Abstract
gp96 (GRP94) elicits antigen-presenting cell (APC) activation and can direct peptides into the cross- presentation pathways of APC. These responses arise through interactions of gp96 with Toll-like (APC activation) and endocytic (cross-presentation) receptors of APC. Previously, CD91, the α2-macroglobulin receptor, was identified as the heat shock/chaperone protein receptor of APC. Recent data indicates, however, that inhibition of CD91 ligand binding does not alter gp96 recognition and uptake. Furthermore, CD91 expression is not itself sufficient for gp96 binding and internalization. We now report that scavenger receptor class-A (SR-A), a prominent scavenger receptor of macrophages and dendritic cells, serves a primary role in gp96 and calreticulin recognition and internalization. gp96 internalization and peptide re-presentation are inhibited by the SR-A inhibitory ligand fucoidin, although fucoidin was without effect on α2-macroglobulin binding or uptake. Ectopic expression of SR-A in HEK 293 cells yielded gp96 recognition and uptake activity. In addition, macrophages derived from SR-A–/– mice were substantially impaired in gp96 binding and uptake. These data identify new roles for SR-A in the regulation of cellular responses to heat shock proteins.
Keywords: antigen-presenting cell/calreticulin/gp96/GRP94/scavenger receptor
Introduction
Investigations into the identity of the tumor-specific transplantation antigens of MethA sarcoma led to the discovery of gp96 (GRP94), the endoplasmic reticulum (ER) heat shock protein (Hsp) 90 molecular chaperone, as a potent tumor antigen (Srivastava et al., 1986; Srivastava and Maki, 1991). Subsequent studies indicated that a number of chaperones, including GRP170, Hsp110, Hsp90, Hsp70 and calreticulin (CRT) could elicit anti-tumor immune responses in both prophylactic and therapeutic immunotherapy settings (Udono and Srivastava, 1993, 1994; Basu and Srivastava, 1999; Wang et al., 2001). Recent research has focused on the mechanism(s) by which these proteins elicit anti-tumor responses, with particular emphasis being placed on their interactions with antigen-presenting cells (APCs).
It is well established that complexes of Hsps and chaperone proteins with synthetic peptides can direct peptides into the MHC class-I antigen-presentation pathways of APCs (Suto and Srivastava, 1995; Singh-Jasuja et al., 2000b; Berwin et al., 2002b). By this process, chaperone–peptide complexes are thought to elicit tumor-directed CD8+ cytotoxic T lymphocyte (CTL) responses (Srivastava and Udono, 1994; Tamura et al., 1997; Yamazaki et al., 1999; Srivastava and Amato, 2001). In the case of Hsp70, substantial experimental evidence has been provided to suggest that tumor-derived, Hsp70-associated peptides can function as tumor antigens (Udono and Srivastava, 1993; Milani et al., 2002; Noessner et al., 2002). For gp96, recent evidence indicates that gp96-mediated tumor suppression can occur through peptide-independent activation of anti-tumor immune responses (Baker-LePain et al., 2002). Indeed, the question of whether Hsps function as peptide binding proteins in vivo has recently come under question (Baker-LePain et al., 2003; Reits et al., 2003).
In a manner independent of bound peptides, gp96, Hsp70 and Hsp60 elicit dendritic cell maturation, activation and associated cytokine secretion (Asea et al., 2000, 2002; Binder et al., 2000a; Kol et al., 2000; Singh-Jasuja et al., 2000a; Vabulas et al., 2001, 2002; Zheng et al., 2001; Milani et al., 2002; Panjwani et al., 2002). At present, the APC receptors responsible for mediating the diverse interactions of Hsps with APC are poorly understood. Several recent studies have identified candidate receptors for the different Hsps. Initially, CD91 [LDL receptor-related protein (LRP)/α2-macroglobulin (α2M) receptor] was reported to be the unique receptor responsible for the re-presentation of peptides associated with gp96, Hsp70, Hsp90 and CRT (Binder et al., 2000b; Basu et al., 2001). However, it now appears unlikely that CD91 is the unique chaperone receptor; its expression does not positively correlate with chaperone binding capacity and both CD40 and the scavenger receptor LOX-1 have been demonstrated to function in Hsp70 internalization (Becker et al., 2002; Berwin et al., 2002a; Delneste et al., 2002). Here we identify scavenger receptor class-A (SR-A) as a predominant endocytic receptor for the chaperones gp96 and CRT.
SR-A is expressed on macrophages and dendritic cells and was originally identified as a clearance receptor for acetylated low-density lipoprotein (AcLDL) (Kodama et al., 1988; Platt and Gordon, 1998). Subsequent work has demonstrated that SR-A serves multiple roles in innate immunity, including the receptor-mediated phagocytosis of bacteria and the internalization of lipopolysaccharide and lipoteichoic acid (Hampton et al., 1991; Peiser et al., 2000; Peiser and Gordon, 2001). In addition, SR-A performs a signaling role in cells, acting through Gi/o (Whitman et al., 2000; Post et al., 2002) and the protein tyrosine kinase Lyn (Miki et al., 1996). We report that cell surface binding and uptake of gp96 and CRT into macrophages is efficiently blocked by the SR-A ligands fucoidin and carrageenan (Radsak et al., 2003). Using genetic models we demonstrate that ectopic expression of SR-A confers cell surface binding and uptake of gp96 and CRT, whereas genetic deletion of SR-A impairs gp96 and CRT binding and uptake. Data are also provided demonstrating that SR-A can direct bound ligands to an endosomal compartment previously shown to be involved in antigen re-presentation.
Results
Identification of the gp96/CRT receptor
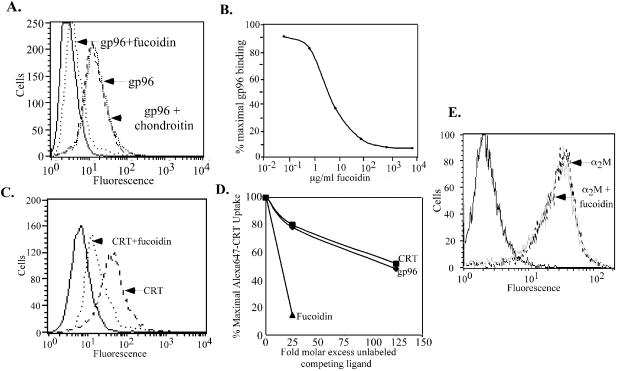
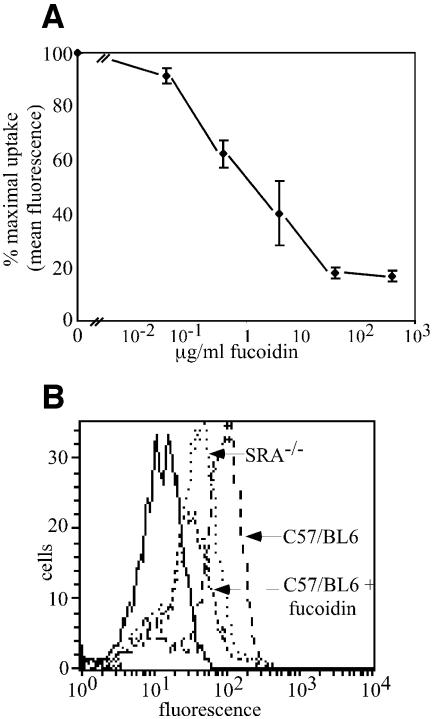
Binding of gp96 to APC cell surface receptors is saturable, of high affinity and is necessary for the trafficking of chaperone-associated peptides into the MHC class-I re-presentation pathway (Binder et al., 2000b; Singh-Jasuja et al., 2000b; Berwin et al., 2002a,b). To investigate the mechanism of chaperone uptake into APC, gp96 and CRT were covalently labeled with fluorophores and their interactions with APC examined (Wassenberg et al., 1999; Berwin et al., 2002b). Both proteins were observed to bind to cell surface receptors of APC, and this binding was competed by the polyanionic SR-A ligands fucoidin (Figure 1A–C; Radsak et al., 2003) and carrageenan (data not shown), but not by the polyanion chondroitin sulfate (Figure 1A). Fucoidin-dependent inhibition of gp96 binding to both elicited peritoneal macrophages (Figure 1B) and RAW264.7 macrophages (data not shown) was dose-dependent, with half-maximal inhibition at ∼4 µg/ml fucoidin; ∼90% of the total cellular binding activity was fucoidin-sensitive (Figure 1B). Similarly, CRT binding to macrophages was efficiently competed by fucoidin (Figure 1C). Competition studies demonstrated that cell surface binding of fluor-conjugated gp96 and calreticulin could be partially inhibited by the presence of increasing concentrations of unlabeled chaperone proteins (Figure 1D). The basis for the partial competition of binding observed with unlabeled chaperone proteins, as contrasted with the efficient competition observed with fucoidin, is not yet clear. Such data do suggest, however, the existence of multiple modes of chaperone protein binding to APC cell surface receptors.
Fig. 1. The SR-A inhibitory ligand fucoidin blocks binding of gp96 and calreticulin to peritoneal macrophages. (A) Fucoidin, but not chondroitin sulfate (75 µg/ml), inhibits the binding of fluorescein(Fl)-conjugated gp96 to elicited peritoneal macrophages. Fucoidin (75 µg/ml) or chondroitin sulfate (75 µg/ml) was added to cultures of adherence-selected peritoneal murine macrophages in the presence of 8 µg/ml Fl–gp96 for 30 min on ice. Cells were subsequently washed, fixed and processed for flow cytometry. (B) Fucoidin inhibits gp96 binding to elicited peritoneal macrophages in a dose-dependent manner. Fl–gp96 binding to peritoneal macrophages was performed in the presence of increasing concentrations of fucoidin, the cells fixed, and analyzed by flow cytometry. (C) Fucoidin (250 µg/ml) inhibits the binding of fluorescein-conjugated calreticulin (20 µg/ml) to elicited peritoneal macrophages. Experiments were conducted as in (A). (D) Uptake of Alexa-conjugated calreticulin is partially inhibited by non-conjugated gp96 or unconjugated calreticulin (CRT). Cellular internalization of Alexa-conjugated calreticulin was assayed in the presence of increasing concentrations of non-conjugated gp96 or calreticulin. The partial inhibition of CRT internalization observed with protein ligands is contrasted by that seen with fucoidin (filled triangles). (E) The binding of the CD91 ligand α2M to elicited peritoneal macrophages is not inhibited by fucoidin. Alexa-conjugated α2M binding to peritoneal macrophages was performed in the presence of a 10-fold molar excess of fucoidin, as in (A). Solid lines in (A), (C) and (E) represent autofluorescence.
The CD91/LRP ligand α2M has previously been reported to block gp96-dependent peptide re-presentation in macrophages (Binder, 2000). The fucoidin sensitivity of α2M binding to macrophages was thus examined and, as previously reported (Imber et al., 1982), no inhibition of α2M binding by fucoidin was observed (Figure 1E). These data indicate that gp96 and α2M are recognized by distinct receptors.
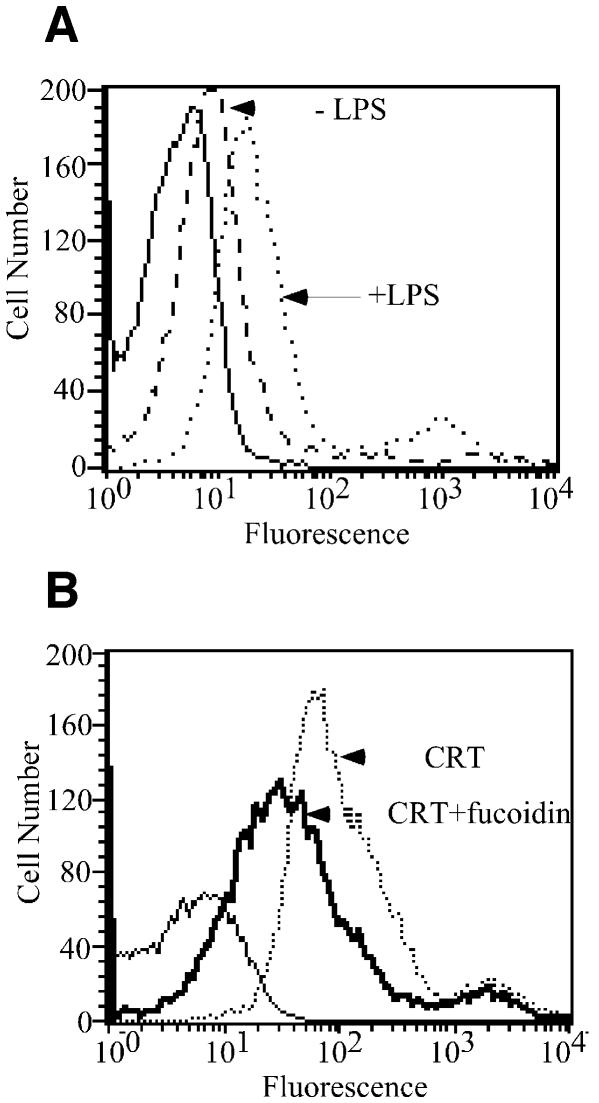
Fucoidin and carrageenan are ligands for several receptor families, including members of the scavenger receptor superfamily (Peiser and Gordon, 2001). To distinguish between candidate classes of scavenger receptors, we investigated the effects of endotoxin-elicited receptor up-regulation on gp96 and CRT binding to RAW264.7 cells. As has been reported previously, in RAW264.7 cells, lipopolysaccharide (LPS)-induced up-regulation of a fucoidin-binding receptor is consistent with the class-A, but not class-B, scavenger receptors (Fitzgerald et al., 2000; Baranova et al., 2002). Binding of CRT (Figure 2A) and gp96 (data not shown) increased ∼3-fold (geometric mean fluorescence) upon endotoxin treatment of RAW264.7 cells. Significantly, the endotoxin-elicited increase in CRT binding capacity was fucoidin sensitive (Figure 2B).
Fig. 2. Endotoxin stimulation of RAW264.7 macrophages elicits the upregulation of a fucoidin-sensitive gp96/CRT receptor. (A) RAW264.7 macrophages were treated with 10 ng/ml LPS for 16 h. Following treatment, cells were washed and the binding of fluorescein-conjugated CRT examined, as described in the legend to Figure 1. (B) CRT- binding to the up-regulated receptor is fucoidin-sensitive. As in (A), LPS stimulated RAW264.7 macrophages were incubated with 5 µg/ml fl–CRT in the presence or absence of 25 µg/ml fucoidin and binding histograms determined by flow cytometry.
SR-A-mediated gp96 and CRT internalization
Scavenger receptors bind and internalize a diverse array of ligands including modified LDL, fucoidin, apoptotic cells, bacterial LPS and lipoteichoic acid, and poly(I) (reviewed in Peiser and Gordon, 2001). Although structurally unrelated, these ligands share the common property of being polyanionic, as are gp96 and CRT (Krieger and Herz, 1994). Not all polyanions are SR ligands, however, as exemplified by chondroitin sulfate. To evaluate SR-A as a candidate receptor for gp96 and CRT, we used HEK 293 cells, which do not express SR-A. 293 cells were stably transfected with the SR-AII cDNA under an inducible tet promoter. These cells are referred to as HEK-SRAtet (Post et al., 2002). It should be noted that SR-AII is a splicing variant of the gene that encodes SR-AI, SR-AII and SR-AIII; SR-AIII is not expressed at the cell surface; no functional difference between SR-AI and SR-AII has been reported (reviewed in Peiser and Gordon, 2001).
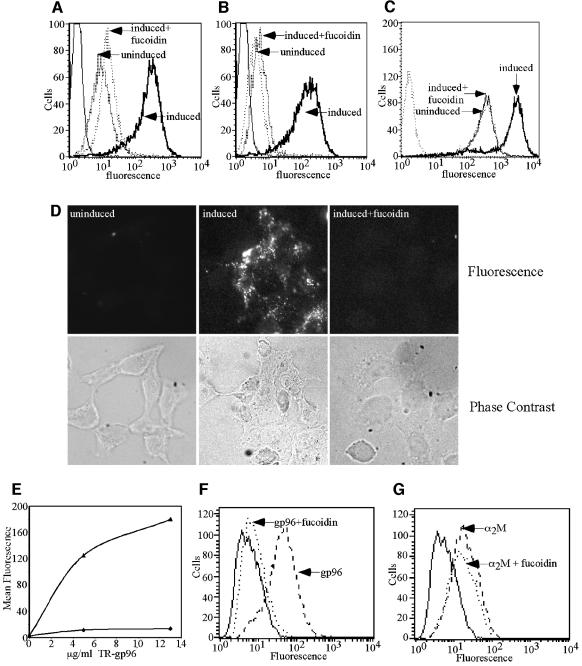
gp96 and CRT uptake was examined in uninduced- (absence of SR-A expression) and induced- (SR-A-expressing) HEK-SRAtet cells, with competition by fucoidin used as a control for ligand specificity. We observed that SR-A expression markedly enhanced (>50-fold) gp96 (Figure 3A and D) and CRT (Figure 3B) internalization. No significant uptake of gp96 or CRT was observed in uninduced HEK-SRAtet cells. As a positive control, an established SR-A ligand, AcLDL, was used in parallel (Figure 3C). SR-A-mediated uptake of both gp96 and CRT was abrogated by the addition of an ∼5× molar excess of fucoidin (Figure 3A, B and D). gp96 uptake was both receptor expression- and ligand concentration-dependent (Figure 3E). The EC50 of fucoidin-sensitive gp96 uptake by induced (SR-A-expressing) HEK-SRAtet cells was 22 ± 12 nM (SD, n = 3), indicative of highly efficient uptake and complementary to previously observed high affinity cell surface binding of gp96 to macrophages (Berwin et al., 2002a). To extend these findings to APC, we examined the effects of fucoidin on chaperone binding and uptake in elicited macrophages. Using an established ligand uptake protocol (Berwin et al., 2002b), we observed that the SR-A ligand fucoidin inhibited gp96 (Figure 3F), but not α2M uptake (Figure 3G).
Fig. 3. SR-A expression is sufficient for the binding and internalization of gp96 and CRT. HEK 293 cells bearing SR-AII under control of a tetracycline inducible promoter (HEK-SRATET) were examined for gp96 and CRT uptake in the uninduced and induced state (see Materials and methods). Both populations of cells were incubated with 12 µg/ml Fl–gp96 (A), Fl–CRT (B) or 2.5 µg/ml Alexa488–AcLDL (C) at 37°C to assess receptor-mediated uptake. Where indicated, 75 µg/ml fucoidin was included to block SR-A-dependent uptake. Following the uptake period, cells were trypsinized and chaperone uptake assessed by flow cytometry. (D) Confocal microscopy (and corresponding phase contrast pictures, below) of Fl–gp96 internalization by HEK-SRATET cells (as in A) when uninduced (not expressing SR-A; left), induced (expressing SR-A; middle) and induced, in the presence of fucoidin (right). (E) Uptake of TR–gp96 by HEK-SRATET cells is saturable and SR-A expression-dependent (uninduced cells, diamonds; induced cells, triangles). LPS-stimulated RAW264.7 macrophages (as in Figure 2) were assayed for their ability to internalize 8 µg/ml Fl–gp96 (F) or Fl–α2M (G) in the presence or absence of 175 µg/ml fucoidin, as described in the legend to Figure 1. Uptake was analyzed by flow cytometry. Solid lines in (A), (B), (F) and (G) are autofluorescence.
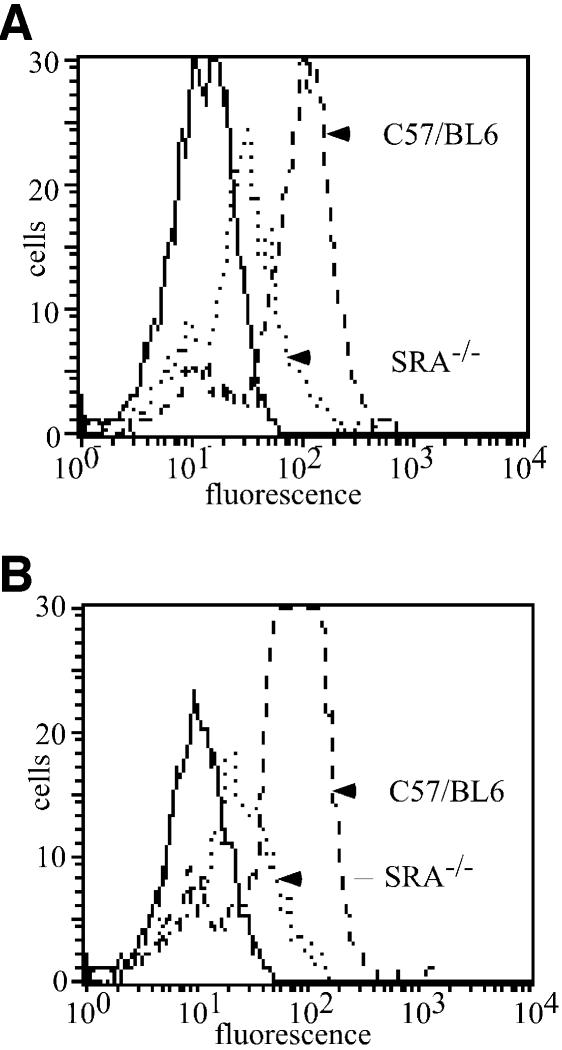
Having established that ectopically expressed SR-A is capable of mediating the internalization of gp96 and CRT and that inhibitory ligands of SR-A compete for gp96 and CRT binding and uptake, we examined the contribution of endogenous SR-A to chaperone uptake in APC using the genetic model of elicited macrophages derived from SR-A knockout (SRA–/–) mice (C57/BL6 background) (Suzuki et al., 1997; Kunjathoor et al., 2002). SR-A–/– macrophages were impaired in their ability to bind both gp96 (Figure 4A) and CRT (Figure 4B) as compared to macrophages derived from C57/BL6 mice, exhibiting an ∼50% reduction in binding. Concomitantly, the ability of fucoidin to compete binding of chaperones to SR-A–/– macrophages was decreased.
Fig. 4. SR-A–/– macrophages are deficient in gp96 and CRT binding. Elicited peritoneal macrophages from C57/BL6 and SR-A–/– (C57/BL6 background) mice were assayed for their ability to bind and internalize gp96 and CRT. Cell surface binding experiments were performed for 30 min at 4°C; TR–gp96 (A) TR–CRT (B). Binding of either chaperone to SR-A–/– macrophages was ∼50% that observed for C57/BL6-derived macrophages. Solid lines represent autofluorescence.
We then assessed whether the impairment of chaperone binding to SR-A–/– APC led to a deficiency in chaperone uptake. Fluorescently labeled gp96 (Figure 5A) or CRT (data not shown) were incubated with peritoneal macrophages at 37°C in the presence of varying fucoidin concentrations. Fucoidin competed ∼80% of chaperone binding, with half-maximal inhibition observed at ∼2 µg/ml fucoidin. Extending the binding studies, we then tested SR-A–/– macrophages for their ability to internalize chaperone proteins. SR-A–/– derived macrophages displayed ∼50% of the CRT uptake activity observed in C57/BL6-derived macrophages (Figure 5B). Similar results were observed for gp96 (data not shown). These studies, utilizing genetic models, further indicate that SR-A functions in the recognition and internalization of gp96 and CRT.
Fig. 5. SR-A mediates gp96 and CRT uptake. (A) Fluorescently labeled gp96 was incubated with elicited peritoneal macrophages for 30 min, 37°C in the presence of the indicated concentrations of fucoidin. Subsequently cells were washed fixed, and chaperone uptake assayed by FACS analysis. (B) SR-A–/– macrophages were tested for their ability to endocytose CRT, relative to wild-type (C57Bl/6-derived) macrophages. Analogous to the experiments performed in (A), chaperone internalization experiments were performed for 7 min at 37°C. SR-A–/– macrophages display ∼50% of the receptor-mediated chaperone uptake of control macrophages. Similar results were observed for gp96.
SR-A directs re-presentation of gp96-associated peptides
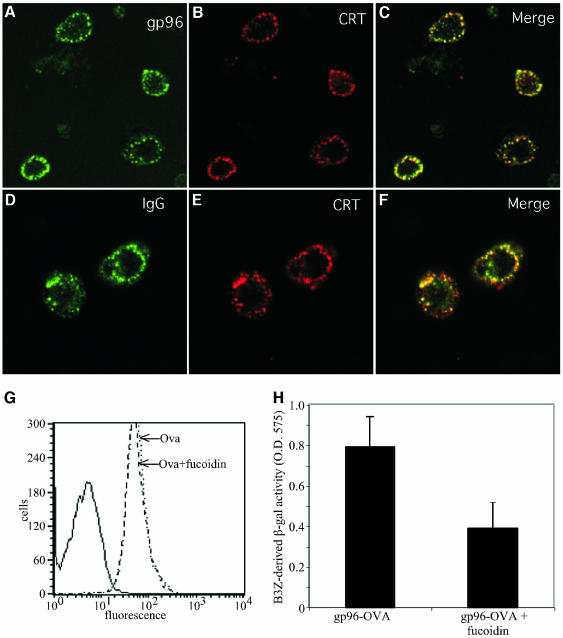
FcγR mediates the uptake of IgG–antigen complexes, with the antigen subsequently cross-presented on cell surface MHC class-I molecules (Regnault et al., 1999; Guyre et al., 2001). Previously, we demonstrated that Fc receptors, and their accompanying cargo, are directed into a subset of early endosomes that are also accessed by receptor-internalized gp96 (Mellman, 1996; Regnault et al., 1999; Guyre et al., 2001; Berwin et al., 2002b). Existing evidence suggests that this compartment can participate in a process of antigen (peptide) exchange onto mature MHC class-I molecules (Kleijmeer et al., 2001; Berwin et al., 2002b). As would be predicted from results indicating that gp96 and CRT share a common endocytic receptor, CRT and gp96 co-localize upon uptake to a peripheral endosomal subpopulation (Figure 6A–C; Berwin et al., 2002b) and, correspondingly, CRT also co-localizes with FcγR upon uptake (Figure 6D–F). To test whether SR-A-mediated uptake is responsible for trafficking chaperones into the re-presentation pathway, we determined whether fucoidin would inhibit gp96-dependent peptide re-presentation. To this end, the Kb ovalbumin peptide epitope SIINFEKL (Ova) was complexed with gp96 and re-presentation of this peptide on macrophage MHC class-I molecules assessed using the reporter T-cell hybridoma B3Z (Karttunen et al., 1992; Shastri and Gonzalez, 1993) and the Kb/Ova-specific antibody 25-D1.16 Ab (Porgador et al., 1997). Fucoidin inhibited the re-presentation of the gp96-associated Ova 52 ± 14% (Figure 6H), while fucoidin did not inhibit the presentation of Ova peptide alone (Figure 6G and H). These data indicate that SR-A can direct gp96–peptide complexes into an MHC class-I re-presentation pathway.
Fig. 6. SR-A directs gp96 and CRT to an FcR+ endosomal compartment competent for the re-presentation of gp96-associated peptides. (A–F) Fluorescein-labeled gp96 (A and C) or IgG (D and F) and TR–CRT (B,C, E and F) were allowed to bind to the surface of macrophages on ice and the cells were subsequently washed to remove unbound ligand. Cells were then warmed to 37°C for 10 min, to allow ligand internalization, and the cells subsequently fixed and processed for confocal microscopy. Extensive co-localization (yellow) of receptor-internalized gp96 and IgG with CRT was observed (C,F). (G) 100 nM Ova peptide was incubated with C57/BL6-derived peritoneal macrophages in the presence or absence of 250 µg/ml fucoidin for 2 h. Surface presentation of the Ova peptide in the context of MHC class-I molecules was then assessed with the Kb-, Ova-specific monoclonal antibody 25-D1.16. (H) gp96–Ova peptide covalent complexes (50 µg/ml; ∼250 nM gp96) or unconjugated OVA peptide (100 nM or 1 µM) were incubated with C57/BL6-derived peritoneal macrophages in the presence or absence of 250 µg /ml fucoidin for 30 min. The macrophages were then washed and incubated with B3Z reporter T-cells, which recognize Ova in the context of Kb MHC class-I molecules. B3Z activation was scored as β-galactosidase activity.
Discussion
We report that the scavenger receptor SR-A mediates the recognition and internalization of gp96 and CRT in elicited murine macrophages, a murine macrophage cell line and human cells expressing SR-A under control of an inducible promoter. The identification of SR-A as an endocytic receptor for gp96 and CRT further expands the scope of scavenger receptor family protein function in heat shock/chaperone protein processing by APCs. Subsequent to the identification of CD91 (LRP, α2M receptor) as the common endocytic receptor for gp96, Hsp90, Hsp70 and CRT (Binder et al., 2000b; Basu et al., 2001), further studies have identified primary functions for the class A (present study), the class B (CD36) (Panjwani et al., 2000) and the endothelial (LOX-1) scavenger receptors (Delneste et al. 2002) in heat shock/chaperone protein recognition and internalization.
Extending from the observations that CD91 expression did not positively correlate with gp96 cell surface binding or internalization, and that α2M*, the primary physiological ligand for CD91, did not block gp96 cell surface binding or uptake, we proposed the existence of an alternative, CD91-independent pathway for gp96 internalization by APC (Berwin et al., 2002a). The data in the current report identify a primary role for SR-A in such a pathway, a finding of particular significance, as receptor-mediated endocytosis is the mechanism by which gp96-associated antigens gain access to the cross-presentation pathway of APC (Singh-Jasuja et al., 2000b). In further support of this proposal, we observed that >90% of macrophage cell-surface gp96 binding, and >75% of gp96 uptake, can be blocked by the SR-A ligand fucoidin. In contrast, α2M binding to macrophages is not competed by fucoidin. Furthermore, and as determined in SR-A–/– macrophages, over half of the macrophage binding and endocytosis of gp96 and CRT are directly attributable to SR-A. Intriguingly, the data regarding fucoidin inhibition of gp96 and CRT binding and uptake corresponds well with the levels of inhibition observed for other SR-A ligands, including apoptotic cells (Platt et al., 1996) and Escherichia coli (Peiser et al., 2000). These data thus suggest that there exists on macrophages redundant SR-A ligand (fucoidin) binding/uptake activity (with another fucoidin-binding receptor accounting for ∼30% of the chaperone binding), or that a compensatory receptor(s) is upregulated upon SR-A inactivation. With regard to either scenario, it is known that the anti-SR-A antibody 2F8 will block adhesion of macrophages to tissue culture plastic (Fraser et al., 1993), yet SR-A–/– macrophages retain the capacity to adhere.
Scavenger receptors can function in antigen cross-presentation (Abraham et al., 1995; Bansal et al., 1999), a finding that places them at the crossroads of the innate and the adaptive cellular immune systems. SR-A seemingly utilizes pattern recognition to bind ligands including apoptotic cells, bacteria, modified lipoproteins, and now resident molecular chaperones of the ER (Peiser and Gordon, 2001). We find that SR-A activity can initiate the process of re-presentation of gp96-associated peptide antigens, while a scavenger receptor on human dendritic cells, LOX-1, has recently been shown to traffic Hsp70-associated peptides into the re-presentation pathway of dendritic cells (Delneste et al., 2002). Delivery of peptides on vehicles such as antibodies and chaperones has previously been shown to be highly effective in raising CD8+ T-cell responses and, in some cases, protective and therapeutic anti-tumor responses (Blachere et al., 1997; Regnault et al., 1999; Castellino et al., 2000; Delneste et al., 2002). Indeed, targeting peptides into APCs via heat shock/chaperone proteins is much more efficacious in generating in vivo responses than using peptide alone (Blachere et al., 1997). Intriguingly, recent data suggest that peptides derived from the intracellular processing of the Hsps themselves may be of immunological significance; Michaelsson et al. (2002) report that a fragment of the Hsp60 signal peptide can be presented by the non-classical MHC class-I molecule HLA-E. Such complexes are not recognized by CD94/NKG2A inhibitory receptors, potentially resulting in the activation of natural killer cell activity. We speculate that peptides generated from other Hsps may render similar effects.
Linked to the ability of chaperones to stimulate the innate immune system is the capacity of chaperone receptors to activate cellular signal transduction pathways. Several reports have indicated chaperones can signal through cell surface Toll-like receptors (TLRs) and CD14, thereby stimulating cell activation and cytokine secretion (Asea et al., 2000, 2002; Vabulas et al., 2001, 2002). However, endotoxin contamination of Hsp preparations continues to be a concern when assessing Hsp-mediated activation of cells (Gao and Tsan, 2003; Reed et al., 2003), particularly where TLRs and CD14 function as shared signaling receptors for both endotoxin and chaperones. Indeed, recent data indicates that cell activation and cytokine production previously ascribed to Hsp70 and gp96 is likely to be due to contaminating endotoxin (Bausinger et al., 2002; Gao and Tsan 2003; Reed et al., 2003); further experiments are clearly required to delineate the role(s) of endotoxin-free heat shock/chaperone proteins in APC signalling. SR-A has previously been documented to signal to intracellular kinases and phospholipases through Gi/o upon binding of another ligand, AcLDL (Hsu et al., 1998; Whitman et al., 2000; Post et al., 2002). Additionally, there is evidence that SR-A can signal through Lyn kinase, a molecule with which it has been co-immunoprecipitated (Miki et al., 1996). Intriguingly, Lyn kinase is involved in ligand-stimulated signaling from Fc receptors (Kovarova et al., 2001; Strzelecka-Kiliszek et al., 2002), which, as described in this report, can be co-localized intracellularly with SR-A ligands and function in antigen uptake and cross-presentation. Additionally, a recent report indicates that gp96 elicits ERK, but not NF-κB, activation in murine macrophages (Reed et al., 2003). However, further studies are required to determine whether SR-A also functions in gp96 signaling in APC. These studies are currently underway.
Heat shock/chaperone protein trafficking and signaling via SR-A may also be of relevance to processes other than tumor immunity. For example, atherosclerotic plaque progression is accompanied by the formation of necrotic lesions bearing a substantial accumulation of macrophages (reviewed in Schwartz et al., 1991). As it is established that necrotic cells release intact ER-derived chaperones (Basu et al., 2000; Berwin et al., 2001), it can now be considered that heat shock/chaperone proteins released from dying cells present in atherosclerotic lesions may recruit macrophages to the site of the lesion, via interactions with SR-A, and thereby contribute to the etiology of atherosclerosis.
In conclusion, we demonstrate a novel endocytic mechanism for the ER chaperones gp96 and CRT, via scavenger receptor SR-A. Other ligands of SR-A, including fucoidin, compete for the binding and uptake of these chaperones, indicating a common binding site. However, these competing ligands are not structurally similar, implicating pattern recognition as a determinant for binding. Ectopic expression of SR-A is sufficient to induce receptor-mediated endocytosis in HEK 293 cells, while studies with SR-A–/– macrophages indicate that SR-A is necessary for wild-type levels of gp96/CRT binding and uptake. Finally, targeting antigens to SR-A via gp96 results in their cross-presentation on APC cell surface MHC class-I molecules. These data expand the role of SR-A as a receptor involved in both the innate and adaptive arms of the cellular immune system.
Materials and methods
Cell culture and transfection
Peritoneal macrophages were elicited in C57/BL6 mice (Jackson Laboratory, Bar Harbor, ME or Charles River, Wilmington, MA) as described previously (Wassenberg et al., 1999; Berwin et al., 2002b). SR-A null mice (Suzuki et al., 1997; Kunjathoor et al., 2002) were back-bred into the C57BL/6J background and were a generous gift of T.Kodama (Tokyo University) and M.W.Freeman (Massachusetts General Hospital, NHLBI Program in Genomics Applications). Mice were sacrificed 4–5 days post-injection and macrophages were obtained by peritoneal lavage. Macrophages were enriched by adherence selection for 1 h in complete DMEM, 10% FCS on either 12-well plates (Corning Glass Works, Corning, NY) for flow cytometry experiments, or 18 mm 1 glass coverslips (VWR Scientific, Inc., Media, PA) for laser scanning confocal microscopy studies. RAW264.7 macrophages were cultured according to published ATCC protocols. Human embryonic kidney (HEK 293) cells expressing the tetracycline response protein (Flp-In™T-REx™-293; Invitrogen; Carlsbad, CA) were maintained in DMEM containing penicillin (10 U/ml), streptomycin (10 µg/ml), 10% FBS (DMEM/FBS), and blasticidin (15 µg/ml) and zeocin (100 µg/ml). The cDNA encoding the type II murine scavenger receptor (provided by A.Daugherty, University of Kentucky) was subcloned into the pcDNA5/FRT/TO vector (Invitrogen) and cotransfected into HEK cells with the POG44 plasmid encoding the flipase gene using Mirus Trans-IT-293 transfection reagent (Panvera, Madison, WI) according to the manufacturer’s protocol. Using this expression system, SR-A is only expressed when an inducing agent (e.g., tetracycline) is added to the cell culture media (Post et al., 2002). Transfected cells (HEK-SRAtet) were selected with hygromycin (100 µg/ml) and inducible expression confirmed by immunostaining adherent cells with monoclonal antibody 2F8 and western blotting of cell lysates with a guinea pig anti-SR-A antibody (Post et al., 2002).
Antibodies and proteins
gp96 and CRT were purified by the method of Wearsch and Nicchitta (1996). Texas Red (TR)- and fluorescein (Fl)-succinymidyl esters, DiOC6 and AlexaFluors were obtained from Molecular Probes (Portland, OR). Protein labeling with the succinymidyl ester conjugates was performed according to manufacturer’s protocols. 2F8 (anti-SR-A) was purchased from Serotec (Oxford, UK). Human α2M was purified and labeled as previously described (Gron and Pizzo, 1998; Berwin et al., 2002a). Fucoidin, carrageenan, PTX and chondroitin sulfate were obtained from Sigma (St Louis, MO). 25-D1.16 Ab (Porgador et al., 1997) was the generous gift of Dr J.Yewdell (NIAID, NIH).
Detection of fluorescent ligand association
Receptor mediated- and fluid phase-uptake studies were performed as described previously (Wassenberg et al., 1999; Berwin et al., 2002a,b). Fluid phase uptake of protein was accomplished by placing cells in 37°C medium containing the labeled protein. Cell surface receptor binding was achieved by incubating the cells at 4°C with the ligand, followed by washing and fixation. Cells were removed from 12-well plates with a cell scraper (Costar Corporation, Cambridge, MA) followed by analysis for fluorescence by flow cytometry (Becton Dickinson, San Jose, CA). Further analysis was performed using Cell Quest (Becton Dickinson, San Jose, CA). HEK-SRAtet cells were incubated for 24 h with tetracycline (0.5 µg/ml) to induce SR-A expression. To assess SR-A-mediated uptake of chaperone proteins, cells were pre-incubated for 2 h in serum-free DMEM and then incubated for 1 or 2 h with fluorescent ligand. Where indicated, the SR-A antagonist fucoidin (75 µg/ml) was added 5 min prior to addition of fluorescent ligand. Unbound ligand was removed by washing cells with PBS, cells suspended by trypsinization, and associated fluorescence determined by flow cytometry. EC50 of gp96 uptake by HEK-SRAtet cells was determined by the aforementioned uptake assay, using 75 µg/ml fucoidin in parallel assays to determine non-specific binding and uptake. The EC50 was determined from a least squares fit of the data to one phase of a hyperbolic curve.
Fluorescent staining and confocal imaging
HEK-SRAtet cells were plated (50 000 cells/well) on two-chambered LAB-TEK slides (Nalge Nunc International, Naperville, IL). To facilitate adhesion of uninduced cells, the slides were treated with BD Cell-Tak (BD Biosciences, Bedford, MA) according to manufacturers’ adsorption protocol. SR-A expression was induced by addition of tetracycline (0.05 µg/ml). After 16 h, the media was removed and cells equilibrated in serum-free DMEM with 0.2% BSA for 2 h. Cells were gently washed twice for 2 min with warm phenol red-free DMEM, and, where indicated, incubated with fucoidin (75 µg/ml) for 10 min before addition of fluorescent ligand. Fluorescent ligands were incubated with cells at 37°C for 1 h. Ligand association was terminated by washing cells twice with ice-cold PBS. Cells were fixed for 5 min at room temperature with 4% paraformaldehyde in PBS, and washed twice for 5 min each with PBS. Cells were then mounted in the embedding medium Mowiol containing 1% n-propyl gallate. Images were digitally captured using a Zeiss LSM 410 confocal microscope (Thornwood, NY). For confocal microscopy analysis of macrophages the cells were incubated with ligands as described in the text. The cells were then washed in PBS and fixed for 10 min at 4°C in 4% paraformaldehyde. Cells were rinsed and mounted in 10% PBS, 90% glycerol, 1 mg/ml phenylenediamine (mounting medium).
Peptide re-presentation
B3Z is a CD8+ T cell hybridoma that expresses LacZ in response to activation of T cell receptors specific for the SIINFEKL peptide (Ova-immunodominant peptide) in the context of H-2Kb MHC class-I molecules (Karttunen et al., 1992). SIINFEKL peptide was crosslinked to gp96 using SPDP (Pierce, Rockford, IL) according to the manufacturer’s protocol. The SIINFEKL-conjugated gp96 was dialyzed extensively against PBS, with the final dialysis buffer used to determine background in the assay. Ova-specific peptide presentation was determined by incubating SIINFEKL-conjugated gp96 or unconjugated Ova with 105 C57/BL6-derived elicited macrophages in the presence or absence of fucoidin, as indicated. Free ligand was then aspirated and the cells washed with medium. The macrophages were then co-cultured with 106 B3Z cells for 4 h. Ova re-presentation was assayed by the measurement of LacZ activity using CPRG (Boehringer Manheim). Alternatively, the Kb-, Ova-specific monoclonal antibody 25-D1.16 Ab (Porgador et al., 1997) was used for FACS analysis of cell surface peptide presentation, as per previously (Berwin et al., 2002a).
Acknowledgments
Acknowledgements
We wish to thank the Duke University Medical Center Comprehensive Cancer Center Shared Flow Cytometry Facility and the University of Kentucky Shared FACS Facility for FACS analyses and would like to acknowledge the support of the DUMC Comprehensive Cancer Center Shared Confocal Microscopy Facility. We are grateful to Drs Jeff Baker, Meredith Rosser and Robyn Reed for advice and reagents. These studies were supported by grants DK53058 (C.V.N.), HL-24066 (S.V.P.), HL-65708 (S.R.P.), and NRSA-1F32CA9016901 and an H.H.Gibson Cancer Fellowship (B.B.).
References
- Abraham R., Singh,N., Mukhopadhyay,A., Basu,S.K., Bal,V. and Rath,S. (1995) Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J. Immunol., 154, 1–8. [PubMed] [Google Scholar]
- Asea A., Kraeft,S.K., Kurt-Jones,E.A., Stevenson,M.A., Chen,L.B., Finberg,R.W., Koo,G.C. and Calderwood,S.K. (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med., 6, 435–442. [DOI] [PubMed] [Google Scholar]
- Asea A., Rehli,M., Kabingu,E., Boch,J.A., Bare,O., Auron,P.E., Stevenson,M.A. and Calderwood,S.K. (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem., 277, 15028–15034. [DOI] [PubMed] [Google Scholar]
- Baker-LePain J.C., Sarzotti,M., Fields,T.A., Li,C.Y. and Nicchitta,C.V. (2002) GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J. Exp. Med., 196, 1447–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain J.C., Reed,R.C. and Nicchitta,C.V. (2003) ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr. Opin. Immunol., 15, 89–94. [DOI] [PubMed] [Google Scholar]
- Bansal P., Mukherjee,P., Basu,S.K., George,A., Bal,V. and Rath,S. (1999) MHC class I-restricted presentation of maleylated protein binding to scavenger receptors. J. Immunol., 162, 4430–4437. [PubMed] [Google Scholar]
- Baranova I., Vishnyakova,T., Bocharov,A., Chen,Z., Remaley,A.T., Stonik,J., Eggerman,T.L. and Patterson,A.P. (2002) Lipopoly saccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect. Immun., 70, 2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. and Srivastava,P.K. (1999) Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J. Exp. Med., 189, 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Binder,R.J., Suto,R. anderson,K.M. and Srivastava,P.K. (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κ B pathway. Int. Immunol., 12, 1539–1546. [DOI] [PubMed] [Google Scholar]
- Basu S., Binder,R.J., Ramalingam,T. and Srivastava,P.K. (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70 and calreticulin. Immunity, 14, 303–313. [DOI] [PubMed] [Google Scholar]
- Bausinger H. et al. (2002) Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur. J. Immunol., 32, 3708–3713. [DOI] [PubMed] [Google Scholar]
- Becker T., Hartl,F.U. and Wieland,F. (2002) CD40, an extracellular receptor for binding and uptake of Hsp70–peptide complexes. J. Cell Biol., 158, 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwin B., Reed,R.C. and Nicchitta,C.V. (2001) Virally induced lytic cell death elicits the release of immunogenic grp94/gp96. J. Biol. Chem., 276, 21083–21088. [DOI] [PubMed] [Google Scholar]
- Berwin B., Hart,J.P., Pizzo,S.V. and Nicchitta,C.V. (2002a) Cutting edge: CD91-independent cross-presentation of GRP94(gp96)-associated peptides. J. Immunol., 168, 4282–4286. [DOI] [PubMed] [Google Scholar]
- Berwin B., Rosser,M.F., Brinker,K.G. and Nicchitta,C.V. (2002b) Transfer of GRP94(Gp96)-associated peptides onto endosomal MHC Class I molecules. Traffic, 3, 358–366. [DOI] [PubMed] [Google Scholar]
- Binder R.J., Anderson,K.M., Basu,S. and Srivastava,P.K. (2000a) Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J. Immunol., 165, 6029–6035. [DOI] [PubMed] [Google Scholar]
- Binder R.J., Han,D.K. and Srivastava,P.K. (2000b) CD91: a receptor for heat shock protein gp96. Nat. Immunol., 1, 151–155. [DOI] [PubMed] [Google Scholar]
- Blachere N.E., Li,Z., Chandawarkar,R.Y., Suto,R., Jaikaria,N.S., Basu,S., Udono,H. and Srivastava,P.K. (1997) Heat shock protein–peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med., 186, 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F., Boucher,P.E., Eichelberg,K., Mayhew,M., Rothman,J.E., Houghton,A.N. and Germain,R.N. (2000) Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J. Exp. Med., 191, 1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delneste Y. et al. (2002) Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity, 17, 353–362. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M.L., Moore,K.J., Freeman,M.W. and Reed,G.L. (2000) Lipopolysaccharide induces scavenger receptor A expression in mouse macrophages: a divergent response relative to human THP-1 monocyte/macrophages. J. Immunol., 164, 2692–2700. [DOI] [PubMed] [Google Scholar]
- Fraser I., Hughes,D. and Gordon,S. (1993) Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature, 364, 343–346. [DOI] [PubMed] [Google Scholar]
- Gao B. and Tsan,M.F. (2003) Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor α release by murine macrophages. J. Biol. Chem., 278, 174–179. [DOI] [PubMed] [Google Scholar]
- Gron H. and Pizzo,S.V. (1998) Non-proteolytic incorporation of protein ligands into human α2-macroglobulin: implications for the binding mechanism of α2-macroglobulin. Biochemistry, 37, 6009–6014. [DOI] [PubMed] [Google Scholar]
- Guyre C.A., Barreda,M.E., Swink,S.L. and Fanger,M.W. (2001) Colocalization of FcγRI-targeted antigen with class I MHC: implications for antigen processing. J. Immunol., 166, 2469–2478. [DOI] [PubMed] [Google Scholar]
- Hampton R.Y., Golenbock,D.T., Penman,M., Krieger,M. and Raetz,C.R. (1991) Recognition and plasma clearance of endotoxin by scavenger receptors. Nature, 352, 342–344. [DOI] [PubMed] [Google Scholar]
- Hsu H.Y., Hajjar,D.P., Khan,K.M. and Falcone,D.J. (1998) Ligand binding to macrophage scavenger receptor-A induces urokinase-type plasminogen activator expression by a protein kinase-dependent signaling pathway. J. Biol. Chem., 273, 1240–1246. [DOI] [PubMed] [Google Scholar]
- Imber M.J., Pizzo,S.V., Johnson,W.J. and Adams,D.O. (1982) Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J. Biol. Chem., 257, 5129–5135. [PubMed] [Google Scholar]
- Karttunen J., Sanderson,S. and Shastri,N. (1992) Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl Acad. Sci. USA, 89, 6020–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijmeer M.J. et al. (2001) Antigen loading of MHC class I molecules in the endocytic tract. Traffic, 2, 124–137. [DOI] [PubMed] [Google Scholar]
- Kodama T., Reddy,P., Kishimoto,C. and Krieger,M. (1988) Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc. Natl Acad. Sci. USA, 85, 9238–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A., Lichtman,A.H., Finberg,R.W., Libby,P. and Kurt-Jones,E.A. (2000) Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol., 164, 13–17. [DOI] [PubMed] [Google Scholar]
- Kovarova M., Tolar,P., Arudchandran,R., Draberova,L., Rivera,J. and Draber,P. (2001) Structure–function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcε receptor I aggregation. Mol. Cell. Biol., 21, 8318–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M. and Herz,J. (1994) Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu. Rev. Biochem., 63, 601–637. [DOI] [PubMed] [Google Scholar]
- Kunjathoor V.V. et al. (2002) Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem., 277, 49982–49988. [DOI] [PubMed] [Google Scholar]
- Mellman I. (1996) Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol., 12, 575–625. [DOI] [PubMed] [Google Scholar]
- Michaelsson J., Teixeira de Matos,C., Achour,A., Lanier,L.L., Karre,K. and Soderstrom,K. (2002) A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J. Exp. Med., 196, 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki S., Tsukada,S., Nakamura,Y., Aimoto,S., Hojo,H., Sato,B., Yamamoto,M. and Miki,Y. (1996) Functional and possible physical association of scavenger receptor with cytoplasmic tyrosine kinase Lyn in monocytic THP-1-derived macrophages. FEBS Lett., 399, 241–244. [DOI] [PubMed] [Google Scholar]
- Milani V., Noessner,E., Ghose,S., Kuppner,M., Ahrens,B., Scharner,A., Gastpar,R. and Issels,R.D. (2002) Heat shock protein 70: role in antigen presentation and immune stimulation. Int. J. Hyperthermia, 18, 563–575. [DOI] [PubMed] [Google Scholar]
- Noessner E. et al. (2002) Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J. Immunol., 169, 5424–5432. [DOI] [PubMed] [Google Scholar]
- Panjwani N., Popova,L., Febbraio,M. and Srivastava,P.K. (2000) The CD36 scavenger receptor as a receptor for Gp96. Cell Stress Chaperones, 5, 391. [Google Scholar]
- Panjwani N.N., Popova,L. and Srivastava,P.K. (2002) Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J. Immunol., 168, 2997–3003. [DOI] [PubMed] [Google Scholar]
- Peiser L. and Gordon,S. (2001) The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect., 3, 149–159. [DOI] [PubMed] [Google Scholar]
- Peiser L., Gough,P.J., Kodama,T. and Gordon,S. (2000) Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain and culture conditions in vitro. Infect. Immun., 68, 1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N. and Gordon,S. (1998) Scavenger receptors: diverse activities and promiscuous binding of polyanionic ligands. Chem. Biol., 5, R193–R203. [DOI] [PubMed] [Google Scholar]
- Platt N., Suzuki,H., Kurihara,Y., Kodama,T. and Gordon,S. (1996) Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl Acad. Sci. USA, 93, 12456–12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porgador A., Yewdell,J.W., Deng,Y., Bennink,J.R. and Germain,R.N. (1997) Localization, quantitation and in situ detection of specific peptide–MHC class I complexes using a monoclonal antibody. Immunity, 6, 715–726. [DOI] [PubMed] [Google Scholar]
- Post S.R., Gass,C., Rice,S., Nikolic,D., Crump,H. and Post,G.R. (2002) Class A scavenger receptors mediate cell adhesion via activation of G(i/o) and formation of focal adhesion complexes. J. Lipid Res., 43, 1829–1836. [DOI] [PubMed] [Google Scholar]
- Radsak M.P., Hilf,N., Singh-Jasuja,H., Braedel,S., Brossart,P., Rammensee,H.G. and Schild,H. (2003) The heat shock protein gp96 binds to human neutrophils and monocytes and stimulates effector functions. Blood, 101, 2810–2815. [DOI] [PubMed] [Google Scholar]
- Reed R.C., Berwin,B., Baker,J.P. and Nicchitta,C.V. (2003) GRP94/gp96 elicits ERK activation in murine macrophages: a role for endotoxin contamination in NF-κB activation and nitric oxide production. J. Biol. Chem., 278, 31853–31860. [DOI] [PubMed] [Google Scholar]
- Regnault A. et al. (1999) Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med., 189, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits E. et al. (2003) Peptide diffusion, protection and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity, 18, 97–108. [DOI] [PubMed] [Google Scholar]
- Schwartz C.J., Valente,A.J., Sprague,E.A., Kelley,J.L. and Nerem,R.M. (1991) The pathogenesis of atherosclerosis: an overview. Clin. Cardiol., 14, 11–16. [DOI] [PubMed] [Google Scholar]
- Shastri N. and Gonzalez,F. (1993) Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J. Immunol., 150, 2724–2736. [PubMed] [Google Scholar]
- Singh-Jasuja H., Scherer,H.U., Hilf,N., Arnold-Schild,D., Rammensee,H.G., Toes,R.E. and Schild,H. (2000a) The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur. J. Immunol., 30, 2211–2215. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H. et al. (2000b) Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J. Exp. Med., 191, 1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P.K. and Amato,R.J. (2001) Heat shock proteins: the ‘Swiss Army Knife’ vaccines against cancers and infectious agents. Vaccine, 19, 2590–2597. [DOI] [PubMed] [Google Scholar]
- Srivastava P.K. and Maki,R.G. (1991) Stress-induced proteins in immune response to cancer. Curr. Top. Microbiol. Immunol., 167, 109–123. [DOI] [PubMed] [Google Scholar]
- Srivastava P.K. and Udono,H. (1994) Heat shock protein–peptide complexes in cancer immunotherapy. Curr. Opin. Immunol., 6, 728–732. [DOI] [PubMed] [Google Scholar]
- Srivastava P.K., DeLeo,A.B. and Old,L.J. (1986) Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc. Natl Acad. Sci. USA, 83, 3407–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelecka-Kiliszek A., Kwiatkowska,K. and Sobota,A. (2002) Lyn and Syk kinases are sequentially engaged in phagocytosis mediated by FcγR. J. Immunol., 169, 6787–6794. [DOI] [PubMed] [Google Scholar]
- Suto R. and Srivastava,P.K. (1995) A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science, 269, 1585–1588. [DOI] [PubMed] [Google Scholar]
- Suzuki H. et al. (1997) A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature, 386, 292–296. [DOI] [PubMed] [Google Scholar]
- Tamura Y., Peng,P., Liu,K., Daou,M. and Srivastava,P.K. (1997) Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science, 278, 117–120. [DOI] [PubMed] [Google Scholar]
- Udono H. and Srivastava,P.K. (1993) Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med., 178, 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H. and Srivastava,P.K. (1994) Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90 and hsp70. J. Immunol., 152, 5398–5403. [PubMed] [Google Scholar]
- Vabulas R.M., Ahmad-Nejad,P., da Costa,C., Miethke,T., Kirschning,C.J., Hacker,H. and Wagner,H. (2001) Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem., 276, 31332–31339. [DOI] [PubMed] [Google Scholar]
- Vabulas R.M. et al. (2002) The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the TLR2/4 pathway. J. Biol. Chem., 277, 20847–20853. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Kazim,L., Repasky,E.A. and Subjeck,J.R. (2001) Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J. Immunol., 166, 490–497. [DOI] [PubMed] [Google Scholar]
- Wassenberg J.J., Dezfulian,C. and Nicchitta,C.V. (1999) Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J. Cell Sci., 112, 2167–2175. [DOI] [PubMed] [Google Scholar]
- Wearsch P.A. and Nicchitta,C.V. (1996) Purification and partial molecular characterization of GRP94, an ER resident chaperone. Protein Expr. Purif., 7, 114–121. [DOI] [PubMed] [Google Scholar]
- Whitman S.C., Daugherty,A. and Post,S.R. (2000) Regulation of acetylated low density lipoprotein uptake in macrophages by pertussis toxin-sensitive G proteins. J. Lipid Res., 41, 807–813. [PubMed] [Google Scholar]
- Yamazaki K., Nguyen,T. and Podack,E.R. (1999) Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J. Immunol., 163, 5178–5182. [PubMed] [Google Scholar]
- Zheng H., Dai,J., Stoilova,D. and Li,Z. (2001) Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J. Immunol., 167, 6731–6735. [DOI] [PubMed] [Google Scholar]