Abstract
Purpose
To identify iris structural alterations associated with intraoperative floppy iris syndrome (IFIS) in patients using systemic α-1-adrenergic receptor antagonists (α-1ARA).
Design
Cross-sectional study.
Participants and Controls
Twenty-nine patients with current or past treatment with any systemic α-1ARA and 22 untreated controls.
Methods
Consecutive eligible patients underwent slit-lamp-adapted optical coherence tomography (SL-OCT, Heidelberg Engineering, GmbH, Dossenheim, Germany) in a masked fashion under standardized lighting conditions.
Main Outcome Measures
Iris thickness at the dilator muscle region (DMR, measured at half of the distance between the scleral spur and the pupillary margin) and at the sphincter muscle region (SMR, 0.75 mm from the pupillary margin), the ratio between the DMR/SMR (to compensate for possible intersubject variability) and pupillary diameter.
Results
Most treated patients were on tamsulosin (27/29). Mean age was similar in study and control groups (70.6 ± 7.6 vs 67.1 ± 9.1 yrs, p=0.061). Photopic pupil diameter was reduced in the study group (2.06 ± 0.5 vs 2.5 ± 0.6 mm, p=0.001). SMR was similar between groups (p=0.53). Significantly lower values were found in treated subjects for the DMR and the DMR/SMR ratio (p<0.001). These differences remained significant after adjusting for pupil diameter (p<0.001). Multiple regression analysis showed that a longer duration of α-1ARA treatment correlated to a reduced DMR/SMR ratio (p=0.001, r=0.47). Age and eye color were not significant in this model.
Conclusion
Patients using systemic α-1ARA have significantly lower values of DMR thickness and DMR/SMR ratio and smaller pupil diameter when compared to age-matched controls. These differences appear to be related to the duration of drug exposure and provide evidence of structural alterations to the iris dilator muscle from this class of agents in IFIS.
Introduction
Benign prostatic hypertrophy (BPH) is a common urologic condition in older men that leads to lower urinary tract symptoms.1 The most common therapeutic options for BPH are the 5-α reductase inhibitors (5ARIs) and α-1 adrenergic receptor antagonists (α-1ARAs).2 5ARIs are uncommonly used due to the propensity for sexual side effects and relatively slow onset of action.3 The α-1ARAs such as terazosin and doxazosin reduce outlet obstruction by relaxing prostatic smooth muscle but have the potential to cause orthostatic hypotension and syncope.4 The selective α-1ARA tamsulosin reduces these adverse effects relative to nonselective agents with comparable efficacy and is agent most commonly prescribed.
Patients with past or present use of α-1ARAs are at risk for intraoperative floppy iris syndrome (IFIS), characterized by progressive intraoperative miosis, billowing of the iris stroma and iris prolapse during cataract surgery. This risk is particularly increased with tamsulosin use and occurs in 62.5% to 93.8% of patients undergoing cataract surgery.5–9 IFIS increases vision-threatening complications of cataract surgery, particularly when surgeons are unaware of the use of these agents.10 Since age is the predominant risk factor for both BPH and cataract, the possibility of IFIS is important to consider in the preoperative evaluation. Approximately 3% of patients undergoing cataract extraction in the United States have a history of tamsulosin use.5,11,12
It is postulated that α-1ARAs affect these receptors in the prostate and iris dilator muscle leading to loss of iris muscle tone.5 Pre-operative cessation does not appear to decrease the risk of IFIS,13 and it has been hypothesized that irreversible atrophy of the iris dilator muscle is responsible for this effect.14,15 There is no current method to predict which patients with a history of α-1ARA use will be affected by IFIS.
Slit-lamp-adapted optical coherence tomography (SL-OCT) is a light-based, noncontact method of obtaining cross-sectional images of the anterior segment that provides rapid and objective information regarding anterior segment dimensions and angle configuration.16,17 We used SL-OCT to evaluate iris structural alterations in patients using systemic α-1ARAs.
Patients and Methods
This non-interventional, cross-sectional study adhered to the tenets of the Declaration of Helsinki, was HIPAA (Health Insurance Portability and Accountability Act) -compliant and approved by the Institutional Review Board of The New York Eye and Ear Infirmary. Written informed consent was obtained from all subjects.
Patients
We prospectively enrolled 51 consecutive male patients (29 patients with current or past treatment with any systemic α-1ARA and 22 untreated controls). All patients had glaucoma or were glaucoma suspects. Data collection included age, race, type and time of using the systemic α-1ARA, iris color (light [blue-green-hazel] vs dark [brown]) and past medical and ocular history. After a complete ophthalmic examination, all eyes presenting previous intraocular surgery were excluded. Other exclusion criteria included exfoliation syndrome, pigment dispersion syndrome, ocular signs or a history of uveitis, previous laser iridoplasty, ocular trauma, history of using any miotic or alpha adrenergic agonist for more than 3 months, or any other condition that could alter iris morphology (eg. iris tumors, iris congenital abnormalities, iridocorneal endothelial syndrome, iris rubeosis, acute angle-closure, current use of mydriatics and medications with vasoactive or cycloplegic properties).
Procedures and data analysis
All patients underwent anterior segment imaging using SL-OCT (optical axial image resolution <25μm and lateral resolution of 20–100μm, Heidelberg Engineering, GmbH, Dossenheim, Germany). A total of 5 high quality cross-sectional images were taken under standardized lighting conditions (300 lux), using a 5×1mm light beam set at the maximum intensity of the device. Quality control parameters were defined as: well centered image, clearly defined scleral spur and absence of artifacts. Patients were instructed to fixate on a target 1 meter from the device to reduce accommodation. All images were taken horizontally through the center of the pupil to avoid interference with the lid margins. Iris crypts were avoided.
Iris thickness measurements were obtained between the pupillary margin and the temporal periphery of the iris for all patients. An anterior segment specialist masked to the patient’s medical history calculated the average iris thickness and pupillary diameter of three high-quality images randomly selected for each eye. The iris thickness was analyzed using the SL-OCT software in 2 different positions based on the iris anatomy: in the dilator muscle region (DMR, measured at half of the distance between the scleral spur and the pupillary margin), and in the sphincter muscle region (SMR, standardized at 0.75 mm from the pupillary margin) (Figure 1). Finally, the ratio between the iris thickness measured in the DMR/SMR was calculated to compensate for possible intersubject variability. Demographic data of each group was compared using independent sample t-test and chi-square test. Iris parameters were compared using a general linear model to correct for interocular dependency. A multiple regression analysis was performed to evaluate the influence of duration of α-1ARA treatment, age and eye color on DMR/SMR ratio values (independent variable) in treated patients.
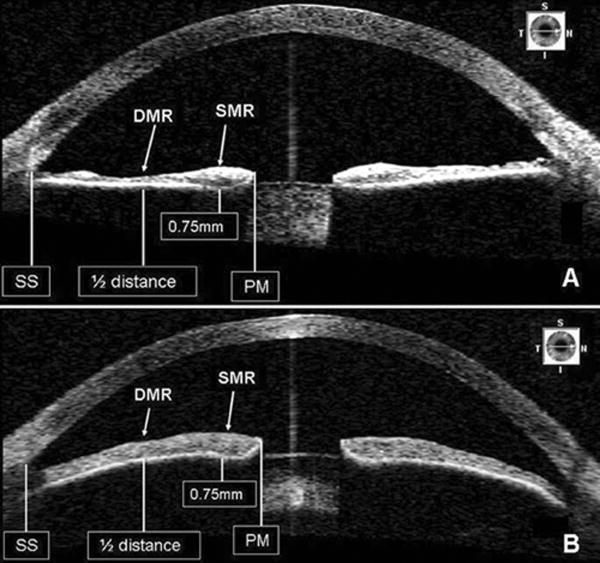
Figure 1.
SL-OCT images demonstrating the standardized positions where iris thickness was measured. Iris thinning at the dilator muscle region (DMR) in a patient on tamsulosin (A) when compared to an age-matched control (B).
SS, scleral spur; PM, pupillary margin; SMR, sphincter muscle region.
Results
Characteristics of patients in both groups are given in table 1. Most patients in the study group were currently under treatment (22/29) and on tamsulosin (27/29). The other two patients were on terazosin. Patients in the study group had been treated with a systemic α-1ARA for 28.2 ± 24.6 months. Regarding glaucoma diagnosis, there were no significant differences between study and control groups for open-angle glaucoma (34% vs 31%), angle-closure glaucoma (38% vs 42%) and glaucoma suspects (28% vs 27%, p=0.29). The proportion of glaucoma patients using prostaglandin analogues in the study and control groups (76% vs 62%) and the mean duration of prostaglandin treatment (52.3 ±15.7 vs 49.2 ±15.4 months) was also similar (p>0.55). There was no difference in mean age between study and control groups (70.6 ± 7.6 vs 67.1 ± 9.1 yrs, p=0.06). Photopic pupil diameter was reduced in the study group (2.06 ± 0.5 vs 2.5 ± 0.6mm, p=0.001). Although there was no difference in iris thickness in the SMR between groups (p=0.53), significantly lower values were found in the study group for the DMR and the DMR/SMR ratio (table 1, p<0.001). These differences remained significant after adjusting for pupil diameter (p<0.001). When compared to the normal distribution of DMR/SMR ratio values in the control group (mean ± 2SD [0.98 ± 0.22]), 63% of the treated patients had a ratio below the inferior limit of the normality. Multiple regression analysis showed that a longer duration of α-1ARA treatment correlated to a reduced DMR/SMR ratio (p=0.001, r=0.47). Age and eye color were not significant in this model.
Table 1.
Characteristics of Patients on Systemic α-1 Adrenergic Receptor Antagonists and Controls*
| Variable | Study Group (n=29, 45 eyes) | Control Group (n=22, 31 eyes) | P value§ |
|---|---|---|---|
| Age (years) | 70.6 ±7.6 | 67.1 ±9.1 | 0.061† |
| Race (C/AD/H) | 25/1/3 | 19/1/2 | 0.541‡ |
| Eye color (light/dark) | 10/19 | 7/15 | 0.741 |
| Photopic pupillary diameter (mm) | 2.06 ±0.5 (1 – 3.4) | 2.50 ±0.6 (1.7 – 3.9) | 0.001 |
| Iris thickness measurements | |||
| Dilator muscle region (μm) | 354.6 ±83.7 (176.7 – 522) | 446.9 ±92.6 (291.3 – 611) | <0.001 |
| Sphincter muscle region (μm) | 473.2 ±76.5 (359.3 – 681.3) | 460.5 ±99.5 (328.3 – 745) | 0.530 |
| DMR/SMR ratio | 0.75 ±0.2 (0.36 – 1.07) | 0.98 ±0.1 (0.79 – 1.23) | <0.001 |
C = Caucasian; AD = African descendent; H = Hispanic; DMR = dilator muscle region; SMR = sphincter muscle region.
Data are given as mean ± standard deviation (range) whenever indicated.
Independent sample t-test.
Chi-square test.
GLM (General linear model).
Discussion
There is a significant difference in iris morphology in patients with a past or current history of α-1ARA use as compared to age-matched controls. Treated patients had significantly decreased DMR thicknesses, lower DMR/SMR ratios and smaller pupillary diameters. These alterations seem to be related to the length of time of α-1ARA usage.
This is the first report demonstrating a structural alteration in the iris dilator muscle region in patients using α-1ARAs. These alterations presumably account for the clinical presentation of IFIS. It is possible that normal smooth muscle tone may be necessary for iris rigidity or integrity. Notably, two patients who had these alterations had documented clinical IFIS in the contralateral eye during cataract extraction. Corroborating previous animal studies that have shown a selective effect of α-1A receptors on the iris dilator smooth muscle,18,19 we found no significant difference in the SMR thickness between treated patients and controls.
Altered iris morphology was present in 63% percent of treated patients compared to controls. This number correlates with the reported prevalence of clinical IFIS in 62.5–93.8% of patients with a history of tamsulosin use.5–9 SL-OCT is a noninvasive way to demonstrate a distinctive change in iris morphology in treated patients. Performed in the sitting position, it is particularly suitable for in vivo imaging of anterior segment structures, which are distinguished on the basis of their varying optical characteristics at the light wavelength used (1310nm). It reproducibly measures various anterior chamber parameters (corneal thickness, anterior chamber depth, anterior chamber angle) and has been used to evaluate such anterior segment pathology as corneal scars/ulcers, blebs, ciliary body cleft and iris lesions16,20–24 Further studies are necessary to determine the accuracy of this methodology in predicting IFIS based on iris imaging with SL-OCT.
This study, although prospective, is limited by its cross-sectional analysis. Since this affected both groups equally, thus we feel that the impact of studying glaucoma patients and suspects on our results is limited. The majority of the treated patients in this study were taking tamsulosin at the time of SL-OCT evaluation and it was impossible to make an inference about the effect of discontinuing tamsulosin on iris morphology. Finally, there is no data in the literature regarding how iris measurements with SL-OCT would correlate with the real iris thickness. However, as all measurements were done using the same device and in the same position for all patients, we believe that it would not significantly alter our results.
Surgeon preparation for the possibility of IFIS limits serious intraoperative complications.10 Despite specific preoperative questioning, many patients fail to report a history of α-1ARA use.25 There is a need for a reliable indicator of the likelihood of IFIS in patients during the preoperative evaluation in at-risk patients. SL-OCT of the anterior segment in patients with a history of α-1ARA use demonstrates an etiologic explanation of clinical IFIS and shows a time-dependent component of structural iris changes. Our findings may provide a platform to further characterize and detect this condition pre-operatively.
Acknowledgments
Financial Support: Supported in part by the Educational Foundation of America, Westport, CT.
Footnotes
Conflicts of Interests: Ritch R: Alcon (c), Allergan (c), Merck (c), Pfizer (c), Ocular Instruments (p); Liebmann JM: Heidelberg (instrument support). None of the other authors have any conflict of interest regarding the present study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marklund-Bau H, Edell-Gustafsson U, Spangberg A. Bothersome urinary symptoms and disease-specific quality of life in patients with benign prostatic obstruction. Scand J Urol Nephrol. 2007;41:32–41. doi: 10.1080/00365590601068926. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED, Wilson SS, McConnell JD, et al. MTOPS Research Group. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. J Urol. 2006;175:1422–6. doi: 10.1016/S0022-5347(05)00708-1. [DOI] [PubMed] [Google Scholar]
- 3.Naslund MJ, Miner M. A review of the clinical efficacy and safety of 5alpha-reductase inhibitors for the enlarged prostate. Clin Ther. 2007;29:17–25. doi: 10.1016/j.clinthera.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Lowe FC. Role of the newer alpha(1)-adrenergic-receptor antagonists in the treatment of benign prostatic hyperplasia-related lower urinary tract symptoms. Clin Ther. 2004;26:1701–13. doi: 10.1016/j.clinthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg. 2005;31:664–73. doi: 10.1016/j.jcrs.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan S, Radomski S, Chung J, et al. Intraoperative floppy-iris syndrome during cataract surgery in men using alpha-blockers for benign prostatic hypertrophy [letter] J Cataract Refract Surg. 2007;33:1826–7. doi: 10.1016/j.jcrs.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Chang DF, Osher RH, Wang L, Koch DD. Prospective multicenter evaluation of cataract surgery in patients taking tamsulosin (Flomax) Ophthalmology. 2007;114:957–64. doi: 10.1016/j.ophtha.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Takmaz T, Can I. Clinical features, complications, and incidence of intraoperative floppy iris syndrome in patients taking tamsulosin. Eur J Ophthalmol. 2007;17:909–13. doi: 10.1177/112067210701700607. [DOI] [PubMed] [Google Scholar]
- 9.Blouin MC, Blouin J, Perreault S, et al. Intraoperative floppy-iris syndrome associated with alpha1-adrenoreceptors: comparison of tamsulosin and alfuzosin. J Cataract Refract Surg. 2007;33:1227–34. doi: 10.1016/j.jcrs.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Schwinn DA, Afshari NA. alpha(1)-Adrenergic receptor antagonists and the iris: new mechanistic insights into floppy iris syndrome. Surv Ophthalmol. 2006;51:501–12. doi: 10.1016/j.survophthal.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Chadha V, Borooah S, Tey A, et al. Floppy iris behaviour during cataract surgery: associations and variations. Br J Ophthalmol. 2007;91:40–2. doi: 10.1136/bjo.2006.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagnier PP, Girman CJ, Garraway M, et al. International comparison of the community prevalence of symptoms of prostatism in four countries. Eur Urol. 1996;29:15–20. doi: 10.1159/000473711. [DOI] [PubMed] [Google Scholar]
- 13.Parssinen O, Leppanen E, Keski-Rahkonen P, et al. Influence of tamsulosin on the iris and its implications for cataract surgery. Invest Ophthalmol Vis Sci. 2006;47:3766–71. doi: 10.1167/iovs.06-0153. [DOI] [PubMed] [Google Scholar]
- 14.Takmaz T, Can I. Intraoperative floppy iris syndrome: do we know everything about it? J Cataract Refract Surg. 2007;33:1110–2. doi: 10.1016/j.jcrs.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 15.Masket S, Belani S. Combined preoperative topical atropine sulfate 1% and intracameral nonpreserved epinephrine hydrochloride 1:4000 [corrected] for management of intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2007;33:580–2. doi: 10.1016/j.jcrs.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 16.Leung CK, Chan WM, Ko CY, et al. Visualization of anterior chamber angle dynamics using optical coherence tomography. Ophthalmology. 2005;112:980–4. doi: 10.1016/j.ophtha.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Memarzadeh F, Li Y, Chopra V, et al. Anterior segment optical coherence tomography for imaging the anterior chamber after laser peripheral iridotomy. Am J Ophthalmol. 2007;143:877–9. doi: 10.1016/j.ajo.2006.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wikberg-Matsson A, Uhlen S, Wikberg JE. Characterization of alpha(1)-adrenoreceptor subtypes in the eye. Exp Eye Res. 2000;70:51–60. doi: 10.1006/exer.1999.0753. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Koss MC. Studies of alpha-adrenoceptor antagonists on sympathetic mydriasis in rabbits. J Ocul Pharmacol Ther. 2003;19:255–63. doi: 10.1089/108076803321908374. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Leung CK, Cheung CY, et al. Repeatability and reproducibility of anterior chamber angle measurement with anterior segment optical coherence tomography. Br J Ophthalmol. 2007;91:1490–2. doi: 10.1136/bjo.2007.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalev-Landoy M, Day AC, Cordeiro MF, Migdal C. Optical coherence tomography in anterior segment imaging. Acta Ophthalmol Scand. 2007;85:427–30. doi: 10.1111/j.1600-0420.2007.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prata TS, Palmiero PM, De Moraes CG, et al. Slit-lamp-adapted optical coherence tomography for assessment of an overhanging filtering bleb. Acta Ophthalmol. doi: 10.1111/j.1755-3768.2008.01417.x. In press. [DOI] [PubMed] [Google Scholar]
- 23.Burés-Jelstrup A, Navarro R, Mateo C, et al. Detection of ciliary body detachment with anterior segment optical coherence tomography. Acta Ophthalmol. 2008;86:810–1. doi: 10.1111/j.1600-0420.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- 24.Prata TS, Palmiero PM, De Moraes CG, et al. Imaging of a traumatic cyclodialysis cleft in a child using slit-lamp-adapted optical coherence tomography. Eye. doi: 10.1038/eye.2008.277. In press. [DOI] [PubMed] [Google Scholar]
- 25.Chang DF, Braga-Mele R, Mamalis N, et al. ASCRS Cataract Clinical Committee. Clinical experience with intraoperative floppy-iris syndrome: results of the 2008 ASCRS member survey. J Cataract Refract Surg. 2008;34:1201–9. doi: 10.1016/j.jcrs.2008.04.014. [DOI] [PubMed] [Google Scholar]