Abstract
Background:
We hypothesize that a smaller posterior fossa (PF) CSF space may be a risk factor for hemifacial spasm (HFS).
Objective:
We conducted a case-control 3-dimensional magnetic resonance (MR) volumetric study in patients with HFS and determined the clinical predictive factors of PF CSF volume.
Methods:
Patients with clinically diagnosed HFS and controls matched for age, sex, race, and hypertension underwent MRI/magnetic resonance angiography examination. The PF CSF space was segmented and quantified on a heavily T2-weighted high-resolution 3-dimensional MR volume slab, centered over the porus acusticus.
Results:
Eighty-two study subjects (41 patients and 41 controls) were included. The mean PF CSF volume in patients with HFS and controls was 17,303.0 ± 3,900.0 vs 19,216.0 ± 3,912.0 mm3. The mean volume in patients with HFS was 11.4% smaller than in controls (p = 0.015). Analysis of differences between individually matched pairs and controls also revealed that PF CSF for controls was larger than that for patients with HFS (p = 0.007). A multivariate linear regression analysis revealed that a small PF CSF volume was associated with HFS (p = 0.01). Decreasing age (p = 0.001) and female gender (p < 0.0005), but not hypertension (p = 0.892), were also found to be predictors of a low PF CSF volume.
Conclusions:
Our results showed that the posterior fossa (PF) CSF volume was lower in patients with HFS compared with matched controls. HFS, female gender, and younger age were associated with smaller PF CSF volume. These observations could explain the strong female preponderance in both clinic- and population-based epidemiologic studies.
GLOSSARY
- CISS
= constructive interference at steady state;
- FA
= flip angle;
- HFS
= hemifacial spasm;
- MR
= magnetic resonance;
- MRA
= magnetic resonance angiography;
- NEX
= number of excitations;
- NVC
= Neurovascular contact;
- PF
= posterior fossa;
- TE
= echo time;
- TR
= repetition time.
Neurovascular contact (NVC) of the facial nerve has been postulated to be the underlying cause of hemifacial spasm (HFS), a socially embarrassing and at times disabling neurologic condition that significantly impacts quality of life.1–4 However, etiologic controversy remains because nonsymptomatic NVC has been reported in 10% to 20% of healthy controls.5 It is also puzzling why there is a female dominance in this condition.1 The average prevalence rate of HFS in America and Norway is around 10 per 100,000 population.6,7 Even though there are no published epidemiologic studies in Asia, most movement disorder neurologists share the common observation that HFS seems more common among Asians compared with whites. This is indirectly supported by the relative larger sample size of reported HFS databases in Asian countries8 and a higher than expected proportion of Asian subjects with HFS in white centers.1
Structural differences of the posterior cranial fossa and temporal bone between Asians and whites have been demonstrated.9 There have also been reports where bony abnormalities (which could result in narrowing of the posterior fossa [PF]) have been found in some cases of HFS.1 These observations led us to hypothesize that a smaller PF CSF space in combination with NVC may be a risk factor for HFS. To address this, we conducted a case-control 3-dimensional magnetic resonance (MR) volumetric study in patients with HFS and determined the clinical predictive factors of PF CSF volume.
METHODS
Clinical.
Patients who were clinically diagnosed with HFS by a movement disorders neurologist and had consented for MRI/magnetic resonance angiography (MRA) evaluation to exclude structural and vascular abnormalities in the PF as part of clinical care were initially included. Controls were volunteers who were invited to participate (90% participation rate) in imaging research of HFS. They were closely matched for age, sex, race, and hypertension (1-to-1 matching) and provided written informed consent for the imaging research study. The Singapore General Hospital ethics committee approved this study and gave waiver of consent for the image analysis for research in patients with HFS.
All subjects underwent a standardized MRI protocol, comprising of 3-dimensional sequences focused over the PF: constructive interference at steady state (CISS) (repetition time [TR] 12, echo time [TE] 6, flip angle [FA] 70, number of excitations [NEX] 2, 282 × 512 matrix, 0.75-mm partitions, 56 slices) and time-of-flight MRA (TR 35, TE 7.2, FA 20, NEX 1, 210 × 512 matrix, 0.8-mm partitions, 96 slices). A screening fluid-attenuated inversion recovery (TR 9,000 msec, TE 110 msec, inversion recovery 2,500 msec, NEX 1) scan of the brain was also obtained to exclude the presence of brainstem and bony abnormalities, or space-occupying lesions in the PF. All the sequences were acquired in the axial plane, parallel to the bicommissural line.
3-Dimensional MR volumetric analysis.
The 3- dimensional CISS MR images of data sets were anonymized and transferred to a personal computer workstation, and the PF volumes were measured using the commercial image analysis software Analyze 8.1 (AnalyzeDirect Inc., Overland Park, KS). Using the DicomTool 8.1, the data sets were loaded to generate 1 × 1 × 1-mm isotropic cubic voxels. The Volume Render and Oblique Sections functions were used to ensure that all images were aligned to the true horizontal plane. The hyperintense CSF in the PF was segmented out using the Threshold tool in the Region of Interest (ROI) module.
The outer margins of PF CSF were defined by the dural contours of the PF. The inner margins were defined by the outline of the brain structures in the PF, including the brainstem and cerebellum. Thence, the cranial nerves and vasculature were included within the PF CSF space, but the fourth ventricle was excluded from it (figure 1). All image sections were individually reviewed and the segmented PF CSF was manually edited to ensure that all erroneously included areas without the dural contours and within the outlines of the PF structures were excluded from the volumetric analysis. The PF CSF was sampled on 20 slices, with the midpoint of the volume slab centered over the porus acusticus.
Figure 1 Segmentation of posterior fossa CSF space
The segmented posterior fossa CSF space is represented by the hyperintense space between the outer red line, defining the dural contours, and the inner red line, defining the brain outline. This is demonstrated on representative axial constructive interference at steady state magnetic resonance images from the superior-most (A), mid (B), and inferior-most (C) sections from the 2-cm volume slab centered over the porus acusticus, depicted as a box over the midsagittal image of the brainstem in D.
Statistical analysis.
The χ2 test and Student t test were used to compare the categorical and continuous variables. A logistic regression analysis was performed with PF CSF volume as the dependent variable and with age, sex, HFS/control, and hypertension as independent variables. Significance was defined at p < 0.05. Based on a pilot study with a mean PF CSF volume of 20,000 mm3 (SD of 4,000 mm3) in controls and an anticipated 20% lower volume in patients with HFS, the number of patients required is 17 age- and sex-matched pairs of controls and patients with HFS for a 2-sided test with α = 0.05 and power of 80%.
RESULTS
There were 82 study subjects, comprising 41 patients and 41 controls, with 25 women (61%) and 16 men (39%) in each group (HFS and control). The mean ages of patients with HFS and controls were 55.0 ± 10.0 (40–78) and 56.0 ± 10.0 (40–77) years. The mean PF CSF volumes in patients with HFS and controls were 17,303.0 ± 3,900.0 (10,947.0–26,951.0) and 19,216.0 ± 3,912.0 (10,996.0–30,070.0) mm3. The mean volume in patients with HFS was 11.4% smaller than in controls (p = 0.015).
Analysis of differences between individually matched pairs and controls also revealed that PF CSF for controls was larger than for patients with HFS (p = 0.007 by Wilcoxon signed rank test). The median (range) difference between the paired volumes of controls and patients with HFS was 2,189 (−5,952 to 8,764) mm3, and the mean difference was 1,913 mm3 (95% confidence interval 651–3,175 mm3). Images of a patient with HFS and a small PF CSF volume and a control with a large PF CSF volume were highlighted in figure 2.
Figure 2 Posterior fossa CSF volume in a patient with HFS and a control
Comparison of 3-dimensional volume rendered constructive interference at steady state images of segmented posterior fossa CSF in a 58-year-old male control subject (left) and a 56-year-old female patient with hemifacial spasm (HFS) (right) when viewed (A) posteriorly, superiorly, and left and (B) below and right of the midline. The control subject has a large posterior fossa CSF volume of 30,070 mm3 compared with a small volume of 10,947 mm3 in the patient with HFS.
The scatterplot demonstrated that the volume in controls was higher than in patients with HFS, because more points lie above the y = x equality line (figure 3).
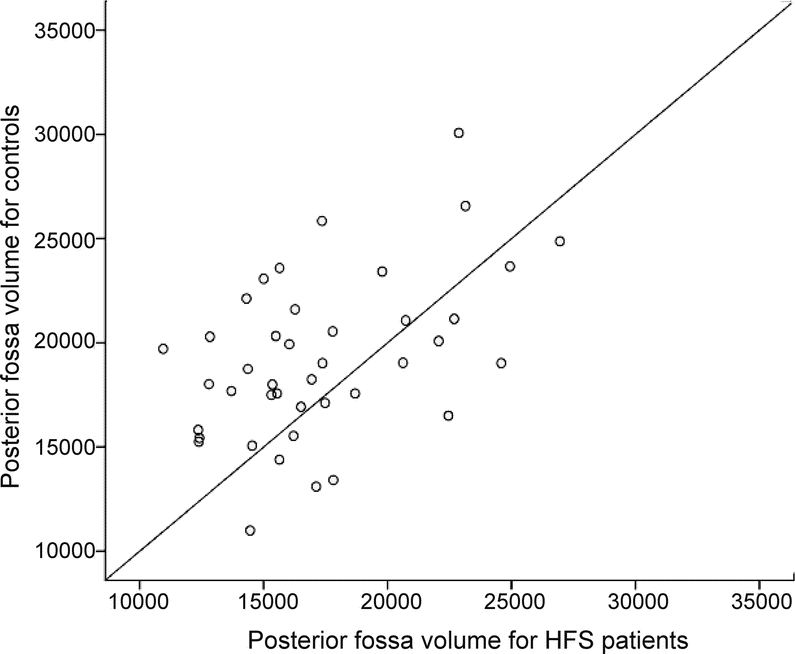
Figure 3 Scatterplot of CSF volumes in patients with HFS and controls
Scatterplot showing the differences in posterior fossa CSF volume (mm3) between matched pairs of patients with hemifacial spasm (HFS) and controls.
A multivariate linear regression analysis with age, sex, hypertension, and HFS disease and control groups as independent factors and with PF CSF volume as the dependent factor revealed that a small PF CSF volume was associated with HFS (p = 0.01). Younger age (p = 0.001) and female gender (p < 0.0005) but not hypertension (p = 0.892) were also found to be predictors for a low PF CSF volume.
DISCUSSION
Vascular compression on the root exit zone of the facial nerves is postulated to be a major etiologic cause of HFS, and microvascular decompression surgery is accepted as an effective treatment option to medical therapy. However, there has been ongoing debate on the actual predisposing factors in HFS. It is unclear why there is a greater prevalence of HFS in Asians and females. It is also puzzling why some people do not develop the condition even though they have vascular abnormalities on imaging.
There have been several case reports of HFS occurring in patients with contralateral space-occupying lesions at the cerebellopontine angles and bony abnormalities such as petrous bony hyperplasia (e.g., from Paget disease) and Chiari malformation, suggesting that narrowing of the PF and crowding of the neurovascular structures may predispose to the occurrence of HFS. A study using indirect measures (petrous bone angle) of narrowing of the PF on 5-mm-thick section computerized tomographic (CT) brain scans also supported this hypothesis.10
We conducted a case-control MR volumetric study using high-resolution 3-dimensional image data sets to directly and accurately quantify the PF CSF space in patients with HFS. The heavily T2-weighted CISS sequence contains high CSF-brain contrast, which allows accurate volumetric segmentation based on thresholding. Centering this volume slab at the porus acusticus allows a precise assessment of the capacity of the CSF space around the root exit zone of the facial nerve, the critical area where neurovascular conflict is believed to occur.
Our results showed that the PF CSF volume was approximately 11% lower in patients with HFS compared with matched controls. The median difference between individually paired PF CSF volumes of controls and patients with HFS was 2,189 mm3. In the logistic regression analysis, HFS, female gender, and younger age were associated with smaller PF CSF volume. These observations could explain the strong female preponderance in both clinic and population-based epidemiologic studies.1–6 Our study revealed that older subjects with larger PF CSF volume, conceivably secondary to age-related involutionary changes, would be at reduced risk of HFS. However, HFS in older patients could also be confounded by a greater prevalence of ectatic or tortuous vessels, especially in patients with hypertension. We were unable to assess the impact of tortuous arteries on the PF CSF volume in our study. Our findings also provide indirect support that a smaller PF CSF volume could be a reason for the greater prevalence of HFS among Asians than among whites.
ACKNOWLEDGMENT
The authors thank Bien-Soo Tan, MD, Department of Diagnostic Radiology, Singapore General Hospital, for support in the purchase of the Analyze 8.1 program.
DISCLOSURE
Dr. L. Chan, K. Ng, S. Fook-Chong, and Dr. Y. Lo report no disclosures. Dr. E.-K. Tan has received speaker honoraria from Boehringer Ingelheim; and has received research support for clinical trials in movement disorders sponsored by Eisai, Schering Plough, and Allergan.
Address correspondence and reprint requests to Eng-King Tan, MD, FRCP(UK), Department of Neurology, Singapore General Hospital, Outram Road, Singapore 169608, Republic of Singapore gnrtek@sgh.com.sg
Supported by a grant from the National Medical Research Council.
Disclosure: Author disclosures are provided at the end of the article.
Received March 9, 2009. Accepted in final form June 15, 2009.
REFERENCES
- 1.Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve 1998;21:1740–1747. [DOI] [PubMed] [Google Scholar]
- 2.Stamey W, Jankovic J. The other Babinski sign in hemifacial spasm. Neurology 2007;69:402–404. [DOI] [PubMed] [Google Scholar]
- 3.Chan LL, Lo YL, Lee E, Fook-Chong S, Tan EK. Ventrolateral medullary compression in hypertensive patients with hemifacial spasm. Neurology 2005;65:1467–1470. [DOI] [PubMed] [Google Scholar]
- 4.Engh JA, Horowitz M, Burkhart L, Chang YF, Kassam A. Repeat microvascular decompression for hemifacial spasm. J Neurol Neurosurg Psychiatry 2005;76:1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felber S, Birbamer G, Aichner F, Poewe W, Kampfl A. Magnetic resonance imaging and angiography in hemifacial spasm. Neuroradiology 1992;34:413–416. [DOI] [PubMed] [Google Scholar]
- 6.Nilsen B, Le KD, Dietrichs E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology 2004;63:1532–1533. [DOI] [PubMed] [Google Scholar]
- 7.Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol 1990;47:1233–1234. [DOI] [PubMed] [Google Scholar]
- 8.Tan EK, Chan LL. Young onset hemifacial spasm. Acta Neurol Scand 2006;114:59–62. [DOI] [PubMed] [Google Scholar]
- 9.Low WK, Fenton JE, Fagan PA, Gibson WP. Racial considerations in acoustic neuroma removal with hearing preservation via the retrosigmoid approach. Acta Otolaryngol 1995;115:783–786. [DOI] [PubMed] [Google Scholar]
- 10.Kamiguchi H, Ohira T, Ochiai M, Kawase T. Computed tomographic analysis of hemifacial spasm: narrowing of the posterior fossa as a possible facilitating factor for neurovascular compression. J Neurol Neurosurg Psychiatry 1997;62:532–534. [DOI] [PMC free article] [PubMed] [Google Scholar]




