Abstract
Aims
MYH is a DNA glycosylase in the base excision repair pathway. Germ-line biallelic mutations in the MYH gene are associated with the development of multiple colorectal adenomas and colorectal carcinoma (CRC). A slightly increased risk of CRC is suggested in monoallelic MYH mutation carriers. The aim was to characterize the histopathological features of carcinomas from biallelics and monoallelics.
Methods and results
Clinicopathological features of 57 colorectal carcinomas from 50 patients identified in familial CRC registries were recorded. These included 16 cancers from 14 MYH biallelics; 25 cancers from 22 MYH monoallelics; and 16 cancers from 14 controls. Carcinomas in biallelics demonstrated tubular, papillary or cribriform patterns as the predominant histological subtype, and main histological groups differed according to mutation status (P = 0.0053). All biallelic cancers were low grade, with high-grade tumours more common in monoallelics and controls (P = 0.002). Synchronous polyps were observed in 75% of biallelics, 33% of monoallelics and 43% of controls (P = 0.035). Serrated carcinoma was the predominant type in 12% (3/25) of the monoallelics but in none of the biallelics or controls. MYH immunohistochemistry failed to distinguish between groups.
Conclusions
Neither pathological features nor immunohistochemistry could predict the MYH mutation status of CRCs in this study.
Keywords: colorectal cancer, pathology, polyps, mutYh, MYH
Introduction
Oxidative damage of DNA results in the generation of 8-oxo-guanine, which, when incorporated into DNA, mispairs with adenine residues. MYH, or MutYH, is a DNA glycosylase in the base excision repair pathway responsible for the repair of oxidative DNA damage. Mutations in the MYH gene impair the DNA base excision repair process, resulting in the accumulation of G:C→T:A mutations in genes such as adenomatous polyposis coli (APC) and Kirsten rat sarcoma (KRAS), both of which have major roles in colorectal tumorigenesis. 1,2 Germ-line biallelic MYH mutations are clinically associated with the development of multiple colonic adenomas and colorectal carcinoma (CRC)3 as well as upper gastrointestinal neoplasms. 4,5 An attenuated polyposis phenotype is generally described, with various studies demonstrating biallelic MYH mutations in approximately 30% of patients with between 10 and 100 polyps and no germ-line APC mutation. 3,6 However, the number of polyps can exceed 100, as seen in classic familial adenomatous polyposis (FAP).3 The exact risk of CRC associated with monoallelic mutation carriers remains uncertain; most large studies have described an odds ratio of <1.5.7–13 No specific clinical or pathological features have been described that predict the presence of an MYH mutation, unlike the Bethesda criteria, which are useful in identifying CRC with high-frequency microsatellite instability (MSI-H).14 In a preliminary pathological review of histology slides from patients with biallelic and monoallelic mutations at our institution, a serrated appearance was noted in some cases. We undertook a detailed review to investigate whether there were specific pathological features that were characteristic of MYH-associated cancers. In addition, a recent study has described the utility of immunohistochemistry using an MYH antibody on paraffin-embedded material from CRCs to predict the presence of mutations.15 We also sought to confirm this finding in our cohort.
Materials and methods
CRCs and polyps from 50 patients (29 female, 21 male) were reviewed for this study. Thirty-six individuals with MYH mutations, including 14 biallelic and 22 heterozygous mutation carriers, were identified from the Familial Gastrointestinal Cancer Registry (FGICR) and from the Ontario Familial Colon Cancer Registry (OFCCR). The FGICR is a clinic-based programme based at Mount Sinai Hospital, Toronto that acts as a Canadian referral centre for subjects and family members at high risk of inherited gastrointestinal cancer and polyposis syndromes. Cases from the FGICR included four biallelic mutation carriers with 10–100 adenomas, and prior negative germ-line APC testing; the MYH mutation status of the cases has been reported previously.6
A total of 32 MYH mutation carriers (10 biallelic and 22 heterozygous carriers) were identified from OFCCR, one of six sites of the Colorectal Cancer Family Registry, a National Cancer Institute-supported consortium of registries dedicated to the study of genetic and epidemiological factors in CRC.16 The OFCCR recruited individuals diagnosed with colorectal carcinoma in the Province of Ontario between 1 July 1997 and 30 June 2000 aged 24–70 years at diagnosis identified through the population-based Ontario Cancer Registry.17 Cases of FAP were excluded from this registry. Fourteen controls were randomly selected from CRC patients from the OFCCR who did not have pathogenic MYH mutations, and were either wild-type or harboured non-pathogenic polymorphisms. The MYH mutation testing of mutation carriers and controls has been previously reported.7
For cases and controls, original pathology reports were reviewed for each CRC specimen to confirm the diagnosis of invasive malignancy, Original pathological slides and tumour blocks, where available, were obtained for each specimen for central review. Tumour microsatellite instability analysis and immunohistochemistry for mismatch repair genes MSH2, MLH1, MSH6 and PMS2 were available for most cases.
PATHOLOGICAL FEATURES
All available haematoxylin and eosin slides from each case were reviewed. The main histological subtype of each carcinoma was recorded (>50% of the tumour). Any secondary patterns were also noted. Serrated carcinoma was diagnosed using published criteria encompassing a number of features. 18, 19 These included a serrated growth pattern in well-differentiated areas in which the serrations lack fibrovascular cores. In poorly differentiated carcinomas, a serrated pattern may not be apparent and trabecular and lace-like patterns may be seen. The cells have a characteristic morphology including abundant eosinophilic cytoplasm and vesicular nuclei with chromatin condensation at the nuclear envelope. Mucinous differentiation is common in serrated carcinomas and the cells retain the cytoplasmic eosinophilia and are often arranged in papillary rods or as cell balls floating in mucin. Mucinous carcinomas were defined according to the World Health Organization criteria, i.e. tumours composed of ≥50% extracellular mucin.20 Additional pathological features recorded included site, size, grade, nature of the tumour margin, tumour-infiltrating lymphocytes [high = ≥12 intraepithelial lymphocytes per high-power field (HPF; x40); low = 5–11 intraepithelial lymphocytes per HPF (x40)], a Crohn’s like lymphocytic response [four or more nodular lymphoid aggregates in a low-power field (x4) in the submucosa or serosa at the advancing edge of the tumour],21 pT status, lymph node status and the presence or absence of the following features: additional polyps, an adenomatous edge to the tumour, intraglandular (‘dirty’) necrosis within the tumour, extraglandular necrosis, a poorly differentiated component at the invasive edge, a desmoplastic response, lymphatic invasion, venous invasion and perineural invasion.
IMMUNOHISTOCHEMISTRY
Tumour slides were stained with Rabbit polyclonal anti-MYH antibody (Abeam, Cambridge, UK) at 1:200 dilution and counterstained with haematoxylin. Both nuclear and cytoplasmic immunoreactivity was assessed in normal colonic mucosa and in carcinomas and scored using the Allred method.22
STATISTICAL ANALYSIS
Frequencies of observed pathological patterns and MYH mutation status were compared using χ2 test and Fisher’s exact test. Comparisons of continuous variables (age, tumour size) was done by analysis of variance. Associations between histological patterns and specific mutation status (biallelic or monoallelic) and multivariate analysis was determined by logistic regression modelling. Correlations between pathological variables were assessed by calculation of Spearman correlation coefficients. All analyses were performed using SAS 9,1 (SAS Institute, Cary, NC, USA).
Results
The study group included 57 carcinomas. These comprised 16 tumours from patients with biallelic mutations (including homozygotes and compound heterozygotes), 25 from patients with monoallelic mutations and 16 from those with benign MYH polymorphisms (controls). Details of the mutations are provided in Table 1.
Table 1.
MYH mutations
| Patients, n | Carcinomas, n | |
|---|---|---|
| Biallelics | 14 | 16 |
| Y165C/Y165C | 2 | 3 |
| G382D/G382D | 3 | 3 |
| Y165C/G382D | 3 | 3 |
| Y165C/891+3A>C | 1 | 1 |
| Y165C/Y90X | 1 | 1 |
| G382D/891+3A>C | 1 | 1 |
| Y165C/Q377X | 1 | 2 |
| 1103delC/1103delC | 1 | 1 |
| Y90X/1103delC | 1 | 1 |
| Heterozygotes | 22 | 25 |
| Y165C/− | 5 | 6 |
| G382D/− | 17 | 19 |
| Controls | 14 | 16 |
The mean age (±SD) at diagnosis of the patients was 51 years (±11.3), 59 years (±14.7) and 62 years (±9.0) for those with germ-line biallelic mutations, heterozygous mutations and controls, respectively (P = 0.04).
PATHOLOGICAL FEATURES
Histological subtype
The most commonly observed primary pattern in all groups was tubular, which was seen in 8/16 (50%) of tumours from biallelic carriers, 11/25 (44%) of those from heterozygous carriers and in 4/16 (25%) of those from controls. A serrated pattern was the predominant histological type in 3/25 (12%) of the heterozygotes, but none of the tumours from biallelic or control patients exhibited a predominantly serrated pattern (Figure 1). One of these cases demonstrated high-grade cytology, but retained the features of serrated carcinoma. In comparing the frequencies of predominant histological patterns among biallelic, heterozygous and control tumours, a trend was seen towards a difference between these groups (P = 0.089); however, due to the low number of observations in each category, this did not achieve statistical significance. Therefore, histological patterns were categorized according to typical/usual adenocarcinoma features (tubular, cribriform, papillary, adenocarcinoma not otherwise specified), microsatellite instability features (mucinous, signet ring, undifferentiated/medullary, microglandular goblet cell carcinoma) and serrated features. Using this grouping of histological patters, all (16/16) biallelic tumours demonstrated usual or typical features compared with 80% of heterozygous tumours and 63% of control tumours, whereas the microsatellite instability pattern was seen in 8% of heterozygous tumours and 37% of control tumours (P = 0.0053). A secondary serrated component was seen in 1/16 (6.2%) of the biallelics, in 1/25 (4%) of the heterozygotes and in 2/16 (12.5%) of the controls. Common secondary patterns in all groups included papillary (40.35% of all carcinomas), tubular (22.8% of all carcinomas) and cribriform (17.5%). There was no significant difference between the groups as regards either individual secondary histological patterns (P = 0.65) or secondary histological group (P = 0.59). The histological types are summarized in Table 2.
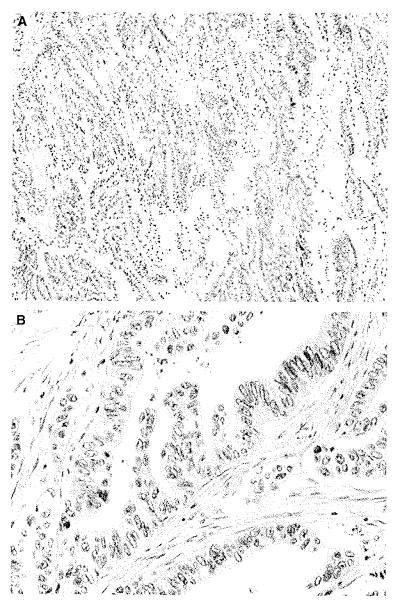
Figure 1.
Serrated carcinoma from a heterozygote. A, The carcinoma has a serrated growth pattern with eosinophilic cytoplasm (H&E). B, Detail of (A) showing vesicular nuclei with chromatin condensation around the nuclear envelope (H&E).
Table 2.
(a) Histological pattern (main) and (b) Histological pattern (secondary)
| Biallelics, n (%) |
Heterozygotes, n (%) |
Controls, n (%) |
P-value* | |
|---|---|---|---|---|
| (a) | ||||
| Serrated | 0 | 3 (12.0) | 0 | |
| Tubular | 8 (50.0) | 11 (44.0) | 4 (25.0) | |
| Papillary | 4 (25.0) | 1 (4.0) | 3 (18.7) | |
| Cribriform | 4 (25.0) | 6 (24.0) | 3 (18.7) | |
| Mucinous | 0 | 2 (8.0) | 4 (25.0) | |
| Medullary | 0 | 0 | 1 (6.3) | |
| Adenocarcinoma NOS | 0 | 2 (8.0) | 0 | |
| Microglandular goblet Cell carcinoma |
0 | 0 | 1 (6.3) | 0.089 |
| Total | 16 | 25 | 16 | |
| Usual pattern | 16 (100.0) | 20 (80.0) | 10 (62.5) | |
| MSI-like pattern | 0 | 2 (8.0) | 6 (37.5) | |
| Serrated pattern | 0 | 3 (12.0) | 0 | 0.0053 |
| (b) | ||||
| Serrated | 1 (6.3) | 1 (4.0) | 2 (12.5) | |
| Tubular | 3 (18.7) | 5 (20.0) | 5 (31.3) | |
| Papillary | 6 (37.5) | 12 (48.0) | 5 (31.3) | |
| Cribriform | 4 (25.0) | 5 (20.0) | 1 (6.2) | |
| Mucinous | 2 (12.5) | 2 (8.0) | 2 (12.5) | |
| Adenocarcinoma NOS | 0 | 0 | 1 (6.2) | 0.65 |
| Usual pattern | 13 (80.5) | 22 (88.0) | 12 (75.0) | |
| MSI-like pattern | 2 (13.3) | 2 (8.0) | 2 (12.5) | |
| Serrated pattern | 1 (6.2) | 1 (4.0) | 2 (12.5) | 0.59 |
Fisher’s exact test.
NOS, Not otherwise specified; MSI, microsatellite instability.
OTHER PATHOLOGICAL FEATURES
Comparing gross pathological features, there was no significant difference between the groups with regard to the location (P = 0.95) or size (P = 0.31) of the tumours. Not surprisingly, there was a significantly higher frequency of synchronous polyps in resection specimens from biallelic mutation carriers compared with heterozygous carriers and controls (P = 0.035). The types of polyps present in biallelic patients included tubular adenomas, tubulovillous adenomas, serrated adenomas, hyperplastic polyps and mixed hyperplastic and adenomatous polyps. However, no adenomatous or hyperplastic polyps were identified in the specimens of 25% of biallelic carriers.
Comparing histological features, all tumours in the biallelic group were low-grade carcinomas, in comparison with 4/25 (16%) tumours in the heterozygous group and 6/16 (37.5%) control tumours that were high grade (P = 0.02). All tumours from biallelic and monoallelic patients showed limited amounts of extraglandular necrosis, whereas 3/16 (18.8%) of control tumours showed extensive extraglandular necrosis.
There was no difference in depth of tumour invasion (T-stage) according to MYH genotype, but there was a slight non-significant trend towards lower rate of lymph node involvement with 2/16 (12.5%) of biallelic tumours having positive lymph nodes compared with 10/24 (42%) and 3/15 (20%) of monoallelic and control tumours, respectively (P = 0.10). Nodal status was unknown in two cases.
Tumours were assessed for mismatch repair (MMR) deficiency by microsatellite instability analysis and/or immunohistochemistry for MMR proteins MLH1, MSH2 and MSH6. Two out of 11 tumours with results available (18%) in biallelic patients demonstrated MMR deficiency compared with 7/24 with results available (29%) of monoallelic and 4/16 (25%) of control tumours mismatch repair (MMR 0.6). Similarly, there was no difference in observed frequencies of MMR-associated pathological characteristics such as tumour-infiltrating lymphocytes or Crohn’s like lymphocytic response. However, there was a significant correlation between microsatellite instability status and Crohn’s-like response and tumour-infiltrating lymphocytes (Spearman ρ = 0.35, P = 0.008). Family history pedigrees for all high-frequency microsatellite instability MYH mutation carriers (biallelic and monoallelic) were reviewed and none of the carriers had a family history suggestive of hereditary non-polyposis colonic cancer (HNPCC) by modified Amsterdam criteria23 (see Table 3 for a summary of all pathological features).
Table 3.
Other pathological features
| Biallelics, n (%) |
Heterozygotes, n (%) |
Controls, n (%) |
P-value | |
|---|---|---|---|---|
| Site | 0.95 | |||
| Right colon | 6/13 (46.2) | 8/22 (36.4) | 5/13 (38.5) | |
| Transverse colon | 0 | 2/22 (9.1) | 0 | |
| Left colon and rectum | 7/13 (53.8) | 12/22 (54.5) | 8/13 (61.5) | |
| Not specified | 3 | 3 | 3 | |
| Grade | 0.002 | |||
| High | 0 | 4/25 (16.0) | 6/16 (37.5) | |
| Low | 16/16 (100.0) | 21/25 (84.0) | 10/16 (62.5) | |
| Tumour margin | ||||
| Expanding | 14/16 (87.5) | 16/25 (64.0) | 12/16 (75.0) | 0.24 |
| Infiltrating | 2/16 (12.5) | 9/25 (36.0) | 4/16 (25.0) | |
| Crohn’s-like lymphocytic respons |
0.64 | |||
| Present | 5/16 (31.3) | 11/24 (45.8) | 5/16 (31.3) | |
| Absent | 11/16 (68.7) | 13/24 (54.2) | 11/16 (68.7) | |
| Unknown | 0 | 1 | 0 | |
| Tumour-infiltrating lymphocytes |
0.63 | |||
| Present | 8/16 (50.0) | 11/25 (44.0) | 8/16 (50.0) | |
| Absent | 8/16 (50.0) | 14/25 (56.0) | 8/16 (50.0) | |
| Microsatellite status | 0.28 | |||
| Stable | 9/11 (81.8) | 17/24 (70.8) | 12/16 (75.0) | |
| Unstable | 2/11 (18.2) | 7/24 (29.2) | 4/16 (25.0) | |
| Unknown | 5 | 1 | 0 | |
| pT | 0.47 | |||
| 1 | 3/16 (18.7) | 4/25 (16.0) | 4/16 (25.0) | |
| 2 | 5/16 (31.3) | 3/25 (12.0) | 1/16 (6.3) | |
| 3 | 8/16 (50.0) | 16/25 (64.0) | 9/16 (56.2) | |
| 4 | 0 | 2/25 (8.0) | 2/16 (12.5) | |
| Lymph node status | 0.1 | |||
| Negative | 14/16 (87.5) | 14/24 (58.3) | 12/15 (80.0) | |
| Positive | 2/16 (12.5) | 10/24 (41.7) | 3/15 (20.0) | |
| Unknown | 0 | 1 | 1 | |
| Lymphatic invasion | 0.12 | |||
| Present | 3/16 (18.7) | 10/25 (40.0) | 2/16 (12.5) | |
| Absent | 13/16 (81.3) | 15/25 (60.0) | 14/16 (87.5) | |
| Venous invasion | 0.43 | |||
| Present | 2/16 (12.5) | 4/25 (16.0) | 5/16 (31.3) | |
| Absent | 14/16 (87.5) | 21/25 (84.0) | 11/16 (68.7) | |
| Perineural invasion | ||||
| Present | 1/16 (6.3) | 3/24 (12.5) | 1/15 (6.7) | 0.85 |
| Absent | 15/16 (93.7) | 21/24 (87.5) | 14/15 (93.3) | |
| Unknown | 0 | 1 | 1 | |
| Intraglandular necrosis | 0.24 | |||
| Marked (>50%) | 1/15 (6.7) | 4/25 (16.0) | 5/16 (31.3) | |
| Mild (<50%) | 14/15 (93.3) | 21/25 (84.0) | 11/16 (68.7) | |
| Unknown | 1 | 0 | 0 | |
| Extraglandular necrosis | 0.02 | |||
| Marked | 0 | 0 | 3/16 (18.8) | |
| Mild | 15/15 (100.0) | 25/25 (100.0) | 13/16 (81.3) | |
| Unknown | 1 | |||
| Poorly differentiated component at invasive edge |
0.63 | |||
| Present | 5/15 (33.3) | 10/25 (40.0) | 4/16 (25.0) | |
| Absent | 10/15 (66.7) | 15/25 (60.0) | 12/16 (75.0) | |
| Unknown | 1 | 0 | 0 | |
| Desmoplastic response | 0.77 | |||
| Marked | 2/15 (13.3) | 5/25 (20.0) | 4/16 (25.0) | |
| Mild/absent | 13/15 (86.7) | 20/25 (80.0) | 12/16 (75.0) | |
| Unknown | 1 | 0 | 0 | |
| Adenomatous edge | 0.78 | |||
| Present | 7/16 (43.7) | 8/24 (33.3) | 5/16 (31.3) | |
| Absent | 9/16 (56.3) | 16/24 (66.7) | 11/16 (68.7) | |
| Unknown | 0 | 1 | 0 | |
| Additional polyps | 0.035 | |||
| Present | 12/16 (75.0) | 8/24 (33.3) | 6/14 (42.9) | |
| Absent | 4/16 (25.0) | 16/24 (66.7) | 8/14 (57.1) | |
| Unknown | 0 | 1 | 2 |
We attempted to determine the utility of MYH immunohistochemistry to detect MYH protein deficiency and to verify the observations of DiGregorio et al.15 MYH immunohistochemistry showed variable degrees of cytoplasmic granular staining in the perinuclear region of normal colonic epithelial cells, adenomas and carcinomas. Nuclear immunoreactivity was not evident. There was no difference in reactivity pattern between biallelics, heterozygotes and controls (Figure 2).
Figure 2.
MYH immunohistochemistry showing a similar pattern of granular cytoplasmic reactivity in malignant and benign epithelium from biallelics (A = tumour, B = normal), heterozygotes (C = tumour, D = normal) and controls (E = tumour, F = normal).
Discussion
MYH-associated polyposis is a recently recognized condition that is associated with intestinal polyposis and an increased risk of CRC in patients with germ-line biallelic mutations and possibly in monoallelic carriers.1,3,7,9,24,25 Given that the inheritance pattern is usually autosomal recessive, a family history may be lacking. A characteristic pathological appearance would therefore be helpful in raising the suspicion of this disorder, as is seen in patients with HNPCC, who have MSI-H carcinomas. Descriptions of the pathological features of carcinomas arising in those with MYH mutations are few and are limited in the number of histological parameters assessed.2,26,27
In this study, the biallelic MYH mutation carriers were significantly younger (51 years), than the heterozygous (59 years) or control (62 years) subjects. These figures concur with the reported ranges for average age of onset of CRC in these groups.2–4,10,28–31
We reviewed 16 invasive cancers from 14 biallelic MYH mutation carriers and 25 cancers from 22 monoallelic carriers. Tumours from biallelic carriers were low grade, demonstrated mild levels of extraglandular necrosis, and were often associated with synchronous adenomatous polyps. The main histological patterns in tumours in biallelic patients were tubular, papillary and cribriform. There was no significant difference in the depth of tumour invasion (T-stage) according to MYH genotype, but there was a non-significant trend to lower frequency of positive lymph nodes in biallelics and higher frequency of pNl disease in monoallelic carriers. This observation for biallelic carriers is in contrast with some studies that report higher-stage tumours in MYH-associated carcinomas,31 but in concordance with others.4 Of the remaining histopathological features that were assessed, carcinomas occurring in patients with biallelic MYH mutations did not manifest a significant difference in comparison with those present in heterozygotes and in controls with wild-type or non-pathogenic polymorphisms.
Genetic factors such as attenuated FAP and HNPCC have been shown to influence tumour location.32,33 Previous series of MYH-associated cancers have reported divergent findings with respect to patterns of tumour location. Some studies have shown more right-sided carcinomas in biallelics. 4,5,27 Conversely, in other studies there is a marked left-sided predominance noted in up to 70% of carcinomas.2,28,29,34 Although we did not observe a significant difference in tumour location according to MYH mutation status, we did note that 46% of tumours from biallelic carriers arose in the caecum or ascending colon. Although an increase in right-sided tumours has been observed in recent years in sporadic carcinomas, this figure is still greater than reported for patients in a similar age range (25.4%) in one recent study based on material from the California tumour registry.35
None of the 16 carcinomas present in biallelics in this series was high grade, in contrast to 16% of those in patients with monoallelic mutations and 37.5% of controls (P = 0.002). The absence of high-grade carcinomas in biallelics has been observed in one other study that included three carcinomas from two patients,27 but other studies do not support this finding.2,15,31 In addition, we observed that 25% of biallelic mutation carriers did not have synchronous polyps in their colectomy specimen. The original phenotype attributed to MYH-associated cancers was derived from series of highly-selected clinic-based polyposis patients, 3,10 and the inconsistent association between MYH and polyps has been previously reported by our group.7 Although polyp counts were derived from partial colectomy specimens and not full colonic assessments, this observation supports the findings of others8,31 that a polyposis phenotype may not be as severe as originally described, or may be absent in some cases.
Preliminary review of a subset of cases from patients with biallelic MYH mutations in our centre suggested that a serrated pattern might be more frequent in these carcinomas. However, none of the biallelic carcinomas had a serrated pattern in the majority of the tumour and only a single case showed a minor serrated component (1/16, 6.3%). Three carcinomas with a predominantly serrated morphology were identified, all from heterozygotes. This gives an incidence of 5.3% for serrated carcinoma, which is close to the reported incidence of up to 7.5%.18,36,37 Although there was no significant difference evident between the groups with regard to a predominantly serrated morphology, it is interesting that all of the cases were observed in heterozygotes. Loss of heterozygosity in chromosome 1p has been noted more frequently in carcinomas arising in heterozygotes, 38 a finding that has also been described in some hyperplastic polyps, some adenomas and as an early event in CRC.39 It may not be surprising that serrated carcinomas are not observed in biallelics, as KRAS mutations are common in MYH-associated tumours,2 but BRAF mutations are not generally present, which are often seen in association with the serrated neoplasia pathway, particularly where hypermethylation is present.36,40,41 The majority of serrated adenocarcinomas are microsatellite stable or have low-level microsatellite instability, and these tend to be associated with traditional serrated adenomas, whereas serrated carcinomas arising in sessile serrated adenomas are frequently microsatellite unstable.18 Of interest, all of the serrated carcinomas in our study were microsatellite stable, and one had an adjacent traditional serrated adenoma.
Microsatellite instability was found in two biallelic cancers in our series. This co-occurrence of biallelic MYH mutations and MSI-H has been reported previously27 and would appear to challenge the suggestion by some that deficiency of MMR and base excision repair pathways is incompatible with cellular survival.2,38 Colebatch et al.27 have demonstrated that one of the three carcinomas in two biallelic patients was microsatellite unstable and all three showed prominent intraepithelial lymphocytes. This tumour had MLH1 inactivation by biallelic promoter methylation rather than somatic mutations secondary to loss of MYH function. The authors concluded that in this case biallelic MYH mutations had played a role in the early stages of colorectal carcinogenesis, but had been superseded by the effects of loss of MLH1 function, and also suggest that MYH mutation testing might be worth considering in younger individuals with MSI-H tumours that have developed because of MLH1 methylation. It is interesting to note that in our series the two biallelic MYH MSI-H cancers did not have features typically associated with MSI-H cancers as the main histological pattern, but rather demonstrated papillary and tubular features, with one tumour having mucinous features as a secondary pattern.
Overall, carcinomas in biallelic MYH mutation carriers do not appear similar to MSI-H tumours, but rather share common histological features with CRC with chromosomal instability (CIN). The histological pattern observed in biallelic MYH cancers may correlate with aspects of molecular carcinogenesis, since both MYH cancers and CIN tumours undergo early somatic inactivation of APC and KRAS.1,2,42 In contrast, although MSI-H tumours can also undergo early mutations in APC, these cancers tend to accumulate mutations in genes with repetitive sequences in the coding region including: TGF-βRII, BAX, IGF-2R, E2F-4 and (β-catenin.43-48 A recent study has shown that when APC mutations are present in sporadic MSI-H CRC, they are likely to be frameshift mutations of short nucleotide repeats, implying that microsatellite instability precedes the development of APC mutations.49 The observed phenotypic similarities between biallelic MYH cancers and CIN tumours may support recent findings by Cardoso et al.,50 which demonstrated a high frequency of aneuploid changes in MYH-associated tumours and similar patterns of chromosomal gains and losses between MYH- and FAP-associated polyps. Cancers found in monoallelic MYH mutation carriers appear to be somewhat more heterogeneous, and the frequency of various histopathological features appears to be similar to that of sporadic CRC.
In this study, MYH immunohistochemistry failed to discriminate between biallelics, heterozygotes and controls. Granular cytoplasmic immunoreactivity in normal colonic mucosa and in CRC was observed with similar frequency in each group. No nuclear reactivity was identified. Sections from these tumours were stained in two other external laboratories with similar results. This contrasts with the findings of DiGregorio et al.,15 who found nuclear and cytoplasmic reactivity in normal colonic mucosa and in sporadic CRC, but loss of nuclear reactivity and prominent cytoplasmic granular reactivity in patients with biallelic MYH mutations. Although we used the same antibody, it is possible that technical factors such as differences in fixation or the age of the paraffin blocks may have influenced the results of our immunohistochemistry. However, to our knowledge, the majority of tumours were fixed in 10% neutral buffered formalin and the presence or absence of immunoreactivity did not appear to be related to the age of the block.
In our experience, therefore, neither immunohistochemical nor histological features can distinguish reliably between carcinomas arising in those with MYH mutations and in sporadic colorectal carcinomas.
Acknowledgements
SPC is supported by a fellowship grant from the Canadian Institute for Health Research. This work was supported by the National Cancer Institute, National Institutes of Health under RFA no. CA-95-011, the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783), and through cooperative agreements with members of the Colon Cancer Family Registry (CFRs) and P.I.s, and the National Cancer Institute of Canada (grant no. 13304 to S.G.). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centres in the CFRs, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CFR.
Abbreviations
- APC
adenomatous polyposis coli
- CIN
chromosomal instability
- CRC
colorectal carcinoma
- FAP
familial adenomatous polyposis
- FGICR
Familial Gastrointestinal Cancer Registry
- HNPCC
hereditary non-polyposis colonic cancer
- HPF
high-power field
- KRAS
Kirsten rat sarcoma
- MMR
mismatch repair
- MSI-H
high-frequency microsatellite instability
- OFCCR
Ontario Familial Colon Cancer Registry
References
- 1.Al Tassan N, Chmiel NH, Maynard J, et al. Inherited variants of MYH associated with somatic G:C->T:A mutations in colorectal tumors. Nat. Genet. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 2.Lipton L, Halford SE, Johnson V, et al. Carcinogenesis in MYH-associated polyposis follows a distinct genetic pathway. Cancer Res. 2003;63:7595–7599. [PubMed] [Google Scholar]
- 3.Sieber OM, Lipton L, Crabtree M, et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N. Engl. J. Med. 2003;348:791–799. doi: 10.1056/NEJMoa025283. [DOI] [PubMed] [Google Scholar]
- 4.Kanter-Smoler G, Bjork J, Fritzell K, et al. Novel findings in Swedish patients with MYH-associated polyposis: mutation detection and clinical characterization. Clin. Gastroenterol. Hepatol. 2006;4:499–506. doi: 10.1016/j.cgh.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP) J. Med. Genet. 2005;42:e54. doi: 10.1136/jmg.2005.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J. Surg. Oncol. 2007;95:499–506. doi: 10.1002/jso.20724. [DOI] [PubMed] [Google Scholar]
- 7.Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J. Natl Cancer Inst. 2004;96:1631–1634. doi: 10.1093/jnci/djh288. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Baudhuin LM, Boardman LA, et al. MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology. 2004;127:9–16. doi: 10.1053/j.gastro.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins MA, Croitoru ME, Monga N, et al. Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol. Biomarkers Prev. 2006;15:312–314. doi: 10.1158/1055-9965.EPI-05-0793. [DOI] [PubMed] [Google Scholar]
- 10.Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39–41. doi: 10.1016/S0140-6736(03)13805-6. [DOI] [PubMed] [Google Scholar]
- 11.Halford SE, Rowan AJ, Lipton L, et al. Germline mutations but not somatic changes at the MYH locus contribute to the pathogenesis of unselected colorectal cancers. Am. J. Pathol. 2003;162:1545–1548. doi: 10.1016/S0002-9440(10)64288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenesa A, Farrington SM, Dunlop MG. Re: Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J. Natl Cancer Inst. 2005;97:320–321. doi: 10.1093/jnci/dji051. [DOI] [PubMed] [Google Scholar]
- 13.Peterlongo P, Mitra N, Sanchez dA, et al. Increased frequency of disease-causing MYH mutations in colon cancer families. Carcinogenesis. 2006;27:2243–2249. doi: 10.1093/carcin/bgl093. [DOI] [PubMed] [Google Scholar]
- 14.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Gregorio C, Frattini M, Maffei S, et al. Immunohistochemical expression of MYH protein can be used to identify patients with MYH-associated polyposis. Gastroenterology. 2006;131:439–444. doi: 10.1053/j.gastro.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 16.Newcombe P, Baron J, Cotterchio M, et al. Colon cancer family registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:2331–2443. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 17.Cotterchio M, McKeown-Eyssen G, Sutherland H, et al. Ontario familial colon cancer registry: methods and first-year response rates. Chronic. Dis. Can. 2000;21:81–86. [PubMed] [Google Scholar]
- 18.Tuppurainen K, Makinen JM, Junttila O, et al. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J. Pathol. 2005;207:285–294. doi: 10.1002/path.1850. [DOI] [PubMed] [Google Scholar]
- 19.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SR, Vogelstein B, Kudo S, et al. Tumours of the colon and rectum. In: Hamilton SR, Aaltonen LA, editors. World Health Organisation classification of tumour. Pathology and genetics of tumours of the digestive system. IARC Press; Lyons: 2000. pp. 103–119. [Google Scholar]
- 21.Jenkins MA, Hayashi S, O’Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 23.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 24.Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int. J. Cancer. 2004;109:680–684. doi: 10.1002/ijc.20054. [DOI] [PubMed] [Google Scholar]
- 25.Jones S, Emmerson P, Maynard J, et al. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C->T:A mutations. Hum. Mol. Genet. 2002;11:2961–2967. doi: 10.1093/hmg/11.23.2961. [DOI] [PubMed] [Google Scholar]
- 26.Chow E, Lipton L, Lynch E, et al. Hyperplastic polyposis syndrome: phenotypic presentations and the role of MBD4 and MYH. Gastroenterology. 2006;131:30–39. doi: 10.1053/j.gastro.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Colebatch A, Hitchins M, Williams R, Meagher A, Hawkins NJ, Ward RL. The role of MYH and microsatellite instability in the development of sporadic colorectal cancer. Br. J. Cancer. 2006;95:1239–1243. doi: 10.1038/sj.bjc.6603421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefevre JH, Rodrigue CM, Mourra N, et al. Implication of MYH in colorectal polyposis. Ann. Surg. 2006;244:874–879. doi: 10.1097/01.sla.0000246937.54435.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int. J. Cancer. 2006;119:807–814. doi: 10.1002/ijc.21905. [DOI] [PubMed] [Google Scholar]
- 30.Peterlongo P, Mitra N, Chuai S, et al. Colorectal cancer risk in individuals with biallelic or monoallelic mutations of MYH. Int. J. Cancer. 2005;114:505–507. doi: 10.1002/ijc.20767. [DOI] [PubMed] [Google Scholar]
- 31.Enholm S, Hienonen T, Suomalainen A, et al. Proportion and phenotype of MYH-associated colorectal neoplasia in a population-based series of Finnish colorectal cancer patients. Am. J. Pathol. 2003;163:827–832. doi: 10.1016/S0002-9440(10)63443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch HT, Smyrk T, McGinn T, et al. Attenuated familial adenomatous polyposis (AFAP). A phenotypically and genotypically distinctive variant of FAP. Cancer. 1995;76:2427–2433. doi: 10.1002/1097-0142(19951215)76:12<2427::aid-cncr2820761205>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 33.Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N. Engl. J. Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 34.Fleischmann C, Peto J, Cheadle J, Shah B, Sampson J, Houlston RS. Comprehensive analysis of the contribution of germline MYH variation to early-onset colorectal cancer. Int. J. Cancer. 2004;109:554–558. doi: 10.1002/ijc.20020. [DOI] [PubMed] [Google Scholar]
- 35.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J. Clin. Gastroenterol. 2007;41:173–177. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 36.Makinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 37.Makinen MJ, George SM, Jernvall P, Makela J, Vihko P, Karttunen TJ. Colorectal carcinoma associated with serrated adenoma - prevalence, histological features, and prognosis. J. Pathol. 2001;193:286–294. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH800>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Kambara T, Whitehall VL, Spring KJ, et al. Role of inherited defects of MYH in the development of sporadic colorectal cancer. Genes Chromosomes Cancer. 2004;40:1–9. doi: 10.1002/gcc.20011. [DOI] [PubMed] [Google Scholar]
- 39.Rashid A, Houlihan PS, Booker S, Petersen GM, Giardiello FM, Hamilton SR. Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology. 2000;119:323–332. doi: 10.1053/gast.2000.9361. [DOI] [PubMed] [Google Scholar]
- 40.Johnson V, Lipton LR, Cummings C, et al. Analysis of somatic molecular changes, clinicopathological features, family history, and germline mutations in colorectal cancer families: evidence for efficient diagnosis of HNPCC and for the existence of distinct groups of non-HNPCC families. J. Med. Genet. 2005;42:756–762. doi: 10.1136/jmg.2005.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol. Cancer. 2006;5:2. doi: 10.1186/1476-4598-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones S, Lambert S, Williams GT, Best JM, Sampson JR, Cheadle JP. Increased frequency of the k-ras G12C mutation in MYH polyposis colorectal adenomas. Br. J. Cancer. 2004;90:1591–1593. doi: 10.1038/sj.bjc.6601747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirabelli-Primdahl L, Gryfe R, Kim H, et al. Beta-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res. 1999;59:3346–3351. [PubMed] [Google Scholar]
- 44.Markowitz S, Wang J, Myeroff L, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 45.Rampino N, Yamamoto H, Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Papadopoulos N, McKinley Al, et al. APC mutations in colorectal tumors with mismatch repair deficiency. Proc. Natl Acad. Sci. USA. 1996;93:9049–9054. doi: 10.1073/pnas.93.17.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza RF, Yin J, Smolinski KN, et al. Frequent mutation of the E2F-4 cell cycle gene in primary human gastrointestinal tumors. Cancer Res. 1997;57:2350–2353. [PubMed] [Google Scholar]
- 48.Souza RF, Appel R, Yin J, et al. Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat. Genet. 1996;14:255–257. doi: 10.1038/ng1196-255. [DOI] [PubMed] [Google Scholar]
- 49.Samowitz WS, Slattery ML, Sweeney C, et al. APC mutations and other genetic and epigenetic changes in colon cancer. Mol Cancer Res. 2007;5:165–170. doi: 10.1158/1541-7786.MCR-06-0398. [DOI] [PubMed] [Google Scholar]
- 50.Cardoso J, Molenaar L, de Menezes RX, et al. Chromosomal instability in MYH- and APC-mutant adenomatous polyps. Cancer Res. 2006;66:2514–2519. doi: 10.1158/0008-5472.CAN-05-2407. [DOI] [PubMed] [Google Scholar]