Abstract
Extensive research within last half a century has indicated that curcumin (diferuloylmethane), a yellow pigment in curry powder, exhibits antioxidant, anti-inflammatory and proapoptotic activities. Whether anti-inflammatory and proapoptotic activities assigned to curcumin, are mediated through its antioxidant mechanism was investigated. We found that TNF-mediated NF-κB activation was inhibited by curcumin; and glutathione reversed the inhibition. Similarly, suppression of TNF-induced AKT activation by curcumin, was also abrogated by glutathione. The reducing agent also counteracted the inhibitory effect of curcumin on TNF-induced NF-κB regulated antiapoptotic (Bcl-2, Bcl-xL, IAP1), proliferative (cyclin D1) and proinflammatory (COX-2, iNOS and MMP-9) gene products. The suppression of TNF-induced AP-1 activation by curcumin was also reversed by glutathione. Also, the direct proapoptotic effects of curcumin were inhibited by glutathione and potentiated by depletion of intracellular glutathione by buthionine sulfoximine. Moreover, curcumin induced the production of reactive oxygen species (ROS) and modulated the intracellular GSH levels. Quenchers of hydroxyl radicals, however, were ineffective in inhibiting curcumin mediated NF-κB suppression. Further, N-acetylcysteine partially reversed the effect of curcumin. Based on these results we conclude that curcumin mediate its apoptotic and anti-inflammatory activities through modulation of the redox status of the cell.
Keywords: Curcumin, Glutathione, Tumor necrosis factor, Nuclear factor-κB, Reactive oxygen species
INTRODUCTION
Curcumin (diferuloylmethane), a dietary pigment responsible for the yellow color of turmeric, is used as a traditional medicine, well documented in ayurveda for the treatment of numerous inflammatory conditions. Extensive research within the last half-a-decade has confirmed that curcumin mediates anti-inflammatory effects through the downregulation of transcription factor nuclear factor-κB (NF-κB) [1,2] tumor necrosis factor (TNF) [3], interleukin-6 (IL-6) [4], interleukin-8 (IL-8) [5], adhesion molecules [6], inducible nitric oxide synthase (iNOS) [7], matrix metalloproteinase-9 (MMP-9) [8], cyclooxygenase-2 (COX-2) [9], and 5-lipoxygenase (5-LOX) [10]. In fact, curcumin has been shown to bind to an active site in 5-LOX, and the two together have been cocrystallized [11]. This phytochemical has also been shown to suppress the proliferation of a wide variety of tumor cells by downregulating c-myc [6], cyclin D1 [12], activator protein-1 (AP-1) [13], phosphatidylinositol-3-kinase/AKT signaling [14], and epidermal growth factor receptor (EGFR) signaling [15]. Curcumin can also induce apoptosis through the modulation of antiapoptotic gene products [2,6] and BID cleavage, cytochrome c release, and caspase-9 activation, leading to caspase-3 activation [16]. More recently, curcumin was found to bind to thioredoxin reductase and alkylate a critical cysteine residue, thus converting the activity of the enzyme to NADPH oxidase [17]. Since thioredoxin reductase is overexpressed in tumor cells, authors suggested that the NADPH- oxidase mediated production of reactive oxygen species (ROS) may be responsible for the ability of curcumin to selectively kill tumor cells [18].
How curcumin mediates all these effects is not fully understood. Besides having anti-inflammatory and growth-modulatory effects, this compound is also one of the most potent antioxidants. According to some reports, curcumin is as much as 10 times more potent than even vitamin E [19]. It has been generally assumed that the antioxidant effects of curcumin are responsible for its anti-inflammatory, antiproliferative, proapoptotic, and chemopreventive effects, although there is currently no evidence to support this. However, there is no evidence so far if this is indeed the case. In the present report, we investigated whether the anti-inflammatory and proapoptotic effects of curcumin are mediated through the antioxidant mechanism. The results to be described indicate that anti-inflammatory and apoptotic effects of curcumin may be due to its ability to perturb the redox balance in the cell.
MATERIALS AND METHODS
Reagents
Curcumin (>95% pure) was purchased from LKT Laboratories (St. Paul, MN). A 25 mM solution of curcumin was prepared in dimethyl sulfoxide, stored as small aliquots at –20 °C, and diluted as needed in cell culture medium. Bacteria-derived human recombinant TNF, purified to homogeneity with a specific activity of 5 × 107 U/mg, was kindly provided by Genentech (South San Francisco, CA). Penicillin, streptomycin, Iscove’s modified Dulbecco’s medium, Dulbecco’s modified Eagle’s medium, and fetal bovine serum were obtained from Invitrogen (Grand Island, NY). Buthionone sulfoximine, glutathione, mannitol N-acetylcysteine and antibody against β-actin were obtained from Sigma-Aldrich (St. Louis, MO). Dichlorodihydrofluorescein diacetate and [5-(and-6)-carboxy-2, 7-dichlorofluoresceindiacetate were purchased from Molecular Probes (Eugene, OR). Antibodies against cyclin D1, iNOS, MMP-9, poly(ADP-ribose) polymerase (PARP), inhibitor of apoptosis protein-1 (IAP1), IAP2, Bcl-2, and Bcl-xL, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against COX-2 was obtained from BD Biosciences (San Diego, CA). Antibodies against IKK-α, and IKK-β, were kindly provided by Imgenex (San Diego, CA).
Cell Lines
Human chronic myeloid leukemia (KBM-5) and human embryonic kidney carcinoma (A293) cells were obtained from the American Type Culture Collection (Manassas, VA). KBM-5 cells were cultured in Iscove’s modified Dulbecco’s medium with 15% fetal bovine serum. A293 cells were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Culture media were also supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin.
Electrophoretic Mobility Shift Assay
To examine NF-κB activation, we performed electrophoretic mobility shift assay (EMSA) as described previously[20]. Briefly, cells were washed with ice-cold phosphate-buffered saline and suspended in 0.4 mol of lysis buffer (10 mM HEPES, pH 7.9,10 mM KC1, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 0.5 mg/ml benzamidine). The cells were allowed to swell on ice for 15 min, after which 25 μl of 10% Nonidet P-40 was added. The tubes were then agitated on a vortex for 10 s and then microcentrifuged for 30 s. The nuclear pellets were resuspended in 25 μl of ice-cold nuclear extraction buffer (20mM HEPES, pH 7.9,0.4M NaCl, 1m M EDTA, 1m M EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 2.0 pg/ml leupeptin, 2.0 pg/ml aprotinin, and 0.5 mg/ml benzamidine), and the tubes were incubated on ice for 15 min with intermittent agitation. This nuclear extract were then microcentrifuged for 5 min at 4°C, and the supernatant was frozen at −70°C. Electrophoretic mobility shift assays (EMSAs) were performed by incubating 15 μg of nuclear extract with 16 fmol of 32P-end-labeled, 45-mer double-stranded NF-κB oligonucleotides from the human immunodeficiency virus long terminal repeat (5′-TTGTTACAA GGGACTTTC CGCTG GGGACTTTC CAGGGAGGCGTGG-3′; boldface indicates NF-κB binding sites) in the presence of 0.5 μg of poly(dI-dC) in a binding buffer (25 mM HEPES, pH 7.9,0.5mM EDTA, 0.5 mM dithiothreitol, 1% Nonidet P-40, 5% glycerol, and 50 mM NaCl) for 30 min at 37 °C. The DNA-protein complex formed was separated from free oligonucleotide on 6.6% native polyacrylamide gels using buffer containing 50 mM Tris, 200 mM glycine, and 1 mM EDTA, pH 8.5.
The specificity of binding was also examined by competition with the unlabeled oligonucleotide. For supershift assays, nuclear extracts prepared from TNF-treated cells were incubated with antibodies against either p50 or p65 of NF-κB for 15 min at 37 °C before the complex was analyzed by EMSA. The dried gels were visualized, and radioactive bands were quantified with a PhosphorImager (Amersham Biosciences, Piscataway, NJ) using ImageQuant software.
IKK Assay
To determine the effect of glutathione (GSH) on curcumin-mediated suppression of TNF-induced IKK activation, IKK assay was performed as described previously [21]. To determine the total amounts of IKK-α and IKK-β in each sample, 50 μg of the whole-cell protein was resolved on 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), electrotransferred to a nitrocellulose membrane, and blotted with antibodies against IKK-α or IKK-β.
Western Blot Analysis
To determine the levels of protein expression, we prepared whole cell extracts [22] and fractionated them by SDS-PAGE. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blotted with the appropriate antibodies, and detected by enhanced chemiluminescence (Amersham Biosciences). The bands obtained were quantified using NIH imaging software (Bethesda, MD).
NF-κB-Dependent Reporter Gene Expression Assay
NF-κB-dependent reporter gene expression was assayed as described [23]. To examine TNF-induced reporter gene expression, we transfected the cells with 0.5 μg of the SEAP expression plasmid and 2 μg of the control plasmid pCMVFLAG1 DNA for 24 h. We then treated the cells for 2 h with GSH and added curcumin at various concentrations. TNF (1 nM) was added after 4 h, and the cell culture medium was harvested collected after 24 h of TNF treatment. The culture medium was then analyzed for SEAP activity, essentially as described by the manufacturer’s instructions (Clontech, Palo Alto, CA), using a Victor 3 microplate reader (Perkin Elmer Life & Analytical Sciences, Boston, MA) with excitation at 360 nm and emission at 460 nm.
AP-1 Activation Assay
To assay AP-1 activation by EMSA, 10 μg of nuclear extract protein was incubated with 16 fmol of 32P-end-labeled AP-1 consensus oligonucleotide (5′-CGCTTGATGACTCAGCCGGAA-3′; bold indicates AP-1 binding site) for 30 min at 37 °C. The resulting DNA-protein complexes were resolved from free oligonucleotide on 6% native polyacrylamide gels [20]. The specificity of binding was examined by competition with unlabeled oligonucleotide. The radioactive bands were visualized and quantified as indicated above.
Live and Dead Assay
To measure apoptosis, we also used a Live and Dead viability/cytotoxicity kit (Molecular Probes, Eugene, OR), which determines intracellular esterase activity and plasma membrane integrity. This assay was performed as indicated [22].
Annexin V Assay
An early indicator of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphatidylserine from the cytoplasmic interface to the extracellular surface. This loss of membrane asymmetry can be detected using the binding properties of annexin V. To identify apoptosis, cells were stained with anti-annexin V antibody conjugated with fluorescein isothiocyanate (FITC). Briefly, KBM-5 cells were preincubated with various concentrations of GSH for 2 h, and then curcumin (50 μM) was added. After treatment with curcumin for 24 h at 37 °C, [24] cells were washed in phosphate-buffered saline, resuspended in 100 μl of binding buffer containing FITC-conjugated anti-annexin V antibody, and analyzed by flow cytometry (FACSCalibur; BD Biosciences). Data were collected from at least 10,000 cells at a flow rate of 250–300 cells/s.
TUNEL Assay
We also assayed cytotoxicity by the TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling) method, which examines DNA strand breaks during apoptosis, using an in situ cell death detection reagent (Roche Diagnostics, Mannheim, Germany). Briefly, KBM-5 cells were preincubated with various concentrations of GSH for 2 h, and then curcumin (50 μM) was added. After treatment with curcumin for 24 h at 37 °C [21], cells were incubated with reaction mixture for 60 min at 37 °C. Stained cells were analyzed by flow cytometry. Data were collected from at least 10,000 cells at a flow rate of 250–300 cells/s.
PARP Cleavage Assay
We examined caspase-3 activation by assaying PARP cleavage. Whole-cell extracts were prepared from treated cells in lysis buffer (20 mM Tris [pH 7.4], 250 mM NaCl, 2 mM EDTA [pH 8.0], 0.1% Triton X-100, 0.01 μg/ml aprotinin, 0.005 μg/ml leupeptin, 0.4 mM phenylmethylsulfonyl fluoride, and 4 mM sodium orthovanadate) [25]. The lysates were spun at 14,000 rpm for 10 min to remove insoluble material, resolved by 10% SDS-PAGE, and probed with anti-PARP antibodies.
Intracellular GSH Assay
To measure intracellular GSH, KBM-5 cells were incubated with the indicated concentrations of buthionine sulfoximine (BSO) or treated with curcumin. Monobromobimane (final concentration, 40 μM) was loaded into cells [26]. Fluorescence emission from cellular sulfhydryl-reacted monobromobimane was recorded using a flow cytometer. Monobromobimane is also known to react with small molecular weight thiols other than GSH but GSH forms the majority of monobromobimane reactive thiols and, for clarity, we address it as GSH levels in the subsequent text. There are several reports in the literature measuring GSH levels using this dye [27–29]. Data were collected from at least 10,000 cells at a flow rate of 250–300 cells/s.
Prooxidant measurements
To detect intracellular ROS, KBM-5 cells were preincubated with 20 μM oxidation-sensitive dichlorofluorescein diacetate (DCF-DA) or oxidation insensitive [5-(and-6)-carboxy-2, 7-dichlorofluorescein diacetate] for 15 min at 37 °C before being treated with various concentrations of curcumin. The oxidized form of the dye (DCF) acts as a control for changes in uptake, ester cleavage, and efflux [30].
After 2 h of incubation, the increase in fluorescence resulting from oxidation of H2DCF to DCF was measured by flow cytometry [31]. Measurements with the oxidation insensitive probe failed to detect any differences in the amount of fluorescence between the different treated groups. The mean fluorescence intensity at 530 nm was calculated. Data were collected from at least 10,000 cells at a flow rate of 250–300 cells/s.
RESULTS
The goal of this study was to investigate the mechanism by which curcumin exhibits anti-inflammatory and proapoptotic effects. Anti-inflammatory effects were examined by investigating the effect of curcumin on NF-κB activation pathway induced by TNF, one of the most potent proinflammatory cytokine.
Glutathione Abrogates Curcumin-Mediated Suppression of TNF-Induced NF-κB Activation Pathway
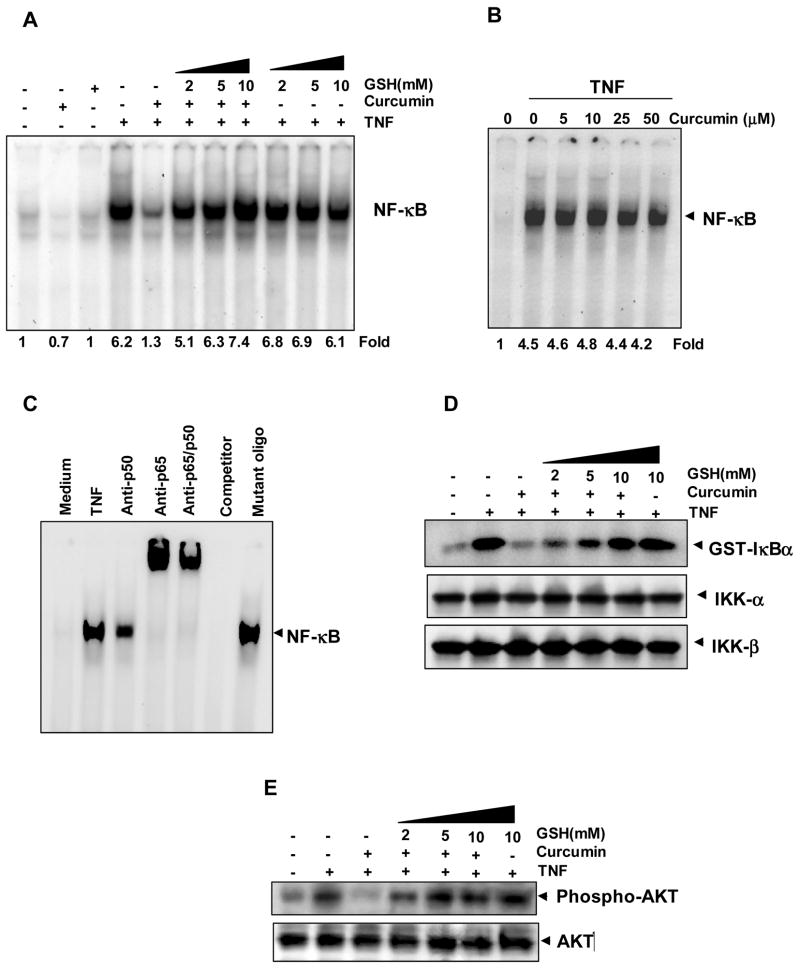
Since curcumin mediates its anti-inflammatory effects primarily through the downregulation of NF-κB, we investigated whether GSH can modulate the effect of curcumin on TNF-induced NF-κB activation. KBM-5 cells were exposed to curcumin in the presence of various concentrations of GSH, and NF-κB was activated by treating the cells with TNF. As indicated by the DNA-binding (EMSA), treatment of cells with TNF induced NF-κB activation, which was inhibited by the addition of curcumin (Fig. 1A). Pretreatment with GSH resulted in an almost-complete blockade of curcumin-mediated suppression of NF-κB activation in a dose-dependent manner. Glutathione alone did not have any effect on TNF-induced NF-κB activation at any of the concentrations used. Fig. 1B shows that curcumin did not modify the DNA-binding ability of NF-κB proteins prepared from TNF-treated cells directly up to 50 μM concentration.
FIGURE 1. Glutathione abrogates curcumin-mediated inhibition of NF-κB.
A, Curcumin inhibited TNF-induced activation of NF-κB and GSH blocked the effect of curcumin in a dose-dependent manner. KBM-5 cells were incubated with indicated concentrations of GSH for 2 h, treated with 50 μM curcumin for 4 h. Then cells were activated with 0.1 nM of TNF for 30 min, and then subjected to EMSA to assay for NF-κB activation. B, The direct effect of curcumin on the NF-κB complex was investigated. Nuclear extracts were prepared from untreated cells or cells treated with 0.1 nM TNF and incubated for 30 min with the indicated concentrations of curcumin. They were then assayed for NF-κB activation by EMSA. C, NF-κB induced by TNF is composed of p65 and p50 subunits. Nuclear extracts from untreated or TNF-treated cells were incubated with the indicated antibodies, unlabeled NF-κB oligo-probe, or mutant oligo-probe and then assayed for NF-κB activation by EMSA. D, Glutathione inhibited curcumin-mediated suppression of IKK activity. KBM-5 cells were preincubated with indicated concentrations of GSH for 2 h, treated with 50 μM concentration of curcumin for 4 h. Then cells were activated with 1 nM TNF for 15 min. Whole-cell extracts were immunoprecipitated with antibody against IKK-α and analyzed by an immune complex kinase assay. To examine the effect of curcumin ad GSH on level of IKK proteins, whole-cell extracts were fractionated on SDS-PAGE and examined by Western blot analysis using anti-IKK-α and anti-IKK-β antibodies. E, Curcumin suppressed TNF-mediated phosphorylation of AKT, as shown by Western blotting; GSH significantly blocked the effect of curcumin. KBM-5 cells were preincubated with indicated concentrations of GSH for 2 h, treated with 50 μM concentration of curcumin for 4 h. Then cells were activated with 1 nM TNF for 10 min. Whole cell extracts were prepared, separated on 10% SDS-PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was performed using phospho AKT antibody. Anti-AKT was used as a loading control.
When nuclear extracts from TNF-activated cells were incubated with antibodies to the p50 (NF-κB1) and p65 (RelA) subunit of NF-κB, the resulting bands was shifted to higher molecular masses, suggesting that the TNF-activated complex consisted of p50 and p65. The addition of excess unlabeled NF-κB (cold oligonucleotide, 100-fold) caused a complete disappearance of the band, whereas mutated oligonucleotide had no effect on DNA binding (Fig. 1C).
Activation of IKK is critical for activation of NF-κB by TNF, and curcumin has been shown to downregulate IKK [2]. We therefore examined whether GSH can modulate the ability of curcumin to inhibit TNF-induced IKK activity. We pretreated KBM-5 cells with various concentrations of GSH for 2 h and then exposed the cells to curcumin for 4 h. We then activated IKK by treating the cells with TNF for 15 min. TNF activated IKK activity, which was completely suppressed by curcumin; GSH blocked this inhibition in a dose-dependent manner (Fig. 1D). Neither GSH nor curcumin affected the expression of IKK-α or IKK-β.
Glutathione Blocks Curcumin-Mediated Suppression of TNF-Induced AKT Activation
TNF has also been shown to be a potent activator of AKT and curcumin has been shown to inhibit it [2]. Although AKT is essential for cell survival, its role in TNF-induced IKK activation is controversial [32]. We investigated whether GSH can affect the ability of curcumin to suppress TNF-induced AKT phosphorylation. As shown in Fig. 1E, TNF induced AKT phosphorylation, which was inhibited by curcumin. GSH blocked the effect of curcumin in a dose-dependent manner. GSH alone, however, did not affect TNF-induced AKT activation.
Glutathione Inhibits Curcumin’s Ability to Suppress TNF-Induced NF-κB Reporter Activity
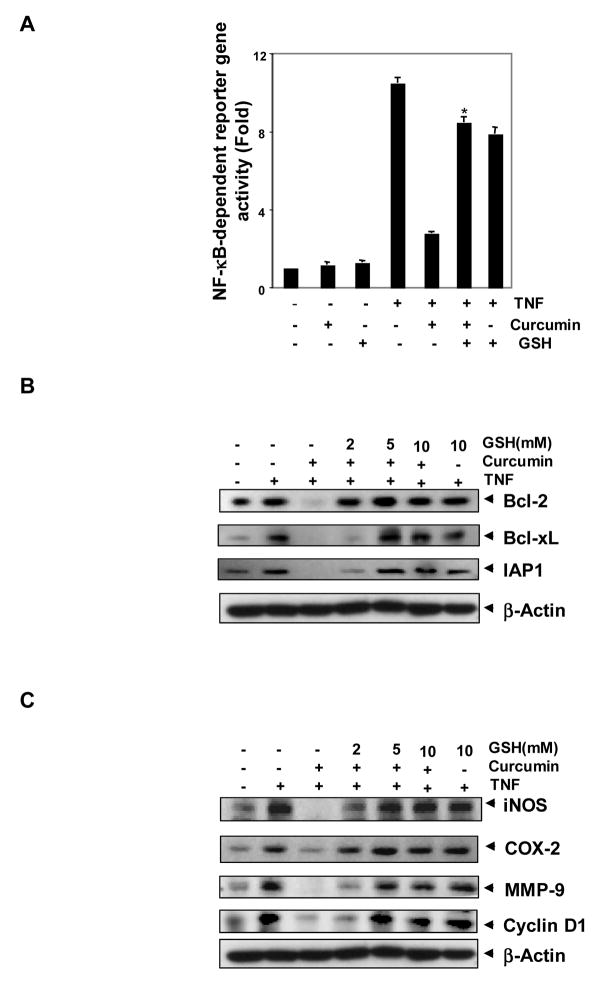
Although we showed by EMSA that GSH modifies the ability of curcumin to suppress NF-κB activation, DNA binding alone is not always associated with NF-κB-dependent gene transcription, suggesting that there are additional regulatory steps. In our reporter gene assay, TNF induced significant NF-κB-dependent reporter (SEAP) activity compared to the control. Curcumin inhibited this TNF-induced NF-κB reporter activity, and pretreatment with GSH significantly blocked the curcumin-mediated suppression of TNF-induced SEAP activity (Fig. 2A).
FIGURE 2. Glutathione blocks curcumin-mediated suppression of NF-κB regulated gene expression.
A, Curcumin significantly suppressed TNF-induced NF-κB reporter gene (SEAP) expression in A293 cells; GSH inhibited the effect of curcumin. A293 cells were transiently transfected with a NF-κB-containing plasmid for 24 h. After transfection, the cells were incubated with 2 mM GSH for 2 h, treated with 10 μM curcumin. Then cells were treated with 1 nM TNF for an additional 24 h. The supernatants of the culture medium were assayed for SEAP activity. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. *p < 0.01, as compared to curcumin plus TNF treated group. B and C, Glutathione blocked curcumin-mediated repression of TNF-induced NF-κB-dependent expression of antiapoptosis (B); proliferation and metastasis-related (C) gene products as shown by Western blotting. KBM-5 cells were incubated with indicated concentrations of GSH for 2 h, treated with 50 μM curcumin for 4 h. Then cells were activated with 1 nM TNF for 16 h. Whole-cell extracts were prepared and subjected to Western blot analysis using the relevant antibodies.
Glutathione Inhibits Curcumin-Mediated Suppression of TNF-Induced NF-κB-Regulated Gene Products
We also examined the effect of GSH on curcumin-mediated suppression of NF-κB down stream events. Because NF-κB regulates the expression of antiapoptotic proteins such as IAP1 [33], Bcl-2 [34], and Bcl-xL [35], we examined whether curcumin can modulate the expression of these antiapoptotic gene products and, if so, whether GSH can block the effect of curcumin. Western blot analysis showed that TNF induced these antiapoptotic proteins, whereas curcumin significantly suppressed them (Fig. 2B). Pretreatment with GSH inhibited the suppressive activity of curcumin. GSH alone had little effect on TNF-induced expression of these antiapoptotic proteins.
NF-κB is known to regulate the expression of proinflammatory and proliferative markers, including iNOS [36], COX-2 [37], MMP-9 [38], and cyclin D1 [39]. To determine whether GSH can inhibit the curcumin to suppress these gene products, cells were pretreated with various concentrations of GSH for 2 h, treated with curcumin for 4 h, and then exposed to TNF. TNF upregulated the levels of these proteins compared to the control, whereas curcumin significantly suppressed the expression of these gene products (Fig. 2C). In the cells pretreated with GSH, curcumin could not suppress gene expression by TNF.
Glutathione Inhibits Curcumin-Mediated Suppression of AP-1 Activity
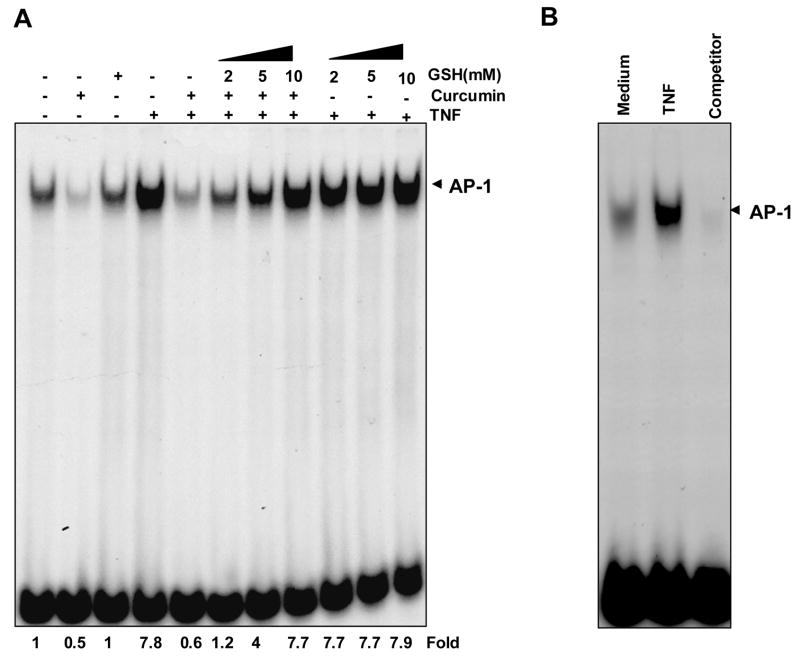
The transcription factor AP-1 regulates the expression of multiple genes essential for cell proliferation, differentiation, and apoptosis [40]. TNF is one of the most potent activators of AP-1 [41] whereas curcumin has been reported to suppress AP-1 activation [42]. To determine whether GSH affects the ability of curcumin to inhibit TNF-induced AP-1 activation, KBM-5 cells were pretreated with GSH for 2 h and then with curcumin for 4 h before AP-1 was activated with TNF. Unstimulated cells showed some basal AP-1 activity, which was suppressed by curcumin (Fig. 3A). TNF induced a several-fold increase in AP-1 levels, whereas curcumin suppressed it completely. Glutathione inhibited curcumin-mediated suppression of AP-1 activity in a concentration-dependent manner. GSH alone did not affect TNF-induced AP-1 activity at any of the concentrations used.
FIGURE 3. Effect of GSH on curcumin-mediated suppression of TNF-induced AP-1 activity.
A, Curcumin suppressed TNF-induced AP-1 activity completely and GSH significantly inhibited the effect of curcumin. KBM-5 cells were incubated with indicated concentrations of GSH for 2 h, treated with 50 μM curcumin for 4 h. Then cells were activated with 0.1 nM of TNF for 30 min, and then subjected to EMSA to assay for AP-1 activation. B, The specificity of binding was examined by competition with unlabeled oligonucleotide. Nuclear extracts from untreated or TNF-treated cells were incubated with the cold unlabeled AP-1 oligo-probe then assayed for AP-1 activation by EMSA.
When nuclear extracts from TNF-activated cells were incubated with excess unlabeled AP-1 (cold oligonucleotide, 100-fold) caused a complete disappearance of the band, suggesting the specificity of probe (Fig. 3B).
Glutathione Inhibits Curcumin-Induced Cell Death
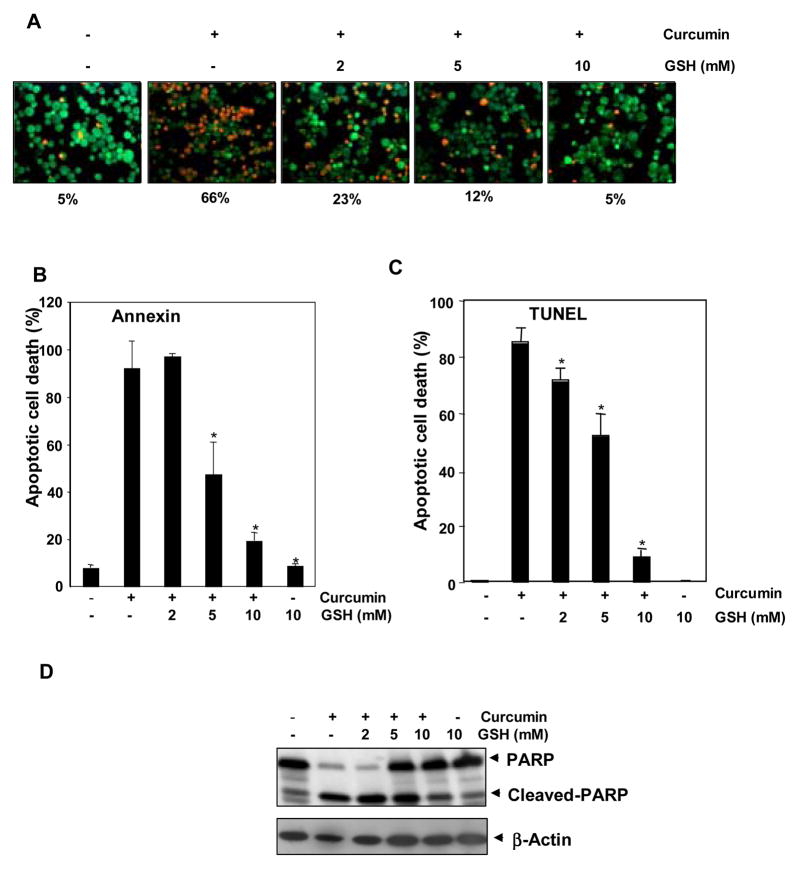
Curcumin is one of the most potent activators of apoptosis in tumor cells [16]. We investigated whether GSH can modulate curcumin’s ability to induce apoptosis. KBM-5 cells were pretreated with various concentrations of GSH for 2 h, after which curcumin was added and cell death was assayed using various techniques. As indicated by esterase staining, curcumin induced apoptosis, and GSH inhibited it in a dose-dependent manner (Fig. 4A). Similar results were obtained using annexin V (Fig. 4B) and TUNEL (Fig. 4C) staining. For instance, TUNEL staining revealed that treatment of cells with curcumin for 24 h induced about 80% cell death, whereas adding GSH almost completely blocked it in a dose-dependent manner. We also monitored curcumin-induced apoptosis by assaying caspase-3 activation, a hallmark of apoptosis. The induction of caspase-3-mediated PARP cleavage by curcumin was significantly inhibited by GSH (Fig. 4D).
FIGURE 4. Effect of GSH on curcumin-induced cell death.
Glutathione inhibited curcumin-induced cell death in a concentration-dependent manner. A, KBM-5 cells were pretreated with indicated concentrations of GSH for 2 h and then treated with 50 μM concentration of curcumin for 16 h. Cells were stained with Live and Dead assay reagent for 30 min and then analyzed under a fluorescence microscope. Red color highlights dead cells, and green color highlights live cells. B, Flow cytometric analysis of annexin V-FITC staining to detect early apoptotic effects. KBM-5 cells were pretreated with indicated concentrations of GSH for 2 h and then treated with 50 μM concentration of curcumin for 24 h. Cells were incubated with anti-annexin V antibody conjugated with FITC and then analyzed with a flow cytometer for early apoptotic effects. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. *p < 0.01, as compared to curcumin treated group. C, Flow cytometric analysis of TUNEL staining to detect apoptotic effects. KBM-5 cells were pretreated with indicated concentrations of GSH for 2 h and then treated with 50 μM concentration of curcumin for 24 h. Cells were fixed, stained with TUNEL reagent. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. *p < 0.01, as compared to curcumin treated group. D, Western blot analysis of PARP cleavage. KBM-5 cells were pretreated with indicated concentrations of GSH for 2 h and then treated with 50 μM concentration of curcumin for 24 h. Whole-cell extracts were prepared and subjected to Western blot analysis using anti-PARP antibody.
Downregulating Endogenous GSH Increases Curcumin-Mediated Cell Death
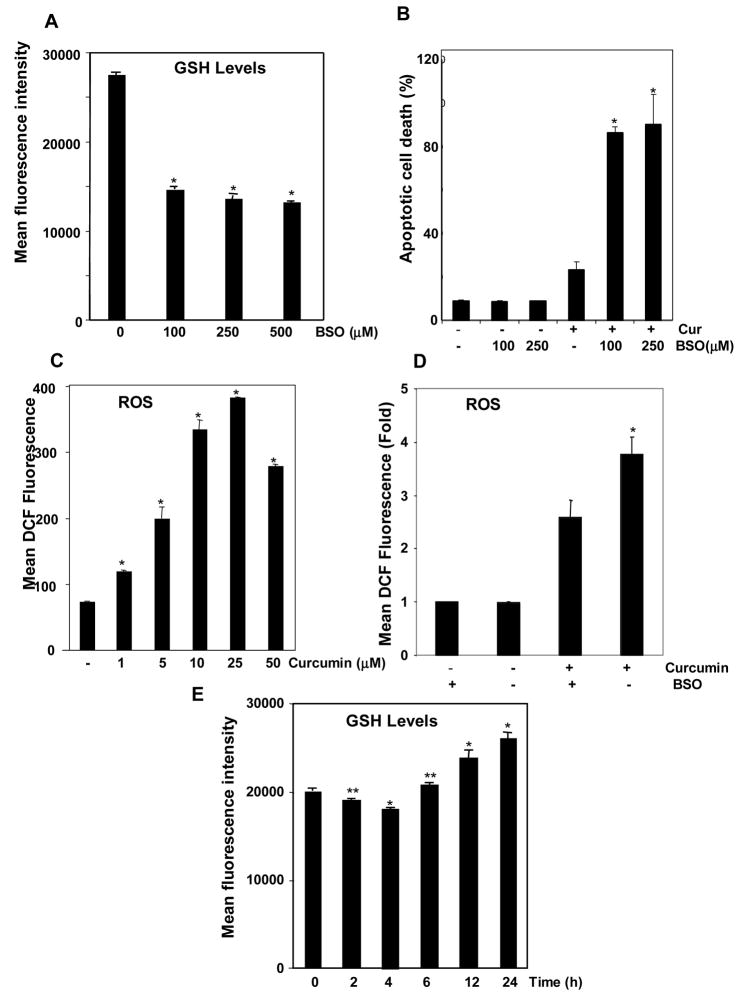
Since exogenous addition of GSH inhibited curcumin-mediated cell death, we determined whether the cytotoxicity of curcumin can be enhanced by downregulating endogenous GSH. Glutathione levels were measured by assaying monobromobimane fluorescence. Endogenous GSH levels were decreased by treating cells with a selective inhibitor of GSH synthesis (BSO) for 24 h (Fig. 5A). Curcumin induced apoptosis, as measured by annexin V-FITC staining and BSO treatment increased curcumin-induced apoptosis significantly (Fig. 5B). No significant differences on either GSH levels or apoptosis was observed between 100 and 250 μM BSO.
FIGURE 5. Depletion of endogenous GSH by pretreatment with BSO increases curcumin-induced cell death.
A, Flow cytometric analysis of monobromobimane, which becomes fluorescent after reacting with GSH, in KBM-5 cells. Glutathione levels were significantly depleted by treatment with BSO. KBM-5 cells were incubated with indicated concentrations of BSO for 24 h. Cells were stained with monobromobimane (40 μM) and acquired on flow cytometer. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. *p < 0.01, as compared to control. B, Decrease in endogenous GSH led to increased cytotoxicity of curcumin, as measured by annexin V staining. KBM-5 cells were incubated with indicated concentrations of BSO for 24 hr, treated with 10 μM concentration of curcumin for 24 h. Cells were incubated with anti-annexin V antibody conjugated with FITC. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. *p < 0.01, as compared to curcumin treated group. C, Curcumin induced generation of ROS in KBM-5 cells. ROS levels are measured using a dye, DCF-DA, which becomes fluorescent after reacting with ROS. Cells were stained with 20 μM DCF-DA for 20 min and then treated with different concentrations of curcumin for 2 h. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. *p < 0.01, as compared to control. D, Depletion of intracellular BSO led to further increase in ROS levels when treated with curcumin as compared to only curcumin treated group. KBM-5 cells were treated with or without BSO (250 μM) for 24 h prior to 20 μM DCF-DA addition. Then cells were incubated in presence or absence of 10 μM curcumin additional for 2h and DCF fluorescence was measured on flow cytometer. Each bar represents mean ± S.D. from three replicates. *p < 0.01, as compared to control. E, Curcumin changed the intracellular GSH levels. KBM-5 cells were treated with 10 μM curcumin for indicated time intervals before staining with 40 μM monobromobimane. Cells were acquired on flow cytometer. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. *p < 0.01, **p < 0.05, as compared to control.
Curcumin Induces ROS Generation in Cells
The evidence presented above suggests that curcumin mediates its effects through the prooxidant pathway. We used a DCF-DA probe to examine whether this mechanism can generate ROS inside cells. Cells were labeled with DCF-DA, treated with various concentrations of curcumin for 2 h, and analyzed by flow cytometry. Curcumin induced a significant increase in ROS levels over the control (Fig. 5C). This effect was observed at concentrations of curcumin as low as 1 μM and increased steadily up to 25 μM curcumin and declined slightly thereafter. ROS levels increased significantly in GSH depleted cells (BSO pretreated cells) upon treatment with curcumin as compared to control cells treated with curcumin (Fig. 5D).
Time course of changes in GSH levels upon treatment with curcumin
KBM-5 cells were treated with 10 μM curcumin for different intervals of time. Cells were stained with monobromobimane and fluorescence was measured on flow cytometer. Curcumin decreased GSH levels after 2 and 4 h of incubation but significant increase was seen at 16 and 24 h time points (Fig. 5E).
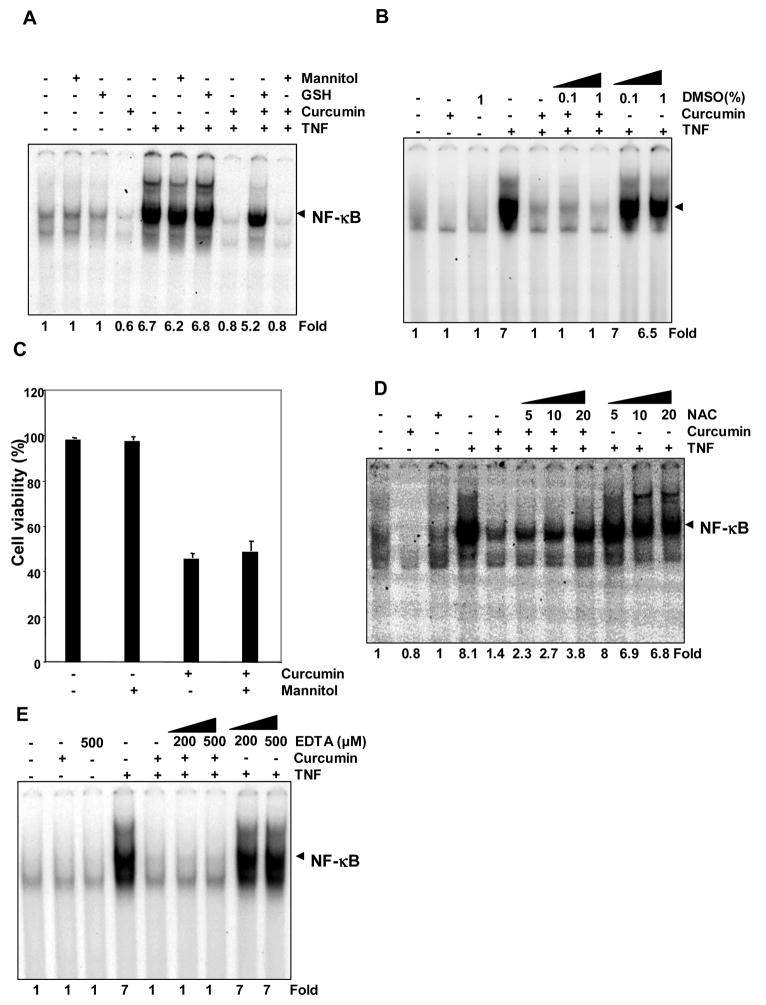
Hydroxyl Radical Quencher Mannitol and DMSO Has No Effect on Curcumin’s Ability to Suppress NF-κB Activation or Induce Apoptosis
We next sought to determine whether curcumin mediates its prooxidant effects through the production of hydroxyl radicals. We pretreated KBM-5 cells with mannitol, a well-known hydroxyl radical scavenger, for 2 h and then with curcumin for 4 h. We then treated the cells with TNF and analyzed for NF-κB activation. Glutathione was used as a positive control in this experiment. Mannitol had no effect on curcumin’s ability to suppress TNF-induced NF-κB activation (Fig. 6A). To confirm above results we used an alternative intracellular hydroxyl radical scavenger DMSO, and we found that DMSO had no effect on curcumin’s ability to suppress TNF- induced NF-κB activation (Fig. 6B). To determine whether mannitol can inhibit curcumin-mediated cell death, cells were pretreated with mannitol for 2 h and then incubated with curcumin. As revealed by a trypan blue exclusion assay, mannitol failed to block the reduction in cell viability caused by curcumin (Fig. 6C).
FIGURE 6. Mannitol and DMSO did not inhibit curcumin-mediated suppression of TNF-induced NF-κB activation.
A, Curcumin completely suppressed TNF-induced NF-κB activation and mannitol failed to block the effect of curcumin. KBM-5 cells were incubated with 10 mM mannitol for 2 h, treated with 50 μM curcumin for 4 h. Then cells were activated with 0.1 nM of TNF for 30 min, and then subjected to EMSA to assay for NF-κB activation. B, Curcumin completely suppressed TNF-induced NF-κB activation and DMSO failed to block the effect of curcumin. KBM-5 cells were incubated with either 0.1or 1% DMSO, treated with 50 μM curcumin for 4 h. Then cells were activated with 0.1 nM of TNF for 30 min, and then subjected to EMSA to assay for NF-κB activation. C, Curcumin induced significant cell death, as shown by trypan blue exclusion, but mannitol did not rescue the cells. KBM-5 cells were pretreated with 10 mM mannitol for 2 h and then treated with 50 μM curcumin for 24 h. Cell viability was measured by trypan blue exclusion. Each bar represents mean ± S.D. from three replicates and two independent experiments were carried out. D and E, Effect of NAC and EDTA on curcumin-mediated suppression of TNF-induced NF-κB activation. KBM-5 cells were incubated with either NAC for 24 h (D) or EDTA for 2 h (E). Then cells were treated with 50 μM curcumin for 4 h and were activated with 0.1 nM of TNF for 30 min. Nuclear extracts were subjected to EMSA for estimation of NF-κB.
NAC and EDTA failed to inhibit curcumin mediated suppression of NF-κB
NAC, a precursor for GSH synthesis was incubated with KBM-5 cells for 2 and 24h before treating the cells with curcumin for 4h. In another set of experiment, cells were treated with EDTA for 2 h prior to the addition of curcumin. We then treated the cells with TNF and analyzed for NF-κB activation. Pretreatment for 24 h with NAC (that increases intracellular GSH levels) lead to about 45% inhibition of curcumin mediated NF-κB suppression (Fig. 6D). However, 2 h prior treatment with NAC failed to inhibit curcumin’s NF-κB suppressive ability (Data not shown). EDTA also could not inhibit curcumin mediated suppression of NF-κB (Fig. 6E).
DISCUSSION
The goal of this study was to determine whether the anti-inflammatory and proapoptotic effects of curcumin are mediated through the antioxidant or prooxidant mechanism. Our results suggest that glutathione can block the ability of curcumin to suppress the TNF-induced activation of NF-κB, IKK, AKT, NF-κB reporter activity, and expression of antiapoptotic, proinflammatory, and proliferative gene products. We also found that curcumin-induced apoptosis can be inhibited by glutathione. Curcumin treatment also led to the production of ROS and changed the intracellular GSH levels. The proapoptotic activity of curcumin has been reported to be inhibited by superoxide dismutase and N-acetyl cysteine in leukemia cells [43], suggesting the involvement of superoxide radicals. In agreement with this report, a specific hydroxyl radical quencher mannitol had no effect on the proapoptotic activity of curcumin. All of this evidence suggests that the proapoptotic effects of curcumin are mediated through the prooxidant pathway. Similarly, the anti-inflammatory activity of curcumin (suppression of NF-κB) was unaffected by mannitol, EDTA or DMSO. Curcumin’s ability to suppress NF-κB was intercepted either by pretreatment of cells with exogenous GSH or through elevating endogenous GSH levels.
The mechanism by which curcumin mediates its prooxidant effects remains unclear. Mitochondria are the major source of ROS in the cell. Evidence from our laboratory and other suggest the role of mitochondria in curcumin induced apoptosis [16,44]. Thus, it is possible that curcumin activates mitochondrial enzymes that lead to production of ROS. The induction of ROS by curcumin could occur through its interaction with thioredoxin reductase [17] and thus changing its activity to NADPH oxidase which could then lead to the production of ROS. Moreover, glutathione has been shown to suppress curcumin-induced ROS production [45]. Several reports suggest that curcumin can induce ROS [18,45,46]. There are also reports which suggest that curcumin quenches ROS production [47,48] and thus acts as an antioxidant. Other reports suggest that curcumin quenches ROS production at low concentrations and induces ROS production at high concentrations [49].
It is not clear which structural group of curcumin is responsible for inducing ROS production. The finding that tetrahydrocurcumin is unable to produce ROS [45] suggests a role for the α,β-unsaturated carbonyl moiety of curcumin in the production of ROS. Curcumin is a Michael acceptor and thus can react with sulfhydryl groups [50]. Curcumin has been shown to be a thiol-modifying agent [51], although it does not oxidize protein thiols but rather alkylates them via a Michael addition [50]. Fang et al. showed that curcumin irreversibly inactivates thioredoxin reductase by alkylating a critical cysteine residue in the catalytic site of the enzyme [17]. This enzyme catalyzes NADPH-dependent reduction of thioredoxins, which play essential roles in substrate reduction, defense against oxidative stress, and redox regulation. Another recent report showed that curcumin also inhibits interleukin-1 receptor-associated kinase (IRAK) by modifying the protein’s cysteinyl sulfhydryl groups in vivo [52].
Whether the effects of GSH on the ability of curcumin to suppress inflammation and induce apoptosis occur through quenching of cellular ROS or through thiol modification is less clear. Curcumin has been shown to induce GSH biosynthesis [5]. It is unlikely that curcumin reacts with GSH directly under our conditions, as Oetari et al., 1996 showed that GSH prevents the instability of curcumin in phosphate buffer at pH 7.4 [53]. These authors have thoroughly studied the stability of curcumin in aqueous solvents in the presence of thiols. We used cell culture medium containing 10% FBS; and these conditions have been shown also to stabilize curcumin [54]. Curcumin has been shown to induce mitochondrial membrane-permeability transition pores through thiol oxidation [55]. Awasthi et al. demonstrated that curcumin forms conjugates with GSH by separating mono- and diglutathionyl adducts of curcumin [56]. This suggests that formation of curcumin-GSH adducts lead to inactivation of parent curcumin’s activity. This is, however, unlikely because glutathionylated curcumin has been reported to be more active than curcumin [57]. We also found that NAC reverses the effect of curcumin, most likely by increasing intracellular GSH contents. That NAC prevents the instability of curcumin in phosphate buffer pH 7.4, has been shown. Consistant with these finding, we found that depletion of intracellular GSH by BSO enhanced curcumin-induced apoptosis. These results are in agreement with Syng-ai et al [18].
Our results are in agreement with previous findings suggesting that ROS are needed for the apoptotic effects of curcumin [18,58–62]. Indeed, we found that depletion of endogenous GSH augmented curcumin-induced cell death in tumor cells. We also ruled out the involvement of hydroxyl radicals in our system. The complexity, however, lies with the suppression of NF-κB activation. It has been shown that ROS are needed for TNF-induced NF-κB activation [63]. Thus, it is not clear how both suppression and activation of NF-κB are mediated through ROS production. It may be that lower levels of ROS result in NF-κB activation, whereas higher levels of ROS suppress NF-κB activation. Another possibility is that the apoptotic effects of curcumin are mediated through ROS generation, whereas the NF-κB-suppressive effects are mediated through thiol modification. NAC, a ROS scavenger, failed to reverse the effect of curcumin mediated NF-κB suppression in 2h (Data not shown). However, 45% reversal was observed at 24 h which correlates with increase in intracellular GSH levels. Indeed, IRAK, a kinase needed for NF-κB activation by interleukin-1 and lipopolysaccharide, has been shown to be modified by curcumin [52]. A third possibility is that curcumin, like vitamin C, acts both as a prooxidant and an antioxidant. While the prooxidant mechanism mediates apoptotic effects, the antioxidant mechanism mediates NF-κB-suppressive effects.
Glutathione also abrogated the ability of curcumin to suppress TNF-induced activation of AP-1, the transcription factor implicated in induction of a number of genes involved in cell proliferation, differentiation, and immune and inflammatory responses [40]. Park et al. showed that curcumin inhibits AP-1 independent of their conserved cysteine residue [64]. Thus, curcumin may exert its antiproliferative effects through the downregulation of AP-1 and cyclin D1, as shown here; these effects also require the production of ROS. Whether in vitro concentrations of curcumin employed here are related to that in vivo, is not clear. Exposure of cells to a drug in vivo is usually much longer than that in vitro. Cheng et al., 2001 showed serum concentration of 1.75 μM [65]. There is little information on curcumin concentrations in tissues but biological responses both in rodents and humans, have been reported. Overall, our results suggest that the intracellular levels of GSH will influence curcumin’s anti-inflammatory and proapoptotic activities. Curcumin has been well proven to be pharmacologically safe in humans. Its proapoptotic and antininflammatory activities described here is applicable to a wide variety of diseases.
Acknowledgments
We would like to thank Pierrette Lo for carefully proofreading the manuscript and providing valuable comments.
This work was supported by the Clayton Foundation for Research (to B.B.A.), Department of Defense U.S. Army Breast Cancer Research Program Grant BC010610 (to B.B.A.), PO1 Grant CA91844 from the National Institutes of Health on lung chemoprevention (to B.B.A.), a P50 Head and Neck SPORE grant from the National Institutes of Health (P50CA97007 to B.B.A.).
ABBREVIATIONS
- GSH
Glutathione
- TNF
Tumor necrosis factor
- NF-κB
Nuclear factor-κB
- ROS
Reactive oxygen species
- EMSA
Electrophorectic mobility shift assay
- DCF-DA
Dichlorodihydrofluorescein diacetate
- FITC
Fluorescein isothiocyanate
- BSO
Buthionine sulfoximine
- SEAP
Secretory alkaline phophatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 3.Jang MK, Sohn DH, Ryu JH. A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF-alpha release from Curcuma zedoaria. Planta Med. 2001;67:550–552. doi: 10.1055/s-2001-16482. [DOI] [PubMed] [Google Scholar]
- 4.Gaddipati JP, Sundar SV, Calemine J, Seth P, Sidhu GS, Maheshwari RK. Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock. 2003;19:150–156. doi: 10.1097/00024382-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 7.Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–1962. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- 8.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251–262. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445–451. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 10.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- 11.Skrzypczak-Jankun E, McCabe NP, Selman SH, Jankun J. Curcumin inhibits lipoxygenase by binding to its central cavity: theoretical and X-ray evidence. Int J Mol Med. 2000;6:521–526. doi: 10.3892/ijmm.6.5.521. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 13.Surh YJ, Han SS, Keum YS, Seo HJ, Lee SS. Inhibitory effects of curcumin and capsaicin on phorbol ester-induced activation of eukaryotic transcription factors, NF-kappaB and AP-1. Biofactors. 2000;12:107–112. doi: 10.1002/biof.5520120117. [DOI] [PubMed] [Google Scholar]
- 14.Hussain AR, Al-Rasheed M, Manogaran PS, Al-Hussein KA, Platanias LC, Al Kuraya K, Uddin S. Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT pathway in acute T cell leukemias. Apoptosis. 2006;11:245–254. doi: 10.1007/s10495-006-3392-3. [DOI] [PubMed] [Google Scholar]
- 15.Korutla L, Kumar R. Inhibitory effect of curcumin on epidermal growth factor receptor kinase activity in A431 cells. Biochim Biophys Acta. 1994;1224:597–600. doi: 10.1016/0167-4889(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 16.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 18.Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–1108. [PubMed] [Google Scholar]
- 19.Patro BS, Rele S, Chintalwar GJ, Chattopadhyay S, Adhikari S, Mukherjee T. Protective activities of some phenolic 1,3-diketones against lipid peroxidation: possible involvement of the 1,3-diketone moiety. Chembiochem. 2002;3:364–370. doi: 10.1002/1439-7633(20020402)3:4<364::AID-CBIC364>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi MM, LaPushin R, Aggarwal BB. Tumor necrosis factor and lymphotoxin. Qualitative and quantitative differences in the mediation of early and late cellular response. J Biol Chem. 1994;269:14575–14583. [PubMed] [Google Scholar]
- 21.Takada Y, Khuri FR, Aggarwal BB. Protein farnesyltransferase inhibitor (SCH 66336) abolishes NF-kappaB activation induced by various carcinogens and inflammatory stimuli leading to suppression of NF-kappaB-regulated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:26287–26299. doi: 10.1074/jbc.M400963200. [DOI] [PubMed] [Google Scholar]
- 22.Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-kappa B activation through inhibition of I kappa B alpha kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol. 2003;171:3278–3286. doi: 10.4049/jimmunol.171.6.3278. [DOI] [PubMed] [Google Scholar]
- 23.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 24.Bharti AC, Takada Y, Shishodia S, Aggarwal BB. Evidence that receptor activator of nuclear factor (NF)-kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB-independent and TRAF6-dependent mechanism. J Biol Chem. 2004;279:6065–6076. doi: 10.1074/jbc.M308062200. [DOI] [PubMed] [Google Scholar]
- 25.Shishodia S, Gutierrez AM, Lotan R, Aggarwal BB. N-(4-hydroxyphenyl)retinamide inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of I(kappa)B(alpha) kinase and nuclear factor-kappaB-regulated gene products. Cancer Res. 2005;65:9555–9565. doi: 10.1158/0008-5472.CAN-05-1585. [DOI] [PubMed] [Google Scholar]
- 26.Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal A, Kaushal P, Agrawal S, Gollapudi S, Gupta S. Thimerosal induces TH2 responses via influencing cytokine secretion by human dendritic cells. J Leukoc Biol. 2007;81:474–482. doi: 10.1189/jlb.0706467. [DOI] [PubMed] [Google Scholar]
- 28.Bouzyk E, Iwanenko T, Jarocewicz N, Kruszewski M, Sochanowicz B, Szumiel I. Antioxidant defense system in differentially hydrogen peroxide sensitive L5178Y sublines. Free Radic Biol Med. 1997;22:697–704. doi: 10.1016/s0891-5849(96)00388-7. [DOI] [PubMed] [Google Scholar]
- 29.Deas O, Dumont C, Mollereau B, Metivier D, Pasquier C, Bernard-Pomier G, Hirsch F, Charpentier B, Senik A. Thiol-mediated inhibition of FAS and CD2 apoptotic signaling in activated human peripheral T cells. Int Immunol. 1997;9:117–125. doi: 10.1093/intimm/9.1.117. [DOI] [PubMed] [Google Scholar]
- 30.Andringa KK, Coleman MC, Aykin-Burns N, Hitchler MJ, Walsh SA, Domann FE, Spitz DR. Inhibition of glutamate cysteine ligase activity sensitizes human breast cancer cells to the toxicity of 2-deoxy-D-glucose. Cancer Res. 2006;66:1605–1610. doi: 10.1158/0008-5472.CAN-05-3462. [DOI] [PubMed] [Google Scholar]
- 31.Kumar SS, Shankar B, Sainis KB. Effect of chlorophyllin against oxidative stress in splenic lymphocytes in vitro and in vivo. Biochim Biophys Acta. 2004;1672:100–111. doi: 10.1016/j.bbagen.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 33.You M, Ku PT, Hrdlickova R, Bose HR., Jr ch-IAP1, a member of the inhibitor-of-apoptosis protein family, is a mediator of the antiapoptotic activity of the v-Rel oncoprotein. Mol Cell Biol. 1997;17:7328–7341. doi: 10.1128/mcb.17.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 35.Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 36.Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, Billiar TR, Geller DA. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 38.Esteve PO, Chicoine E, Robledo O, Aoudjit F, Descoteaux A, Potworowski EF, St-Pierre Y. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem. 2002;277:35150–35155. doi: 10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 41.Brenner DA, O’Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 42.Huang TS, Lee SC, Lin JK. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo ML, Huang TS, Lin JK. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim Biophys Acta. 1996;1317:95–100. doi: 10.1016/s0925-4439(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 44.Uddin S, Hussain AR, Manogaran PS, Al-Hussein K, Platanias LC, Gutierrez MI, Bhatia KG. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene. 2005;24:7022–7030. doi: 10.1038/sj.onc.1208864. [DOI] [PubMed] [Google Scholar]
- 45.Atsumi T, Fujisawa S, Tonosaki K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005;11:236–242. doi: 10.1111/j.1601-0825.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 46.Strasser EM, Wessner B, Manhart N, Roth E. The relationship between the anti-inflammatory effects of curcumin and cellular glutathione content in myelomonocytic cells. Biochem Pharmacol. 2005;70:552–559. doi: 10.1016/j.bcp.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Das KC, Das CK. Curcumin (diferuloylmethane), a singlet oxygen ((1)O(2)) quencher. Biochem Biophys Res Commun. 2002;295:62–66. doi: 10.1016/s0006-291x(02)00633-2. [DOI] [PubMed] [Google Scholar]
- 48.Mishra S, Kapoor N, Mubarak Ali A, Pardhasaradhi BV, Kumari AL, Khar A, Misra K. Differential apoptotic and redox regulatory activities of curcumin and its derivatives. Free Radic Biol Med. 2005;38:1353–1360. doi: 10.1016/j.freeradbiomed.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Wanming D, Zhang D, Liu Q, Kang J. Water-soluble antioxidants improve the antioxidant and anticancer activity of low concentrations of curcumin in human leukemia cells. Pharmazie. 2005;60:57–61. [PubMed] [Google Scholar]
- 50.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurrmann N, Brigelius-Flohe R, Bol GF. Curcumin blocks interleukin-1 (IL-1) signaling by inhibiting the recruitment of the IL-1 receptor-associated kinase IRAK in murine thymoma EL-4 cells. J Nutr. 2005;135:1859–1864. doi: 10.1093/jn/135.8.1859. [DOI] [PubMed] [Google Scholar]
- 53.Oetari S, Sudibyo M, Commandeur JN, Samhoedi R, Vermeulen NP. Effects of curcumin on cytochrome P450 and glutathione S-transferase activities in rat liver. Biochem Pharmacol. 1996;51:39–45. doi: 10.1016/0006-2952(95)02113-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 55.Morin D, Barthelemy S, Zini R, Labidalle S, Tillement JP. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Lett. 2001;495:131–136. doi: 10.1016/s0014-5793(01)02376-6. [DOI] [PubMed] [Google Scholar]
- 56.Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, Seifert WE, Jr, Awasthi YC, Ansari GA. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem Biol Interact. 2000;128:19–38. doi: 10.1016/s0009-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 57.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–314. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- 59.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min do S, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 60.Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26:1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 61.Fujisawa S, Atsumi T, Ishihara M, Kadoma Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004;24:563–569. [PubMed] [Google Scholar]
- 62.Khar A, Ali AM, Pardhasaradhi BV, Begum Z, Anjum R. Antitumor activity of curcumin is mediated through the induction of apoptosis in AK-5 tumor cells. FEBS Lett. 1999;445:165–168. doi: 10.1016/s0014-5793(99)00114-3. [DOI] [PubMed] [Google Scholar]
- 63.Garg AK, Aggarwal BB. Reactive oxygen intermediates in TNF signaling. Mol Immunol. 2002;39:509–517. doi: 10.1016/s0161-5890(02)00207-9. [DOI] [PubMed] [Google Scholar]
- 64.Park CH, Lee JH, Yang CH. Curcumin derivatives inhibit the formation of Jun-Fos-DNA complex independently of their conserved cysteine residues. J Biochem Mol Biol. 2005;38:474–480. doi: 10.5483/bmbrep.2005.38.4.474. [DOI] [PubMed] [Google Scholar]
- 65.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]