Abstract
We investigated the effect of adiponectin (APN) deficiency in the CD4+CD45RBhigh transfer model of colitis. Recombination activating gene (Rag)-1 knockout (KO) and Rag-1 APN KO mice receiving CD4+CD45RBhigh cells developed colitis of comparable severity. Colonic mRNA expression of IL-6 and IL-17 was lower in Rag-1 APN KO mice compared to Rag-1 KO mice. Rag-1 APN KO and Rag-1 KO mice released comparable amounts of IL-6 from colon cultures, whereas release of IL-17 was higher in Rag-1 APN KO compared to Rag-1 KO mice. Expression of TNFα mRNA was comparable in Rag-1 KO and Rag-1 APN KO mice, but protein release was lower in Rag-1 APN KO mice compared to Rag-1 KO mice. Levels of IFNγ and IL-10 at mRNA and protein were comparable in Rag-1 KO and Rag-1 APN KO mice. Higher mRNA expression of VCAM-1 was observed in the colon of healthy APN KO compared to WT mice, while induction of colitis resulted in a comparable increase in VCAM-1 expression in Rag-1 KO and Rag-1 APN KO mice. In conclusion, although APN regulates expression of cytokines and adhesion molecules in the colon, this does not result in alteration of overall colitis severity in the CD4+CD45RBhigh transfer model.
Keywords: Inflammatory bowel disease, colon, inflammation
1. Introduction
White adipose tissue is an important participant in the immune response, largely because it releases adipokines, i.e., cytokines produced by adipocytes. Adiponectin (APN), an adipokine present in blood in the μg/ml range, is most commonly known for its protective role in obesity-related insulin resistance, metabolic syndrome and type-2-diabetes [1]. Reduced APN levels associated with obesity contribute to the endothelial dysfunction, macrophage foam cell formation, and smooth muscle cell migration observed in atherosclerosis and cardiovascular disease [2-4]. Adiponectin has traditionally been characterized as an anti-inflammatory molecule, suppressing production of pro-inflammatory cytokines, such as IFNγ, TNFα and IL-6, while increasing expression of anti-inflammatory ones, such as IL-10 and IL-1Ra [5, 6]
However, outside the context of diseases associated with obesity, an inflammatory status is not necessarily associated with low APN levels. In fact, the opposite is observed, with APN levels increasing during inflammatory conditions that are unrelated to an increase in fat mass [7]. This trend is particularly pronounced in chronic inflammatory/autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, type-1-diabetes and inflammatory bowel disease (IBD) [8-13]. In particular, circulating APN is significantly increased in Crohn's Disease (CD) patients compared to patients with ulcerative colitis and healthy controls [13]. In addition, mRNA and protein levels of APN are significantly upregulated in mesenteric adipose tissue of CD patients compared to healthy controls [14, 15]. The amount of APN is further enhanced in hypertrophied mesenteric adipose tissue compared to normal mesenteric adipose tissue within the same CD patient [14]. These observations indicate that APN production is upregulated under inflammatory conditions in CD, suggesting that this adipokine might participate in modulation of intestinal inflammation in this chronic disorder.
The role of APN in experimental intestinal inflammation remains controversial. In fact, both protection and increased susceptibility to colitis induced by dextran sulfate sodium (DSS)- and 2,4,6-trinitrobenzene sulfonic acid (TNBS) have been reported in APN knockout (KO) mice [16, 17]. In the IL-10 KO model of chronic colitis, which partially reproduces CD pathogenesis, APN deficiency does not play a significant role in modulating spontaneous colonic inflammation [18].
In an attempt to further clarify whether APN is involved in the pathogenesis of IBD, in the present report we used the model of colitis induced by transfer of naïve CD4+CD45RBhigh (RBhigh) lymphocytes into immunocompromised Recombination activating gene (Rag)-1 KO [19]. Following transfer of RBhigh cells into T and B cell deficient Rag-1 KO mice, the naïve RBhigh cells migrate to secondary lymphoid organs where they encounter antigen presenting cells. After priming, the effector T cells are able to enter the colon, where intestinal bacteria further drive activation and proliferation of the T cells. The initial inflammation is amplified by recruitment of the innate immune system resulting in the uncontrolled T-helper (Th)-1 and Th-17 driven expansion of inflammatory cells in the colon [20, 21]. Our present data indicate that although APN regulates expression of cytokines and adhesion molecules in the colon, it does not alter overall colitis severity in the transfer model.
2. Materials and Methods
2.1. Generation of Rag-1 APN KO mice
Care of mice followed institutional guidelines under a protocol approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago. Rag-1 APN KO mice were generated by crossing C57BL6 Rag-1 KO mice from The Jackson Laboratories (Bar Harbor, ME) with C57BL6 APN KO mice from our previously established colony originally obtained from Dr. Lawrence Chan at Baylor College of Medicine (Waco, TX). Mice were genotyped by PCR of tail DNA using primers specific for the genes of interest.
2.2. Hematologic parameters
Total white blood cells, red blood cells, hemoglobin and platelets of healthy 20-week old male WT and APN KO mice were analyzed using the COULTER AC. T diff analyzer.
2.3. FACS Analysis
Single-cell suspensions were prepared from both splenocytes and mesenteric lymph nodes isolated from healthy WT and APN KO mice. Using 1×107 cells per sample, surface marker expression was evaluated using antibodies from BD Pharmingen and Caltag. Analysis was conducted on a FACSCalibur (BD Pharmingen) using the Cell Quest analysis program (BD Pharmingen). Markers evaluated included CD4, CD8, CD45RB, DX5, CD19 and CD11c.
2.4. Cell preparation, sorting and administration
A single-cell suspension of splenocytes isolated from WT mice was prepared and enriched for CD4+ cells using magnetic beads (Miltenyi), followed by labeling with a FITC-conjugated anti-CD4 antibody and a PE-conjugated anti-CD45RB antibody (BD Biosciences) and sorting into RBhigh and CD4+CD45RBlow populations. Sorted cells were consistently >95% pure. Sorted RBhigh cells (4×105/mouse) were injected intraperitoneally into 8-week old Rag-1 and Rag-1 APN KO recipients. Mice were age and sex-matched for each experiment.
2.5. Clinical and histological assessment of colitis
Mice were weighed at the time of cell transfer and once weekly thereafter. Groups of non-transferred wild-type (WT) and APN KO mice were also evaluated as control groups. Mice were euthanized 8 weeks post-transfer by isofluorane inhalation and cervical dislocation. Post mortem, the entire colon was excised and a 1 cm segment of tranverse colon was fixed in 10% buffered formalin for histological analysis. Paraffin sections were stained with haematoxylin/eosin. Sections were evaluated in a blinded fashion by a pathologist (RJC) and scored for inflammatory infiltrate and crypt elongation as previously described [18].
2.6. Serum and tissue collection
Blood samples were obtained from the retro-orbital plexus under isofluorane anesthesia and serum was prepared and stored at −70°C until analysis. Two segments of the colon were removed and snap-frozen in liquid nitrogen and stored at −70°C until analysis. For colon cultures, duplicate segments of the transverse colon were excised, cut longitudinally, washed in PBS containing 1% penicillin and streptomycin and placed in 24-well culture plates containing 1 ml of RPMI with 1% penicillin and streptomycin and incubated at 37°C for 24 h.
2.7. Miscellaneous measurements
Colon culture supernatants were harvested and assayed for the cytokines TNFα, INFγ, IL-6, IL-17, and IL-10 and the chemokines CXCL1, CXCL2 and CCL2 by ELISA (BD Bioscience, San Jose, CA and eBioscience, San Diego, CA). Protein concentration of the supernatant was quantified using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Serum amyloid A (SAA) levels were determined in serum by ELISA (Biosource International, Camarillo, CA). Cytokine and adhesion molecule expression was measured in the colon by PCR using specific primers for TNFα (Integrated DNA Technologies; Coralville, IA) and by real-time RT-PCR using specific primers for IL-6, IL-17, IL-10, VCAM-1 and ICAM-1 (Applied Biosystems). Expression of APN receptors on RBhigh and RBlow cells was determined by real-time RT-PCR using specific primers for AdipoR1 and AdipoR2 (Integrated DNA Technologies; Coralville, IA).
2.8. Statistical analysis
Statistical analyses were performed using XLStat software (Addinsoft, Paris, France). Significance of differences was determined by factorial ANOVA (Fischer's pair wise comparison) and paired Student's T-test. Data are expressed as the mean +/− SEM. Differences were considered significant at p < 0.05.
3. Results
3.1. Characterization of leukocyte subpopulations in WT and APN KO mice
Because different types of immune cells play an important role in mediating and modulating inflammation in the transfer model of colitis, we analyzed hematologic parameters and leukocyte subpopulations in WT and APN KO mice. Results indicate that APN deficiency is not associated with alterations in leukocyte, erythrocyte or platelet counts or hemoglobin levels in peripheral blood of healthy, untreated mice (data not shown). In addition, flow cytometry analysis indicated no significant differences between WT and APN KO mice in the distribution of T cells (CD4 and CD8), B cells (CD19), monocytes/macrophages (CD11c), or NK cells (DX5) in the spleen or mesenteric lymph nodes (data not shown). Furthermore, comparable numbers of RBhigh and memory CD4+CD45RBlow cells were present in WT and APN KO mice.
3.2. Induction of colitis by transfer of RBhigh cells in Rag-1 Ko and Rag-1 APN KO mice
To investigate the role of APN in the transfer model of IBD, we generated double Rag-1 APN KO mice as described in the Materials and Methods section. Adiponectin signals through its two known receptors, AdipoR1 and AdipoR2, which are widely expressed on tissues including muscle, liver, adipose tissue, pancreas, endothelium, and the colon [16, 22], as well as on immune cells such as monocytes, macrophages and lymphocytes [23, 24]. However, whether AdipoR1 and AdipoR2 are expressed by the specific subsets of RBhigh or RBlow cells was not known. Using real-time RT-PCR, we found that both AdipoR1 and AdipoR2 were equally expressed by RBhigh and RBlow cells (data not shown), indicating that APN is likely capable of signaling through its receptors on transferred lymphocytes.
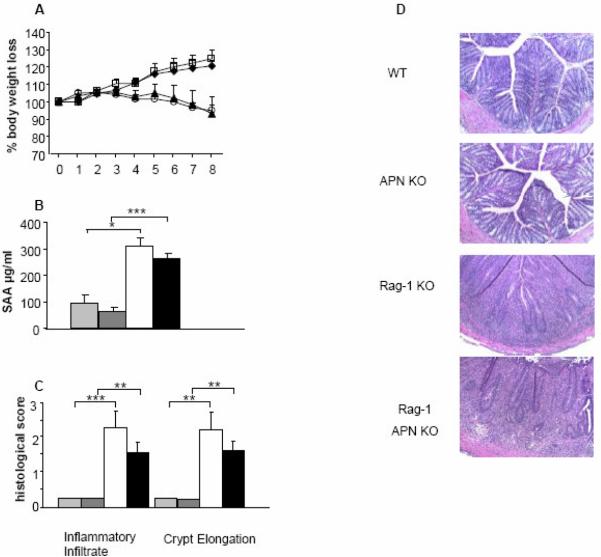
Both Rag-1 KO and Rag-1 APN KO mice were injected with RBhigh cells and monitored for disease development over a period of 8 weeks. As shown in Figure 1A, both Rag-1 KO and Rag-1 APN KO mice lost a significant amount of weight compared to their controls, beginning at week 7 post-transfer for Rag-1 KO mice and week 6 for Rag-1 APN KO mice. The percentage of weight loss did not significantly differ between Rag-1 and Rag-1 APN KO mice throughout the 8-week experiment. Serum levels of the acute-phase protein SAA were measured as a marker of inflammation at the completion of the experiment. A comparable increase in SAA levels was observed in Rag-1 and Rag-1 APN KO mice (Figure 1B). Histological analysis of the colon indicated a significantly higher inflammatory infiltrate accompanied by crypt elongation in mice that had received RBhigh cells compared to healthy controls 8 weeks post-transfer (Figure 1C and D). However, no significant differences were observed between Rag-1 KO and Rag-1 APN KO mice in terms of histological score. These data collectively demonstrate that APN deficiency does not alter disease severity in the transfer model of IBD.
Figure 1. Response of Rag-1 KO and Rag-1 APN KO mice to transfer of RBhigh cells.
A) Body weight loss was recorded weekly after injection of RBhigh cells. Rag-1 KO (black triangles) lost significantly more weight then their respective controls (WT, black diamonds) beginning at week 7 (p<0.05); Rag-1 APN KO mice (open circles) lost a significant amount of weight compared to their respective controls (APN KO, open squares) beginning at week 6 (p<0.01). Percent body weight loss was not significantly different between Rag-1 and Rag-1 APN KO mice throughouthe 8-week experiment. B) Serum SAA levels were measured in WT (light gray bars), APN (dark gray bars), Rag-1 KO (white bars) and Rag-1 APN KO mice (black bars) by ELISA at week 8 post-transfer. C) Histological score of WT, APN KO, Rag-1 KO and Rag-1 APN KO mice at week 8 post-transfer. Colonic sections were scored for inflammatory infiltrate and crypt elongation. D) H&E staining in the colon of control WT and APN KO mice compared with transferred Rag-1 KO and Rag-1 APN KO mice. Data are mean +/− SEM, n= 5-11 mice per group. *p<0.05, **p<0.01, ***p<0.001.
3.3 Colonic cytokine expression and release
To investigate whether APN deficieny is associated with alterations in cytokine production in the transfer model of colitis, mRNA expression in colonic tissue and protein release from colon cultures were evaluated. Mice that received RBhigh cells had a significantly higher expression of TNFα, IL-6, IL-10 and IL-17 compared to their respective non-transferred controls (Figure 2 A-D). Colonic expression of IL-6 and IL-17 (Figure 2 A and B) was significantly lower in transferred Rag-1 APN KO mice compared to transferred Rag-1 KO mice, whereas no significant differences between transferred Rag-1 and Rag-1 APN KO mice were observed for expression of TNFα and IL-10 (Figure 2 C and D).
Figure 2. Cytokine gene expression and protein production in the colon after transfer of RBhigh cells.
Colonic expression of IL-6 (A), IL-17 (B), TNFα (C) and IL-10 (D) was determined by real-time RT-PCR in WT (light gray bars), APN KO (dark gray bars), Rag-1 KO (white bars) and Rag-1 APN KO mice (black bars). Data are expressed as fold induction compared with control WT mice that did not receive RBhigh cells. Colon cultures were prepared at week 8 post-transfer as described in the materials and methods section. Levels of IL-6 (E), IL-17 (F), TNFα (G) and IL-10 (H) were measured in culture supernatants. Data are mean ± SEM, n=5-11 mice per group. *p<0.05; **p<0.01; ***<0.001.
Evaluation of cytokine release from inflamed and non-inflamed colon was performed using colon cultures. A significant induction of TNFα, IL-6, IL-10 and IL-17 (Figure 2 E-H) was observed in transferred mice compared to healthy controls. Significantly lower levels of TNFα (Figure G) were released from colon cultures of transferred Rag-1 APN KO mice compared to transferred Rag-1 KO mice. In contrast, transferred Rag-1 APN KO mice released significantly higher amounts of IL-17 (Figure 2 F) compared to transferred Rag-1 KO mice. Furthermore, no significant differences between the two strains were observed in terms of release of IL-6 and IL-10 (Figure 2 E and H) as well as IFNγ or the chemokines CXCL1, CXCL2 AND CCL2 (data not shown).
3.4. Expression of adhesion molecules
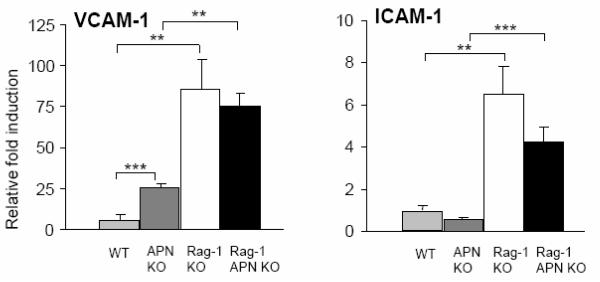
Adiponectin deficiency leads to an increase in leukocyte rolling and adhesion, associated with upregulation of the adhesion molecule VCAM-1 in the vascular endothelium [25]. Since both ICAM-1 and VCAM-1 play important roles in the deregulated trafficking of leukocytes in human IBD [26], expression of these adhesion molecules was evaluated in colonic tissue of control mice and mice that received the transfer of RBhigh cells. As shown in Figure 3, a significant increase in mRNA expression of VCAM-1 was observed in the colon of healthy, non-transferred APN KO compared to WT mice, thus confirming previous reports [25]. However, induction of colitis resulted in a comparable increase in VCAM-1 expression in both Rag-1 KO and Rag-1 APN KO mice. No significant differences were observed between the two strains of mice for expression of ICAM-1 under either control or inflamed conditions.
Figure 3. Colonic gene expression of adhesion molecules after transfer of RBhigh cells.
Colonic expression of VCAM-1 and ICAM-1 in WT (light gray bars), APN KO (dark gray bars), Rag-1 KO (white bars) and Rag-1 APN KO mice (black bars) was determined by real-time PCR. Data are expressed as fold induction compared with control WT mice that did not receive RBhigh cells. n=5-11 mice per group. **p<0.01; ***<0.001.
3.5. Modulation of APN and leptin levels after transfer of RBhigh cells
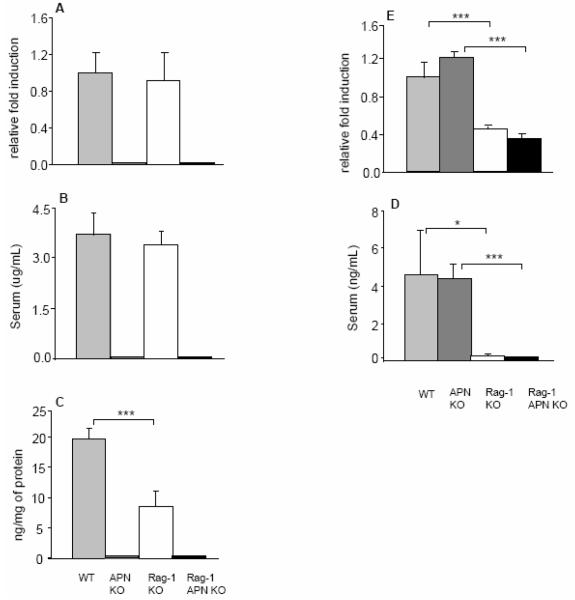
Upregulation of both APN and leptin, another adipokine, has been reported in the hypertrophic mesenteric adipose tissue of patients with CD [13, 15]. To assess whether the transfer model of chronic colitis mimics CD in terms of APN and leptin expression, these adipokines were measured in the serum, colon cultures and mesenteric adipose tissue of control and colitic mice. As shown in Figure 4, no significant differences in circulating APN levels or mRNA expression in mesenteric adipose tissue were observed between healthy WT controls and transferred Rag-1 KO mice (Figure 4A and B). Release of APN from colon cultures was significantly lower in tissue obtained from transferred Rag-1 KO mice compared to their healthy controls (Figure 4C). As a negative control, APN levels were below detection limit in each sample obtained from APN KO or Rag-1 APN KO mice. At variance with APN, both serum levels and mRNA expression of leptin were significantly decreased in transferred Rag-1 KO and Rag-1 APN KO mice compared to their non-transferred controls (Figure 4D and E). Release of leptin from colon cultures was below detection limit in each experimental group (data not shown). These results indicate that the transfer model of colitis does not reproduce observations obtained in CD patients in terms of regulation of APN and leptin levels.
Figure 4. Adipokine levels in Rag-1 KO and Rag-1 APN KO after transfer of RBhigh cells.
Expression of APN and leptin in mesenteric adipose tissue of WT (light gray bars), APN KO (dark gray bars), Rag-1 KO (white bars) and Rag-1 APN KO mice (black bars) was determined by real-time RT-PCR (A and D). Data are expressed as fold induction compared with control WT mice that did not receive RBhigh cells. Adiponectin and leptin levels were measured in serum (B and D) and in colon culture supernatants (C) in WT (light gray bars), APN KO (dark gray bars), Rag-1 KO (white bars) and Rag-1 APN KO mice (black bars) by ELISA. Data are mean +/− SEM, n=5-13 mice per group. *p<0.05, ***p<0.001
4. Discussion
The present study demonstrates that APN deficiency does not alter disease severity in the transfer model of chronic colitis in mice, even though it modulates expression of cytokines and adhesion molecules.
No difference in body weight loss, inflammation and hyperplasia of colonic tissue or levels of acute-phase proteins was observed between Rag-1 KO and Rag-1 APN KO mice 8 weeks post-transfer of RBhigh cells. These results are in agreement with data obtained in the IL-10 KO model of chronic colitis[18], but at variance with results we obtained using the DSS and TNBS models [16]. A possible explanation for these conflicting results between models is that disease development in the IL-10 KO and transfer models of colitis are mostly mediated by T lymphocytes, whereas the innate immune system is the major player in the DSS and TNBS model, which are relatively independent from lymphocyte-mediated responses [27, 28]. This suggests that APN may play a more important role in regulating innate rather than adaptive immune responses.
Modulation of cytokine production by APN has been demonstrated in several reports, although with conflicting results [5, 6, 23, 29, 30]. In the present study we observed a multifaceted regulation of colonic cytokine production in APN KO mice, with differential effects observed at the level of mRNA expression and protein release. In fact, APN deficiency was associated with reduced mRNA expression of IL-6 and IL-17, two cytokines that play critical pro-inflammatory roles in IBD [31]. However, at the protein level APN deficiency did not alter release of IL-6 from colon cultures while it enhanced IL-17 release. A dissociation between mRNA expression and protein release was also observed for TNFα, another critical mediator in IBD [32]. Adiponectin deficiency did not affect mRNA expression of TNFα, but significantly reduced protein release from colon cultures. These data suggest that APN affects TNFα production at the translational or secretion level, at least in the context of colonic inflammation.
Adhesion molecules ICAM-1 and VCAM-1 play important roles in the trafficking of leukocytes in IBD [26]. In the present study, we observed increased mRNA expression of VCAM-1 in the colon of healthy APN KO compared to WT mice, in agreement with previous findings [25]. However, induction of colitis resulted in a comparable increase in VCAM-1 expression in both Rag-1 KO and Rag-1 APN KO mice. There was no difference in ICAM-1 expression in either control or colitic conditions. These data indicate that, although APN is an important contributor to maintaining low expression of VCAM-1 under healthy conditions, this adipokine does not play a major role in modulating expression of adhesion molecules during chronic colitis.
Both APN and leptin are overexpressed in the hypertrophic mesenteric adipose tissue of patients with CD [33]. In addition, circulating levels of APN are increased in CD patients, while circulating leptin levels are lower in CD patients compared to healthy controls [13]. Evaluation of local and systemic APN and leptin levels in the transfer model of colitis indicates that this model does not reproduce the pathogenesis of CD in terms of regulation of adipokine levels, suggesting that different experimental systems, such as TNBS- or DSS-induced colitis, might be more appropriate to study this response [16, 34]
Our data indicate that although APN regulates expression of cytokines and adhesion molecules in the colon, it does not alter overall colitis severity in the transfer model. In addition, these data suggest that the RBhigh transfer model does not mimic CD in terms of regulation of APN and leptin, suggesting it is not an ideal model for studying adipokine regulation in IBD.
Acknowledgments
The authors would like to acknowledge Dr. Lawrence Chan for providing APN KO mice. The authors would also like to thank Christiana McMorran for technical support. This work was supported by National Institutes of Health grant DK-061483 (to GF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–8. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 3.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1290–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 4.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–8. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 5.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 6.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–30. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Sada KE, Yamasaki Y, Maruyama M, Sugiyama H, Yamamura M, Maeshima Y, Makino H. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:1545–52. [PubMed] [Google Scholar]
- 9.Senolt L, Pavelka K, Housa D, Haluzik M. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine. 2006;35:247–52. doi: 10.1016/j.cyto.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, Yung Yu C, Nagaraja HN. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–33. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 12.Perseghin G, Lattuada G, Danna M, Sereni LP, Maffi P, De Cobelli F, Battezzati A, Secchi A, Del Maschio A, Luzi L. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1174–81. doi: 10.1152/ajpendo.00279.2003. [DOI] [PubMed] [Google Scholar]
- 13.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–5. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, Ito T, Nezu R, Tsutsui S, Miyagawa JI, Tamura S, Matsuzawa Y, Shimomura I, Shinomura Y. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005;54:789–96. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul G, Schaffler A, Neumeier M, Furst A, Bataillle F, Buechler C, Muller-Ladner U, Scholmerich J, Rogler G, Herfarth H. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm Bowel Dis. 2006;12:471–7. doi: 10.1097/00054725-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007;132:601–14. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131:853–61. doi: 10.1053/j.gastro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Pini M, Gove ME, Fayad R, Cabay RJ, Fantuzzi G. Adiponectin deficiency does not affect development and progression of spontaneous colitis in IL-10 KO mice. Am J Physiol Gastrointest Liver Physiol. 2008 doi: 10.1152/ajpgi.90593.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 20.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–71. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 21.Ostanin DV, Boa R, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T-Cell Transfer Model of Chronic Colitis: Concepts, Considerations and Tricks of the Trade. Am J Physiol Gastrointest Liver Physiol. 2008 doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi T, Adachi Y, Ohtsuki Y, Furihata M. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol. 2007;40:115–20. doi: 10.1007/s00795-007-0364-9. [DOI] [PubMed] [Google Scholar]
- 23.Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem. 2007;282:21695–703. doi: 10.1074/jbc.M701419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberti L, Gilardini L, Girola A, Moro M, Cavagnini F, Invitti C. Adiponectin receptors gene expression in lymphocytes of obese and anorexic patients. Diabetes Obes Metab. 2007;9:344–9. doi: 10.1111/j.1463-1326.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 25.Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 2007;117:1718–26. doi: 10.1172/JCI29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera-Nieves J, Gorfu G, Ley K. Leukocyte adhesion molecules in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1715–35. doi: 10.1002/ibd.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 28.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 29.Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol. 2006;120:99–105. doi: 10.1016/j.clim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–25. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–8. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller C. Tumour necrosis factor in mouse models of chronic intestinal inflammation. Immunology. 2002;105:1–8. doi: 10.1046/j.1365-2567.2002.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin--implications for inflammatory bowel disease. Mol Nutr Food Res. 2008;52:855–66. doi: 10.1002/mnfr.200700050. [DOI] [PubMed] [Google Scholar]
- 34.Karagiannides I, Kokkotou E, Tansky M, Tchkonia T, Giorgadze N, O'Brien M, Leeman SE, Kirkland JL, Pothoulakis C. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci U S A. 2006;103:5207–12. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]