Abstract
Background:
Trials of IV recombinant tissue plasminogen activator (rt-PA) have demonstrated that longer times from ischemic stroke symptom onset to initiation of treatment are associated with progressively lower likelihoods of clinical benefit, and likely no benefit beyond 4.5 hours. How the timing of IV rt-PA initiation relates to timing of restoration of blood flow has been unclear. An understanding of the relationship between timing of angiographic reperfusion and clinical outcome is needed to establish time parameters for intraarterial (IA) therapies.
Methods:
The Interventional Management of Stroke pilot trials tested combined IV/IA therapy for moderate-to-severe ischemic strokes within 3 hours from symptom onset. To isolate the effect of time to angiographic reperfusion on clinical outcome, we analyzed only middle cerebral artery and distal internal carotid artery occlusions with successful reperfusion (Thrombolysis in Cerebral Infarction 2–3) during the interventional procedure (<7 hours). Time to angiographic reperfusion was defined as time from stroke onset to procedure termination. Good clinical outcome was defined as modified Rankin Score 0–2 at 3 months.
Results:
Among the 54 cases, only time to angiographic reperfusion and age independently predicted good clinical outcome after angiographic reperfusion. The probability of good clinical outcome decreased as time to angiographic reperfusion increased (unadjusted p = 0.02, adjusted p = 0.01) and approached that of cases without angiographic reperfusion within 7 hours.
Conclusions:
We provide evidence that good clinical outcome following angiographically successful reperfusion is significantly time-dependent. At later times, angiographic reperfusion may be associated with a poor risk–benefit ratio in unselected patients.
GLOSSARY
- CI
= confidence interval;
- IA
= intraarterial;
- ICA
= internal carotid artery;
- IMS
= Interventional Management of Stroke;
- mRS
= modified Rankin Score;
- NIHSS
= NIH Stroke Scale score;
- rt-PA
= recombinant tissue plasminogen activator;
- sICH
= symptomatic intracranial hemorrhage;
- TCD
= transcranial Doppler;
- TICI
= Thrombolysis in Cerebral Infarction.
The definitive National Institute of Neurological Disorders and Stroke IV recombinant tissue plasminogen activator (rt-PA) trials and a pooling of major randomized stroke trials of IV rt-PA have demonstrated that longer times from ischemic stroke onset to initiation of IV thrombolysis with rt-PA are associated with lower likelihoods of good clinical outcomes.1–3 More recently, results from the ECASS III randomized trial of IV rt-PA at 3 to 4.5 hours and the SITS-MOST registry of IV rt-PA treatment up to 6 hours from stroke onset reinforced the finding that later treatment is associated with less absolute benefit.4,5 However, how the timing of initiation of IV rt-PA administration relates to timing of actual restoration of blood flow has been unclear.
An understanding of how the time interval from symptom onset to actual angiographic reperfusion influences clinical outcome is critical to clinical decision-making in the setting of intraarterial (IA) revascularization therapies. Newly developed IA approaches to intracranial revascularization, consisting of catheter-guided thrombus removal or dissolution with lytics, offer the potential of restoring blood flow more quickly and effectively than IV thrombolysis, the only Food and Drug Administration–approved therapy for acute ischemic stroke.6–10 However, restoring blood flow to irreversibly damaged brain may risk hemorrhagic transformation and procedural complications, without benefit.11–14 This is especially relevant as recent nonrandomized trials of IA revascularization devices, leading to their 510(k) Food and Drug Administration approval for clot removal, set a precedent for extending the time window for initiation of endovascular treatment to as much as 8 hours from symptom onset, with the achievement of angiographic reperfusion at 1 to 2 hours from procedure onset typically.9,15–17
Therefore, we sought to determine how time from stroke onset to technically successful angiographic reperfusion influenced clinical outcome within the Interventional Management of Stroke (IMS) Phase I and II trials.
METHODS
The IMS I and II trials were NIH/National Institute of Neurological Disorders and Stroke–funded open-label, single-arm, pilot trials designed to assess the safety of combined IV/IA therapy, consisting of low-dose IV rt-PA (0.6 mg/kg) started within 3 hours from stroke symptom onset, followed by IA rt-PA (up to 22 mg) delivered via microcatheter for up to 7 hours from symptom onset, for patients with large ischemic strokes (NIH Stroke Scale score [NIHSS] ≥10). In the IMS II trial, IA rt-PA was infused in the context of low-energy ultrasound via the EKOS MicroLysus® Catheter whenever possible. To limit variability and thereby isolate only the effect of time to technically successful angiographic reperfusion, we selected the subset of cases with angiographic middle cerebral artery (M1 and M2) or distal internal carotid artery (ICA-T) occlusions on baseline angiogram. Specifically, because lesion location influences clinical outcomes, we wanted to analyze a fairly homogeneous group of lesion locations; this would provide more precision for testing our hypothesis. Seven cases with continued intervention beyond 7 hours (a protocol violation in the trial) were prespecified to be excluded in this analysis, both because this time point would have few data points and because the outcomes could be confounded by other unmeasured factors leading to the decision to violate the protocol.
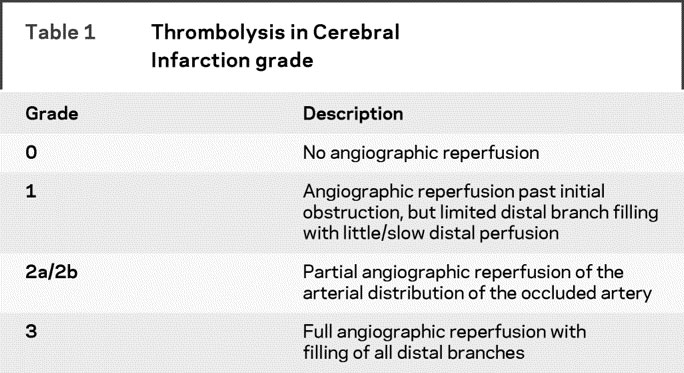
Technically successful angiographic reperfusion was defined as partial or complete restoration of blood flow to the arterial bed of the occluded artery achieved during the interventional procedure, which was measured as Thrombolysis in Cerebral Infarction (TICI) grade 2–3 angiographic reperfusion (table 1).18 The time to angiographic reperfusion was defined as time from stroke onset to procedure termination (minutes). This was expected to be a reasonable definition since angiographic assessments were encouraged after successive 15-minute infusions of IA rt-PA, and termination of the angiographic procedure was recommended based on achievement of TICI 3 flow. We chose to examine angiographic reperfusion (successful restoration of blood flow to the occluded artery and all its visualized distal arterial branches) as the revascularization endpoint, as opposed to recanalization (the restoration of blood flow only at the site of occlusion) as is seen in TCD studies because restoration of flow to the distal vasculature may have a greater influence on the possibility of regaining full neurologic function.19
Table 1 Thrombolysis in Cerebral Infarction grade
Good clinical outcome was defined as modified Rankin Score (mRS) 0–2 at 3 months, an established primary clinical endpoint in acute ischemic stroke trials that measures functional disability.6,9,20 These scores were collected systematically as part of the prospective IMS I and II trials.
We prespecified the following variables as potential predictors of clinical outcome to be considered for adjustment: age (year), baseline NIHSS score, sex, and baseline glucose.3,21–26 Logistic regression was used for multivariable modeling, with stepwise methodology used for variable selection. Goodness of fit was assessed via the Hosmer and Lemeshow test.
Additional exploratory analyses included testing whether symptomatic intracranial hemorrhage (sICH) was associated with later angiographic reperfusion, and testing whether time to technically successful angiographic reperfusion was associated with CT infarct volume. Infarct volumes on 24-hour CT scans were digitally measured using Cheshire 4.4.8 Image analysis software.
RESULTS
Among 161 cases enrolled in the IMS I and II trials, 117 subjects were taken to IA therapy. Ninety-eight of these cases had ICA-T or MCA (M1 or M2) occlusions, and 7 were excluded due to reperfusion at greater than 7 hours as discussed in Methods. Of the remaining 91 subjects with ICA-T or MCA arterial occlusions at the start of the angiographic procedure, 54 cases (59.3%) had technically successful angiographic reperfusion (TICI 2–3) including 6 cases with complete (TICI 3) reperfusion. These 54 cases consisted of 8 ICA-T and 46 MCA (30 M1 and 16 M2) occlusions. Six cases achieved TICI 3 angiographic reperfusion. Angiographic reperfusion times ranged from 208 to 395 minutes, as measured by the end of the angiographic procedure. Median baseline NIHSS scores were 18 (range 10–28) for these 54 cases, compared to 21 (range 10–27) for the 38 cases without angiographic reperfusion (TICI 0–1; p = 0.15). Median age was 65 (range 20–80) for these 54 cases, compared to 63 (range 36–78) for those without angiographic reperfusion (p = 0.38). Good clinical outcomes (mRS 0–2) were seen in 29 of 54 angiographic reperfusion cases (53.7%), compared to 8 of 37 nonreperfusion cases (21.6%).
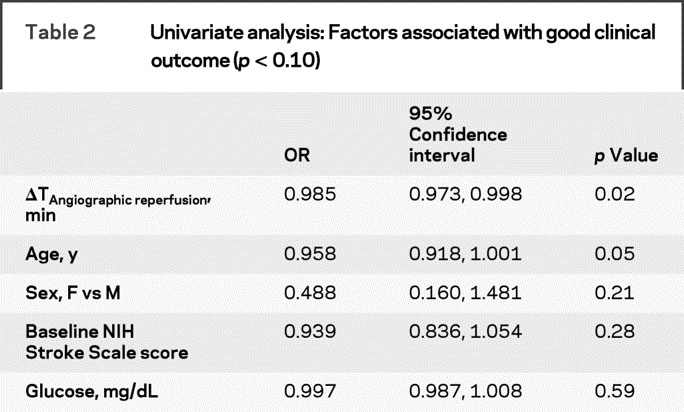
In univariate analysis, as time to angiographic reperfusion (in minutes) increased, the probability of a good clinical outcome decreased (OR 0.985, 95% confidence interval [CI] 0.973–0.998), as shown in figure 1. Using 30-minute time intervals (instead of minutes), the magnitude of the OR was 0.641 (95% CI 0.423–0.922). Also, in univariate analysis, as age (by year) increased, the probability of a good clinical outcome trended toward being decreased (OR 0.958, 95% CI 0.918–1.001). See table 2 for complete univariate analyses. With stepwise regression modeling considering each potential covariate, as both time to technically successful angiographic reperfusion (OR 0.982; 95% 0.969–0.996) and age (OR 0.945; 95% CI 0.899–0.993) increased, probabilities of good clinical outcome were significantly decreased in the final multivariable model. Goodness of fit was confirmed by formal testing. Thus, both unadjusted (p = 0.024) and adjusted (p = 0.012) analyses showed that the probability of good clinical outcome decreased as time to angiographic reperfusion increased.

Figure 1 Probability of good clinical outcome over time to technically successful angiographic reperfusion
The graph above shows the probability of a good clinical outcome over time (with 95% confidence bands) for cases with angiographic reperfusion as predicted by unadjusted logistic regression (p = 0.02). In addition, a horizontal line depicting the rate of good clinical outcome for all cases with ICA-T and MCA occlusions on angiogram that did not show significant angiographic reperfusion is provided as a reference.
Table 2 Univariate analysis: Factors associated with good clinical outcome (p < 0.10)
After obtaining this primary analysis result, we explored the relationship of time to angiographic reperfusion and clinical outcome further. We considered an alternate definition of good clinical outcome, mRS 0–1 (rather than 0–2), and showed an association (OR 0.98; 95% CI 0.968–0.995; p = 0.008). In addition, in an effort to validate our findings, survival methods were applied to the combined cohort of subjects who achieved successful reperfusion and those who did not, as shown in figure 2. Subjects who did not achieve technically successful angiographic reperfusion (TICI 2–3) were considered censored at the termination of the procedure. The corresponding log-rank test indicates that there is a significant difference between subjects with good clinical outcome (mRS 0–2) and those with a poor outcome (mRS >2) in the distribution of the time to reperfusion (p = 0.0002; figure 3). Of note, separate analysis of only complete reperfusion (TICI 3) cases could not be performed due to the small number of these cases (n = 6).

Figure 2 Histogram of reperfusion times

Figure 3 Proportion of subjects without angiographic reperfusion among those with good vs poor clinical outcome
The graph above shows the estimated survival curves (i.e., proportions not reperfused) for subjects with good clinical outcome (modified Rankin Score [mRS] 0–2) and those without good clinical outcome (mRS 3–6). The tick marks denote subjects who did not achieve technically successful angiographic reperfusion by the end of the angiographic procedure and, as such, were censored in the analysis.
Time to angiographic reperfusion did not show a significant association with stroke lesion volume on 24-hour CT infarct scan in univariate analysis (p = 0.59), and neither did potential covariates of age, baseline glucose level, and baseline NIHSS score (p > 0.15). However, CT infarct volumes were significantly larger in cases with poor clinical outcome (mRS > 2) compared those with good clinical outcome (mRS 0–2) using the Wilcoxon 2-sample test (median 82.3 vs 18.3 mL; p = 0.003).
Among the 54 ICA-T or MCA cases with successful angiographic reperfusion, 7 cases had sICH, and all of those had a poor clinical outcome. Median time to angiographic reperfusion was 332 minutes among sICH cases and 311 minutes among non-SICH cases (p = 0.32). Thus, no relationship between time to angiographic reperfusion and sICH was demonstrated in this sample.
DISCUSSION
We provide clinical trial evidence that good clinical outcome following technically successful angiographic reperfusion is time-dependent. For example, achieving reperfusion at 310 minutes, compared to 280 minutes, corresponds to a 10.6% decrease in the probability of a good outcome. This is the same magnitude as the planned effect size of the ongoing IMS III trial, which is comparing standard IV rt-PA to the combined IV/IA approach. The probability of a good clinical outcome progressively declined with time from stroke symptom onset, and approached the same probability of good clinical outcome as those without angiographic reperfusion within 7 hours in this combined IV/IA cohort.
IV rt-PA stroke trials have reliably shown that event-to-needle time for IV therapy affects clinical outcome. This has made it possible to establish a generally accepted time window, beyond which initiation of treatment is considered futile, or even harmful. However, we have not had a detailed understanding of how this relates to the timing of the actual restoration of blood flow. IA therapy entails more direct removal of the clot, making it difficult to translate the IV window to an equivalent window for IA treatment. Our findings suggest that the practice of IA therapy at later times may be of limited benefit while introducing procedural risk.
Prior human studies have addressed vessel patency in a limited manner. Transcranial Doppler (TCD) studies of IV thrombolysis have typically measured recanalization at a single time point and have supported the importance of achieving recanalization, showing better clinical outcomes among those with recanalization compared to those without recanalization.27–30 Studies with 2 time points within 6 hours from stroke onset failed to show that earlier time to recanalization was associated with better outcome, but may have lacked statistical power and were unable to assess the status of the distal vasculature.27,28 One TCD study assessed recanalization continuously through 6 hours after IV tPA but, for the purpose of analysis, considered cases without recanalization during observation as having recanalized at 6 hours, thereby not isolating the role of time (personal communication, A. Alexandrov, 2007).29
Angiographic studies of this topic have been limited by study design and sample size.6,30–32 PROACT II, in particular, recorded only angiographic reperfusion status after the required 2 hours of r-pro-urokinase infusion. Analysis did not show an effect of time to randomization (p = 0.94) or time to IA treatment (p = 0.49) on clinical outcome, but the study included a preponderance of patients treated at later times. The median time to initiation of IA treatment was 5.3 hours, and only one subject had angiographic reperfusion therapy initiated within 3 hours in the PROACT II trial.6,33
Our findings are consistent with animal studies, which suggest a 6-hour time window before irreversible neurologic injury.34 In our analysis, the lower limit of the 95% CI band for probability of a good clinical outcome after technically successful angiographic reperfusion meets the probability of a good clinical outcome for cases without angiographic reperfusion at 350 minutes. However, while this finding is provocative, it should be interpreted with caution. A formal comparison cannot be made because the predictor of interest, time to technically successful angiographic reperfusion, is not valid for cases without angiographic reperfusion, which have no comparable time component. We are unable to precisely identify a “point of no return.”
This analysis may have actually underestimated the effect of time by defining the time of technically successful angiographic reperfusion (TICI 2–3) as the end of the IA procedure. Some subjects may have already achieved TICI 2 angiographic reperfusion spontaneously or due to IV rt-PA bridging therapy, and received continued IA therapy in an attempt to get a better result. Our data represent the latest time that TICI 2–3 angiographic reperfusion could occur, and these good clinical outcomes may have had earlier times to effective angiographic reperfusion than this analysis would indicate.
We considered whether time to angiographic reperfusion related to CT infarct volume as a secondary analysis, and found no significant relationship. One would expect CT infarct volume to be the mechanism linking early angiographic reperfusion and good clinical outcome, and additional analyses did show that subjects with poor outcome did indeed have larger infarct volumes. We surmise that the effect of timing of angiographic reperfusion relationship on CT infarct volume may have been more difficult to demonstrate due to the substantial variability of how individual infarct sizes in specific locations affect clinical outcome. A larger sample size may have been required to show significance.
A further consideration is that our findings were seen in, specifically, a combined IV/IA rt-PA angiographic reperfusion therapy approach. While there is no evidence that hemorrhage rates or procedural risk are different for other endovascular modalities such as mechanical revascularization or IA lytics alone, this finding requires empiric testing in other cohorts. The possibility of a neurotoxic effect of rt-PA cannot be ruled out in this analysis. In analysis of other (often quite small) cohorts, however, it is important to bear in mind the statistical adage that not showing a relationship is not the same as showing that there is no relationship, particularly in small cohorts. Patient groups should be relatively homogeneous at a given time point, which can be problematic in device trials in which patients ineligible for rt-PA, and therefore with other comorbidities, comprise the group treated in less than 3 hours. Variability should be limited by considering similar stroke lesions and adjusting for key confounders of clinical outcome, such as glucose, age, and NIHSS score. Finally, particularly in IA trials, initiation of treatment may not correspond to angiographic reperfusion timing due to variable timing for access, revascularization, and interventionalist skill.
To summarize, potential limitations of this study include an inability to generalize to other treatment paradigms, the possibility of underestimating the role of time, and an inability to identify a precise time to reperfusion that provides no benefit. Moreover, this is an exploratory finding, and it requires validation in larger cohorts with data regarding revascularization status.
Nevertheless, our findings reinforce the notion that acute stroke treatment must emphasize speed. Angiographic reperfusion strategies that might restore blood flow more rapidly, such as mechanical intervention or the combination of IV rt-PA with GP IIb/IIIa inhibitors, should be prioritized as the most promising therapies.10,35 Treatment paradigms that integrate rapidly accessible and widely available therapies, as with the combined IV/IA treatment paradigm,20 may be preferable. Finally, stroke systems of care for delivery of IV rt-PA and transfer to neurointerventional suites must be efficient. Without efficient transfers, the added time for transfer to the angiographic suite may negate the benefits of potentially higher angiographic reperfusion rates with IA therapy, as is known to be the case for angiographic reperfusion therapies of myocardial infarction.36
Further risk stratification, based on MRI and CT diffusion-perfusion assessments of “physiologic time” and other parameters, may be helpful for extending the time window in the future, based on promising data from the DEFUSE and EPITHET trials.37–39
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by S.D.Y.
DISCLOSURE
Dr. Khatri receives royalties from publishing The Stroke Center Handbook (Taylor & Francis Group, 2006–2008); receives research support from the NIH/NINDS NS059843; and received an honorarium for contributing to Continuum. Dr. Abruzzo has served as a consultant to Starfire Medical Inc., Boston Scientific Neurovascular, Microvention Inc., and the Council On New Technologies And Clinical Therapies; has received research support in form of materials from Boston Scientific Neurovascular, Cordis Endovascular, Microvention Incorporated, and EV3 Incorporated; has received research support from the University of Cincinnati Neuroscience Institute and the Neuroradiology Education and Research Foundation; and holds stock in Merlin Medical Incorporated (Singapore), for which his spouse serves as Vice President of Regulatory and Clinical Affairs. Dr. Yeatts receives research support from Boehringer-Ingelheim (Biostatistical Support) and the NIH [NINDS R01 NS057127 (coinvestigator), NINDS U01 NS054630 (coinvestigator), NINDS U01 NS059041 (biostatistical support), NINDS K23 NS059843-01 (biostatistical support), NIDA P50 DA016511-04 (biostatistical support), NIDA K24 DA000435-07 (biostatistical support), and NIDA R25 DA020537 (biostatistical support)]. Dr. Nichols reports no disclosures. Dr. Broderick has served on scientific advisory boards for Johnson & Johnson and Wyeth Pharmaceuticals; has received non-industry-sponsored funding for travel; holds US Patent 7,135,305, issued November 15, 2006, and a closely related patent in 2007; has received honoraria from Genentech; has held corporate appointment with Novo and Genentech; has received research support in the form of materials from Genentech, Novo Nordisk, Schering Plough, Concentric, EKOS, and Johnson & Johnson; and receives research support from the NIH [NINDS U01 NS052220 (PI); NINDS P50 NS44283 (PI); NINDS R01 NS39512 (coinvestigator); and NINDS #R01 NS36695 (coinvestigator)]. Dr. Tomsick serves on the editorial boards of American Journal of Neuroradiology and Stroke; treats stroke in his clinical practice; and has provided expert testimony in multiple medicolegal actions.
APPENDIX
Clinical Coordinating Center: Judith Spilker and Janice A. Carrozzella (University of Cincinnati); Canadian Coordinating Center: Michael D. Hill, Karla Ryckborst, Andrew Demchuk (University of Calgary); Statistical Coordinating Center: Yuko Palesch, Renee Martin (Medical University of South Carolina). Clinical sites, investigators, and coordinators: University of Cincinnati, University of Calgary, University of Pittsburgh, Oregon Health Sciences, University of Texas–Houston, Case Western Reserve University, University of California–San Francisco, University of California–Los Angeles, Wayne State University, St. Luke’s Hospital of Kansas City, Vancouver General Hospital, University of Alberta–Edmonton, Erlanger Health Care System, University of British Columbia, Henry Ford Hospital, Emory University, St. Luke’s Hospital–Mayo, One Site Sutter General Hospital, Queens University. CT infarct volume measurements: Gowri Ramadas (University of Cincinnati).
Address correspondence and reprint requests to Dr. Pooja Khatri, Department of Neurology, University of Cincinnati Academic Health Center, 260 Stetson St., Ste 2308, PO Box 670525, Cincinnati, OH 45267-0525 pooja.khatri@uc.edu
*The IMS I and II Investigators are listed in the appendix.
Supported by NIH K23 NS059843 (P.K.) and NIH/NINDS R01NS39160 (J.P.B.). The IMS I and II trials, the source of the data, were funded by the NIH/NINDS (IMS I); EKOS Corporation in partnership with NIH R44HL64434 (IMS II); and Genentech, which supplied study drug, and Johnson and Johnson, which supplied study catheters (IMS I and II).
Disclosure: Author disclosures are provided at the end of the article.
Received November 24, 2008. Accepted in final form June 29, 2009.
REFERENCES
- 1.Hacke W, Donnan G, Fieschi C, et al, ATLANTIS Trials Investigators, ECASS Trials Investigators, NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–774. [DOI] [PubMed] [Google Scholar]
- 2.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology 2000;55:1649–1655. [DOI] [PubMed] [Google Scholar]
- 3.NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, et al, ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 5.Wahlgren N, Ahmed N, Eriksson N, et al, for the SITS-MOST Investigators. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST). Stroke Epub 2008. [DOI] [PubMed]
- 6.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II study: a randomized controlled trial: Prolyse in acute cerebral thromboembolism. JAMA 1999;282:2003–2011. [DOI] [PubMed] [Google Scholar]
- 7.Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–911. [DOI] [PubMed] [Google Scholar]
- 8.IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke 2007;38:2127–2135. [DOI] [PubMed] [Google Scholar]
- 9.Smith WS, Sung G, Starkman S, et al, MERCI Trial Investigators. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–1438. [DOI] [PubMed] [Google Scholar]
- 10.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–973. [DOI] [PubMed] [Google Scholar]
- 11.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86. [DOI] [PubMed] [Google Scholar]
- 12.Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator: The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Molina CA, Alvarez-Sabin J, Montaner J, et al. Thrombolysis-related hemorrhagic infarction: a marker of early angiographic reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke 2002;33:1551–1556. [DOI] [PubMed] [Google Scholar]
- 14.Khatri P, Wechsler LR, Broderick JP. ICH associated with revascularization therapies. Stroke 2007;38:431–440. [DOI] [PubMed] [Google Scholar]
- 15.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke: Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 16.Jahan R. Hyperacute therapy of acute ischemic stroke: intraarterial thrombolysis and mechanical revascularization strategies. Tech Vas Interv Radiol 2005;8:87–91. [DOI] [PubMed] [Google Scholar]
- 17.McDougall C, Clark W, Mayer T, et al. The Penumbra Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in acute ischemic stroke. Presented at the International Stroke Conference, New Orleans, LA, 2008.
- 18.Higashida R, Furlan A, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol 2003;14:S493–S494. [DOI] [PubMed] [Google Scholar]
- 19.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T, IMS-I Investigators. Revascularization end points in stroke interventional trials: recanalization versus angiographic reperfusion in IMS-I. Stroke 2005;36:2400–2403. [DOI] [PubMed] [Google Scholar]
- 20.Khatri P, Hill MD, Palesch YY, et al, for the IMS III Investigators. Methodology of the Interventional Management of Stroke (IMS) III Trial. Int J Stroke 2008;3:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston KC, Connors AF Jr, Wagner DP, Haley EC Jr. Predicting outcome in ischemic stroke: external validation of predictive risk models. Stroke 2003;34:200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henon H, Godefroy O, Leys D, et al. Early predictors of death and disability after acute cerebral ischemic event. Stroke 1995;26:392–398. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler LR, Roberts R, Furlan AJ, et al, PROACT II Investigators. Factors influencing outcome and treatment effect in PROACT II. Stroke 2003;34:1224–1229. [DOI] [PubMed] [Google Scholar]
- 24.König IR, Ziegler A, Bluhmki E, et al. Virtual International Stroke Trials Archive (VISTA) Investigators: predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke 2008;39:1821–1826. [DOI] [PubMed] [Google Scholar]
- 25.Kissela B, Lindsell CJ, Kleindorfer D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke 2009;40:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savitz SI, Schlaug G, Caplan L, Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke 2005;36:1447–1451. [DOI] [PubMed] [Google Scholar]
- 27.Ribo M, Alvarez-Sabin J, Montaner J, et al. Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue angiographic reperfusion techniques. Stroke 2006;37:1000–1004. [DOI] [PubMed] [Google Scholar]
- 28.Wunderlich MT, Goertler M, Postert T, et al, Duplex Sonography in Acute Stroke (DIAS) Study Group, Competence Network Stroke. Recanalization after intravenous thrombolysis: does a recanalization time window exist? Neurology 2007;68:1364–1368. [DOI] [PubMed] [Google Scholar]
- 29.Molina CA, Montaner J, Abilleira S, et al. Time course of tissue plasminogen activator-induced recanalization in acute cardioembolic stroke: a case-control study. Stroke 2001;32:2821–2827. [DOI] [PubMed] [Google Scholar]
- 30.Christou I, Alexandrov AV, Burgin WS, et al. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial Doppler correlates with clinical recovery from ischemic stroke. Stroke 2000;31:1812–1816. [DOI] [PubMed] [Google Scholar]
- 31.Bendszus M, Urbach H, Ries F, Solymosi L. Outcome after local intra-arterial fibrinolysis compared with the natural course of patients with a dense middle cerebral artery on early CT. Neuroradiol 1998;40:54–58. [DOI] [PubMed] [Google Scholar]
- 32.Zaidat OO, Suarez JI, Sunshine JL, et al. Thrombolytic therapy of acute ischemic stroke: correlation of angiographic recanalization with clinical outcome. AJNR Am J Neuroradiol 2005;26:880–884. [PMC free article] [PubMed] [Google Scholar]
- 33.Wechsler LR, Roberts R, Furlan AJ, et al. Factors influencing outcome and treatment effect in PROACT II. Stroke 2003;34:1224–1229. [DOI] [PubMed] [Google Scholar]
- 34.Zivin JA. Factors determining the therapeutic window for stroke. Neurology 1998;50:599–603. [DOI] [PubMed] [Google Scholar]
- 35.Pancioli AM, Brott TG. Therapeutic potential of platelet glycoprotein IIb/IIIa receptor antagonists in acute ischemic stroke: scientific rationale and available evidence. CNS Drugs 2004;18:981–988. [DOI] [PubMed] [Google Scholar]
- 36.Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med 2007;357:1631–1638. [DOI] [PubMed] [Google Scholar]
- 37.Neumann-Haefelin T, Steinmetz H. Time is brain: is MRI the clock? Curr Opin Neurol 2007;20:410–416. [DOI] [PubMed] [Google Scholar]
- 38.Albers GW, Thijs VN, Wechsler L, et al, DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early angiographic reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 39.Davis SM, Donnan GA, Parsons MW, et al, EPITHET investigators. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 2008;7:299–309. [DOI] [PubMed] [Google Scholar]


