Abstract
During their fall migration, Eastern North American monarch butterflies (Danaus plexippus) use a time-compensated sun compass to aid navigation to their overwintering grounds in central Mexico. It has been assumed that the circadian clock that provides time compensation resides in the brain, although this assumption has never been examined directly. Here we show that the antennae are necessary for proper time-compensated sun compass orientation in migratory monarch butterflies, that antennal clocks exist in monarchs, and that they likely provide the primary timing mechanism for sun compass orientation. These unexpected findings pose a novel function for the antennae and open a new line of investigation into clock-compass connections that may extend widely to other insects that use this orientation mechanism.
Eastern North American monarch butterflies, Danaus plexippus, undergo one of the most magnificent long-distance migrations observed in animals. Each fall in the northern United States and southern Canada, migratory monarchs travel distances up to 4000 kilometers to arrive at their overwintering grounds in central Mexico (1, 2). The navigational abilities of the migrants include the use of a time-compensated sun compass (3–5). Previous studies show that a circadian clock provides the internal timing device that allows the butterflies to correct their flight orientation, relative to skylight parameters, to maintain a southerly flight bearing, as the sun moves across the sky during the day (3–5).
The circadian clock mechanism in the monarch butterfly has been recently elucidated (6). It relies on a negative transcriptional feedback loop that involves the transcription factors CLOCK (CLK) and CYCLE (CYC), which drive the expression of period (per), timeless (tim) and a vertebrate-like cryptochrome, designated cry2. The translated PER, TIM and CRY2 proteins form complexes in the cytoplasm and after the appropriate time delay translocate back into the nucleus where CRY2 represses CLK:CYC-mediated transcription (6–8). A Drosophila-like CRY also exists in the butterfly, designated CRY1, which functions as a blue light photoreceptor to synchronize (entrain) the circadian clockwork to the prevailing light-dark conditions (6). Four cells in the dorsolateral region of the central brain (the pars lateralis) house the major circadian clocks in butterfly brain (6, 9).
Because circadian rhythms in locomotor activity and the timing of adult eclosion in insects such as silkmoths (10) and Drosophila (11) are under the control of brain clocks, it has been assumed that the clock involved in time-compensated sun compass orientation in monarchs is also located in the brain (6, 9, 12). This assumption has never been examined directly. The location of the sun compass, on the other hand, is more firmly established. It resides in the central complex, a midline structure in the brain of insects, based on electro-physiological studies in locusts and crickets (13, 14), and genetic studies in Drosophila (15).
In addition to the brain, circadian clocks are also found in the antennae of insects (16–19) and likely exist in the antennae of monarch butterflies. Insect antennal clocks are believed to modulate olfactory reception within the antennae themselves (20, 21), and there has been scant evidence that antennal clocks directly influence brain mechanisms. However, Urquhart presented anecdotal evidence almost 50 years ago suggesting a role of the antennae in the flight orientation of migratory monarchs (2) that had not been pursued since. In view of this historical observation, we wanted to rigorously examine a role of the antennae in sun compass orientation.
To begin, we compared time-compensated sun compass orientation of intact migrants with migrants whose antennal flagellum had been surgically removed (Fig. S1) (22). Both intact and antennae-less migratory monarchs were housed indoors in either a 12-hour light:12-hour dark (LD) cycle that was timed to coincide with prevailing lighting conditions or a 6 hour-delayed LD cycle. Six to eight days later, the butterflies housed in either lighting cycle were tethered, and over the next 26 days individual flight direction and group orientation were examined in butterflies flown outdoors in a flight simulator (22, 23) (Fig. S2).
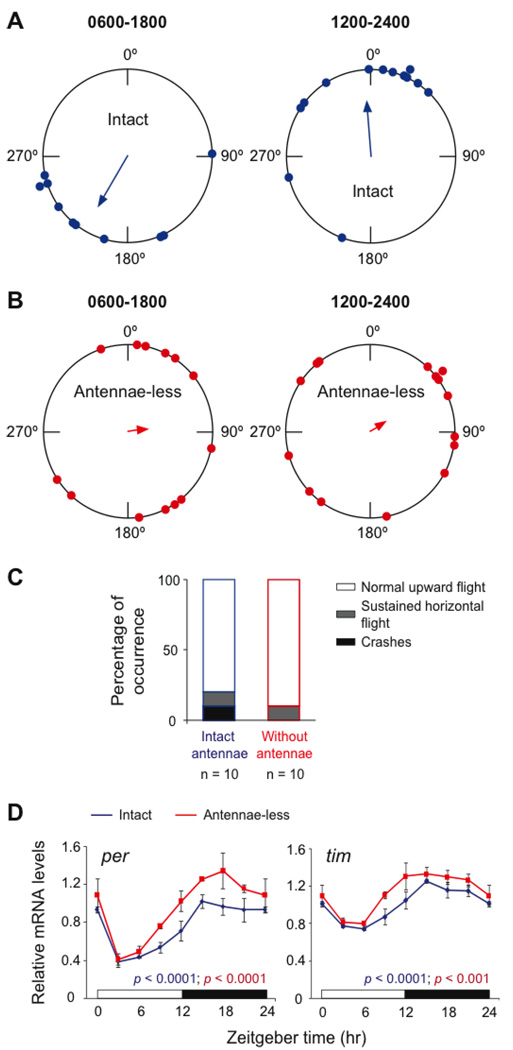
Intact monarch migrants housed under LD conditions exhibited directional flight that was oriented as a group significantly to the southwest with a mean vector (α) of 211º (n = 10, r = 0.67, p < 0.01; Rayleigh test; Fig. 1A, left panel), in close agreement with previous reports (3, 4, 24). The group of intact migrants housed under the 6-hour delayed LD cycle were also oriented significantly, but in the northwesterly direction, with an α of 355º (n = 13, r = 0.63, p < 0.005; Fig. 1A, right panel). The clockwise shift in the direction of orientation in the 6 hour-delayed LD group, relative to the LD group, was that expected for a time-compensated sun compass that has been delayed by several hours. However, the magnitude of the difference between the two groups (a clockwise shift of 144º; F1,21 = 31.92, p < 0.0001; Watson-Williams test) was greater than expected for the 6-hour shift (<132º; the speed of the Sun’s azimuth varied from 14º to 22º per hour during the study period), but within the range of directions found in a large number of phase-delayed migrants (24).
Figure 1.
Antennae are necessary for time-compensated sun compass orientation. (A) Flight orientation of intact migrants under different lighting conditions. Butterflies were flown between 1100 and 1500 hours from 24 September to 18 October 2008. The large circle represents the 360° of possible directions (0° = north); small solid circles on the perimeter represent the flight orientation of individual butterflies. The arrow indicates the mean vector; arrow length, r value. Left, orientation data of butterflies in LD. Right, orientation data of butterflies in 6-hour delayed LD. (B) Orientation of antennae-less migrants under the different lighting conditions. (C) Free-flight behavior of intact migrants (left bar) and those without antennae (right bar). (D) Temporal profiles of per and tim mRNA levels in brains of monarchs with antennae (blue) and without antennae (red). Values are mean ± SEM of 3 brains. Points at CT0 are replotted at CT24 to show 24-hour trend. Horizontal bars: open, light; black, darkness. p-values, one-way ANOVA.
Remarkably, group flight was disoriented in the antennae-less monarchs studied under either lighting cycle (Fig. 1B). Individual antennae-less migrants housed under either LD or the 6 hour-delayed LD cycle each exhibited significant directional flight. However, orientation of each of the two groups was randomly distributed over the 360° of direction (n = 13, r = 0.23, p > 0.05 for the LD group and n = 15, r = 0.209, p > 0.05 for the 6 hour-delayed LD group) (Fig. 1B). Antennae-less migrants flew as strongly and consistently as intact butterflies in the flight simulator, and the proportion of antennae-less migrants eliminated from analysis because of non-directional flight did not differ from intact migrants (22).
Because the antennae have been shown to mediate flight stability in moths through mechanosensors located at the base of the antennae (25), we also analyzed the effect of antennal flagellum amputation on the free-flight performance of migrant monarchs (22). We found that the majority of intact and antennae-less migrants exhibited normal upward flight (8/10 and 9/10, respectively) (Fig. 1C). Thus, the effect of antennal amputation on flight orientation of migrating monarch butterflies was not the consequence of disrupted flight stability. Taken together, our results show that the antennae are necessary for time-compensated sun compass orientation in migratory monarch butterflies.
The disorientation of flight behavior in the antennae-less butterflies suggested that the timing component of the time-compensated sun compass mechanism was disrupted when antennal input was lacking. Is it possible that the antennae are necessary for the proper workings of the molecular clocks in the brain? To test this, we examined the temporal patterns of clock gene expression in the brains of butterflies with and without antennae. Using the quantitative real-time polymerase chain reaction (qPCR), we analyzed the temporal expression patterns of the clock genes per and tim (22); these genes were chosen because they exhibit the most robust mRNA cycling among the clock genes previously studied in monarch heads (6). The butterflies were studied in LD to match the condition used for the flight orientation experiments.
The levels of per and tim mRNA in brains of intact and antennae-less butterflies cycled in phase (Fig. 1D), as previously described in monarch heads and in DpN1 cells, a monarch butterfly cell line that contains a light-driven clock (6). Thus, compared to the brains from butterflies with intact antennae, the relative timing of per and tim mRNA levels to the LD cycle were unaltered in antennae-less butterflies. These results suggest that the brain clocks are functioning in synchrony in LD without attached antennae and raise the interesting prospect that the clock for time compensation may actually reside in the antennae, and not in the brain.
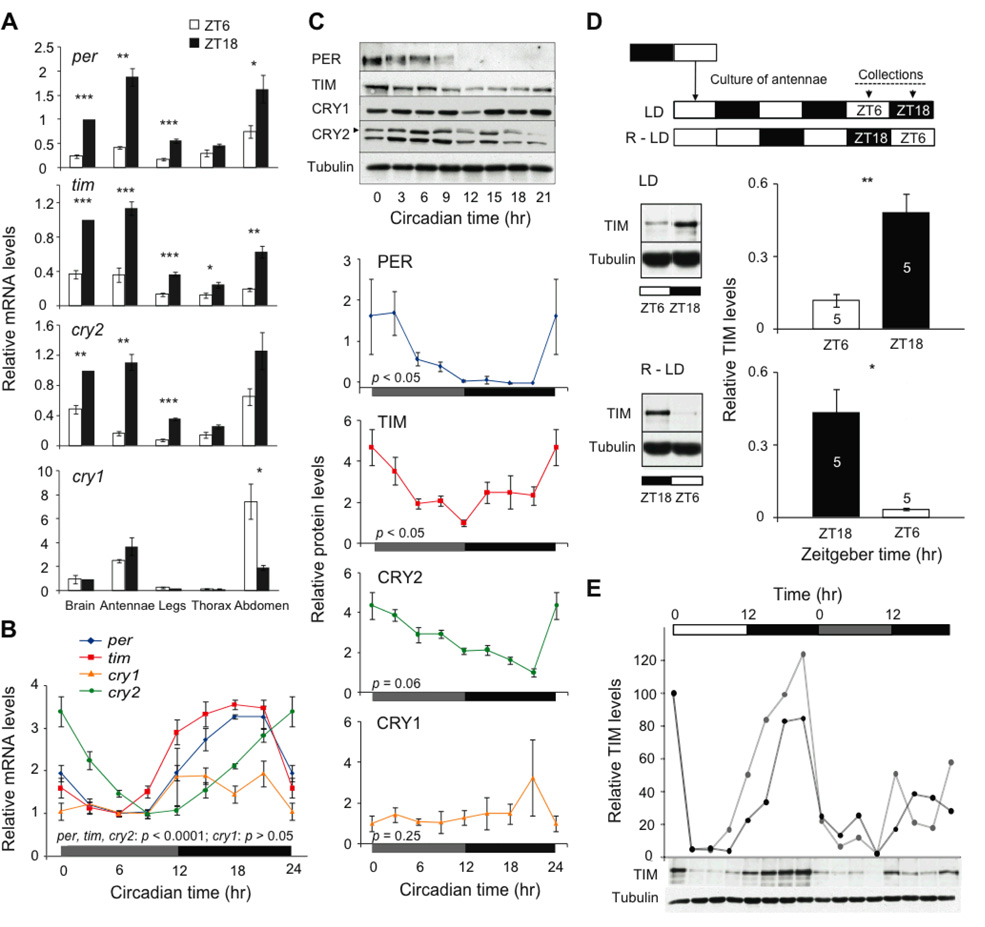
Accordingly, we next surveyed rhythmic clock gene mRNA and protein expression in the antennae, as well as in other peripheral tissues (legs, thorax, and abdomen) of monarch butterflies. Using qPCR, we found that the mRNA levels of per, tim, and cry2 were significantly lower at mid-light (ZT6) than at mid-dark (ZT18) in the antennae and the legs, similar to those in the brain (Fig. 2A). The expression of tim was also significantly reduced at ZT6 compared to ZT18 in the thorax and the abdomen, and per expression showed a similar oscillation in the abdomen. There were no significant differences in cry1 mRNA levels in most tissues examined (Fig. 2A). At the protein level (22), the antenna was the only peripheral tissue of those examined to express clock protein abundance patterns similar those found in the brain (Fig. S3).
Figure 2.
Circadian clocks in monarch antennae. (A) Clock gene expression in monarch tissues. Tissues were collected at ZT 6 (white bars) and ZT18 (black bars). Values are normalized to those in the brain at ZT18 and are mean ± SEM of 4 animals. p-values, Student’s t-test: ***p < 0.001; **p < 0.01; *, p < 0.05. (B) Clock gene mRNA profiles in antennae. Values are relative to the minimal level for each gene and are the mean ± SEM of 4 antennae. Points at CT0 are replotted at CT24. Horizontal bars: gray, subjective day; black, subjective night. p-values, one-way ANOVA. (C) Circadian profiles of clock protein abundance in antennae. Top, representative autoradiographs in DD. Arrowhead, CRY2 band; the lower band is non-specific, as shown previously (6). Bottom four graphs, quantification of relative protein levels. Values are normalized to the minimal level of protein expression and are mean ± SEM of 3–4 antennae. p-values, one-way ANOVA. (D) Light-sensitivity of antennae in culture. Top, experimental scheme in LD or in phase-reversed LD (R-LD). Arrows, collection times. Bottom, western blot analyses. Left, representative blots; right, quantifications. Open bars, mid-light; black bars, mid-dark. Values are mean ± SEM of 5 antennae. p-values, Student’s t-test: **, p < 0.01; *, p < 0.05. (E) Daily and circadian patterns of TIM abundance in two sets of cultured antennae (gray and black lines). Top, lighting conditions. Bottom, representative western blot.
Focusing on antennae, a more detailed temporal analysis showed that per, tim and cry2 mRNA levels exhibited robust circadian rhythms in constant darkness (DD), (Fig. 2B), similar to those previously described in monarch brains (Fig. 1D) and/or in DpN1 cells (6). PER and TIM also exhibited significant circadian oscillations in abundance (Fig. 2C). In addition, PER showed temporal changes in electrophoretic mobility corresponding to changes in phosphorylation (6) (Fig. 2C). CRY2 showed a temporal trend in abundance that was not significant (Fig. 2C). Circadian cycling of mRNA and protein levels of these core clock components in vivo suggests the presence of circadian clocks in the monarch butterfly antenna.
To show that the monarch antennae actually house light-entrained and tissue-autonomous circadian clocks, we examined whether the antennal clocks are reset by light and continue to oscillate when explanted in vitro (22). The light-sensitivity of isolated antennae was evaluated by maintaining antenna in culture in two oppositely phased LD cycles and probing TIM abundance by western blot. In both lighting conditions, TIM expression was significantly lower during the light phase (at ZT6) than in the dark (at ZT18) (Fig. 2D). These data show that the LD oscillation in TIM abundance persists in vitro in a phase-appropriate manner, suggesting that the antennal clocks can be directly entrained by light, even when disconnected from the brain. We also investigated the ability of the antennae to maintain self-sustained circadian oscillations by analyzing the temporal abundance of TIM in LD and during the first day in DD from individual antennae maintained in culture. TIM levels oscillated in LD and continued to oscillate though with reduced amplitude on first day in DD (Fig. 2E). Thus, monarch antennae possess light-sensitive circadian clocks, which could function, independently from the brain, as time compensation components for sun compass orientation.
If antennal clocks are involved in sun compass orientation in migrants, then altering their rhythmicity in vivo should alter time-compensated sun compass orientation. Blocking light input to antennal clocks should alter their rhythmicity in two ways. First, antennal clocks would continue to oscillate, but would gradually drift out of their normal phase relationship with the prevailing lighting cycle (i.e., “free-running” clocks). Second, after several days without light input, the individual free-running clocks would eventually desynchronized from each other because of the innate difference in free-running period length (26).
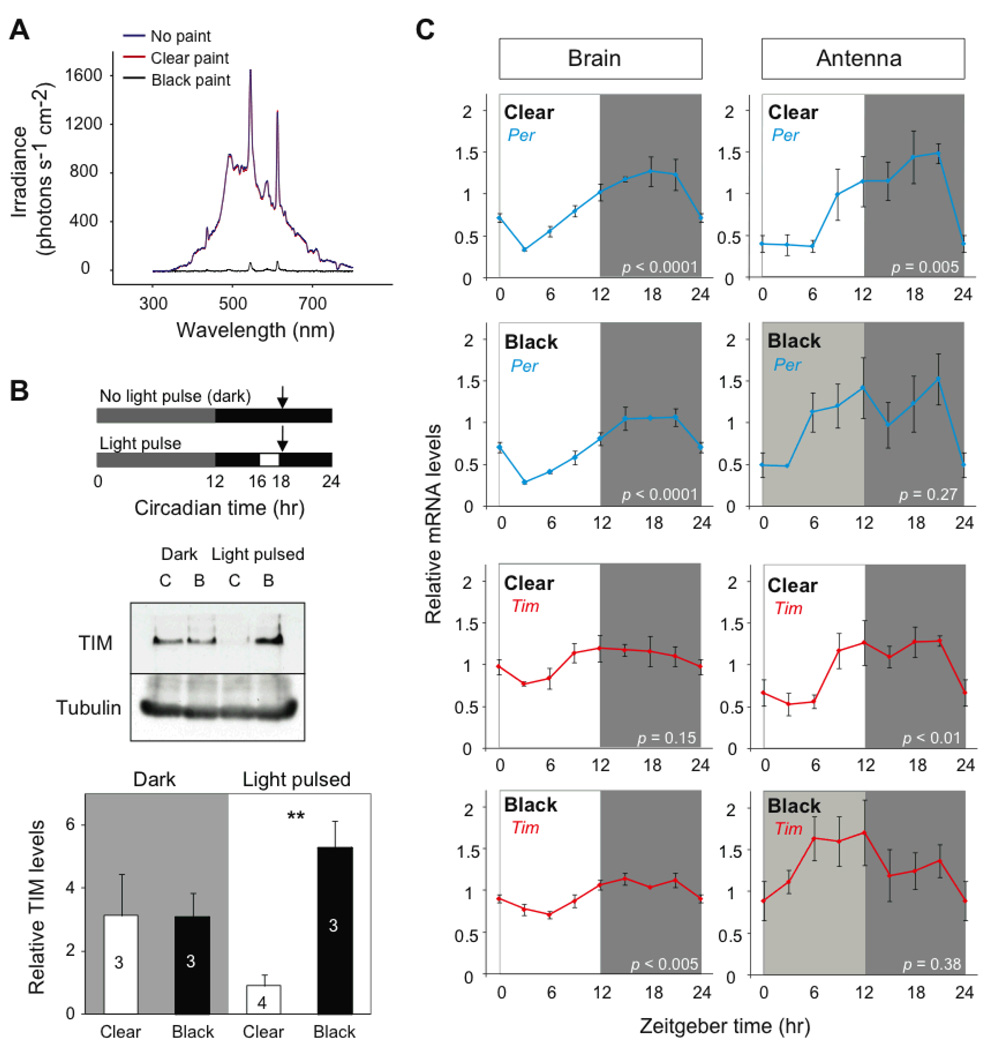
To prevent light input to intact antennae, we painted the flagellum with an enamel-based black paint (Fig. S1) (22), which blocked antennal perception of full-spectrum light (from 300nm to 800nm; Fig. 3A); the control, enamel-based clear paint, did not reduce either the intensity or wavelengths of light that could pass through the antenna (Fig. 3A). We verified the efficiency of black paint to block light input in vivo by examining the light-induced decline in TIM abundance in painted antennae. As expected, clear- and black-painted antennae in DD had similar TIM abundance during mid-subjective night (the period the lights normally would have been off in LD) (Fig. 3B). However, when both groups were exposed to a 2-hour light pulse from CT16–18, TIM abundance was substantially lower in the clear-painted antennae compared to those painted black (Fig. 3B). Thus, black paint blocks light input to the antennal clocks.
Figure 3.
Blinding antennal clocks alters their timing. (A) Irradiance curves for different painting conditions. Light measurements were taken under full-spectrum light through plastic that was either painted or not. (B) Light-sensitivity of TIM abundance. Top, experimental paradigm. Painted antennae were harvested at CT18 (arrows). Middle, blot of TIM levels from pooled antennae painted clear (C) or black (B) from butterflies in dark or light pulsed. Bottom, quantifications. Values are mean ± SEM of 3–4 antennae. White bars, clear-painted antennae; black bars, black-painted antennae. p-values, Student’s t-test: **, p < 0.005. (C) Temporal patterns of per and tim expression in brains (left column) and antenna (right column) from butterflies with the antennae painted clear or black. Values are mean ± SEM of 3 animals, except for the three points without error bars that represent the mean of 2 animals. Box shading: open, light; dark gray, night; and light gray, subjective day. p-values, one-way ANOVA.
Light-sensitivity of the antennae is unlikely to be mediated by opsins, because qPCR of the three opsin genes in monarchs (ultraviolet, blue, and long wavelength; (9)), showed that they were not detected in the antennae (Fig. S4). CRY1 is likely the photoreceptor for the light-input pathway to the antennal clocks and causing TIM degradation (6). In contrast to Drosophila CRY, monarch CRY1 is not degraded by light in either the brain (6) or the antennae (Fig. S5). However, knocking down CRY1 expression by RNA interference in DpN1 cells blocks the ability of light to degrade TIM, showing the monarch CRY1 is a light sensor (6).
To examine how the rhythmicity of antennal clocks is altered by black paint, we quantified by qPCR the expression of the clock genes per and tim from both clear- and black-painted antennae of butterflies maintained in LD for 11 days after painting (Fig. 3C). In clear-coated antennae, per and tim mRNA levels exhibited robust daily rhythms that were in phase with each other. In the same animals, per was also rhythmically expressed in the brain, with the phase of the oscillation similar to that found in the antennae. The daily pattern of tim in the brain, however, was similar but not significantly rhythmic. The lack of a significant oscillation for tim was likely due to the combined effects of individual variation and the shallow amplitude rhythms normally found in the brain (Fig. 1D, right; reference 6). Predictably, per and tim mRNA levels were not significantly rhythmic in the black-painted antennae and exhibited peak levels occurring earlier and for a broader duration than in the clear-painted antennae (Fig. 3C), while these genes cycled normally in the brains of the same butterflies.
Painting the antennae black thus appears to specifically block LD entrainment of the antennal clocks. The lack of overall rhythmicity observed in black-painted antennae is likely due to desynchrony among the free-running clocks after 11 days in virtual DD. This apparent arrhythmicity could be due to desynchrony of antennal clocks within the antenna of individual butterflies, between the two antennae, among the antennae of the different butterflies, or to a combination of these effects. Thus, sufficient synchrony among free-running clock gene oscillations may exist after several days to synchronize group flight orientation (see below).
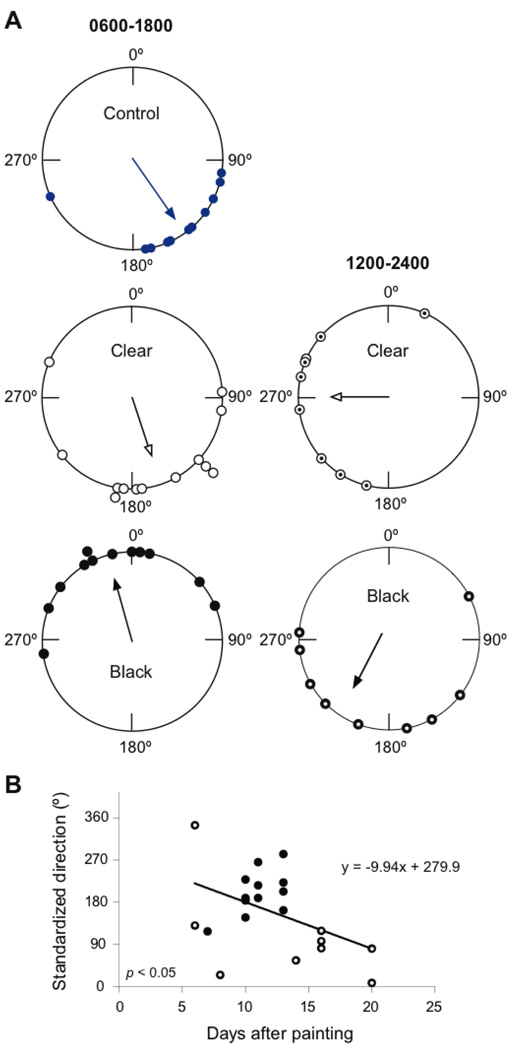
To test a potential role of antennal clocks in time-compensated sun compass orientation of migratory monarchs, we analyzed the flight orientation of migrants with either clear- or black-painted antennae housed in LD or placed from LD into the 6-hour phase delayed LD cycle after painting. We found that the control migrants, without antennal painting, and migrants with clear-painted antennae both oriented significantly to the south/southeast with an α of 144º and 162º, respectively (n = 11, r = 0.80, p < 0.0001 and n = 13, r = 0.66, p < 0.005; Fig. 4A). The mean flight orientation did not differ significantly between groups (F1,22 = 0.77, p > 0.05). Such a south/southeasterly orientation direction has been reported previously for flight simulator and free-flight studies of migrating monarchs late in the season (27, 28). The migrants in our study oriented in the southwesterly direction at the beginning of the fall (Fig. 1; top panel, left), but oriented to the southeast later in the season (Fig. 4A, left panel). Nonetheless, migrants with clear-painted antennae and housed under 6-hour shifted LD showed the appropriate shift in their orientation towards the west (α = 269 º, n = 9, r = 0.63, p < 0.05; Fig. 4A, right panel); the direction and magnitude of the group orientation differences between the two groups with clear-painted antennae (a clockwise shift of 107°, F1,20=17.48; p < 0.001) were those expected for a time-compensated sun compass delayed by 6 hours. Thus, although clear-painted antennae lack olfactory reception (see Fig. S6), they have normal light reception for entraining antennal clocks, and, correspondingly, those migrants show proper time-compensated sun compass orientation.
Figure 4.
Blinding antennal clocks alters sun compass orientation. (A) Flight orientation of migrant butterflies with intact antennae (control, upper) and with antennae painted clear (middle) or black (lower). Butterflies were flown between 1100 and 1500 hours from 20 October to 16 November 2008. Left, butterflies housed in LD. Right, butterflies housed in 6-hour delayed LD. (B) Relationship between orientation angle and day of study. Individual orientation directions were standarized to the mean vector of control butterflies (144º = 0º) and assumed to be drifting from the mean in a counterclockwise direction over time. Black dots, LD 0600–1800; open dots, 1200–2400. p-value, linear regression analysis.
A completely different situation was found for flight orientation in the migrants with black-painted antennae. As a group, those migrants housed under LD oriented significantly to the north/northwest (α = 344º, n = 12, r = 0.73, p < 0.001; Fig. 4A, left panel), an orientation direction that was 182 º different from that of the group of migrants with clear-painted antennae (F1,23 = 61.17, p < 0.00001). Migrants with black-painted antennae and housed under the 6-hour shifted LD cycle did not exhibit significant group orientation (n = 9, r = 0.5, p > 0.05; Fig. 4A, right panel), but there was a trend to orient to the southwest. A second difference between the group maintained in LD and the 6-hour shifted LD group was related to the interval from antennal painting to analysis in the flight simulator. While the butterflies were flown randomly, retrospectively, the groups differed in distribution over time since painting (Fig. S7).
If the altered flight orientation directions of the migrants with black-painted antennae were a general reflection of the timing of free-running antennal clocks, there should be a correlation between the day of study and the direction of oriented flight; that is, there should be either a progressive increase or a decrease in the angle of orientation with increasing days of study after antennal painting. Because a short free-running period length in DD had been described previously for the timing of adult eclosion behavior in monarchs (see Fig. S3, panel B in reference 6) and the per and tim mRNA oscillations measured after 11 days in black-painted antennae were advanced in their peak levels (Fig. 3C), we calculated the drift in orientation angle over the 14 days of study in a counterclockwise direction relative to mean orientation of control butterflies. Consequently, we observed a significant linear correlation between day of study and orientation angle (Fig. 4B). The slope of the regression line (y = −9.9x + 279.9) predicted a free-running period length for the antennal clocks of 23.3 hours, in accordance with the short free-running period found for adult eclosion.
These flight simulator data are consistent with a free-running timing mechanism in the black-painted antennae, influencing sun compass orientation. The orientation findings in migrants with black-painted antennae contrast with those of the antennae-less butterflies in which antennal clocks have been removed and no residual group orientation is apparent (Fig. 1B), although these migrants were studied later after antennal removal (Fig. S2).
The altered flight directions of migrants with black-painted antennae could also reflect the combined effects of free-running antennal clocks and entrained brain clocks on sun compass orientation. Indeed, flight orientation of migrants with black-painted antennae differed between the LD group and the 6-hour shifted LD group (Fig. 4A, lower left and right), suggesting a role of brain clocks. Thus, circadian information from the antennae and the brain may be integrated downstream from the actual clocks themselves, perhaps at an integration site somewhere in the central complex or its output pathways controlling motor behavior.
In conclusion, we discovered that the antennae are necessary for proper time-compensated sun compass orientation in migratory monarch butterflies. Our results are consistent with a major role of antennal clocks in the timing of sun compass orientation in migratory monarchs. The antennae may function alone, without any influence from brain clocks, or antennal output may influence the integration of timing information from brain circadian clocks within the sun compass structure or at the level of its output pathways. Both possibilities suggest the existence of a crucial but hitherto unknown neural circuit between the antennae and the central complex system.
The role of the antennae in the clock-compass circuitry underlying sun compass orientation could have broad implications, as a similar process may extend to other insects (such as bees, ants, and locusts) that use this orientation mechanism. Furthermore, our results add to the growing list of important non-olfactory functions (e.g., of gravity, wind and sound sensing) housed in the antennae of insects (25, 29, 30).
Supplementary Material
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Summary: The antennae contain circadian clocks that provide a timing mechanism for time-compensated sun compass orientation.
Supporting Online Material, www.sciencemag.org, Materials and Methods, Figs. S1–S7, References
References and notes
- 1.Brower LP. Journal of the Lepidopterist's Society. 1995;49:304. [Google Scholar]
- 2.Urquhart FA. The monarch butterfly. Toronto, Canada: University of Toronto Press; 1960. [Google Scholar]
- 3.Froy O, Gotter AL, Casselman AL, Reppert SM. Science. 2003;300:1303. doi: 10.1126/science.1084874. [DOI] [PubMed] [Google Scholar]
- 4.Mouritsen H, Frost BJ. Proc Natl Acad Sci U S A. 2002;99:10162. doi: 10.1073/pnas.152137299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez SM, Taylor OR, Jander R. Nature. 1997;387:29. [Google Scholar]
- 6.Zhu H, et al. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Mol Biol Evol. 2007;24:948. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, et al. Curr Biol. 2005;15:R953. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Sauman I, et al. Neuron. 2005;46:457. doi: 10.1016/j.neuron.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Truman JW, Riddiford LM. Science. 1970;167:1624. doi: 10.1126/science.167.3925.1624. [DOI] [PubMed] [Google Scholar]
- 11.Hardin PE. Curr Biol. 2005;15:R714. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Reppert SM. Cell. 2006;124:233. doi: 10.1016/j.cell.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Heinze S, Homberg U. Science. 2007;315:995. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- 14.Sakura M, Lambrinos D, Labhart T. J Neurophysiol. 2008;99:667. doi: 10.1152/jn.00784.2007. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, et al. Nature. 2006;439:551. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 16.Merlin C, Francois MC, Queguiner I, Maibeche-Coisne M, Jacquin-Joly E. Insect Mol Biol. 2006;15:137. doi: 10.1111/j.1365-2583.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 17.Merlin C, et al. J Biol Rhythms. 2007;22:502. doi: 10.1177/0748730407307737. [DOI] [PubMed] [Google Scholar]
- 18.Plautz JD, Kaneko M, Hall JC, Kay SA. Science. 1997;278:1632. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 19.Schuckel J, Siwicki KK, Stengl M. Cell Tissue Res. 2007;330:271. doi: 10.1007/s00441-007-0471-x. [DOI] [PubMed] [Google Scholar]
- 20.Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Curr Biol. 2004;14:638. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Saifullah AS, Page TL. J Biol Rhythms. 2009;24:144. doi: 10.1177/0748730408331166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Materials and methods are available as supporting material on Science online.
- 23.Reppert SM, Zhu H, White RH. Curr Biol. 2004;14:155. doi: 10.1016/j.cub.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Gegear RJ, Casselman A, Kanginakudru S, Reppert SM. BMC Biol. 2009;7:14. doi: 10.1186/1741-7007-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sane SP, Dieudonne A, Willis MA, Daniel TL. Science. 2007;315:863. doi: 10.1126/science.1133598. [DOI] [PubMed] [Google Scholar]
- 26.Dunlap JC. Cell. 1999;96:271. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 27.Stalleicken J, et al. J Exp Biol. 2005;208:2399. doi: 10.1242/jeb.01613. [DOI] [PubMed] [Google Scholar]
- 28.Calvert WH. Journal of the Lepidopterist's Society. 2001;55:162. [Google Scholar]
- 29.Kamikouchi A, et al. Nature. 2009;458:165. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 30.Yorozu S, et al. Nature. 2009;458:201. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.We thank Lauren Foley and Amy Casselman for assistance; and Fred Gagnon, Carol Cullar and Orley Taylor for supplying monarch butterflies. We also thank David Weaver, Quan Yuan and members of the Reppert laboratory for their inputs and comments. Supported by NIH grant R01 GM086794
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.