Abstract
Patients with defective ectodysplasin A (EDA) have X-linked hypohidrotic ectodermal dysplasia (XLHED; OMIM#305100), a condition comprising hypotrichosis, inability to sweat, abnormal teeth, and frequent pulmonary infections. The XLHED dogs show the same clinical signs as humans with the disorder, including frequent respiratory infections that can be fatal. The respiratory disease in humans and dogs is thought to be due to the absence of tracheal and bronchial glands which are a vital part of the mucociliary clearance mechanism. In our XLHED model, the genetically missing EDA was replaced by post-natal intravenous administration of recombinant EDA resulting in long-term, durable corrective effect on adult, permanent dentition. After treatment with EDA, significant correction of the missing tracheal and bronchial glands was achieved in those dogs that received higher doses of EDA. Moreover, successful treatment resulted in the presence of esophageal glands, improved mucociliary clearance, and the absence of respiratory infection. These results demonstrate that a short-term treatment at a neonatal age with a recombinant protein can reverse a developmental disease and result in vastly improved quality of life.
Keywords: ectodermal dysplasia, respiratory disease, animal model or dog therapy, recombinant protein
INTRODUCTION
X-linked hypohidrotic/anhidrotic ectodermal dysplasia (XLHED) in humans (MIM #305100), manifests by hypoplasia of hair and eccrine sweat glands [Bears et al., 1987; Clarke, 1987; Clarke et al., 1987]. Other milder clinical signs of XLHED are sparse or absent hair, absent and malformed teeth, skin hyperpigmentation around the eyes, wrinkled, dry skin, atopy, recurrent benign infections, and increased susceptibility to bronchitis and pneumonia [Clarke et al., 1987; Gilenkrantz et al., 1989; Nordgarden et al., 2001; Reed et al., 1970; Siegel et al., 1990; Soderholm et al., 1985].
There is significant morbidity and mortality in affected children due to hyperthermia because of their inability to sweat and due to an increased risk of respiratory tract infections because they lack nasal, tracheal, and bronchial glands [Bears et al., 1987; Clarke et al., 1987; Kere et al., 1996; Reed et al., 1970; Spiegel et al., 1990; Soderholm et al., 1985]. As in human patients with XLHED, affected dogs lack respiratory glands and are at an increased risk for pulmonary disorders [Casal et al., 2005, 2007]. Over the years we have been maintained an XLHED dog colony; almost all affected dogs exhibited chronic nasal discharge and nasal crusts, and mild to fatal bronchopneumonia was seen in almost half of the affected dogs [Casal et al., 2005].
Analogous to experiments performed in Tabby mice, the mouse homologue of human and canine XLHED [Gaide et al., 2003], recombinant ectodysplasin A (Fc:EDA1) was administered neonatally to XLHED pups in an attempt to reverse the disease. Previously, we had shown that postnatal administration of Fc:EDA1 has a prominent effect on permanent dentition and significantly improved long-term resistance to eye and airways infections [Casal et al., 2007]. The findings presented here demonstrate that the absence of pulmonary disease is a direct consequence of restoration of tracheal and bronchial glands after postnatal administration of Fc:EDA1 and thus improved/normalized mucociliary clearance.
MATERIALS AND METHODS
Treatment and clinical evaluation of XLHED dogs
Two affected pups received a single injection of 2 mg (7.1 mg/kg) of Fc:EDA1 [Gaide et al 2003] IV via the jugular vein 2 days after birth (T2×1 protocol). One XLHED pup was injected with 1 mg Fc:EDA1 on 2 (3.5 mg/kg), 5 (2.2 mg/kg), 8 (2.8 mg/kg), and 11 (1.8 mg/kg) days after birth (T1×4 protocol) and four XLHED dogs received a higher dose of 2 mg (7 to 2 mg/kg with the doses decreasing with increasing age) Fc:EDA1 on days 2, 5, 8, 11, and 14 after birth (T2×5 protocol). All dogs were cared for according to the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals and in the International Guiding Principles for Biomedical Research Involving Animals.
All dogs were observed daily and physical examinations were performed once weekly or as needed if abnormal clinical signs were seen. Mucociliary clearance studies were performed as described [Casal et al., 2005].
Histopathologic evaluation
Complete necropsies were performed in all dogs including an untreated normal and an untreated affected control. The samples were reviewed by one investigator (EAM) blinded to the treatment group. Tissues were harvested at necropsy from the proximal trachea and esophagus as well as bronchi with adjacent pulmonary tissue. Each sample was fixed in 10% neutral buffered formalin, routinely processed and sectioned at 5 microns and stained with hematoxylin and eosin.
RESULTS
Clinical evaluation
No nasal discharge, sneezing, coughing or respiratory distress was seen in the treated XLHED dogs or the normal male sibling. The untreated XLHED dog had chronic nasal discharge, and one episode of clinical signs compatible with bronchopneumonia. Mucociliary clearance was significantly improved in the one dog that received 2 mg of the recombinant protein at 2 days of life and in 3 of 4 dogs that received the T2×5 treatment protocol, but not in the dog with the T1×4 treatment (Table I). Adult dentition was also corrected in the same dogs.
Table I.
Summary of Clinicopathologic Findings in Treated and Untreated XLHED Dogs
| Dog | Therapy Protocol |
Age at Necropsy |
Teeth | Tracheal Glands |
Bronchial Glands |
Esophageal Glands |
BG numbers* |
Mucociliary clearance** |
|---|---|---|---|---|---|---|---|---|
| E120 | Normal untreated |
3.5 yr | Normal | +++ | +++ | +++ | 19.5±8.4 | 15.0 ± 1.4 |
| E215 | Affected untreated |
7 mo. | Affected | None | None | None | 0±0 | 8.9 ± 2.6 |
| E201 | T1×4 | 1 yr | No correction |
+ | None | + | 0±0 | 8.0 ± 4.6 |
| E192 | T2×1 | 1 yr | Corrected | + | None | +++ | 0±0 | 12.3 ± 2.4 |
| E193 | T2×1 | 1 yr | Partial | ++ | None | None | 0±0 | 8.1 ± 1.6 |
| E140 | T2×5 | 2.8 yr | Corrected | +++ | +++ | +++ | 16±6 | 14.5 ± 0.1 |
| E151 | T2×5 | 2.5 yr | No correction |
+ | + | ++ | 2.5±1.1 | 10.9 ± 1.3 |
| E173 | T2×5 | 2 yr | Corrected | ++ | +++ | +++ | 23±0 | 12.1 ± 2.5 |
| E174 | T2×5 | 2 yr | Corrected | ++ | +++ | + | 16±6 | 18.3 ± 3.3 |
number of glands per bronchus in 1-cm lung section
mm/min as measured in ref 11.
Histopathologic evaluation (Table I)
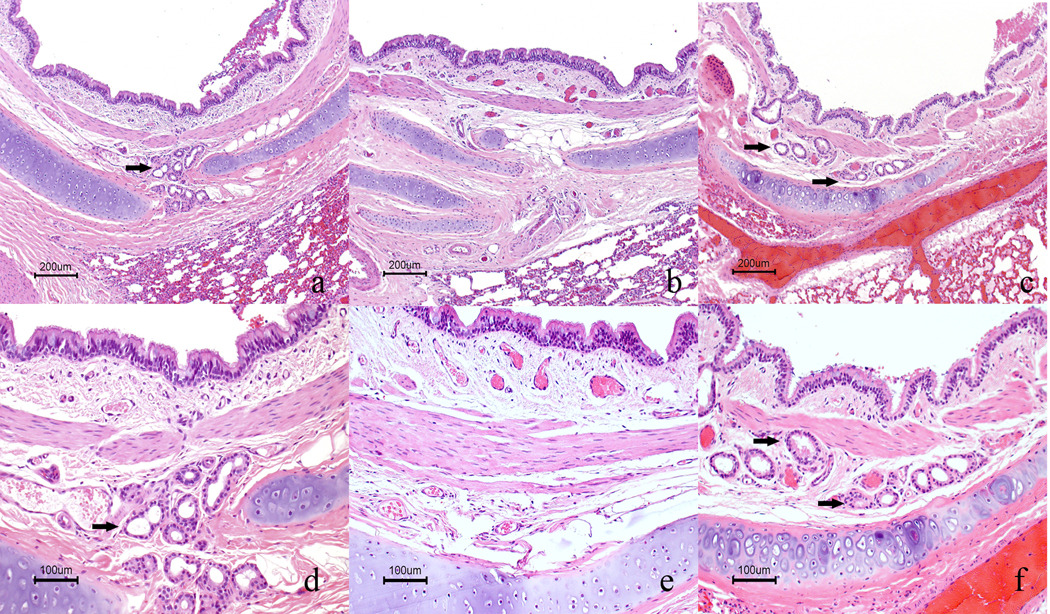
In the normal dog (E120), numerous seromucinous glands were located in the submucosa of all bronchi. Bronchial glands were completely absent, as predicted, in the untreated control dog (E215). The three dogs that received either the T2×1 or T1×4 treatment (E192, E193, and E201) had no bronchial glands. Three dogs that received a total dose of 10 mg (T2×5 treatment) (E140, E173, and E174) had bronchial glands subjectively equal to or slightly fewer than the normal dog (Fig 1). The fourth dog that received the 10 mg treatment (E151) had some scattered bronchial glands but fewer than those seen in the other 10 mg-dosed dogs. The bronchial glands were used for statistical evaluation due to even distribution in all tissue sections.
Figure 1.
Correction of bronchial glands in XLHED treated dogs. a. Bronchus of normal control dog (E120) with numerous seromucinous glands (arrows). b. Untreated XLHED dog (E215) with complete absence of bronchial glands. c. Treated XLHED dog (E140) with numerous bronchial glands. H&E; Bar = 200 µm. Higher magnification of normal (d), untreated XHLED (e) and treated XHLED dog (f), respectively. H&E, Bar= 100 µm.
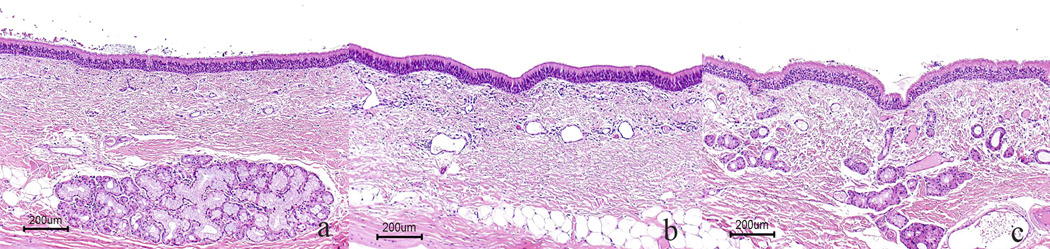
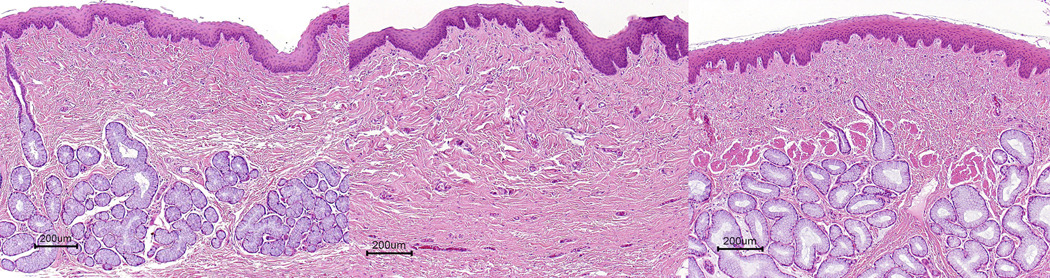
The normal control dog (E120) had numerous tracheal and esophageal glands unevenly distributed within each section. No glands were present in either tissue in the untreated dog (E215). In general, the treated dogs had both esophageal and tracheal glands but the glands were sporadically distributed in the tissues (Fig. 2, Fig 3). Dogs in all treatment groups had tracheal glands, which varied from 10–49% of the control (E151, E192, E201) to 50–75% (E173, E174, E193). E140 had numerous tracheal glands, which were indistinguishable from the normal dog (E120). Esophageal glands were equal in number to that of the control dog in E140, E173, and E192. No esophageal glands were present in E193. E174 and E201 had few glands (10–49%), while E151 had moderately fewer glands than the control (50–74%).
Figure 2.
Correction of tracheal glands in XHLED dogs. a. Tracheal mucosa of normal dog (E120) with numerous seromucinous glands. b. Complete absence of glands in untreated XLHED dog (E215). c. Numerous glands in treated XHLED dog (E140). H&E, Bar = 200 µm.
Figure 3.
Correction of esophageal glands in XHLED treated dogs. a. Normal control dog (E120) with numerous seromucinous glands. b Untreated dog with XHLED (E215) with complete lack of glands lining the esophagus (E215). c. Treated XLHED dogs (E140) with numerous glands. H&E, Bar = 200 µm
DISCUSSION
Chronic respiratory infections including rhinitis, tracheitis, bronchitis, and pneumonia are common in patients with XLHED [Beahrs et al., 1971; Clarke 1987; Clarke et al., 1987; Clarke et al., 1987; Kere et al., 1996; Reed et al., 1970; Soderholm et al., 1985]. The rhinitis has often been described as a foul smelling nasal discharge with a tendency towards crusting, which may lead to obstruction [Al-Jassim et al., 1987; Beahrs et al., 1971; Reed et al., 1970; Siegel et al., 1990]. Early observations pointed out the lack of mucus in XHLED patients, especially when compared to normal individuals who generally produce excessive amounts of mucus in the presence of a respiratory infection, and it was thought the mucous glands had atrophied in affected patients [Beahrs et al., 1971]. Respiratory cilia were described as being absent or defective [Beahrs et al., 1971], but later studies confirmed that the cilia were normal in their beating frequency and pattern, but their number was reduced [Al-Jassim et al., 1987]. Other studies examining XLHED patients with respiratory diseases were able to demonstrate the absence of mucous glands in the respiratory tract as well as the esophagus, suggesting that excessive dryness of these tissues led to the lack of protective functions and thus all of clinical findings [Beahrs et al., 1971; Clarke 1987; Clarke et al., 1987; Reed ,., 1970]. Histological evaluations of one affected patient confirmed the lack of mucous glands in pharynx, larynx, trachea, large and small bronchi, and upper esophagus [Reed et al., 1970]. An early paper described the possibility of asthma as a cause for the frequent respiratory tract infections in XHLED patients [Beahrs et al., 1971], but later studies showed that the lack of mucus production was directly related to the increased tendency for asthma [Siegel et al., 1990].
Pulmonary disease has not been described as a sequela to ectodermal dysplasia in the Tabby mouse. Our XLHED dogs, however, have consistently shown signs of respiratory tract disease, which were fatal in two cases. Earlier studies demonstrated the lack of tracheal and bronchial glands, decreased mucociliary clearance, and frequent pneumonia [Casal et al., 2005]. In this same study we were able to show that the respiratory immunity did not appear to be impaired. Interestingly, one paper describes an increased tendency for respiratory infections in human carrier females [Clarke et al., 1987]. None of our female carrier dogs have had respiratory infections to our knowledge.
Previously, we had shown that neonatal treatment with recombinant EDA resulted in correction of secondary dentition and improvement of lacrimation, sweating ability, and mucociliary clearance [Casal et al., 2007]. Studies performed in fetal Tabby mice demonstrated that the earlier the treatment was initiated in life, the more complete the correction from affected phenotype to wild-type normal [Gaide et al., 2003]. Many of the typical manifestations were corrected such as the ability to sweat and dental abnormalities or were greatly improved (hair abnormalities). However, evaluating the respiratory components of XLHED is difficult in mice, as they do not show clinical signs of pulmonary disease. The XLHED dog provides a model in which many of the clinical signs mimic those found in humans much more accurately [Casal et al., 2007]. Of the first 20 affected dogs born in our facility, 6 (35%) died before weaning of pulmonary disease. Normal preweaning losses during the same time frame were 3 (7%) of 44 phenotypically normal littermates. Of the remaining 14 affected dogs, 5 males were adopted out to private homes. All of the XLHED dogs had frequent crusty nasal discharge. Four of the dogs in private homes, each developed two bouts of pneumonia throughout their lifetime and one was treated for three episodes. The 9 remaining adult XLHED dogs developed respiratory illnesses early in life and most improved as they aged. However, one male and one female died of bronchopneumonia. These previous observations demonstrate the significance of pulmonary disease in individuals affected with XLHED and the need for treatment that is aimed at restoring function rather than just treating secondary illness.
Clinical signs of respiratory disease are present in almost all of the affected dogs particularly during adolescence and are also very common in humans affected with XLHED. The results presented here demonstrate that partial to complete restoration of the tracheal and bronchial glands is possible after neonatal administration of recombinant EDA. The appearance of the respiratory glands greatly improved mucociliary clearance. Improved clearance correlated with the presence of numerous glands in the respiratory tract. Interestingly, the treated XLHED dogs never showed signs of respiratory illness, nor did they experience nasal discharge. This was also true for those dogs in which mucociliary clearance was not detectably improved. Taken together, these data suggests that the presence of even a few glands in the respiratory tract is already sufficient to achieve clinical benefit.
ACKNOWLEDGEMENTS
We thank Jürg Tschopp (University of Lausanne) and Mark Haskins (University of Pennsylvania) for their support and critical reading of the manuscript. We would also like to thank Patricia O’Donnell, and the veterinary students for the expert care and assistance with the experiments and Vivian Staci from the nuclear medicine facility (New Bolton Center, University of Pennsylvania). This work was supported by NIH grants AR049817 and RR02512, the National Foundation for Ectodermal Dysplasias (to MLC), the Swiss National Research Foundation and the NCCR Molecular Oncology (to PS).
Footnotes
This manuscript was presented at the International Conference on Ectodermal Dysplasia, Charleston, South Carolina, March 10–12, 2008.
REFERENCES
- Al-Jassim AH, Swift AC. Persistent nasal crusting due to hypohidrotic ectodermal dysplasia. J Laryngol Otol. 1996;110:379–382. doi: 10.1017/s0022215100133687. [DOI] [PubMed] [Google Scholar]
- Beahrs JO, Lillington GA, Rosan RC, Russin L, Lindgren JA, Rowley PT. Anhidrotic ectodermal dysplasia: predisposition to bronchial disease. Ann Intern Med. 1971;74:92–96. doi: 10.7326/0003-4819-74-1-92. [DOI] [PubMed] [Google Scholar]
- Casal ML, Lewis JR, Mauldin EA, Tardivel A, Ingold K, Favre M, Paradies F, Demotz S, Gaide O, Schneider P. Significant correction of disease after postnatal administration of recombinant EDA in canine X-linked ectodermal dysplasia. Am J Hum Genet. 2007;81:1050–1056. doi: 10.1086/521988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal ML, Mauldin EA, Ryan S, Scheidt J, Kennedy J, Moore PF, Felsburg PJ. Frequent respiratory tract infections in the canine model of X-linked ectodermal dysplasia are not caused by an immune deficiency. Vet Immunol Immunopath. 2005;107:95–104. doi: 10.1016/j.vetimm.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. Hypohidrotic ectodermal dysplasia. J Med Genet. 1987;24:659–663. doi: 10.1136/jmg.24.11.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Phillips DI, Brown R, Harper PS. Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child. 1987;62:989–996. doi: 10.1136/adc.62.10.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Sarfarazi M, Thomas NS, Roberts K, Harper PS. X-linked hypohidrotic ectodermal dysplasia: DNA probe linkage analysis and gene localization. Hum Genet. 1987;75:378–380. doi: 10.1007/BF00284112. [DOI] [PubMed] [Google Scholar]
- Gaide O, Schneider P. Permanent correction of an inherited ectodermal dysplasia with recombinant EDA. Nat Med. 2003;9:614–618. doi: 10.1038/nm861. [DOI] [PubMed] [Google Scholar]
- Gilgenkrantz S, Blanchet-Bardon C, Nazzaro V, Formiga L, Mujica P, Alembik Y. Hypohidrotic ectodermal dysplasia. Clinical study of a family of 30 over three generations. Hum Genet. 1989;81:120–122. doi: 10.1007/BF00293886. [DOI] [PubMed] [Google Scholar]
- Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, Chen EY, Ezer S, Saarialho-Kere U, de la Chapelle A, Schlessinger D. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- Nordgarden H, Jensen JL, Storhaug K. Oligodontia is associated with extra-oral ectodermal symptoms and low whole salivary flow rates. Oral Dis. 2001;7:226–232. [PubMed] [Google Scholar]
- Reed WB, Lopez DA, Landing B. Clinical spectrum of anhidrotic ectodermal dysplasia. Arch Dermatol. 1970;102:134–143. [PubMed] [Google Scholar]
- Siegel MB, Potsic WP. Ectodermal dysplasia: the otolaryngologic manifestations and management. Int J Pediatr Otorhinolaryngol. 1990;19:265–271. doi: 10.1016/0165-5876(90)90006-d. [DOI] [PubMed] [Google Scholar]
- Soderholm AL, Kaitila I. Expression of X-linked hypohidrotic ectodermal dysplasia in six males and in their mothers. Clin Genet. 1985;28:136–144. doi: 10.1111/j.1399-0004.1985.tb00373.x. [DOI] [PubMed] [Google Scholar]