin gestations accounting for 10 to 17% of all perinatal mortality1,2. The natural history of untreated TTTS is well established with mortality rates up to 90%1,3,4. The incidence of this serious complication has been increasing in parallel with the increasing incidence of multiple gestations5.
Numerous treatment options for TTTS have been reported, including amnioreduction (AR)6–12, intertwin septostomy13–16, non-selective fetoscopic laser17–20 selective fetoscopic laser photocoagulation (SFLP)21–28, and cord coagulation29–32, all of which have improved survival rates compared to the untreated natural history of TTTS. It has been controversial which of these treatment options would provide the best outcome in specific cases of TTTS. AR has been associated with an average survival of 50%33, with large international registries reporting 60 to 65% survival34,35. Saade et al. suggested the reason AR works is because it inadvertently creates an intertwin septostomy36. This inadvertent septostomy has been suggested to account for the “single amnioreduction paradox” in which TTTS appears to stop in up to 20% of patients after only a single AR36. Consistent with this view, a prospective randomized trial comparing AR to septostomy found the same 65% survival in each arm of the study16. The problem with septostomy has been the risk of creating a mono-amniotic gestation and the attendant risk of cord entanglement with double fetal demise37. Cord coagulation, either by fetoscopic bipolar cautery or radiofrequency ablation, at best can only achieve a 50% survival and is unacceptable to many parents as it necessitates the sacrifice of one fetus.
Fetoscopic laser photocoagulation for TTTS was first described by De Lia et al., as a means of functionally converting a monochorionic placenta to a dichorionic one by occluding vascular connections between the twins normally present in monochorionic placentas17,18. While these vascular connections are not the cause of TTTS, they are a necessary prerequisite for the syndrome. This technique as originally described, and still in use in some centers, is non-selective in nature, meaning all vessels found to cross the intertwin membrane were photocoagulated17,18. Survival with this approach is reported to be 53 to 56%17–18, comparable to survival rates observed with AR. An intriguing finding which stimulated interest in this approach was the observation that newborn survivors treated by fetoscopic laser appeared to have a lower incidence of head ultrasound or MRI abnormalities (5–7% vs. 14–25%)5,17–20. Subsequently, Quintero et al., recognizing there is no anatomic relationship between the position of the intertwin membrane and the vascular equator, described a selective technique of fetoscopic laser photocoagulation in which only vessels demonstrated to be communicating between the two twins are treated21. This selective fetoscopic laser technique is thought to preserve non-communicating vessels and has been associated with survival rates of 62 to 77%21–28.
In order to eliminate the up to 20% of patients with TTTS that may respond to a single AR, the so-called single “amnioreduction paradox”, and to compare the impact on 30-day neonatal survival in patients at the most severe end of the spectrum of TTTS, we conducted a prospective randomized clinical trial on subjects with TTTS presenting prior to 22 weeks’ gestation, stages II, III or IV, who failed to respond to AR, and randomized either to SFLP or serial AR.
Materials and Methods
Study Design
This trial was a 5-year multicenter prospective randomized controlled trial to examine the effect of SFLP compared to serial AR on perinatal mortality in severe twin-twin transfusion syndrome. Patients meeting entry criteria (see Table 1) and consenting to enter the trial were randomized to one of the two treatment arms with equal likelihood.
Primary and Secondary Outcomes
The primary outcome variable to be assessed in this trial comparing AR to SFLP is the 30-day neonatal survival of donors and recipients. In addition, secondary outcome variables to be assessed include the number of pregnancies in each arm with one or more twins surviving; and the overall survival to 30 days (total number of twins surviving to 30 days divided by the total number of fetuses treated).
Demographics
Subjects were pregnant women 18 years or older, diagnosed with TTTS at less than 22 weeks’ gestation, and randomized and treated prior to 24 weeks’ gestation. The subjects must have had no contraindication to general anesthesia or abdominal surgery, history of preterm labor, or uterine anomalies (see Table 1).
Sample Size Considerations
Subjects were assigned to AR or SFLP with equal probability (1:1 odds). The plan consisted of three interim analyses at accrual of 1/4, 2/4, and 3/4 of the planned sample size. The criteria for stopping were based on the O’Brien-Fleming stopping rules38. The primary null hypothesis to be tested was that the survival rates of donor/recipient twins at 30 days after birth and no treatment failure are the same between the two treatment groups. With a two-sided Type I error of 0.025 for each of the two primary outcomes and a power of 0.80, it would take 146 patients to detect an increase from a 50% survival rate with AR to a 75% survival rate with SFLP. These estimates were based on pooled reports of overall survival to delivery from published series of patients treated by AR or SFLP5,12,17–28.
Study Procedures
Pre Screening Criteria
Prior to randomization, each subject underwent a diagnostic evaluation which included ultrasound to confirm monochorionic diamniotic gestation with like sex twins, single placental mass, and a thin intertwin membrane. In addition, there had to be a “stuck” donor twin defined as a twin with a deepest vertical pocket of ≤ 2 cm and a recipient co-twin with polyhydramnios defined with a deepest vertical pocket of > 8 cm, with or without Doppler or echocardiographic changes, and presenting prior to 22 weeks’ gestation. The empty bladder in the donor twin should not be seen to fill during the ultrasound examination (Quintero stage II) unless there are Doppler velocimetry changes in umbilical artery, umbilical vein, or ductus venosus waveforms (Quintero Stage III). In addition, structural anomalies and CNS abnormalities were specifically excluded. A transvaginal ultrasound was performed after the qualifying AR to exclude foreshortened cervix and length < 2.0 cm excluded the mother from the trial. To ensure uniformity, determination of projected gestational age was based on the method used by the NICHD-sponsored Maternal Fetal Medicine Units Network39.
A single diagnostic and therapeutic qualifying amniocentesis was performed on the polyhydramniotic sac with sufficient fluid removed to reduce the deepest vertical pocket to < 5 cm. This initial qualifying AR was performed by a participating investigator per a standard protocol. A follow-up ultrasound was performed within one hour of the procedure to detect evidence of inadvertent microseptostomy which, if detected, excluded the patient from eligibility for randomization.
Final Screening
If the follow-up ultrasound obtained 12 to 24 hours after the qualifying amniocentesis visualized a decompressed bladder in the donor twin which is not seen to fill during the examination and a deepest vertical pocket of < 2 cm in the donor sac, the subject was considered a candidate for entry into the trial and offered randomization. If the donor deepest vertical pocket was > 2 cm or the bladder was not decompressed and/or seen to fill during the follow-up ultrasound examination 12 to 24 hours later, the subject was considered a responder and not a candidate for randomization (see Table 1).
Pre-Treatment Studies
Prior to initiation of treatment, baseline studies including ultrasound, fetal echocardiogram, and MRI were obtained and recorded on blinded tapes/films and sent to the Data Coordinating Center (DCC). In the SFLP arm, maternal screening for anesthesia by an anesthesiologist and pre-operative CXR, EKG, and CBC were obtained. An ultrasound and echocardiogram were obtained on each fetus at least weekly. Each of these surveillance tests had a checklist of data to be obtained with the examination.
Randomization Plan
Randomization was centralized via faxed case report forms and stratified by cluster and gestational age group. AR was performed at the local clinic. SFLP subjects were sent to either Cincinnati, CHOP, or UCSF based on geographical location.
Selective Fetoscopic Laser Photocoagulation (SFLP)
Mothers randomized to the SFLP arm of the study underwent the procedure under epidural anesthesia and intravenous sedation for comfort. Although most of the procedures could be performed by a single percutaneous port, some cases with an anterior placenta required a mini-laparotomy to expose the surface of the uterus. A 4 mm incision was made in the skin to allow ultrasound-guided placement of a 3.3 mm 3-port fetoscope that has a lens port as well as two working ports; one for the 600μ laser endostat and the other for the Level I for rapid infusion of physiologic saline, if needed, to clear the amniotic cavity. The chorionic plate of the placenta was mapped three times: 1) trace every vessel that leaves the placental cord insertion of the recipient twin to its termination in a cotyledon or in an anastomosis to a vessel crossing to the donor twin’s umbilical cord insertion, 2) intraoperatively mark and record location of all connecting vessels for subsequent photocoagulation, and 3) verify that no connecting vessels were missed. All procedures were digitally recorded and a permanent record of vascular anatomy was made for each procedure. All recorded SFLP procedures were independently externally reviewed to ensure quality control. The vessels were photocoagulated using 60 watts power with the endostat positioned at 1 cm from the surface of the vessel. The amniotic fluid volume was reduced such that the deepest vertical pocket was < 5 cm. Antibiotics were instilled into the amniotic cavity prior to trocar removal.
During a 9-month period, the trial was placed on pause when the principal investigator moved from Philadelphia to Cincinnati. During this pause, the NIH and DSMB requested that we incorporate the use of the new Storz remote head fetoscope into the trial as standard equipment at all 3 laser centers. This required IRB approval at all centers because this device at the time was not FDA approved. Up to that time, all three centers were using the Storz 3-3 mm fetoscope with standard head for camera attachments.
Aggressive Amnioreduction (AR)
Mothers who were randomized to the aggressive serial AR arm of the study underwent ultrasound examination on a Monday, Wednesday, Friday schedule. AR was performed whenever the recipient twin sac deepest vertical pocket was ≥ 8.0 cm. The volume of fluid removed during these procedures was sufficient to reduce the deepest vertical pocket to ≤ 5 cm. The AR procedure was standardized by protocol to require use of a 20-gauge needle and vacuum suction bottle. The patient was assessed for an inadvertent microseptostomy by checking the deepest vertical pocket around the donor within 1 hour of the procedure. All fetuses delivered as clinically indicated.
Definition of Treatment Failure
A failure of therapy was defined as the progression of non-immune hydrops or cardiac failure with imminent fetal demise despite therapy in one of the two treatment arms. A fetus would have to meet at least two of the four following criteria in order to meet the definition of cardiac failure despite therapy: 1) development or progression of severe AV valve (mitral or tricuspid) regurgitation, 2) reversal of diastolic flow in the ductus venosus, 3) reversal of diastolic flow in the umbilical artery of the recipient twin, or 4) development or progression of severe biventricular dysfunction. In the event that a subject from either arm of the study was declared a treatment failure, she was offered the treatment of her choice outside the trial. Being declared a treatment failure was treated statistically as equivalent to a death for the purposes of proving endpoint analysis.
Statistical Considerations
Interim Analysis and Early Trial Closure
The Data Safety and Monitoring Board (DSMB) reviewed the trial for efficacy for the primary outcome at the interim analyses performed after 38 randomized patients were evaluable. Adverse events and the primary outcome of perinatal mortality were the only outcome variables considered when deciding to close the trial.
Primary Analysis
The primary outcome variables, survival of the donor and recipient twins at 30 days after birth, were analyzed using a logistic regression model with treatment (AR, SFLP) as one of a list of predictor covariates. Other covariates included gestational age at diagnosis, Quintero stage (II, III, or IV), marital status, cohort, transportation for SFLP, and an echocardiographic score based on the Cardiovascular Profile Score (CVPS) described by Huhta42. Adjustments for pairing and clustering, as is done in studies of paired organs such as eyes and kidneys, were unnecessary because the donor and recipient results were analyzed separately.
The primary null hypothesis to be tested is that there is no difference in success rates between pregnancies randomized to AR or SFLP treatment. The primary alternative hypothesis is that SFLP is superior to serial AR. For power analysis we assumed values of 50% success rate with AR and a 75% success rate with SFLP, a difference considered significant in a clinical sense.
Results
The study was stopped early, after 42 subjects were randomized, at the request of the investigators. Referring physicians were increasingly unwilling to refer eligible subjects for evaluation to participating centers in which SFLP was only available through randomization in the trial. At the same time that the investigators’ meeting was held to decide to stop the trial, the Trial Oversight Committee, charged with evaluating all adverse events and serious adverse events, detected a statistical trend in adverse outcome affecting the recipient twin in one treatment arm. The Trial Oversight Committee was blinded to the treatment received in each arm. A recommendation was made to the DSMB that the trial be stopped to allow biostatistical analysis of this adverse trend. The DSMB concurred with the decision to stop the trial.
Enrollment
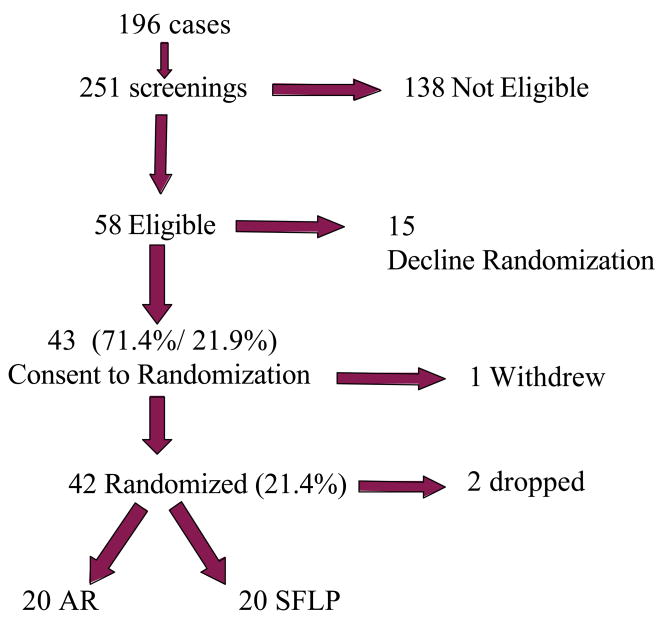
196 cases (where each case consisted of three individuals; mother, donor, and recipient) were screened a total of 251 times or an average of 1.28 screens per case (see Figure 1). Patients in early stages of TTTS were often screened on more than one occasion until they either met criteria or were ineligible. Of the 196 cases, 58 (29.6%) were deemed eligible by meeting all inclusion criteria (54 cases) or passing via protocol deviation [4 cases had a visible bladder (stage I) but had Doppler changes upstaging them to stage III]. Twelve patients refused a qualifying AR. Of the 46 patients who consented to an AR, only 4/46 (8.7%) were ineligible because of a positive therapeutic response to the AR. Of the 4, 2 had evidence of amniotic fluid around the donor within 1 hour of the AR, consistent with septostomy. Of the 58 cases eligible, 43 (21.9% of screened, 74.1% of eligible) consented to enrollment. Only 42 cases (21.4% of screened) actually got randomized to a treatment arm. One patient dropped out of each arm leaving 20 in the AR arm and 20 in the SFLP arm. Primary analyses were based on an Intent-to-Treat principle where the case is assigned to the treatment strategy whether or not it actually received the treatment.
Figure 1.
196 subjects were screened a total of 251 times with 138 subjects not found to be eligible. Of the 58 found to be eligible for the trial, 15 declined randomization. Of the 58 eligible, 43 consented to randomization but 1 withdrew. 21 subjects were randomized to each arm of the study and a single subject dropped out of each arm.
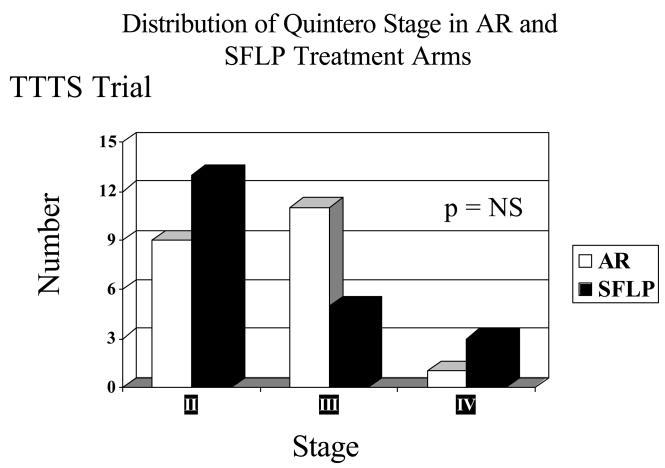
There is no statistically significant difference in stage distribution between AR and SFLP arms using the Quintero staging system (see Figure 2). The mean gestational age at delivery is 30 and 4.7/7 weeks for patients treated by SFLP and 30 and 2/7 weeks for AR. These differences are not statistically significant. Excluding loss due to PPROM within 7 days of procedure, the mean gestational age at delivery is 32 and 3.8/7 weeks for patients in the SFLP arm and 31 and 2/7 weeks for patients in the AR arm. These differences are not statistically significant.
Figure 2.
The distribution by stage using the Quintero staging system is equally distributed among stages II, III, and IV in both the amnioreduction (AR) and selective fetoscopic laser photocoagulation (SFLP) groups. There were no stage I patients in the trial. There is no statistically significant difference in stage distribution between amnioreduction (AR) and selective fetoscopic laser photocoagulation (SFLP).
Primary Outcome by Treatment
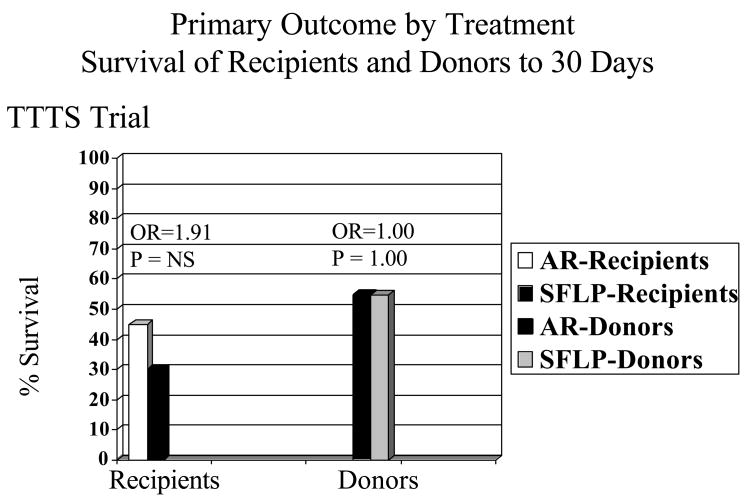
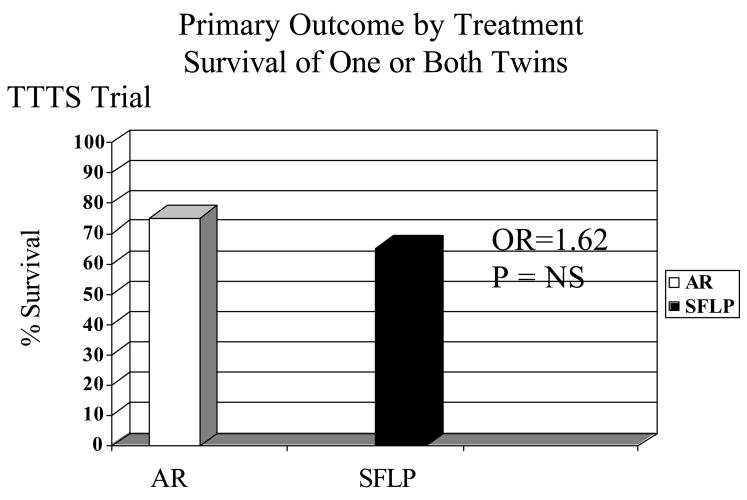
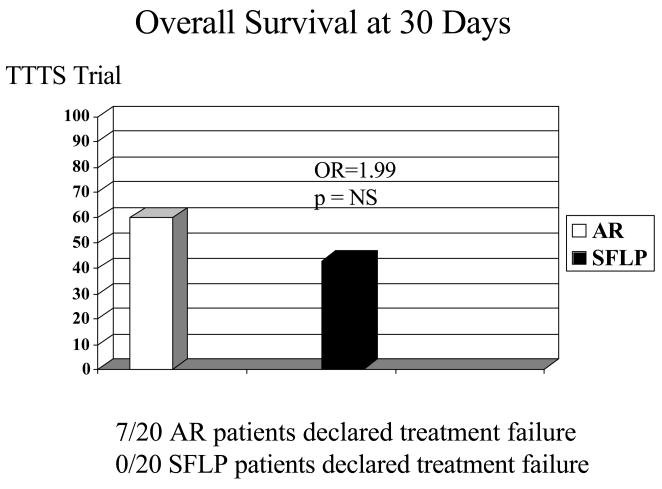
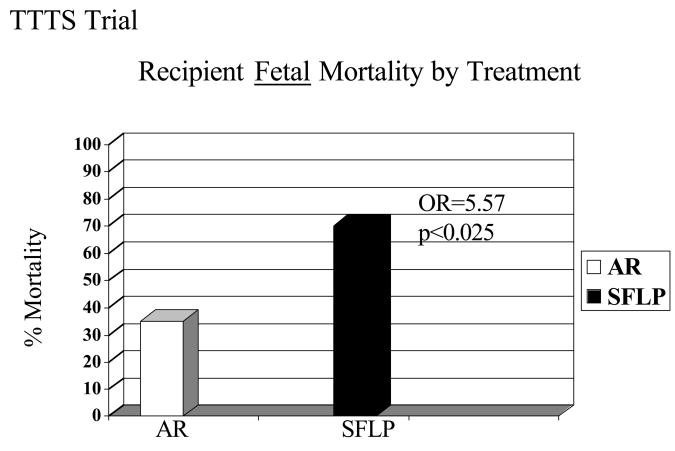
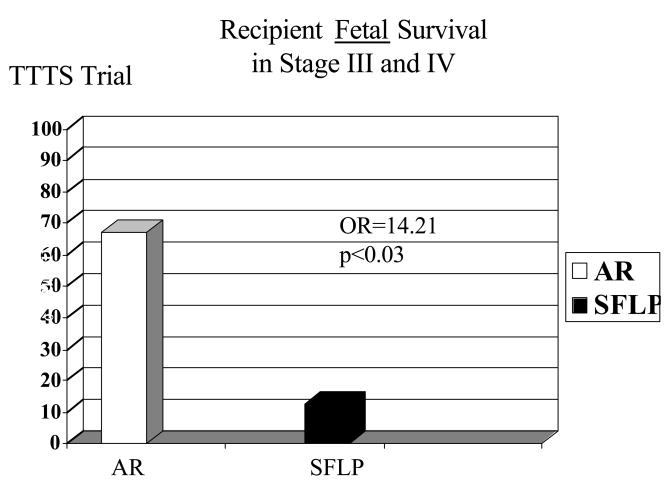
There is no significant difference in the primary outcome of 30-day postnatal survival between SFLP or AR treatment for donors in the AR arm (55%, 11/20) versus the SFLP arm (55%, 11/20) (p=1, OR=1.00, 95%CI=0.24 to 4.14) or recipients in the AR arm (45%, 9/20) versus the SFLP arm (30%, 6/20) (p=0.51, OR=1.88, 95%CI=0.44 to 8.64) (see Figure 3). There is no significant difference in 30-day survival of one or both twins on a per pregnancy basis between AR 75% (15/20) and SFLP 65% (13/20) (p=0.73, OR=1.62, 95%CI=0.34 to 8.09) (see Figure 4). There is no significant difference in overall 30-day survival (newborns divided by fetuses treated) between AR and SFLP at 60% (24/40) vs. 45% (18/40) (p=0.18, OR=2.01, 95%CI=0.76 to 5.44) (see Figure 5). There is a statistically significant increase in fetal mortality among recipient twins treated by SFLP 70% (14/20) versus AR 35% (7/20) (p=0.03, OR=5.31, 95%CI=1.19 to 27.6) (see Figure 6). Within stages III and IV there is a significant difference in 30-day survival for AR of 67% versus SFLP of 12.5% (p,0.03, OR=14.21) (see Figure 7). There are more treatment failures in the AR treatment arm (7) versus the SFLP arm (0) (p=0.0083, OR=Infinity), resulting in no significant overall differences between the treatment arms at 30 days (see Figure 5). In 7 of 7 AR cases meeting criteria for treatment failure, it was due to recipient findings of hydrops (4/7) or development or progression of severe AV valve regurgitation and development or progression of severe biventricular dysfunction despite AR treatment (3/7). Neonatal death occurred in 6 of the 7 recipients in the AR arm meeting criteria for treatment failure and offsetting the increased fetal recipient mortality in the SFLP arm.
Figure 3.
The survival to 30 days of age when broken down by recipient and donor shows no significant difference in survival between AR) or SFLP groups for either recipients or donors.
Figure 4.
There is no statistically significant difference in survival of 1 or both twins to 30 days of life.
Figure 5.
Despite the differences observed in recipient fetal mortality, there were no statistically significant differences in overall survival to 30 days of age. The reason was that while there were more fetal recipient deaths in the SFLP arm than in the AR arm of the study, more recipients were declared treatment failures in the AR arm (7 of 20), versus the SFLP arm (0 of 20). Neonatal death occurred in 6 of the 7 cases declared treatment failures.
Figure 6.
There is a statistically significantly increased fetal mortality in recipients treated by SFLP (70%) versus those treated by AR (35%, p < 0.025, OR=5.31, 95% CI= 1.19 to 27.6)
Figure 7.
The difference in fetal recipient survival is more pronounced among recipients in stages III and IV that were treated by AR (63%) versus SFLP (12.5%, p < 0.03). Despite these differences in fetal recipient survival, there is no difference in survival at 30 days because 7/20 recipients in the AR arm met criteria for treatment failure (in which 6 of the 7 were neonatal deaths) while 0/20 in the SFLP arm did so.
Efficacy Adjusted for Covariates
A straightforward logistic regression of recipient success based on treatment, after adjusting for all other covariates of interest [stage, marital status, echocardiographic score, long-distance transportation, gestational age, and cohort (pre/post-pause for equipment changes)], shows a non-significant difference between SFLP and AR in the full model, but with SFLP having a worse outcome which is lost during backward variable selection. For donors there are only negligible differences between treatment arms in the full model which is also lost during backward variable selection.
The most predictive models for recipients used only their echocardiographic score (OR=3.025/point, p=0.0552). The score was based on the 10-point Cardiovascular Prognostic Score (CVPS) described by Huhta42 using as many parameters as possible with the measures and observations available from ultrasound and echocardiograms. Missing values were replaced with means in computing the score. For donors, the most predictive models were stage (OR=0.446/stage, p=0.1249) and gestational age (OR=1.052/day, p=0.0987). In addition, fitting models including each covariate alone with treatment in the predictor list had similar results.
Additional Approaches
Several alternative analysis approaches were explored because of the small sample size and to further examine the roles of the covariates. The use of alternative analysis methods also helped to show if results are robust to variations in approach. With conditional logistic regression, the results show that echocardiographic score is a useful predictor for recipients (OR=2.84, p=0.0576) in the same direction as before. No other covariates or treatment are significant using this approach. We also created new treatment categorizations to more accurately reflect the AR cases which experienced treatment failure and went on to receive other treatments. Similarly, we examined different definitions of outcome such as survival to thirty days ignoring treatment failure. One situation has strong and interesting results: when the outcome is survival to birth, the recipient twins are under a significant disadvantage under SFLP (OR=0.124, p=0.0084) along with a slight negative effect for later gestational age (OR=0.95, p=0.1428). The reason that it does not show up in the primary analysis is that recipient twins under AR tend to make up for the difference with treatment failures instead. Analyses using variations on the intent-to-treat principle such as only including subjects who actually received the assigned treatment or by treatment actually received are comparable.
Finally, one question that cannot be adequately addressed by the analyses above is the influence the status of the co-twin has on the outcome. A time-to-event, proportional hazards (a.k.a. Cox) model was fit to the data with time-varying covariates, one of them being the status of the co-twin. The hypothesis is that the demise of the co-twin might reduce the competition and improve the chances of success. As it turns out, however, treatment failure or death of the co-twin means a poorer prognosis for the twin (Hazard Ratio = 32.6 for recipients and 12.88 for donors, p = 0.0001 and 0.0003, respectively). This result argues against the removal of competition and rather towards a sharing of predicament. In other words, if the co-twin is in trouble, both are in trouble.
Safety Analyses
In this study, adverse events and serious adverse events were common and often closely related to the primary outcome which is death and/or treatment failure. Every adverse event whether serious or not, related or not, anticipated or not, was reported to the Trial Oversight Committee and the IRBs. Due to the broad definition of adverse events these were frequent in both arms of the study. There were no maternal deaths and no serious maternal adverse events related to the operative procedure, even in the 7 cases in which a minilaparotomy was used to expose the surface of the uterus.
No patient in the study had a chorioamniotic separation as a result of the qualifying AR. In one case there was uterine bleeding occurring during an SFLP procedure (4.7%) which did not require maternal transfusion but did compromise visualization and necessitated the procedure being stopped. There was a single case of spinal headache (2.3%) resulting from placement of an epidural catheter which responded to blood patch. Maternal hospitalization at any time during the remainder of the pregnancy required for preterm labor, short cervix, preterm premature rupture of membranes (PPROM), or to monitor for fetal growth occurred in 9.5% of cases in both the AR and SFLP arms of the study. The specific incidence of PPROM occurring prior to 28 weeks’ gestation was 0% in the AR arm and 4.8% in the SFLP arm. The incidence of preterm labor requiring tocolysis was 4.8% in the AR arm and 0 in the SFLP arm. The incidence of delivery prior to 28 weeks’ gestation was 4.8% (n=1) in the AR arm and 9.5% (n=2) in the SFLP arm. These differences are not statistically significant.
Comments
The results of this trial show no statistically significant difference in overall neonatal survival to 30 days of life or neonatal survival of one or both twins in the same pregnancy in cases of severe TTTS treated by either AR or SFLP. Despite these overall results, there is a statistically significantly worse fetal survival observed among recipient twins in pregnancies treated by SFLP compared to those treated by AR. This apparent conundrum can be accounted for by recipient fetal losses in the SFLP arm being offset by an increase in treatment failures among recipient subjects in the AR arm. These results suggest that, in these highly selected cases of severe TTTS, neither treatment is superior to the other. Once TTTS reaches this severity, the mortality among recipients will be considerable, but the losses may occur at different times depending on treatment. The impact of TTTS severity on fetal survival is further supported by the significantly worse fetal survival among recipient twins in stages III and IV compared to stage II. One of the strongest predictors of recipient demise is echocardiographic evidence of TTTS cardiomyopathy. The losses of fetal recipients treated by SFLP usually occur within 24 hours of the procedure. In contrast, the recipients treated by AR are not lost following the procedure, but there is progressive TTTS cardiomyopathy as reflected by more recipients in the AR arm meeting criteria to be declared treatment failures. In every case, it was due to findings in the recipient twin which met criteria for treatment failure. Taken together these data suggest a disproportionate impact of TTTS cardiomyopathy on recipient survival in advanced stages of TTTS no matter what treatment they receive.
The Quintero staging system was found to be an independent predictor of outcome among recipient twins with worse survival in recipients in stages III and IV compared to stage II. This was found to be the case despite the Quintero staging system being heavily weighted toward the impact of TTTS on the donor. Stage II is based on absence of visualization of urine in the bladder in the donor. In stage III, the most commonly observed abnormality in critical Doppler waveforms is absent end diastolic flow in the umbilical artery of the donor twin. Other critical Doppler changes which occur in the recipient twin in TTTS such as the loss or reversal of the a wave in the ductus venosus, pulsatile umbilical vein, and absent or reversed diastolic flow in the recipient twin’s umbilical artery are late changes only observed in more advanced cases of TTTS. Similarly hydrops, required for stage IV, is usually seen in the recipient twin which was the case in each of our stage IV subjects. TTTS cardiomyopathy, which specifically affects the recipient twin, appears to be one of the most important contributing factors in recipient mortality in advanced cases of TTTS. The results of this trial suggest that in advanced cases of TTTS, the recipient survival may be compromised no matter what treatment the patient receives. Consistent with these findings in the trial, Shah et al. recently reported the use of the cardiovascular prognostic score (CVPS) as an indicator of the severity of TTTS cardiomyopathy demonstrating a negative impact on recipient survival41.
Despite differences in patient selection (22 vs 26 weeks’ gestation) and response to qualifying AR versus primary therapy, the NIH-sponsored trial has comparable overall survival of one or both twins to those reported in the Eurofoetus trial20. The survival with AR is significantly better in the NIH trial (60%) than in the Eurofoetus trial (41%) and consistent with single institution series6–12, a prospective randomized trial15,16, and registries (60–65%)34,35. This may be due to the standardized aggressive protocol used for serial AR in the NIH trial. Conversely, the survival in the NIH trial for SFLP-treated TTTS is significantly less than reported previously using this technique both in single institution series as well as in the Eurofoetus trial19–28. One possible explanation for the poorer survival observed in the SFLP arm could be a lack of proficiency of the surgeons performing the SFLP in the three laser centers in the trial. However, every SFLP procedure was recorded and independently reviewed by fetal surgeons not participating in the trial with significant experience with the SFLP technique. This review independently confirmed the adequacy of the procedures. Minor differences in technique were identified among the laser centers in which one center frequently used a mini-laparotomy to access the uterus while the other two centers used an entirely percutaneous approach. The placental mapping and the SFLP technique used were the same in all three centers. The results in these centers with SFLP, before and after the NIH trial, support the view that problems with technical proficiency do not account for differential fetal survival of recipients observed in this trial40. The more likely explanation, as noted above, is the severity of TTTS cardiomyopathy.
The outcome of this trial does not conclusively answer the question of which treatment, AR or SFLP, is the superior treatment modality. The results do suggest, however, that no matter which treatment is employed, survival is likely to be compromised if treatment is initiated later in the disease progression, particularly among recipients. TTTS cardiomyopathy appears to be an important factor in recipient survival in TTTS. The Cincinnati modification of the Quintero staging system to incorporate echocardiographic changes into stage III, as suggested by Harkness et al., may be useful to more appropriately stratify recipient risk40. Comparisons between this trial and the Eurofoetus trial must be drawn cautiously given the significant differences in the subjects that were treated in each.
Additional trials in TTTS are needed to define the best treatment for a given case of TTTS. It is unfortunate that the state of community equipoise which resulted in a decline of subjects screened for this trial may also prevent randomized trials in TTTS in the future. An alternative strategy may be rigorously designed and controlled prospective cohort studies. The impact of TTTS on neuroimaging, echocardiographic assessment, and long-term neurodevelopmental outcome of the subjects in this trial awaits analysis of the follow-up assessments of survivors.
Acknowledgments
This work was supported by a grant from the National Institute of Child Health and Human Development R01 HD41149 (T. M. C.). Statistical work was partially supported by NICHD MRDDRC at CHOP, 3P30 HD26979-04S2 (D. S.).
The authors gratefully acknowledge the expert technical assistance of the following:
Victoria Shokrollah, MT, Trial Coordinator, The Children’s Hospital of Philadelphia
Roberta Ballard, MD, Consultant, The Children’s Hospital of Philadelphia
Lori J. Howell, RN, MS, Study Coordinator, The Children’s Hospital of Philadelphia
Jamie Koh, Study Coordinator, The Children’s Hospital of Philadelphia
Joy Macdonald, RN, Study Coordinator, The Children’s Hospital of Philadelphia
Joy N. Williams, RN, BSN, Study Coordinator, The Children’s Hospital of Philadelphia
Kim Lyons, RN, BSN, CCRC, Trial Coordinator, Cincinnati Children’s Hospital Medical Center
Jennifer L. Mason, RN, BSN, Study Coordinator, Cincinnati Children’s Hospital Medical Center
Sabine Bousleiman, RN, Study Coordinator, Columbia-Presbyterian Medical Center
Barb Ross, Study Coordinator, Eastern Virginia Medical School
Patty Trail, Study Coordinator, Eastern Virginia Medical School
Elaine Haney, BS, MT, Study Coordinator, Evanston Northwestern Healthcare
Elvira Kolchinshaky, RDMS, Study Coordinator, Mount Sinai Medical Center
Robin Bisgaard, RN, Study Coordinator, University of California, San Francisco
Jody Farrell, RN, Site Coordinator, University of California, San Francisco
Jane Berg, Study Coordinator, University of Colorado Health Sciences Center
Jennifer M. Lee-Rollins, MS, Study Coordinator, University of Texas Medical Branch
Kristi Nelson, RN, Study Coordinator, University of Utah
Lindsey Hancock-Wolosiewicz, Study Coordinator, William Beaumont Hospital
With special appreciation to the following groups of individuals (listed alphabetically):
Data Coordinating Center
Tina Alvarado-Taylor, Randomization Specialist
Mignon Davis, Database Designer
Tracy DeMeio, Data Manager
Jeff Dragone, Safety Officer
Katherine Gibbs, BFA, Database Manager
Julie Distefano-Pappas, Data Manager
Kathy Ma, Biostatistician
Wendy Sieferheld, Biostatistician
Margie Tartaglione, Data Manager
Marc Taylor, Data Entry, Data
Clare Weiler, Clinical Research Director/Monitor
Sanyi Zhao, Biostatistician
Data Safety Monitoring Board
Nicholas M. Fisk, PhD, Member, Imperial College School of Medicine
John C. Fletcher, PhD, 1st Chairman, University of Virginia School of Medicine (deceased)
Kurt Hecher, MD, Member, Barmbek Hospital, Hamburg, Germany
James Goldberg, MD, 2nd Chairman, Pacific Medical Center, San Francisco, CA
Haywood Brown, MD, Duke University
Richard Martin, MD, Rainbow Babies and Children’s Hospital, Cleveland, OH
Trial Oversight Committee
Vinod Bhutani, MD, Chairman, Stanford University School of Medicine
Arnold Cohen, MD, Albert Einstein Medical Center
Robert Levy, MD, Children’s Hospital of Philadelphia
David J. Margolis, MD, University of Pennsylvania Medical Center
Kim Olthoff, MD, University of Pennsylvania
Donna Sylvester, RN, Children’s Hospital of Philadelphia
Joseph Zorc, MD, Children’s Hospital of Philadelphia
Abbreviations
- TTTS
twin-twin transfusion syndrome
- AR
amnioreduction
- SFLP
selective fetoscopic laser photocoagulation
- OR
odds ratio
- CI
confidence interval
- PPROM
preterm premature rupture of membranes
- CVPS
cardiovascular profile score
Footnotes
Presented at the 27th Annual Clinical Meeting of the Society for Maternal–Fetal Medicine, San Francisco, CA, Feb. 5-10, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weir PE, Ratten GJ, Beischer NA. Acute polyhydramnios-a complication of monozygous twin pregnancy. Br J Obstet Gynaecol. 1979;86:849–53. doi: 10.1111/j.1471-0528.1979.tb10710.x. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg LH, Hurley VA, Desmedt E, Beischer NA. Acute polyhydramnios in twin pregnancies. Austral NZ J Obstet Gynecol. 1990;30:196–200. doi: 10.1111/j.1479-828x.1990.tb03211.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheschier NC, Seeds JW. Polyhydramnios and oligohydramnios in twin gestations. Obstet Gynecol. 1988;71:882–4. [PubMed] [Google Scholar]
- 4.Saunders NJ, Snijders RJ, Nicolaides KH. Twin-twin transfusion syndrome during the 2nd trimester is associated with small intertwin haemoglobin differences. Fetal Diag Ther. 1991;6:34–6. doi: 10.1159/000263622. [DOI] [PubMed] [Google Scholar]
- 5.Callahan TL, Hall JE, Ettner SL, Christiansen CL, Greene ML, Crowley WF. The economic impact of multiple-gestation pregnancies and the contribution of assisted-reproduction techniques to their incidence. N Engl J Med. 1994;331:244–9. doi: 10.1056/NEJM199407283310407. [DOI] [PubMed] [Google Scholar]
- 6.Moise KJ., Jr Polyhydramnios: problems and treatment. Sem Perinatol. 1993;17:197–209. [PubMed] [Google Scholar]
- 7.Rodestal A, Thomassen PA. Acute polyhydramnios in twin pregnancy. A retrospective study with special reference to therapeutic amniocentesis. Acta Obstet Gynecol Scand. 1990;69:297–300. doi: 10.3109/00016349009036150. [DOI] [PubMed] [Google Scholar]
- 8.Urig MA, Clewell WH, Elliott JP. Twin-twin transfusion syndrome. Am J Obstet Gynecol. 1990;163:1522–6. doi: 10.1016/0002-9378(90)90618-h. [DOI] [PubMed] [Google Scholar]
- 9.Mahony BS, Petty CN, Nyberg DA, Luthy DA, Hickok DE, Hirsch JH. The “stuck twin” phenomenon: ultrasonographic findings, pregnancy outcome, and management with serial amniocenteses. Am J Obstet Gynecol. 1990;163:1513–22. doi: 10.1016/0002-9378(90)90617-g. [DOI] [PubMed] [Google Scholar]
- 10.Elliott JP, Urig MA, Clewell WH. Aggressive therapeutic amniocentesis for treatment of twin-twin transfusion syndrome. Obstet Gynecol. 1991;77:537–40. [PubMed] [Google Scholar]
- 11.Pinette MG, Pan Y, Pinette SG, Stubblefield PG. Treatment of twin-twin transfusion syndrome. Obstet Gynecol. 1993;82:841–6. [PubMed] [Google Scholar]
- 12.Reisner DP, Mahony BS, Petty CH, Nyberg DA, Porter TF, Zingheim RW, et al. Stuck twin syndrome: outcome in thirty-seven consecutive cases. Am J Obstet Gynecol. 1993;169:991–5. doi: 10.1016/0002-9378(93)90041-g. [DOI] [PubMed] [Google Scholar]
- 13.Saade GR, Olson G, Belfort MA, Moise KJ. Amniotomy: a new approach to the “stuck twin” syndrome. Am J Obstet Gynecol. 1995;172:429–34. [Google Scholar]
- 14.Saade GR, Belfort MA, Berry DL, Bui TH, Montgomery LD, Johnson A, et al. Amniotic septostomy for the treatment of twin oligohydramnios-polyhydramnios sequence. Fetal Diagn Ther. 1998;13:86–93. doi: 10.1159/000020812. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JR, Rossi KQ, O’Shaughnessy RW. Amnioreduction versus septostomy in twin-twin transfusion syndrome. Am J Obstet Gynecol. 2001;185:1044–7. doi: 10.1067/mob.2001.117640. [DOI] [PubMed] [Google Scholar]
- 16.Saade G, Moise K, Forman K, et al. A randomized trial of septostomy versus amnioreduction in the treatment of twin oligohydramnios polyhydramnios sequence (TOPS) Am J Obstet Gynecol(Society for Maternal-Fetal Medicine. Oral presentation abstract 3) 2003;187 [Google Scholar]
- 17.De Lia JE, Cruikshank DP, Kaye WR. Fetoscopic neodymium: YAG laser occlusion of placental vessels in severe twin-twin transfusion syndrome. Obstet Gynecol. 1990;75:1046–53. [PubMed] [Google Scholar]
- 18.De Lia JE, Kuhlmann RS, Harstad TW, Cruikshank DP. Fetoscopic laser ablation of placental vessels in severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 1995;172:1202–11. doi: 10.1016/0002-9378(95)91480-3. [DOI] [PubMed] [Google Scholar]
- 19.Ville Y, Hyett J, Hecher K, Nicolaides KH. Preliminary experience with endoscopic laser surgery for sever twin-twin transfusion syndrome. N Engl J Med. 1995;332:224–7. doi: 10.1056/NEJM199501263320404. [DOI] [PubMed] [Google Scholar]
- 20.Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351:136–44. doi: 10.1056/NEJMoa032597. [DOI] [PubMed] [Google Scholar]
- 21.Quintero RA, Morales WJ, Mendoza G, Allen M, Lalter C, Giannina G, et al. Selective photocoagulation of placental vessels in twin-twin transfusion syndrome: evolution of a surgical technique. Obstet Gynecol Surv. 1998;53:597–603. doi: 10.1097/00006254-199812010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Hecher K, Plath H, Bregenzer T, Hansmann M, Hackeloer BJ. Endoscopic laser surgery versus serial amniocenteses in the treatment of severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 1999;180:717–24. doi: 10.1016/s0002-9378(99)70278-4. [DOI] [PubMed] [Google Scholar]
- 23.Quintero RA, Dickinson JE, Morales WJ, Bornick PW, Bermudez C, Cincotta R, et al. Stage-based treatment of twin-twin transfusion syndrome. Am J Obstet Gynecol. 2003;188:1333–40. doi: 10.1067/mob.2003.292. [DOI] [PubMed] [Google Scholar]
- 24.Hecher K, Diehl W, Zikulnig L, Vetter M, Hackeloer BJ. Endoscopic laser coagulation of placental anastomoses in 200 pregnancies with sever mid-trimester twin-to-twin transfusion syndrome. Eur J Obstet Gynecol Reprod Biol. 2000;92:135–9. doi: 10.1016/s0301-2115(00)00437-1. [DOI] [PubMed] [Google Scholar]
- 25.Huber A, Wiehl W, Bregenzer T, Hackeloer BJ, Hecher K. Stage-related outcome in twin-twin transfusion syndrome treated by fetoscopic laser coagulation. Obstet Gynecol. 2006;108:333–7. doi: 10.1097/01.AOG.0000225945.17022.6b. [DOI] [PubMed] [Google Scholar]
- 26.Quintero RA, Comas C, Bornick PW, Allen MH, Kruger M. Selective vs non-selective laser photocoagulation of placental vessels in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2000;16:230–6. doi: 10.1046/j.1469-0705.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 27.Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin-twin transfusion syndrome. Am J Perinatol. 1999;19:550–5. doi: 10.1038/sj.jp.7200292. [DOI] [PubMed] [Google Scholar]
- 28.Crombleholme TM, Livingston J, Polzin W, Gottliebson W, et al. Multimodality and sequential therapy for twin-twin transfusion syndrome (TTTS): a stage and gestational age based approach to the use of amnioreduction (AR), selective fetoscopic laser photocoagulation (SFLP), and radio frequency ablation (RFA) Am Obstet Gynecol. 2006;195(Suppl):669. (abstr) [Google Scholar]
- 29.Robyr R, Yamamoto M, Ville Y. Selective fetocide in complicated monochorionic twin pregnancies using ultrasound-guided bipolar cord coagulation. Br J Obstet Gynecol. doi: 10.1111/j.1471-0528.2005.00746.x. (in press) [DOI] [PubMed] [Google Scholar]
- 30.Crombleholme TM, Robertson F, Marx G, Yarnell R, D’Alton ME. Fetoscopic cord ligation to prevent neurologic injury in monozygous twins. Lancet. 1996;348:191. doi: 10.1016/s0140-6736(96)24029-2. [DOI] [PubMed] [Google Scholar]
- 31.Danner C, Shahabi S, Thomas D, et al. Selective fetocide by embolization in twin-twin transfusion syndrome. A report of two cases. J Reprod Med. 1997;42:747–50. [PubMed] [Google Scholar]
- 32.Deprest JA, Van Ballaer PP, Evrard VA, Peers KH, Spitz B, Steegers EA, et al. Experience with fetoscopic cord ligation. Eur J Obstet Gynecol Reprod Biol. 1998;81:157–64. doi: 10.1016/s0301-2115(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 33.Moise KJ., Jr Polyhydramnios: problems and treatment. Semin Perinatol. 1993;17:197–209. [PubMed] [Google Scholar]
- 34.Mari G, Roberts A, Detti L, Kovanci E, Stefos T, Bahado-Singh RO, et al. Perinatal morbidity and mortality rates in severe twin-twin transfusion syndrome. Results of the international amnioreduction registry. Am J Obstet Gynecol. 2001;185:708–15. doi: 10.1067/mob.2001.117188. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson JE, Evans SF. Obstetric and perinatal outcomes from the Australian and New Zealand twin-twin transfusion syndrome registry. Am J Obstet Gynecol. 2000;182:706–12. doi: 10.1067/mob.2000.104236. [DOI] [PubMed] [Google Scholar]
- 36.Saade GR, Ludomirsky A, Fisk NJ. Feto-fetal transfusion. In: Fisk NM, Moise KJ Jr, editors. Fetal Therapy and Transplacental. Cambridge UK: Cambridge University Press; 1997. pp. 225–51. [Google Scholar]
- 37.Cook TL, O’Shaughnessy R. Iatrogenic creation of a monoamniotic twin gestation in severe twin-twin transfusion syndrome. J Ultrasound Med. 1997;16:853–5. doi: 10.7863/jum.1997.16.12.853. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 39.Meis PJ for the NICHD Maternal-Fetal Medicine Units Network. Ultrasound determination of gestational age alters pregnancy outcome measurements. Paediatric and Perinatal Epidemiology. 2001;15:A23. [Google Scholar]
- 40.Harkness UF, Crombleholme TM. Twin-twin transfusion syndrome: where do we go from here? Semin Perinatol. 2005;29:296–304. doi: 10.1053/j.semperi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Shah AD, Border WL, Crombleholme TM, Michelfelder EC. The relationship between cardiovascular status and recipient twin outcomes in twin-twin transfusion syndrome: Assessment by fetal cardiovascular profile score; Presented at the American Heart Association Annual Meeting; November 12–15 2006; Chicago IL. [Google Scholar]
- 42.Huhta J. Right ventricular function in the human fetus. J Perinat Med. 2001;29:390–8. doi: 10.1515/JPM.2001.054. [DOI] [PubMed] [Google Scholar]
- 43.Fisk NM, Galec P. Twin-twin transfusion syndrome – as good as it gets? N Engl J Med. 2004;351:182–4. doi: 10.1056/NEJMe048047. [DOI] [PubMed] [Google Scholar]