Abstract
Background
Excessive glutamatergic neurotransmission may contribute to the pathophysiology of major depressive disorder (MDD). Recent evidence suggests that riluzole and other agents that target glutamate neurotransmission may show antidepressant activity.
Methods
Ten patients with treatment resistant depression had riluzole added to their ongoing medication regimen for six weeks, followed by an optional six-week continuation phase. Depression and anxiety severity were assessed using the Hamilton depression rating scale (HDRS) and the Hamilton anxiety rating scale (HARS). Linear mixed models were used to test for a linear trend in HDRS and HARS scores across time with treatment.
Results
HDRS and HARS scores declined significantly following the initiation of riluzole augmentation therapy. The effect of riluzole was significant at the end of the first week of treatment and persisted for the twelve-week duration of the study.
Conclusion
These data suggest that riluzole augmentation produces antidepressant and anxiolytic effects in patients with treatment resistant depression.
Keywords: riluzole, major depressive illness, treatment resistant, glutamate, treatment
Although there are now more than 20 different antidepressant medications available in the US, treatment-resistant depression remains a common problem, with up to half of depressed patients failing to respond sufficiently to adequate trials of antidepressant medications (Fava and Davidson 1996; Trivedi et al 2006). These high rates of treatment-resistance may reflect the fact that existing antidepressants target the monoaminergic systems almost exclusively. The limited success of these treatments provides a strong impetus for the development of novel antidepressant medications with unique targets of action.
Emerging evidence implicates the glutamatergic system in the pathogenesis, pathophysiology and treatment of mood disorders (Kugaya and Sanacora 2005). Stress-related increases in extracellular glutamate (Moghaddam 1993), alterations in hippocampal glutamate receptor subunit gene expression (Bartanusz et al 1995) and excitotoxic-like effects of excessive glutamate transmission (Sapolsky 2000) serve as potential mediators between stress and mood disorders. Increasing evidence also suggests that drugs which reduce glutamatergic tone play a role in the treatment of depression (Sanacora et al 2003). Riluzole, an agent currently approved by the US Food and Drug Administration for the treatment of amyotrophic lateral sclerosis, has been shown to inhibit excitotoxic injury in experimental models (Risterucci et al 2006) through direct effects on the glutamatergic system, resulting in decreased glutamate release (Risterucci et al 2006; Stefani et al 1997; Wang et al 2004) and increased glutamate uptake (Azbill et al 2000; Frizzo et al 2004).
Recently, open-label monotherapy trials of riluzole revealed antidepressant and anxiolytic effects in individuals with major depression and generalized anxiety disorder (Mathew et al 2005; Zarate et al 2004). Riluzole was also beneficial when added to lithium treatment in bipolar depression (Zarate et al 2005). The aim of this study was to explore whether riluzole would be well tolerated and effective when added to ongoing antidepressant treatments in individuals with treatment-resistant major depression currently receiving traditional monoaminergic antidepressants.
Methods
Men and women between the ages of 21 and 65 with a SCID (First et al 1995) diagnosis of major depressive disorder were recruited to the study through referrals to the Yale Depression Research Program. Both inpatients and outpatients were included in the study after completing an informed consent process approved by the Yale University IRB. Subjects were required to have a score of greater than 21 on the 24-item HDRS at the time of evaluation despite at least six weeks of treatment with a stable dose of an antidepressant medication. Other medications were permitted if a stable dose had been maintained for at least four weeks at the time of evaluation and throughout the course of the study.
Patients meeting criteria for substance abuse or dependence (excluding nicotine) in the six months prior to the study were excluded, as were any patients with a comorbid psychiatric diagnosis other than an anxiety disorder. Because hepatotoxicity is known to be associated with riluzole treatment, subjects with a history of liver disease or found to have baseline liver enzyme elevations more than twice above normal limits were also excluded.
After the initial screening process, baseline assessments were obtained. Each subject then initiated treatment with 50mg of riluzole twice a day added to their ongoing treatment regimen. Although lower than the dose used in a recent monotherapy trial of riluzole in depression (Zarate et al 2004), this dose was selected because it is the dose recommended for use in patients with ALS, with higher doses believed to cause an increase in adverse events (Riluzole package insert). Patients were maintained on the starting dose as tolerated, with reductions made at the discretion of the treating physician if side effects were reported. Patients were treated openly for six weeks and offered the opportunity to continue for an additional six weeks if they believed they were experiencing a benefit from the medication.
Subjects were evaluated on a weekly basis with the HDRS and HARS by unmasked raters with at least two years of experience. Clinical response was defined as a 50% decrease from baseline on the HDRS, with clinical remission defined as a score of less than or equal to 8. Statistical analyses were performed only on subjects completing at least two weeks of riluzole treatment. Linear mixed models were used to test for a linear trend in HDRS and HARS, where time was modeled as a within-subject explanatory variable and subject was used as the clustering factor. Post-hoc analyses were performed using Dunnett’s procedure where levels measured at individual time points were compared to baseline levels.
Results
Seven men and eight women were consented to participate in the study. Two subjects were withdrawn from the study prior to receiving any riluzole due to abnormal baseline laboratory values. Unapproved changes to their standing medications caused the withdrawal of one subject before the end of study week one, and two others before the end of study week two. This resulted in six men (2 inpatients) and four women (1 inpatient) who completed the six-week protocol and were included in the analyses. Their mean age was 47.9± 8.1 years and mean age of illness onset was 19.2 ±6.0 years. All subjects either had greater than two lifetime episodes of depression or had experienced the current episode for more than 2 years. The mean duration of their current depressive episode was 5.0 ± 7.0 years. All but one subject was in stage 3 or higher of treatment resistance according to the Thase and Rush staging levels, defined as a failure to respond to at least 2 different classes of antidepressant medications with at least one tricyclic antidepressant trial(Thase and Rush 1997). Over the six-week trial, subjects received a mean riluzole dose of 95.0 ± 17.1 mg/day.
Riluzole augmentation of traditional antidepressant medications produced a significant improvement in HDRS [F(1,54)=8.61, P<0.005] (Figure 1) and HARS [F(1,54)=7.82, P<0.007] scores over the period of the study. After six weeks of treatment, mean HDRS scores in the 10 completers decreased by 36% (9.6 points) and mean HARS scores by 31% (5.8 points). Significant reductions from baseline scores were seen as early as week 1 for both the HDRS [t=-3.64, df=49, Dunnett-adjusted p<0.01] and the HARS [t=-4.09, df=49, adjusted p<0.001] and remained significant for every week thereafter (all adjusted P<.05). The intent-to-treat analysis (n=13) also showed a significant effect of treatment by time for both the HDRS [F (1,68)=4.71, P = 0.0335] and the HARS [F(1,67)=4.72,P = 0.0333]. At the end of week six, four subjects (40%) met HDRS criteria for response and of those, three met criteria for remission. The effect of treatment on HDRS [F(1,43)=3.92, P<.05] and HARS [F(1,43)=4.22, P<.05] scores remained significant in separate analyses excluding two subjects who were previously described in a case report (Sanacora et al 2004).
Figure: 1.
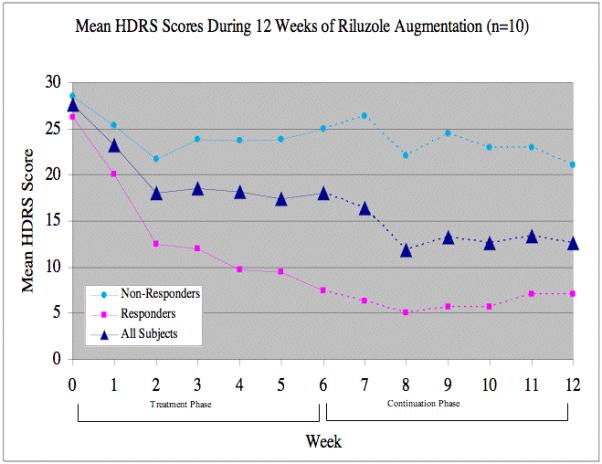
Following the initial course of the study, six subjects elected to continue with riluzole augmentation for an additional six weeks. These six included the three subjects who achieved remission during the first six weeks of treatment, and all three remained in remission at the end of the twelve-week period. The fourth subject meeting response criteria after the initial six weeks lived out of state and chose not to continue with the trial. One subject not meeting response criteria after six weeks went on to meet response criteria at weeks 11 and 12. The remaining two subjects showed limited improvement during the six-week extension.
The most frequent adverse event reported during the study was fatigue, which required that the dose of riluzole be lowered in two subjects after the fourth week of treatment. LFTs were monitored throughout the study and no significant elevations were observed during the twelve week trial. One subject experienced visual hallucinations one week after initiating riluzole augmentation, which required a change in her standing medication regimen. However, this subject had a history of similar visual symptoms when taking other psychotropic medications in the past (Pittenger et al 2006).
Discussion
In this open-label study, riluzole augmentation of traditional monoaminergic antidepressants resulted in a rapid improvement of symptoms in individuals with treatment-resistant MDD. Significant decreases in symptom severity ratings were evident within the first week of treatment. While riluzole appeared to be exceedingly effective in some individuals, generating a rapid improvement which was maintained for several months, it had only limited benefit in others.
Growing evidence of altered glutamatergic transmission in the pathophysiology of MDD suggests that riluzole’s ability to lower extracellular glutamate levels may be related to its mechanism of antidepressant action. However, riluzole has also been shown to have other effects which may contribute to its antidepressant properties, including increasing brain derived neurotrophic factor (BDNF) expression and promoting hippocampal cell proliferation (Katoh-Semba et al 2002; Mizuta et al 2001) through pathways that may or may not involve its effects on the glutamatergic system.
Since this study consisted of an open-label design with a small sample size, its results need to be interpreted with caution. This study supports the general safety of riluzole when used as an augmenting agent for the treatment of depression. However, close monitoring of subjects taking this drug is important. In total, these findings highlight the need for larger, controlled studies designed to explore the benefits of combining glutamatergic modulators such as riluzole with traditional monoaminergic antidepressants for use in individuals with treatment-resistant depression.
Acknowledgment
Supported in part by the VA CT Research Enhancement Award Program [REAP] research center (JHK, GS), NARSAD (SK, AS), K12RR17594 from the NCRR (AS)
Footnotes
Some of these data were previously presented in the form of a case report.
References
- Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–80. doi: 10.1016/s0006-8993(00)02430-6. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Aubry JM, Pagliusi S, Jezova D, Baffi J, Kiss JZ. Stress-induced changes in messenger RNA levels of N-methyl-D-aspartate and AMPA receptor subunits in selected regions of the rat hippocampus and hypothalamus. Neuroscience. 1995;66:247–52. doi: 10.1016/0306-4522(95)00084-v. [DOI] [PubMed] [Google Scholar]
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatric Clinics of North America. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-Patient edition (SCID-I/P, version 2.0) Biometric Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Frizzo ME, Dall’Onder LP, Dalcin KB, Souza DO. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cellular & Molecular Neurobiology. 2004;24:123–8. doi: 10.1023/B:CEMN.0000012717.37839.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R, Asano T, Ueda H, Morishita R, Takeuchi IK, Inaguma Y, et al. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB Journal. 2002;16:1328–30. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–19. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162:2379–81. doi: 10.1176/appi.ajp.162.12.2379. [DOI] [PubMed] [Google Scholar]
- Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S. Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neurosci Lett. 2001;310:117–20. doi: 10.1016/s0304-3940(01)02098-5. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. Journal of Neurochemistry. 1993;60:1650–7. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Naungayan C, Kendell SF, Coric V, Malison R, Krystal JH, et al. Visual hallucinations from the addition of riluzole to memantine and bupropion. Journal of Clinical Psychopharmacology. 2006;26:218–20. doi: 10.1097/01.jcp.0000203228.64117.9f. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Coccurello R, Banasr M, Stutzmann JM, Amalric M, Nieoullon A. The metabotropic glutamate receptor subtype 5 antagonist MPEP and the Na(+) channel blocker riluzole show different neuroprotective profiles in reversing behavioral deficits induced by excitotoxic prefrontal cortex lesions. Neuroscience. 2006;137:211–20. doi: 10.1016/j.neuroscience.2005.08.054. Epub 2005 Oct 20. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Fenton L, Coric V, Krystal JH. Riluzole augmentation for treatment-resistant depression. American Journal of Psychiatry. 2004:161. doi: 10.1176/appi.ajp.161.11.2132. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Rothman DL, Mason GF, Krystal JH. Clinical studies implementing glutamate neurotransmission in mood disorders. In: Moghaddam B, Wolf ME, editors. Glutamate and Diorders of Cognition and Motivation. Vol. 1003. 2003. pp. 292–308. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological Psychiatry. 2000;48:755–65. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Differential inhibition by riluzole, lamotrigine, and phenytoin of sodium and calcium currents in cortical neurons: implications for neuroprotective strategies. Exp Neurol. 1997;147:115–22. doi: 10.1006/exnr.1997.6554. [DOI] [PubMed] [Google Scholar]
- Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. Journal of Clinical Psychiatry. 1997;58(Suppl 13):23–9. [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. see comment. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Wang KY, Wang WC. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes) Neuroscience. 2004;125:191–201. doi: 10.1016/j.neuroscience.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. American Journal of Psychiatry. 2004;161:171–4. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biological Psychiatry. 2005;57:430–2. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]


