Abstract
The development of antiretroviral drugs over the past couple of decades has been commendable due to the identification of several new targets within the overall Human Immunodeficiency Virus (HIV) replication cycle. However, complete control over HIV/Acquired Immune Deficiency Syndrome is yet to be achieved. This is because the current anti-HIV drugs, although effective in reducing plasma viral levels, cannot eradicate the virus completely from the body. This occurs because most anti-HIV drugs do not accumulate in certain cellular and anatomical reservoirs including the Central Nervous System (CNS). Insufficient delivery of anti-HIV drugs to the CNS is attributed to their low permeability across the blood-brain-barrier (BBB). Hence, low and sustained viral replication within the CNS continues even during prolonged antiretroviral drug therapy. Therefore, developing novel approaches that are targeted at enhancing the CNS delivery of anti-HIV drugs are required. In this review, we discussed the potential of nanocarriers and the role of cell-penetrating peptides in enhancing drug delivery to the CNS. Such drug delivery approaches could also lead to higher drug delivery to other cellular and anatomical reservoirs where the virus harbor than with conventional treatment, thus providing an effective therapy to eliminate the virus completely from the body.
Keywords: Acquired Immune Deficiency Syndrome (AIDS), anti-HIV drugs, Blood-brain-barrier (BBB), Central nervous system (CNS), Macrophage, Nanocarrier systems, Viral reservoirs
1. Introduction
Human Immunodeficiency Virus (HIV) is the primary cause of Acquired Immuno Deficiency Syndrome (AIDS) which still remains the cause of significant mortality globally [1, 2]. HIV infection principally results in destruction of white blood cells (lymphocytes), which constitute an important element of the body’s immune defense. This decline in lymphocyte level in the more advanced stages of infection, is responsible for the profound immune suppression that characterizes the advanced stage of AIDS [3]. Antiretroviral therapy for HIV infection has transformed this disease from a terminal illness to a chronic, yet manageable condition and has significantly reduced HIV-related mortality. Further, the weakened immune system also makes the individual more susceptible to “opportunistic infections”, such as tuberculosis [4, 5], toxoplasmosis, Kaposi’s sarcoma as well as other such cancers [6].
2. Mechanism of Viral Infection
Once inside the body, HIV can then enter the human cell in three important steps:
Attachment of the HIV surface gp120 glycoprotein to the CD4 receptor located on the cell membrane. These receptors are expressed by the monocyte derived macrophages and T-lymphocytes
Interaction of the gp120 protein and CD4 complex with a coreceptor
Virus-cell membrane fusion mediated by transmembrane gp41 protein
Upon internalization, the viral enzymes, viz. reverse transcriptase (RT) and integrase are released into the cytosol of the host cell. The viral RNA is then transcribed into a double-stranded DNA with the help of enzyme RT, followed by integration of viral DNA into the host genome resulting in formation of a provirus. Provirus formation is followed by a transcription step, wherein the unspliced viral RNA leaves the nucleus and, with the help of the host translation machinery, viral proteins are formed from unspliced transcript.
Involvement of the central nervous system (CNS) in HIV-infected individuals is common. The CNS serves as a sanctuary for HIV-1 that is capable of reactivating the infection. Important brain structures such as microglia, macrophages and possibly neurons, play a major role in viral persistence in the CNS. Direct injury to the brain resulting from HIV infection can lead to milder form of cognitive impairment and dementia in the more severe cases [7].
HIV-1 entry in the CNS begins with the infection of three different types of cells, which are the principal components of the body’s immune system. These are the CD4+ T lymphocytes, macrophages and monocytes [8]. These cell types act as a latent viral reservoir, which can cause the re-establishment of infection despite low or negligible plasma virus levels. The CD4+ T lymphocytes and monocytes primarily serve as the port of entry for HIV-1 into the CNS. A tight barrier of endothelial cells, known as the blood-brain-barrier (BBB), separates the CNS from the peripheral system. This barrier selectively regulates the transport of cells and other substances from the blood to the brain. According to one mechanism, infected monocytes facilitate transmigration of leukocytes through this BBB by means of adhesion molecules and release chemokines, leukotrienes, and tumor necrosis factor-alpha (TNF-α), which are responsible for disruption of the BBB integrity [9]. Subsequent to their entry, these monocytes further differentiate into macrophages, which are considered to be one of the main sources of productive HIV-1 infection. HIV-1 infection of other brain cells (astrocytes, oligodendrocytes and neurons) also occurs, albeit in a nonproductive fashion. Although HIV-1 nucleic acid was detected in the neuronal tissue in vivo during HIV infection, the subsequent other studies were unsuccessful in identifying the presence of HIV-1 nucleic acids or structural proteins within neurons [10–12]. Recently, Nogués et al have demonstrated that HIV-1 can actively infect human neurons in vivo. In their study, HIV-1-infected neurons were detected in the brain cortex in 50% of the subjects using light microscopy and in situ hybridization [13]. However, the general consensus remains that HIV-1 infection of neurons does occur, but neurons do not contribute extensively towards its progression. Astrocytes do not express the CD4 receptor, but they express the strain-specific CXCR4 receptor and the CCR5 co-receptor which can be recognized by HIV-1 leading to their infection [14]. Infection of astrocytes in vivo may also occur, albeit with a lower efficiency than what occurs within T cells and macrophages [14]. Infected astrocytes may assist in viral propagation and sustenance in the brain, thus serving as a sanctuary [15]. The mechanism of infection of oligodendrocytes is unclear since they do not express the CD4 receptors either [16].
3. Neurodegeneration due to HIV Infection
Infection of the central nervous system (CNS) by HIV-1 infection can lead to encephalitis that presents clinically as HIV-1-associated dementia (HAD) and HIV-associated neurocognitive disorders (HAND) compromises brain function and presents clinically as HAD [17, 18]. In the post-HAART era, HAND characterizes the neurological complications of acquired immuno deficiency syndrome (AIDS) that include HAD-related impairments. HAD remains the most severe form of HAD while minor cognitive and motor disorder is also observed [MCMD] [17]. Typically, HAND includes subcortical events, consisting of cognitive, behavior and motor dysfunction [19]. Symptoms of neurocognitive impairment in HAND include impaired short-term memory, reduced concentration, learning capability and reduced psychomotor skills that are often accompanied by behavioral symptoms such as personality changes, apathy and social withdrawal [17, 20]. However, a more subtle form of CNS dysfunction, MCMD is present in about 30% of the HIV-1 infected patients [21]. It is characterized by loss of memory, decrease in computational skills and other higher cortical functions [22]. One potential explanation for the development of MCMD is that, a low level of viral replication found in most successful ART regimens, leads to slower progressive neurodegeneration [23].
Despite the advent of anti-retroviral therapy (ART), at least 11.2% of HIV-1 patients suffer from HAD at the late stage of the disease [24]. Significant neuropathological damage occurs in the course of HIV infection of CNS, leading to severe neurological manifestations. This occurs due to direct as well as indirect effects of virus on the brain and neuronal cells. For example, HIV-1 TAT causes neurotoxicity by increasing cellular calcium levels and reactive oxygen species, and caspase activation of the apoptotic pathway [25]. TAT also increases the permeability of the BBB, leading to the infiltration of infected cells into the CNS [26]. Another viral protein, HIV-1 Vpr, arrests cells in G2/M cell cycle phase which causes neuronal cell death [27]. The HIV-1 envelope glycoprotein gp120 also has a neurotoxic effect, due to interaction with NMDA receptors [28]. gp-120 induced toxicity is induced by the double-stranded RNA activation of protein kinase, a stress kinase, which has a downstream signaling effect on the NMDA receptor resulting in subsequent neurotoxicity [29]. Indirect neurodegeneration occurs due to the persistent infection of monocytes, lymphocytes and microglia in the brain. These infected cells release cytokines, reactive oxygen species and other neurotoxins resulting in neuronal apoptosis. Some of the neurotoxins are TNF-α, arachidonic acid, quinolinic acid and nitric oxide [30]. Such inflammatory cascades beginning with the HIV-1-infected and immune activated microglial cells in turn likely lead to glial activation and changes in glial inflammatory responses, ultimately resulting in neurodegeneration.
Current HIV-1 treatment regimen consists of a combination therapy of one or more drugs that inhibit different enzymes in the HIV replication cycle (discussed in-depth in subsequent section). These drugs are always used in a combination of at least two and often three or four agents, and are referred to as “Highly Active Antiretroviral Therapy” (HAART) [31]. With the advent of HAART, the incidence of severe forms of dementia has been less frequent [19]. On the contrary, neurological deficits in the form of neurocognitive disorders and peripheral neuropathies are more common in clinical population, due to the increased life span of HIV infected individuals [32]. These deficits occur possibly due to non-reversible loss of neurons, or alternatively, due to continual neuronal damage occurring in patients despite being on combination therapy [33]. The resulting neurocognitive impairment in these individuals is characterized by symptoms of depression, and motor abnormalities such as slow movement, gait abnormality, lack of limb co-ordination and hyperreflexia [19, 34]. HIV patients failing HAART may also be affected by demyelinating leucoencephalopathy which is characterized by the infiltration of infected monocytes/macrophages in the brain and subsequent white matter destruction. Furthermore, 15% of the HIV-infected patients suffer from vacuolar myelopathy, which results due to vacuolization in the lateral and posterior columns of the spinal cord (Table 1) [35].
Table 1.
CNS complications due to HIV infection.
| Condition | Cause |
|---|---|
| Neuronal cell death | Cell-cycle arrest at G2/M phase caused by HIV-1 Vpr protein |
| Indirect neurodegeneration | Cytokines, reactive oxygen species, secreted by infected monocytes, lympocytes and microglia |
| Neurocognitive impairement (e.g. motor and behavioral abnormalities) | Non-reversible loss of neurons |
| Peripheral neuropathy | |
| Demyelinating leucoencephalopathy | HAART failure in HIV-1 infected patients |
| Vacuolar myelopathy | Vacuolization in lateral and posterior columns of the spinal cord |
Prior to initiation of antiretroviral therapy, neurological complications ensue due to direct effect of viral products. However, in patients who are already on drug therapy, cognitive impairments occur due to an ongoing low-level of inflammation resulting from immune activation [36]. Delivery of antiretrovirals to the CNS may thus be important in order to treat the underlying persisting infection within the CNS. Therapy should also aim for initiating antiretroviral treatment early on during the disease state, to minimize the neuronal loss. HIV is now considered as a chronic illness necessitating long-term management. Effective treatment paradigms for the HIV-1 associated neurocognitive deficits are yet to be devised, and an important consideration is the accurate determination of the consequences of therapy to treat these deficits. Assessing the viral load in the cerebrospinal fluid (CSF) may be a useful indicator of treatment effects in CNS. Ances et al. correlated the reduction in CSF viral load (< 50 copies of virus/ml of CSF), in patients with the initiation of HAART at prescribed doses of antiretroviral drugs. This viral reduction correspondingly improved the patient performance in neurophysiological tests [19].
4. Current Antiretroviral Therapies and Their Mechanism of Action
There are several potential targets in the HIV-1 replication cycle, upon which different anti-HIV drugs could act, as constituents of a HAART regimen. Currently there are about 20 anti-HIV drugs approved by the U.S Food and Drug Administration for clinical use and nearly 30 drugs are in preclinical trial stage (Table 2) [37]. Several problems exist with currently used regimens of general anti-HIV therapy which further complicate the delivery of anti-HIV drugs to the CNS (Table 3). Some of these problems are:
Table 2.
Current antiretroviral therapies and their mechanism of action.
| Class of anti-HIV drug | Mechanism of Action | Examples |
|---|---|---|
| Entry inhibitors | Inhibit HIV-1 entry into healthy CD4+ cells | Maraviroc |
| Fusion inhibitors | Prevent HIV from binding to T-cell surface | Enfuvirtide |
| Nucleoside reverse transcriptase inhibitors | Block the viral enzyme reverse transcriptase (RT) and inhibit DNA synthesis | Lamivudine, Zidovudine, Stavudine, Didanosine, Zalcitabine and Abacavir |
| Non-nucleoside reverse transcriptase inhibitors | Block the formation of new virions by preventing the conversion of viral RNA to DNA | Etravirine, Delaviridine, Efavirenz and Nevirapine |
| HIV integrase strand transfer inhibitors | Block the enzyme integrase and prevent the integration of the viral DNA into the host genome | Raltegravir |
| Protease inhibitors | Block the protease enzyme and prevent viral replication and viral assembly | Saquinavir, Ritonavir, Indinavir, Nelfinavir, Lopinavir, Amprenavir and Tipranavir |
Table 3.
Problems with current anti-HIV therapy.
| Problems associated with anti-HIV drug therapy |
|---|
| Poor absorption and limited bioavailability |
| Polypharmacy |
| Requirement of high dose and chronic therapy |
| Lack of compliance |
| Drug resistance |
| High plasma protein binding |
| Substrate for efflux transporters |
| Limited penetration across the BBB |
4.1 Low Oral Bioavailability
Although oral dosage forms of anti-HIV therapy offer ease of administration, the administered drug undergoes extensive first pass metabolism through this route. In the case of RT inhibitors, the time to reach peak blood/plasma concentration (Tmax) is typically within an hour, while their bioavailability is often variable (60–90%) depending on their site of absorption [38, 39]. The expression of multidrug resistant efflux proteins (MRP) such as P-glycoprotein (P-gp) on the gastrointestinal tract further decreases their oral bioavailability and reduces the amount of drug that can actually reach the CNS. Confounding the above factor is the high protein binding of most anti-HIV drugs that prevent their diffusion across the BBB [40].
One other major problem with anti-HIV drug therapy is that of resistance. The process of HIV replication is rapid and error-prone (~ 10 billion viral particles are produced on a daily basis), while generating at least one mutation per genome. These genetic mutations enable the virus to develop resistance to anti-HIV drug therapy, especially when monotherapy is employed [41]. Resistance to drug therapy has become a common occurrence and newly infected patients stand a chance of acquiring an HIV-resistant strain [42].
4.2 Long-term Drug Therapy
Antiretroviral therapy has significantly reduced AIDS-related morbidity and mortality. In addition to combining drugs from different classes, it is critical that the therapy remains uninterrupted to prevent the development of resistance [43]. However, prolonged treatment with these drugs has resulted in several side-effects, including muscular dystrophy, metabolic disorders, and peripheral neuropathy.
Additionally, it is estimated that at least 5 years of continuous antiretroviral therapy is required in order to eliminate the latent viral reservoir completely. However, compliance issues then become a complication and are often problematic [44]. In addition, the virus residing within the macrophages remains protected from the effect of antiretroviral drugs [45]. This viral reservoir continues to persist even after early initiation of antiretroviral therapy thus making antiretroviral therapy extremely challenging.
4.3 Anti-HIV gene therapy
While anti-retroviral drugs only control the level of viral replication, an important advantage of gene therapy is that it is able to operate by replacing the pool of cells with those resistant to HIV, thereby preventing further viral replication. Vesicular stomatitis virus G glycoprotein pseudotyped HIV-1-based virus-like particles (VLPs) have shown to eliminate HIV-1 infected cells, including the non-HIV replicating monocyte derived macrophages [7]. Gene therapy also has several disadvantages, however, which include the insertion of the gene at a wrong location in the cellular DNA, gene transfer in a wrong cell-type, selection of the right vector for the gene delivery, and the infrequent expression or no expression of the inserted gene [7]. Despite these technical difficulties, a gene therapy product for reduction of HIV levels has entered the phase two clinical trials and has demonstrated efficacy. The murine leukemia virus based gene has been successful in reducing the HIV levels in infected individuals with a subsequent increase in CD4+ count.
5. Role of BBB in the CNS Delivery of Anti-HIV Drugs
Unlike the peripheral cells, endothelial cells of the BBB are characterized by lack of fenestrations, lesser pinocytic activity and by the presence of intracellular tight junctions [46, 47]. The tight junctions between endothelial cells of the BBB give rise to a high endothelial electrical resistance (1500–2000Ω · cm2)resulting in very low paracellular permeability [48]. This vasculature also impedes the access of other agents and drugs that might be beneficial for the treatment of CNS diseases [49]. The BBB is therefore considered as a major impediment in the CNS permeation of therapeutic drugs. Drugs may be transported through the BBB by either passive or active transport. Greater CNS efflux than influx has been demonstrated with certain anti-HIV drugs (e.g. 3′-azido-3′-deoxythymidine, 2′,3′-dideoxyinosine and zidovudine), suggesting the involvement of efflux transporters [50, 51].
Specific transporters are expressed on the endothelial cells of the BBB that transport many lipophilic drugs entering the brain back to the blood. A multitude of influx and efflux transporters from several families have been detected. These include the multi-drug resistant protein (MDR), multi-drug resistance-associated protein (MRP), system L-transporters (LAT), organic anion transporter (OAT), organic cation transporter (OCT), monocarboxylate transport system (MCT), concentrative nucleoside transporter (CNT), and equilibriative nucleoside transporter (ENT) [52]. The most important amongst these is the ATP-binding cassette transporter, P-gp. P-gp is an energy-dependant transporter, encoded by the MDR1 gene and is highly localized on the apical surface of the endothelial cells of the brain capillaries [53, 54]. The poor passage of these drugs, particularly the PIs across the BBB is mainly attributed to their P-gp mediated efflux. In humans, P-gp is also expressed on the kidneys, hepatocytes, testes and on intestinal cells. P-gp expressed in intestinal cells is responsible for reduced oral bioavailability of PIs [54].
5.1 Approaches for overcoming the BBB to enhance CNS delivery
Due to the ineffectiveness of existing therapies in the delivery of anti-HIV drugs to the CNS, aggressive research has been directed towards the development of new strategies for effective delivery of drugs to the brain for the treatment of the CNS infection of HIV. These strategies could involve either modifying the drug, disrupting the BBB, or by developing novel drug delivery systems.
For example, augmenting the delivery of drugs to the brain by coupling drugs to molecules that have the ability to penetrate this tight vasculature has been investigated [55]. This includes chemically modifying the functional groups of drugs to make them more lipophilic. This approach has been used to deliver an analogue of the analgesic leucine-enkephalin to the brain. By the attachment of leucine-enkaphalin to 1,4-dihydrotrigonellyl on its ‘N’ terminus and a lipophilic ester (Lpf) on the ‘C’ terminus, it was possible to alter the polarity of the peptide and enhance its delivery to the brain [56]. This change in polarity rendered the peptide more lipophilic, thereby increasing its transport across the BBB, as measured by the prolonged tail-flick latency in rats following an intravenous injection of the modified peptide. However, this ‘prodrug’ approach could be an extensive undertaking with an uncertain future.
In another approach hypertonic solution of urea or mannitol is infused to temporarily disrupt the BBB [57]. This method drains out the fluid from the cells, causing them to shrink and allowing the tight junctions to open temporarily. Although, this method has been approved and is currently employed in the management of brain tumors in certain patients, it is risky, especially for long-term therapy. Moreover, infusion of hyperosmotic solutions in the brain induced seizures in experimental animals as well as chronic neuropathological changes [58, 59]. BBB can be disrupted via various other mechanisms such as by using low-frequency ultrasound (260 Hz). The disruption presumably in this case occurs due to interaction between ultrasonic waves, microbubbles and the brain microvasculature [60]. Further, BBB disruption can also be caused by certain chemicals (e.g. etoposide) and by thermal shock [61, 62].
For therapeutic compounds that are not synthetically open to lipophilic modification or are too large for diffusion, other means of blood-to-brain entry have to be investigated. Attaching an active drug to a vector that accesses a specifically catalyzed transport mechanism creates a Trojan horse-like deception that tricks the BBB into welcoming the drug through its gates. This approach enables the delivery of specific agents to the brain by attaching them to ligands that can traverse through the BBB. The therapeutic agent or drug is fused to a molecular Trojan horse, typically, a cell-penetrating peptide or a monoclonal antibody that binds to a specific receptor, such as insulin or the transferrin receptors on the BBB [63–65]. Various biomolecules, such as plasmid DNA, neurotrophic factors and therapeutic enzymes have been delivered to the CNS using this technology [66].
6. Nanocarriers for Delivery across BBB
Recently, nanocarrier systems such as micelles, liposomes, nanoparticles (NPs), dendrimers, etc. have been under investigation for delivery of therapeutic agents to the CNS [67]. These nanocarriers may protect the drug from enzymatic degradation. In addition, the surface of nanocarriers can be engineered by the attachment of specific ligands to enhance their targeting to the CNS, thus reducing toxicity.
6.1 Liposomes
Liposomes are lipid vesicles consisting of either one or more phospholipid bilayers. They comprise of a polar core for encapsulation of hydrophilic drugs, while amphiphilic and lipophilic drugs are solubilized within the phospholipid bilayer. Conventional liposomes are characterized by lower plasma circulation times on account of their uptake by the reticuloendothelial system. As a result, there is a need to modify their surface using polymers such as hydrophilic poly(ethylene glycol) to enhance their blood circulation times or by conjugating them to specific antibodies in order to improve their CNS targeting potential [68]. Encapsulation of the antiretroviral drug, foscarnet within liposomes resulted in a thirteen-fold increase in drug accumulation within the rat brains as compared to drug in solution (Table 4) [69]. Due to their complex structural order, which occurs as a result of hydrophobic interactions, liposomes are relatively unstable in circulation (half-life ~ 4.2 h) [70]. Liposomes also have a low drug loading capacity for water-soluble drugs, possibly because the inner volume (~15 μl) of the liposomes represents only a small fraction of the total liposome suspension [71].
Table 4.
Areas under the curve of free and liposome-encapsulated foscarnet (PFA) in different tissues following the administration of a single intravenous dose (10 mg/kg) in rats.
| Tissues | Liposomal PFA1 | Free PFA1 | Ratio L-PFA/free PFA |
|---|---|---|---|
| Lymph Nodes | 163.5 | 20.3 | 8.1 |
| Brain | 40.8 | 3.1 | 13.2 |
| Eyes | 86.9 | 22.9 | 3.8 |
| Spleen | 1151.4 | 0.8 | 1495.3 |
| Liver | 62.5 | 1.2 | 52.1 |
| Lungs | 59.7 | 1.5 | 39.8 |
Values expressed as nmol PFA/g tissue/h, were calculated from the mean values of tissue distribution profile using the trapezoidal rule. Reproduced with permission from Dusserre et al. AIDS (1995) 9: 833–41.
6.2 Dendrimers
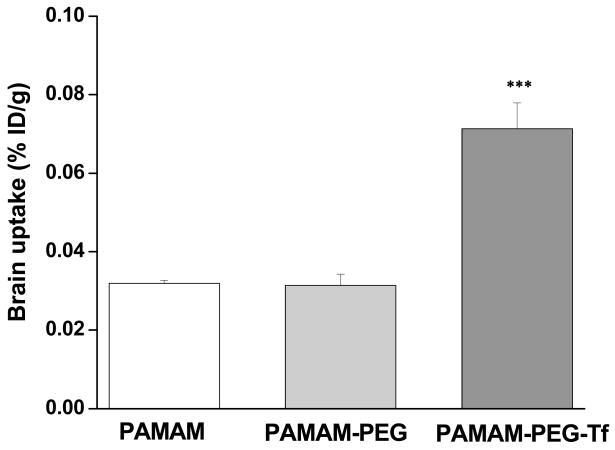
Dendrimers are versatile and highly branched nanocarriers of 5–20 nm size. The surfaces of dendrimers can be easily functionalized due to the availability of multiple reactive functional groups. Dendrimers have been investigated for the delivery of drugs across the BBB. Polyether-co-polyester dendrimers containing the cytotoxic drug methotrexate were conjugated to D-glucosamine to enhance the BBB permeability in order to deliver high amounts of drug to the CNS [72]. Huang et al. observed an increase in the brain uptake of DNA complexed with transferrin-conjugated polyamidoamine (PAMAM) dendrimers (Figure 1). Transferrin was selected as a ligand since the brain capillaries are specifically known to express transferrin receptors [73]. In another study, lamivudine-loaded mannosylated PAMAM dendrimers were evaluated for their in vitro antiretroviral activity in HIV-infected MT2 cells. It was observed that following encapsulation within dendrimers, lamivudine exhibited greater antiretroviral activity as a result of 21-fold higher drug uptake when compared to drug in solution. The viral p24 levels reduced by 2.6-fold as a result of loading within dendrimers, as compared to drug in solution [74]. One of the limitations of dendrimers is the variability of release mechanisms and the short-term of release kinetics for dendrimer-based drug delivery platforms. Drugs encapsulated within dendrimers tend to be released rapidly, expelling their payload prematurely before the macromolecules can reach their target sites [75].
Figure 1.
Brain uptake of 125I-labeled dendrimers 48 h after i. v. administration of PAMAM/DNA, PAMAM-PEG/DNA, and PAMAM-PEG-Tf/DNA complexes into Balb/c mice at a dose of 50 μg DNA/mouse. Brain uptake is plotted as % of injected dose per g tissue. ***P < 0.001 compared with the PAMAM/DNA complex. Data are expressed as mean ± SE (n = 4). Reproduced with permission from Huang et al. FASEB J (2007) 21:1117–25
6.3 Micelles
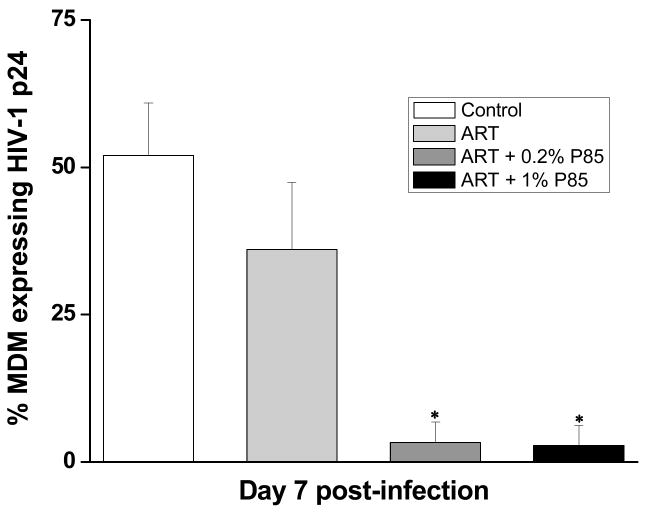
Polymeric micelles have demonstrated great promise as intracellular drug delivery systems. One unique example of polymeric micelles are the Pluronic block copolymers that can inhibit drug efflux transporters and enhance drug transport to the CNS [76]. Due to their versatility, Pluronics are increasingly being used for micellar drug delivery. They are readily available and are FDA approved for use. Furthermore, Pluronics do not demonstrate any toxicity to the BBB, and thus, exhibit a great potential in the development of novel modalities for CNS drug delivery. Pluronics such as P85 inhibit the P-gp efflux transporters widely expressed on BBB. Co-administration of P85 with anti-HIV drugs like zidovudine, lamivudine and nelfinavir enhanced their antiretroviral efficacy in a severe combined immunodeficiency (SCID) mouse model of HIV-encephalitis [77]. Specifically, seven days after administration, a decrease in the percentage of MDM cells expressing HIV-1 p24 was demonstrated within the combined drug and Pluronic group. The percentage of p24 expressing MDM was 36% in the drug solution group alone while it was 3.3% and 2.8%, respectively for 1% and 2% solutions of P85 co-administered with the drug (Figure 2). Micelles can be chemically modified for coupling of ligands to target specific biological structures [78]. However, the instability of the Pluronic micelles unless cross-linked, in circulation until they reach to the target tissue, remains an issue.
Figure 2.
Effect of triple drug combination of zidovudine, lamivudine and nelfinavir (ART), co-administered with Pluronic P85 (0.2% and 1%) on viral replication in HIVE SCID mice. The percent of MDM-expressing HIV-1 p24 in mice brain was averaged for each treatment group by histological analysis. Bar values represent mean ± s.e.m. Reproduced with permission from Spitzenberger et. al. J Cereb Blood Flow Metab (2007) 27: 1033–42.
6.4 Nanoparticles
Nanoparticles (NPs) are solid colloidal particles typically in the size range of 100–300 nm, in which therapeutic agents either alone or in combination can be entrapped or chemically linked to the surface [79]. Currently, NPs are gaining wide interest as carriers for the CNS delivery of various therapeutic agents, including protease inhibitors [80]. This is because NPs offer more stability to the encapsulated drug in biological fluids and against enzymatic metabolism as compared to other colloidal systems, such as liposomes or micelles [81]. Due to their small size they can often be taken up by cells where the uptake of larger particles is precluded. Drugs that have been successfully delivered to the CNS using NPs include loperamide, tubocurarine and doxorubicin [81–83]. By encapsulating the drug within NPs, it can be prevented from being effluxed out, thus facilitating its CNS entry [84].
Development of NPs formulated from polylactide homopolymers (PLA) and poly(lactide-co-glycolide) (PLGA) offers an advantage for the CNS delivery of therapeutic agents. PLGA and PLA are the FDA approved polymers for human use. These polymers are extensively used in biomedical, drug delivery and tissue engineering applications because they are biodegradable, biocompatible and do not induce any inflammatory responses after injection [85]. Further, their degradation products, i.e. glycolic acid and lactic acid, are eventually converted to carbon dioxide and water through the Kreb’s cycle and finally eliminated [86]. One of the main advantages of PLA- and PLGA-based delivery systems over other carriers is that a wide variety of hydrophilic and hydrophobic drugs can be entrapped within their matrix, along with the ability to release the entrapped drug in a sustained manner for several weeks [87]. The small size of NPs (> 100 nm) enables them to permeate the BBB and is particularly attractive for the CNS drug delivery. However, the efficiency of transport of unmodified NPs across the BBB could be significantly lower and may not result in therapeutic dosing of the drug to the CNS. Therefore, several surface modifications are proposed to enhance their transcytosis across the BBB.
Engulfment of nanoparticles and the drug by the reticuloendothelial system (RES) is a common occurrence with nanotherapeutic formulations. Uptake by the brain parenchyma can be increased by chemically modifying the drug itself or encapsulating the drug in a carrier that increases BBB permeability, bioavailability and/or receptor affinity. In order to limit the uptake by the RES, chemical alteration such as PEGylation can be employed. By imparting hydrophilicity to the nanoparticle surface in this manner, interaction with peripheral organs can be restricted and can thus confer long circulating properties to the nanocarriers.
Further enhancement in brain parenchymal delivery can be achieved via alternate non invasive delivery routes. Intranasal delivery of therapeutic agents bypasses the BBB and is an effective, non-invasive technique of brain delivery. The transport occurs via the olfactory and trigeminal neural pathway, and may occur due to diffusion within perineuronal channels, perivascular spaces, or lymphatic channels directly connected to brain tissue [88]. The active agents have been found to reach the brain within minutes. Intranasal administration of peptide T, a viral entry inhibitor is currently being investigated to reduce the cognitive impairment associated with HIV.
7. Targeted Delivery of NPs
The delivery of drug-encapsulated NPs can be further enhanced by conjugation to specific targeting agents. Targeted delivery methods, which are independent of the ligand-receptor interactions on the cell surface, are currently being used for the delivery of the drug of interest. The targeted delivery of a drug to the brain depends upon the characteristic properties of the (i) vector, (ii) linker and the (iii) drug itself, and how each one of these individually interacts with the brain. The brain specificity of the vector is an important attribute for targeted CNS drug delivery. Vectors that can selectively bind to receptors or transporters solely expressed on the endothelium of the BBB have been used for the targeted delivery of anti-HIV agents and other drugs to the brain [89]. The suitability of the vector is also governed by its pharmacokinetic profile. Other desirable properties include non-toxicity, ease of conjugation and stability in circulation [90].
A variety of linkers can be used to conjugate the vector to the carrier for brain delivery. High coupling efficiency of the linker to the vector and the drug or the delivery system is preferred to maximize the amount of drug that reaches the brain [90]. Multifunctional linkers are commonly used to increase coupling efficiency. Coupling of the carrier to the vector is generally performed in multiple steps to prevent the undesirable formation of multi-molecular aggregates. The linker used should be non-toxic and be stable in plasma [91].
Once inside the body the drug conjugate is subject to the lytic action of a multitude of enzymes. Depending upon the site of cleavage, the drug may be generated as a separate molecule, or is lysed along with the linker still attached. In this case, the activity of the drug-linker conjugate is lower than the activity of the native drug. This problem can be partly avoided by the use of nanocarriers for targeted delivery in which the drug is entrapped in its native form and conjugation occurs on the surface of the delivery system.
To increase the efficiency of NP uptake by the CNS, certain modifications have been investigated, including conjugation of NPs to specific ligands such as thiamine and transferrin [92, 93]. These conjugated NPs interact with the thiamine and transferrin receptors expressed at the BBB, thereby increasing their binding and uptake across the BBB. The most important advantage of receptor-mediated transcytosis is the specificity of delivery, which minimizes undue exposure to other organs. Additionally, specific delivery of larger drug molecules or particles is possible through this mechanism. However, some disadvantages in this method exist. A key disadvantage of receptor mediated transcytosis is due to saturation of the receptor binding sites when excess ligand molecules are present, which may prevent pharmacologically relevant amounts of drug from reaching the CNS. Furthermore, the level of receptors may be altered in disease state thereby leading to varying levels of drug absorption.
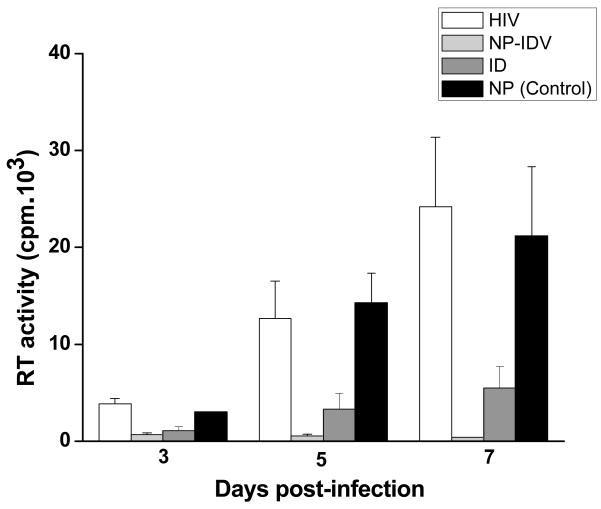
The small size of NPs also makes them favorable candidates for cell-based antiretroviral therapies. NPs can be effectively packaged within micron size cells for systemic administration that can provide sustained drug effect in areas of active viral replication. Lipoid-80 coated indinavir nanaocrystals prepared by high pressure homogenization were highly effective in reducing the level of infection in HIV-infected macrophages as compared to indinavir solution (Figure 3) [94]. In the NP-treated group, viral concentrations were reduced below the detection limits, as compared to the viral levels in the control group. For in vivo efficacy, indinavir nanocrystals were packaged into bone-marrow-derived macrophages (BMMs) and then injected into HIV-infected mice, it was observed that the BMMs migrated into HIV infected areas, such as the brain, liver, spleen and lungs and delivered indinavir at levels far greater than the therapeutic dose typically required to inhibit viral replication in these tissues [95]. The antiretroviral effects, as determined by the decrease in HIV-1 p24 viral levels, were sustained for weeks after a single injection of the formulation.
Figure 3.
Anti-retroviral activities of indinavir-loaded NPs (NP–IDV). A single dose of soluble indinavir (IDV) sulfate or NP–IDV was administered to monocyte-derived macrophages (MDM) 5 days before HIV challenge. The MDM were then analyzed at days 3, 5 and 7 post-infection. Reverse transcriptase (RT) activity was used to assay progeny virion production 7 days after viral exposure in all experimental groups. Both treated cultures showed a significant prevention of viral replication as compared to untreated HIV-1 infected MDM (*P < 0.05) at all time points. Significant anti-retroviral activities were seen in NP–IDV treated MDM when compared to soluble IDV sulfate (#P < 0.05). Reproduced with permission from Dou et. al. Virology (2007) 358: 148–58.
7.1 Cell Penetrating Peptide- Mediated Drug Delivery
Another technique involves the use of cell-penetrating peptides (CPPs) to enhance the CNS delivery. CPPs contain a sequence of highly basic amino acids which confer a positive charge on the peptide. They interact with the cell surface via a receptor independent mechanism. Furthermore, CPPs can transport the molecules that are tagged to them across the cell membrane, into the cytoplasm and to the nucleus [96, 97], and this effect is independent of the cell type. The most commonly studied peptides are the HIV-1 trans-activating transcriptional activator (TAT) peptide, Herpes Simplex Virus type-1 transcription factor (HSV VP-22) peptide, antennapedia and penetratin [98].
7.2 TAT Peptide-Mediated Drug Delivery
The HIV-TAT peptide was discovered in 1988, when it was found that TAT protein is able to migrate from quiescently infected cells producing the protein to uninfected cells and initiate viral replication. Later, it was found that the TAT-protein consisted of the sequence known as protein transduction domain (PTD) which is responsible for this translocation. Since then, TAT peptide-mediated translocation has been an area of intense research. The TAT peptide, derived from human immunodeficiency virus type-1 (HIV-1) possesses an arginine-rich sequence making it highly positively charged. TAT peptide is a small polypeptide of 86 amino acids and a cysteine rich region [99]. The basic region of TAT peptide consisting of two lysine and six arginine residues is essential for efficient cellular uptake [100]. It has been observed that TAT peptide permeates the cell membrane in a receptor and transporter independent mechanism. TAT peptide can permeabilize the cell by forming an inverted micelle by destabilizing the phospholipid bilayer by interacting with the negatively charged phospholipids of plasma membrane [101].
Two primary mechanisms that have been proposed to explain the cellular uptake of TAT-peptide are endocytosis and macropinocytosis. However, regardless of the discrepancy between uptake mechanisms, strong experimental evidence has proven the effectiveness of TAT conjugation in the delivery of tagged entities. The main benefit of TAT coupling is that, along with efficient delivery of molecules, biological activity of the coupled molecule is preserved. Further, the size of the molecule being transported is also not a rate-limiting factor.
TAT conjugation was used to facilitate the delivery of biomacromolecules across the BBB. Schwarze et al. delivered TAT-conjugated protein to the brain along with other tissues. In this study, FITC-labeled TAT peptide was fused with β-Gal protein to form a TAT-β-Gal fusion protein and this complex was intraperitoneally injected into mice [102]. The conjugate was found to localize into the brain parenchyma within 20 minutes of injection while the β-gal activity was found in the brain after 8 hours of injection [102]. It was also demonstrated that the BBB did not become leaky and remained intact during this period. This finding established the basis for application of peptide-based therapy to target the brain.
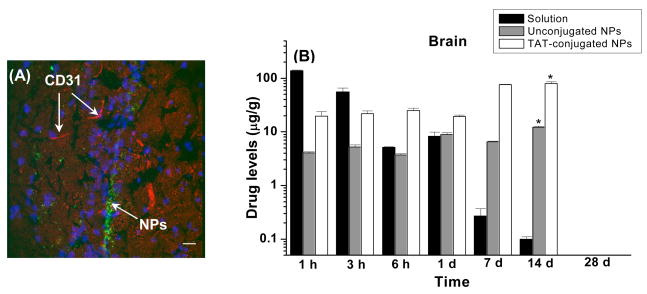
In a recent study, Peetla et al. have shown greater biophysical interactions of TAT-conjugated NPs with a model endothelial membrane than of unconjugated or scrambled TAT peptide-conjugated NPs, and these interactions correlated with the delivery of the encapsulate ritonavir in human vascular endothelial cells. The results thus suggested that biophysical interactions of the conjugated TAT with cell membrane lipids play a role in cellular uptake of NPs [103]. Rao et al. have shown that drug loaded in TAT peptide-conjugated NPs bypass the efflux action of P-glycoprotein and increase the uptake and transport of the encapsulated ritonavir in P-gp over-expressing multidrug resistant MDCK cell line (Table 5). Further, the study also showed that the conjugated NPs are transported to the CNS without disrupting the integrity of the BBB (Figure 4A), and enhanced the CNS bioavailability of ritonavir, and maintained its level in the brain over two weeks. The brain drug levels were significantly lower with unconjugated NPs; and with drug in solution, the levels declined rapidly with time (Figure 4B) [26]. The drug levels in the brain achieved with conjugated NPs were estimated to be above the therapeutic dose of the drug required in the CNS (25 ng/ml of ritonavir in cerebrospinal fluid) to suppress viral replication [104]. Thus, due to the transport properties of TAT, it can ferry different types of therapeutics including proteins to the brain, thereby serving as a “Trojan horse”.
Table 5.
Apparent permeability (Papp) and uptake of ritonavir in MDCK-MDR1 and MDCK-wt cells.
| Cell Type | Papp (cm/sec) × 10−5 | Uptakea (μg/mg protein) | ||||
|---|---|---|---|---|---|---|
| Drug in Solution | Unconjugated NPs | TAT-conjugated NPs | Drug in Solution | Unconjugated NPs | TAT-conjugated NPs | |
| MDCK-MDR1 | 0.05 | 1.97 | 2.4 | 0.04 ± 0.01 | 0.59 ± 0.14 | 3.63 ± 0.75 |
| MDCK-wt | 0.60 | 0.81 | 3.0 | 0.29 ± 0.05 | 0.53 ± 0.02 | 0.83 ± 0.09 |
Papp was calculated from the transport studies(n=6).
Data are mean ± s.e.m (n = 6). p < 0.05 for the TAT-conjugated group between MDCK-MDR1 and MDCK-wt cells. Reproduced with permission from Ref. Rao et al. Biomaterials, (2008) 29:4429–38.
Figure 4.
(A) Localization of 6-coumarin-loaded TAT-conjugated NPs in mice brains, 1 d after intravenous injection of NPs at a dose of 250 mg/kg. This dose of NPs is equivalent to the 45 mg/kg dose of ritonavir used in vivo study to determine brain uptake of the drug. Sections were observed using a confocal microscope equipped for fluorescence at 40X magnification under an oil immersion lens. Image demonstrates the localization of TAT-conjugated NPs within the brain ventricles. Magnification bar = 25 μm. Blue fluorescence is due to DAPI-staining of the nuclei, red fluorescence is due to CD-31 antibody-staining of the blood capillaries, and green fluorescence is due to the NP localization. Reproduced with permission from Rao et. al., Biomaterials 29:33 4429–38. (B): Brain levels of ritonavir in FVB/Ntac mice injected intravenously with either ritonavir solution or ritonavir-loaded unconjugated or TAT peptide-conjugated NPs at a drug dose of 45 mg/kg. Data are represented as mean ± s.e.m. (n = 4). * p < 0.05 compared between the TAT-conjugated NPs group with the unconjugated NPs and solution groups. Reproduced with permission from Rao et al. Biomaterials, (2008) 29:4429–38.
An important consideration in the development of delivery strategies that are targeted towards the CNS is the neurotoxicity of the targeting agent. At higher concentrations, TAT-peptide displays cytotoxicity and cell death. However, severe neuronal damage in mice was observed only in the case of supraphysiological concentrations of TAT-peptide (LD50 values between 13.5 μg – 180 μg) [105]. Rao et al. have demonstrated that only nanogram amounts of the peptide are sufficient to deliver therapeutic levels of ritonavir to the brain [26], hence may not pose a particular concern.
8. Conclusions
The CNS has been implicated as a hidden reservoir for HIV-1 that results in significant neurological complications. TAT peptide-conjugated NPs have the potential to achieving the therapeutic dose of anti-HIV drugs to the brain, thus could be effective in controlling the viral replication in the CNS and eventually preventing neurological complications associated with HIV infection. A well developed CNS drug delivery system could potentially be used in the treatment of other neurological conditions such as Alzheimer or Parkinson’s disease.
9. Expert Opinion
Recent advances in anti-HIV drug therapy have resulted in significant reduction in the plasma viral levels, but are not quite as effective in eliminating the viral load in the principal anatomical and cellular viral reservoirs, namely the CNS and macrophages. As a result, individuals on combination therapy experience symptoms of motor abnormalities and neurocognitive impairments that are associated with a chronic infection occurring within these sites. Developing drugs that can effectively diffuse in various cellular and tissue compartments where the virus harbors is one way to completely cure the HIV-infected patients. However, this would require extensive undertaking and had to undergo the same evaluation and approval process as new drug entities. A better alternative to the above approach could be developing effective drug delivery systems for the existing drugs which have been tested and proven effective in reducing the viral load. These drug delivery systems should be able to carry anti-HIV drugs in therapeutic doses and maintain the level that is effective in preventing viral replication.
Apart from the CNS, HIV-1 virus also resides in certain macrophage-rich organs such as the lymphoid tissue, testes and spleen. Although greater degree of efforts have been directed towards improving the CNS delivery of anti-HIV drugs, it is quite possible that the same strategy might also be effective in improving the bioavailability of these drugs to the other tissue compartments where the virus is harbored. This is because of the involvement of the same issue as the CNS delivery i.e. poor permeability of drugs. Hence cell penetrating peptides, such as TAT peptide, conjugated to drug loaded cargos, could also be effective in transporting drugs to other body compartments including to those where the virus harbor. Therefore, a detailed study of pharmacokinetic and pharmacodynamics of drugs with nanocarriers would be required to determine the effective dosing and duration of therapy required to eliminate the virus completely from the body. With sustained drug delivery NPs, such a therapy could be once in few weeks. Thus, one can foresee in the future a therapy that is targeted towards effectively controlling the viral replication in the entire body. Such a comprehensive approach of treatment could prove effective also in controlling the spread of HIV infection.
Acknowledgments
Declaration of interest
The study reported here is funded by grants R21 MH067525 from the National Institute of Mental Health (to VL) and R01 NS048837 from the National Institute of Stroke and Neurological Disorders (to AG) of the National Institutes of Health. KSR is supported by a Pre-doctoral Fellowship from the American Heart Association, Heartland Affiliate (Grant # 0710119Z).
List of Abbreviations
- AIDS
Acquired Immune Deficiency Syndrome
- BBB
Blood-brain-barrier
- CNS
Central nervous system
- CPP
Cell-penetrating peptide
- HAART
Highly Active Antiretroviral Therapy
- HAND
HIV-1-associated neurocognitive disorder
- HIV
Human immunodeficiency virus
- IDV
Indinavir
- NPs
Nanoparticles
- PIs
Protease Inhibitors
- P-gp
P-glycoprotein
- RT
Reverse transcriptase
- TAT
Trans-activating transcriptor
Bibliography
- 1.CDC. HIV/AIDS Statistics and Surveillance. 2006 Available at: http://www.cdc.gov/hiv/topics/surveillance/basic.htm#ddaids.
- 2.UNAIDS. AIDS Epidemic Update. 2007 Available at: http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 3.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–23. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 4.Fleury S, de Boer RJ, Rizzardi GP, et al. Limited CD4+ T-cell renewal in early HIV-1 infection: effect of highly active antiretroviral therapy. Nat Med. 1998;4:794–801. doi: 10.1038/nm0798-794. [DOI] [PubMed] [Google Scholar]
- 5.Sachsenberg N, Perelson AS, Yerly S, et al. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paparizos VA, Kyriakis KP, Kourkounti S, et al. The Influence of a HAART regimen on the expression of HIV-associated Kaposi sarcoma. J Acquir Immune Defic Syndr. 2008;49:111. doi: 10.1097/QAI.0b013e31816d9d2b. Letter United States. [DOI] [PubMed] [Google Scholar]
- 7.Kaul M. HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–94. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folks T, Kelly J, Benn S, et al. Susceptibility of normal human lymphocytes to infection with HTLV-III/LAV. J Immunol. 1986;136:4049–53. [PubMed] [Google Scholar]
- 9.Nottet HS, Persidsky Y, Sasseville VG, et al. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–95. [PubMed] [Google Scholar]
- 10.An SF, Groves M, Giometto B, et al. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. 1999;98:481–7. doi: 10.1007/s004010051113. [DOI] [PubMed] [Google Scholar]
- 11.Bagasra O, Lavi E, Bobroski L, et al. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. Aids. 1996;10:573–85. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Wiley CA, Achim CL, Christopherson C, et al. HIV mediates a productive infection of the brain. Aids. 1999;13:2055–9. doi: 10.1097/00002030-199910220-00007. [DOI] [PubMed] [Google Scholar]
- 13.Canto-Nogues C, Sanchez-Ramon S, Alvarez S, et al. HIV-1 infection of neurons might account for progressive HIV-1-associated encephalopathy in children. J Mol Neurosci. 2005;27:79–89. doi: 10.1385/JMN:27:1:079. [DOI] [PubMed] [Google Scholar]
- 14.Gorry PR, Ong C, Thorpe J, et al. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–73. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- 15.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. Aids. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wiley CA, Schrier RD, Nelson JA, et al. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–93. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008;20:33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- 18.Letendre S, McCutchan JA, Ellis RJ. Neurologic Complications of HIV Disease and Their Treatment. Top HIV Med. 2008;16:15–22. [PubMed] [Google Scholar]
- 19.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. • Review article focusing on neurocognitive impairments associated with HIV infection of the CNS. [DOI] [PubMed] [Google Scholar]
- 20.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinkin CH, Castellon SA, Atkinson JH, et al. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54 Suppl 1:S44–52. doi: 10.1016/s0895-4356(01)00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiseman MB, Sanchez JA, Buechel C, et al. Patterns of relative cerebral blood flow in minor cognitive motor disorder in human immunodeficiency virus infection. J Neuropsychiatry Clin Neurosci. 1999;11:222–33. doi: 10.1176/jnp.11.2.222. [DOI] [PubMed] [Google Scholar]
- 23.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–90. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 24.Maschke M, Kastrup O, Esser S, et al. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–80. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186 Suppl 2:S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 26.Rao KS, Reddy MK, Horning JL, et al. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–38. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart SA, Poon B, Song JY, et al. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–11. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corasaniti MT, Strongoli MC, Piccirilli S, et al. Apoptosis induced by gp120 in the neocortex of rat involves enhanced expression of cyclooxygenase type 2 and is prevented by NMDA receptor antagonists and by the 21-aminosteroid U-74389G. Biochem Biophys Res Commun. 2000;274:664–9. doi: 10.1006/bbrc.2000.3160. [DOI] [PubMed] [Google Scholar]
- 29.Alirezaei M, Watry DD, Flynn CF, et al. Human Immunodeficiency Virus-1/Surface Glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci. 2007;27:11047–55. doi: 10.1523/JNEUROSCI.2733-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghafouri M, Amini S, Khalili K, et al. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. • Review article describing the neuropathology of HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook JE, Dasgupta S, Middaugh LD, et al. Highly active antiretroviral therapy and human immunodeficiency virus encephalitis. Ann Neurol. 2005;57:795–803. doi: 10.1002/ana.20479. [DOI] [PubMed] [Google Scholar]
- 32.Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 34.Robinson-Papp J, Byrd D, Mindt MR, et al. Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch Neurol. 2008;65:1096–101. doi: 10.1001/archneur.65.8.1096. • An important clinical study describing the predictors of cognitive impairments in patients on antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rumbaugh JA, Nath A. Developments in HIV neuropathogenesis. Curr Pharm Des. 2006;12:1023–44. doi: 10.2174/138161206776055877. [DOI] [PubMed] [Google Scholar]
- 36.Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16:94–8. [PubMed] [Google Scholar]
- 37.Tan H, Rader AJ. Identification of putative, stable binding regions through flexibility analysis of HIV-1 gp120. Proteins. 2008 doi: 10.1002/prot.22196. [DOI] [PubMed] [Google Scholar]
- 38.Kwei GY, Novak LB, Hettrick LA, et al. Regiospecific intestinal absorption of the HIV protease inhibitor L-735,524 in beagle dogs. Pharm Res. 1995;12:884–8. doi: 10.1023/a:1016269206048. [DOI] [PubMed] [Google Scholar]
- 39.Lin JH, Chen IW, Vastag KJ, et al. pH-dependent oral absorption of L-735,524, a potent HIV protease inhibitor, in rats and dogs. Drug Metab Dispos. 1995;23:730–5. [PubMed] [Google Scholar]
- 40.Bocedi A, Notaril S, Narciso P, et al. Binding of Anti-HIV Drugs to Human Serum Albumin. 2004:609–14. doi: 10.1080/15216540400016286. [DOI] [PubMed] [Google Scholar]
- 41.Greene JN, Poblete SJ, Krieff D. New directions in antimicrobial therapy. Chest Surg Clin N Am. 1999;9:39–61. vii–viii. [PubMed] [Google Scholar]
- 42.Markowitz M, Mohri H, Mehandru S, et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report. Lancet. 2005;365:1031–8. doi: 10.1016/S0140-6736(05)71139-9. [DOI] [PubMed] [Google Scholar]
- 43.Ho D. Residual pool of HIV after prolonged combination therapy. 5th Conference on Retroviruses and Opportunistic Infections; Chicago, IL. February 3; 1998. [Google Scholar]
- 44.Ho DD. Therapy of HIV infections: problems and prospects. Bull N Y Acad Med. 1996;73:37–45. •• Review discussing the limitations of currently existing antiretroviral therapy. [PMC free article] [PubMed] [Google Scholar]
- 45.Crowe SM, McGrath MS, Elbeik T, et al. Comparative assessment of antiretrovirals in human monocyte-macrophages and lymphoid cell lines acutely and chronically infected with the human immunodeficiency virus. J Med Virol. 1989;29:176–80. doi: 10.1002/jmv.1890290306. [DOI] [PubMed] [Google Scholar]
- 46.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–77. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 48.Crone C, Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981;77:349–71. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhuri JD. Blood brain barrier and infection. Med Sci Monit. 2000;6:1213–22. • Review examining the different mechanisms by which HIV infects the brain. [PubMed] [Google Scholar]
- 50.Takasawa K, Terasaki T, Suzuki H, et al. In vivo evidence for carrier-mediated efflux transport of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine across the blood-brain barrier via a probenecid-sensitive transport system. J Pharmacol Exp Ther. 1997;281:369–75. [PubMed] [Google Scholar]
- 51.Wang Y, Sawchuk RJ. Zidovudine transport in the rabbit brain during intravenous and intracerebroventricular infusion. J Pharm Sci. 1995;84:871–6. doi: 10.1002/jps.2600840717. [DOI] [PubMed] [Google Scholar]
- 52.Tsuji A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx. 2005;2:54–62. doi: 10.1602/neurorx.2.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schinkel AH. The roles of P-glycoprotein and MRP1 in the blood-brain and blood-cerebrospinal fluid barriers. Adv Exp Med Biol. 2001;500:365–72. doi: 10.1007/978-1-4615-0667-6_60. [DOI] [PubMed] [Google Scholar]
- 54.Thiebaut F, Tsuruo T, Hamada H, et al. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989;37:159–64. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- 55.Dwibhashyam VSNM, Nagappa A. Strategies for enhanced drug delivery to the central nervous system. Indian J Pharm Sci. 2008;70:145–53. doi: 10.4103/0250-474X.41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prokai-Tatrai K, Prokai L, Bodor N. Brain-targeted delivery of a leucine-enkephalin analogue by retrometabolic design. J Med Chem. 1996;39:4775–82. doi: 10.1021/jm960356e. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda M, Bhattacharjee AK, Kondoh T, et al. Synergistic effect of cold mannitol and Na(+)/Ca(2+) exchange blocker on blood-brain barrier opening. Biochem Biophys Res Commun. 2002;291:669–74. doi: 10.1006/bbrc.2002.6495. [DOI] [PubMed] [Google Scholar]
- 58.Neuwelt EA, Hill SA, Frenkel EP. Osmotic blood-brain barrier modification and combination chemotherapy: concurrent tumor regression in areas of barrier opening and progression in brain regions distant to barrier opening. Neurosurgery. 1984;15:362–6. doi: 10.1227/00006123-198409000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Salahuddin TS, Johansson BB, Kalimo H, et al. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions: a light microscopic and immunohistochemical study. Neuropathol Appl Neurobiol. 1988;14:467–82. doi: 10.1111/j.1365-2990.1988.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 60.Hynynen K. Focused ultrasound for blood†“brain disruption and delivery of therapeutic molecules into the brain. Expert Opinion on Drug Delivery. 2007;4:27–35. doi: 10.1517/17425247.4.1.27. [DOI] [PubMed] [Google Scholar]
- 61.Oztas B, Kucuk M. Intracarotid hypothermic saline infusion: a new method for reversible blood-brain barrier disruption in anesthetized rats. Neuroscience Letters. 1995;190:203–6. doi: 10.1016/0304-3940(95)11542-5. [DOI] [PubMed] [Google Scholar]
- 62.Spigelman MK, Zappulla RA, Johnson J, et al. Etoposide-induced blood-brain barrier disruption. Journal of Neurosurgery. 1984;61:674–8. doi: 10.3171/jns.1984.61.4.0674. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Schlachetzki F, Pardridge WM. Global non-viral gene transfer to the primate brain following intravenous administration. Mol Ther. 2003;7:11–8. doi: 10.1016/s1525-0016(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 64.Pardridge WM. Brain Drug Targeting: The Future of Brain Drug Development. Cambridge, U.K.: Cambridge University Press; 2003. • Book discussing the strategies for targeted delivery of drugs to the central nervous system. [Google Scholar]
- 65.Coloma MJ, Lee HJ, Kurihara A, et al. Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res. 2000;17:266–74. doi: 10.1023/a:1007592720793. [DOI] [PubMed] [Google Scholar]
- 66.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Current Opin Pharmacol. 2006;6:494–500. doi: 10.1016/j.coph.2006.06.001. • This paper discusses the application of genetically engineered peptides as molecular Trojan horses for brain drug delivery. [DOI] [PubMed] [Google Scholar]
- 67.Tiwari SB, Amiji MM. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 2006;3:219–32. doi: 10.2174/156720106776359230. [DOI] [PubMed] [Google Scholar]
- 68.Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2:99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dusserre N, Lessard C, Paquette N, et al. Encapsulation of foscarnet in liposomes modifies drug intracellular accumulation, in vitro anti-HIV-1 activity, tissue distribution and pharmacokinetics. AIDS. 1995;9:833–41. doi: 10.1097/00002030-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23:1384–95. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 71.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. • A comprehensive review on liposomal brain drug delivery. [DOI] [PubMed] [Google Scholar]
- 72.Dhanikula RS, Argaw A, Bouchard JF, et al. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy and intratumoral transport capability. Mol Pharmaceutics. 2008;5:105–16. doi: 10.1021/mp700086j. [DOI] [PubMed] [Google Scholar]
- 73.Huang RQ, Qu YH, Ke WL, et al. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Faseb J. 2007;21:1117–25. doi: 10.1096/fj.06-7380com. [DOI] [PubMed] [Google Scholar]
- 74.Dutta T, Jain NK. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta. 2007;1770:681–6. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 75.Wolinsky JB, Grinstaff MW. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv Drug Deliv Rev. 2008;60:1037–55. doi: 10.1016/j.addr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 76.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54:759–79. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 77.Spitzenberger TJ, Heilman D, Diekmann C, et al. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. J Cereb Blood Flow Metab. 2007;27:1033–42. doi: 10.1038/sj.jcbfm.9600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Webb K, Caldwell K, Tresco PA. Fibronectin immobilized by a novel surface treatment regulates fibroblast attachment and spreading. Crit Rev Biomed Eng. 2000;28:203–8. doi: 10.1615/critrevbiomedeng.v28.i12.350. [DOI] [PubMed] [Google Scholar]
- 79.Lockman PR, Mumper RJ, Khan MA, et al. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm. 2002;28:1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 80.Ojewole E, Mackraj I, Naidoo P, et al. Exploring the use of novel drug delivery systems for antiretroviral drugs. Eur J Pharm Biopharm. 2008:697–710. doi: 10.1016/j.ejpb.2008.06.020. •• This article reviews the potential of novel drug delivery systems for the future effective treatment of HIV/AIDS. [DOI] [PubMed] [Google Scholar]
- 81.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 82.Alyautdin RN, Tezikov EB, Ramge P, et al. Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanoparticles: an in situ brain perfusion study. J Microencapsul. 1998;15:67–74. doi: 10.3109/02652049809006836. [DOI] [PubMed] [Google Scholar]
- 83.Tosi G, Costantino L, Rivasi F, et al. Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J Control Release. 2007;122:1–9. doi: 10.1016/j.jconrel.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Kaur IP, Bhandari R, Bhandari S, et al. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127:97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 85.Dechy-Cabaret O, Martin-Vaca B, Bourissou D. Controlled ring-opening polymerization of lactide and glycolide. Chem Rev. 2004;104:6147–76. doi: 10.1021/cr040002s. [DOI] [PubMed] [Google Scholar]
- 86.Yoo JY, Kim JM, Seo KS, et al. Characterization of degradation behavior for PLGA in various pH condition by simple liquid chromatography method. Biomed Mater Eng. 2005;15:279–88. [PubMed] [Google Scholar]
- 87.Zhang Z, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27:4025–33. doi: 10.1016/j.biomaterials.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9 Suppl 3:S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tadayoni BM, Friden PM, Walus LR, et al. Synthesis, in vitro kinetics and in vivo studies on protein conjugates of AZT: Evaluation as a transport system to increase brain delivery. Bioconjugate Chemistry. 1993;4:139–45. doi: 10.1021/bc00020a006. [DOI] [PubMed] [Google Scholar]
- 90.Temsamani J, Rousselle C, Rees AR, et al. Vector-mediated drug delivery to the brain. Expert Opinion on Biological Therapy. 2001;1:773–82. doi: 10.1517/14712598.1.5.773. [DOI] [PubMed] [Google Scholar]
- 91.Hermanson GT. Bioconjugate Techniques. London: Academic Press; 1996. [Google Scholar]
- 92.Lockman PR, Oyewumi MO, Koziara JM, et al. Brain uptake of thiamine-coated nanoparticles. J Control Release. 2003;93:271–82. doi: 10.1016/j.jconrel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 93.Mishra V, Mahor S, Rawat A, et al. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J Drug Target. 2006;14:45–53. doi: 10.1080/10611860600612953. [DOI] [PubMed] [Google Scholar]
- 94.Dou H, Morehead J, Destache CJ, et al. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages. Virology. 2007;358:148–58. doi: 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 95.Dou H, Destache CJ, Morehead JR, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108:2827–35. doi: 10.1182/blood-2006-03-012534. •• Proof-of-concept study describing the use of macrophages loaded with indinavir nanoparticles for targeting HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–5. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 97.Richard JP, Melikov K, Vives E, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–90. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 98.Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 2004;9:1012–9. doi: 10.1016/S1359-6446(04)03279-9. [DOI] [PubMed] [Google Scholar]
- 99.Zheng L, Yang YD, Lu GC, et al. Extracellular HIV Tat and Tat cysteine rich peptide increase CCR5 expression in monocytes. J Zhejiang Univ Sci B. 2005;6:668–72. doi: 10.1631/jzus.2005.B0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–7. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 101.Derossi D, Calvet S, Trembleau A, et al. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–93. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 102.Schwarze SR, Ho A, Vocero-Akbani A, et al. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. •• Breakthrough study describing the potential of TAT-conjugation to facilitate delivery of macromolecules to the brain. [DOI] [PubMed] [Google Scholar]
- 103.Peetla C, Rao KS, Labhasetwar V. Relevance of biophysical interactions with model membrane in predicting cellular uptake: Study with TAT peptide-conjugated nanoparticles. Mol Pharmaceutics. 2009 doi: 10.1021/mp900011h. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kravcik S, Gallicano K, Roth V, et al. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–5. [PubMed] [Google Scholar]
- 105.Sabatier JM, Vives E, Mabrouk K, et al. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991;65:961–7. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]