Abstract
Background:
The neuropeptide calcitonin gene-related peptide (CGRP) plays a key role in migraine pathophysiology. In this large phase 3 clinical trial, we sought to confirm the efficacy of telcagepant, the first orally bioavailable CGRP receptor antagonist.
Methods:
Adults with migraine with or without aura (International Headache Society criteria) treated a moderate or severe attack with oral telcagepant 50 mg (n = 177), 150 mg (n = 381), 300 mg (n = 371), or placebo (n = 365) in a randomized, double-blind trial. The 5 co-primary endpoints were pain freedom, pain relief, and absence of photophobia, absence of phonophobia, and absence of nausea, all at 2 hours postdose. The key secondary endpoint was 2–24 hour sustained pain freedom. The prespecified primary efficacy analyses evaluated the 150 mg and 300 mg groups; the 50-mg group was included on an exploratory basis to further characterize the dose response but was not prespecified for analysis. Tolerability was assessed by adverse experience reports.
Results:
Telcagepant 300 mg was more effective (p ≤ 0.001) than placebo on all primary endpoints and the key secondary endpoint, as was telcagepant 150 mg (p ≤ 0.05). Telcagepant 300 mg showed a slight numeric advantage over telcagepant 150 mg on most measures. Telcagepant 50 mg values were numerically intermediate between placebo and telcagepant 150 mg and 300 mg. The percentages of patients with adverse experiences were 32.2% for telcagepant 50 mg, 32.0% for telcagepant 150 mg, 36.2% for telcagepant 300 mg, and 32.2% for placebo.
Conclusions:
This study confirmed previous findings that telcagepant 300 mg was effective at relieving pain and other migraine symptoms at 2 hours and providing sustained pain freedom up to 24 hours. In this study, telcagepant 150 mg was also effective. Telcagepant was generally well tolerated.
GLOSSARY
- CGRP
= calcitonin gene-related peptide.
Migraine is a common neurologic disorder characterized by episodic, often disabling headache, sensory, autonomic, and cognitive symptoms. The introduction in the 1990s of serotonin (5-HT)1B/1D receptor agonists, the triptans, revolutionized acute therapy for migraine attacks. Triptans are effective in the majority of patients with migraine1 and generally well-tolerated though not in all patients. Because of their potential vasoconstrictive 5-HT1B-mediated effects, triptans are contraindicated in patients with coronary artery disease, cerebrovascular disease, or significant risk factors for either.2 There is a need for well-tolerated migraine-specific medications that are applicable to all patient populations.
Calcitonin gene-related peptide (CGRP) is a neuropeptide with receptors found on meningeal blood vessels, trigeminal ganglion and afferents, the periaqueductal gray and other brainstem nuclei, the amygdala, and the hypothalamus.3 Elevated CGRP levels have been observed in jugular venous blood during some studies of spontaneous and nitroglycerine-provoked migraine attacks,4–6 but not all,7 and IV administration of CGRP can provoke migraine-like attacks in migraineurs.8
CGRP receptor antagonists are not direct vasoconstrictors,9–11 and may thereby confer an advantage over triptans in the acute treatment of migraine. Proof-of-concept for the efficacy and tolerability of olcegepant, an IV CGRP receptor antagonist, in acute migraine has been reported.12 Telcagepant (MK-0974), an oral CGRP receptor antagonist, was effective and well-tolerated for the acute treatment of migraine attacks in a phase 2b adaptive design trial13 and a phase 3 placebo- and active-controlled trial.14 The current study is a placebo-controlled phase 3 trial performed to confirm the efficacy and tolerability of telcagepant in the acute treatment of migraine.
METHODS
Patients.
Patients were eligible for the study if they were ≥18 years of age, had a history of migraine with or without aura for at least 1 year (International Headache Society criteria)15 and had experienced 1 to 8 moderate or severe migraine attacks per month in the 2 months prior to the screening visit, and were in good general health. Patients with a history or clinical evidence of cardiovascular disease or uncontrolled hypertension were excluded. Patients taking migraine preventative medications were allowed to enter the study provided that their prescribed daily dose had not changed during the 3 months prior to screening. Due to potential interactions with telcagepant, patients taking strong CYP3A4 inhibitors or inducers within 1 month of the screening visit were ineligible, and these medications were not permitted during the study.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the ethical review committee for each site and each patient provided written informed consent. The trial was registered at ClinicalTrials.gov (NCT00432237).
Study design.
In this randomized, double-blind, placebo-controlled, parallel-group, outpatient study, patients treated a single migraine attack. The study (Merck Protocol 016) was conducted at 83 sites in the United States, Europe, and Latin America from March 2007 through November 2007. Patients were randomized to telcagepant 50 mg, telcagepant 150 mg, telcagepant 300 mg, or placebo in a 1:2:2:2 ratio. Patients were instructed to administer study medication when they experienced a migraine attack with moderate or severe headache. Patients had the option of taking a blinded optional second dose if they still had moderate or severe headache 2 hours after dosing or if they experienced headache recurrence within 48 hours post-initial dose. Patients randomized to telcagepant 50 mg or placebo as their initial treatment were allocated to receive the same treatment for their optional second dose, while those initially randomized to telcagepant 150 mg or 300 mg were allocated to receive either a repeat of their initial treatment or placebo in an equal ratio. The efficacy of the optional second dose is not discussed since the sample sizes were relatively small. A prespecified meta-analysis across all of the telcagepant phase 3 studies is planned.
Telcagepant and matching placebo were supplied in liquid-filled soft elastic capsules (1 capsule for each dose with matching placebo). Since the telcagepant 300-mg capsule had a different appearance from the 50-mg and 150-mg capsules, each study treatment was packaged using a double-dummy design (e.g., patients assigned to telcagepant 150 mg also received placebo matching telcagepant 300 mg). The formulation of telcagepant used in this study had a tmax of approximately 1–2 hours and a t½ of approximately 5–8 hours. It is anticipated that the final clinical formulation will be a tablet, with similar pharmacokinetic properties to the capsule formulation.
Patients were allocated using a computer-generated randomized allocation schedule prepared by a blinded statistician at Merck, using a block size of 7. Numbered containers were used to implement allocation. Personnel at each study site used a central interactive voice response system to determine which container should be given to which patient. All study personnel, including investigators, study site personnel, patients, and Merck staff, remained blinded to treatment allocation throughout the study. Unblinding took place after data collection was complete.
Procedure.
Study eligibility was assessed at a screening visit where physical examinations, vital signs, laboratory screens, and ECGs were performed. Eligible patients were randomized and provided with study medication. Patients were instructed to take study drug when they experienced a moderate or severe migraine headache. If the patient continued to have a moderate or severe headache 2 hours after dosing, or experienced headache recurrence from 2 to 48 hours after dosing, they could elect to take a blinded optional second dose of study treatment (see above), to take their own rescue medication (e.g., nonsteroidal anti-inflammatory drugs, triptans, opioids, antiemetics), or to take no further treatment.
During the 48 hours following the initial dose of study medication, patients recorded subjective assessments and use of additional medications in a paper diary, as detailed below. Patients also recorded information about any adverse experiences that occurred up to the time they returned to the clinic. Patients were instructed to return to the study site for follow-up within approximately 7 days after treatment with study medication. At this visit, the study site staff reviewed the diary, assessed medication compliance, and conducted safety assessments.
Assessments.
Headache severity was assessed using a 4-grade scale (no pain, mild pain, moderate pain, severe pain) at baseline (0 hour = time of taking study medication) and at 0.5, 1, 1.5, 2, 2.5, and 24 hours postdose. The presence or absence of phonophobia, photophobia, nausea, vomiting, and ratings of functional disability (4-grade scale: normal, mildly impaired, severely impaired, requires bedrest) were recorded at the same time points as the headache severity ratings. For those patients who reported pain relief (reduction of pain to mild or none) or pain freedom (no pain) at 2 hours postdose, the presence or absence of headache worsening (recurrence) within 2–24 hours and 24–48 hours was recorded. Use of rescue medication (including the optional second dose) within 48 hours after the initial dose of study medication was also recorded. Patients also completed the Migraine-Specific Quality-of-Life Questionnaire16 24 hours after the initial dose of study medication.
Tolerability and safety were assessed via spontaneous adverse experience reports and routine prestudy and poststudy physical and laboratory examinations, ECGs, and vital signs.
Statistical analysis.
The five co-primary hypotheses were that at least one of the 150-mg or 300-mg telcagepant doses is superior to placebo, as measured by the percentage of patients at 2 hours reporting: 1) pain freedom; 2) pain relief; 3) absence of photophobia; 4) absence of phonophobia; and 5) absence of nausea. Based on a sample size of 450 patients randomized per treatment group for telcagepant 150 mg, 300 mg, and placebo (assumed to yield 382 evaluable patients per treatment group) and using a 1-sided significance level of 0.025, the study had greater than 90% power to simultaneously demonstrate significance on the five co-primary endpoints. The hypotheses, and hence the power calculation, did not apply to the telcagepant 50-mg dose, which was included in the study to further explore efficacy at the lower end of the dose range and thus had a smaller planned sample size of 225 randomized patients.
Secondary endpoints were as follows: 1) 2- to 24-hour sustained pain freedom (pain free from 2 to 24 hours without the use of rescue medication, including the optional second dose); 2) total migraine freedom at 2 hours (no pain, photophobia, phonophobia, nausea, or vomiting); and 3) 2- to 24-hour total migraine freedom (2- to 24-hour sustained pain freedom with no photophobia, phonophobia, nausea, or vomiting from 2 to 24 hours). The key secondary endpoint was 2- to 24-hour sustained pain freedom.
The full analysis set was the primary population for assessing efficacy. For each co-primary endpoint, the full analysis set included all treated patients who had a baseline headache severity score and at least one postdose measurement occurring at or prior to 2 hours postdose. Non-baseline missing data were imputed using a last observation carried forward approach. Patients were counted in the treatment group to which they were randomized. Patients who took the initial telcagepant dose (50 mg, 150 mg, or 300 mg) were considered to be a part of the same treatment group (50 mg, 150 mg, or 300 mg) regardless of the optional second dose.
The response rates and odds ratios in the telcagepant 150 mg, 300 mg, and placebo groups were estimated using a logistic model with categorical terms for treatment, geographic region (United States, non-United States), and baseline headache severity (moderate or severe), with age included as a continuous covariate. To control for multiplicity, a step down closed testing procedure17 was applied to the five co-primary hypotheses and the key secondary hypothesis, each at a significance level of 0.05. The hierarchy of hypothesis testing, numbered in order of testing, is shown in table e-1 on the Neurology® Web site at www.neurology.org.
Additional prespecified exploratory analyses were performed involving the above measures at additional time points (e.g., pain relief at time points other than 2 hours, 2- to 48-hour sustained pain freedom) or different measures (e.g., functional disability, migraine quality of life). Findings of interest from these analyses are also presented. Since telcagepant 50 mg was primarily included in the study to further explore efficacy at the lower end of the dose range, no formal statistical comparisons between telcagepant 50 mg and placebo were performed.
An exploratory step-down trend test for dose was conducted for the 2-hour pain freedom and pain relief measures using a logistic regression model with categorical terms for geographic region and baseline headache severity, and with dose (0 mg/placebo, 50 mg, 150 mg, 300 mg) and age as continuous variables. The trend test was based on the estimate of the slope coefficient for the dose level variable. All 4 treatment groups were included in the trend test at the first step to evaluate whether there was substantial evidence of a dose-related trend in efficacy across all doses included in this study. To further evaluate the contribution of individual doses to the trend analysis result, a sequential procedure that evaluated progressively smaller subsets of the treatment groups was employed. Specifically, if the test was significant at the 0.05 level, then the test was repeated excluding the top dose and so on in a stepwise fashion until there was only one dose remaining or until nonsignificance was observed.
All patients who were randomized and took study treatment were included in the safety assessment. All adverse experiences reported up to 14 days following study treatment were included. The percentages of patients with any adverse experiences, any serious adverse experiences, and the most commonly occurring adverse experiences were calculated for each treatment group. In order to fully characterize the tolerability profile of telcagepant, a separate analysis was also performed using adverse experiences within the first 48 hours after dosing, on the assumption that those occurring soonest after dosing were most likely to be attributable to study drug.
RESULTS
Patient accounting and demographics.
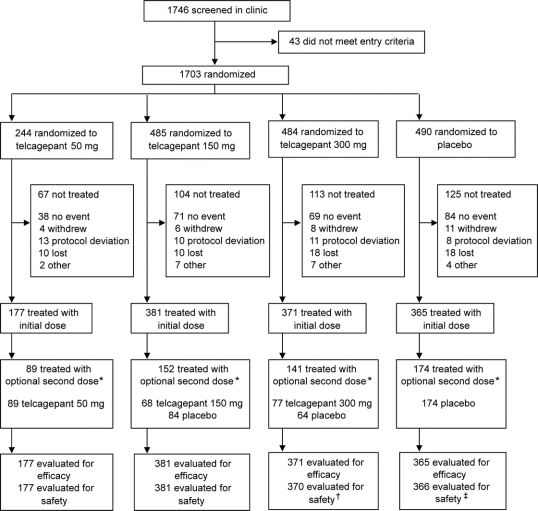
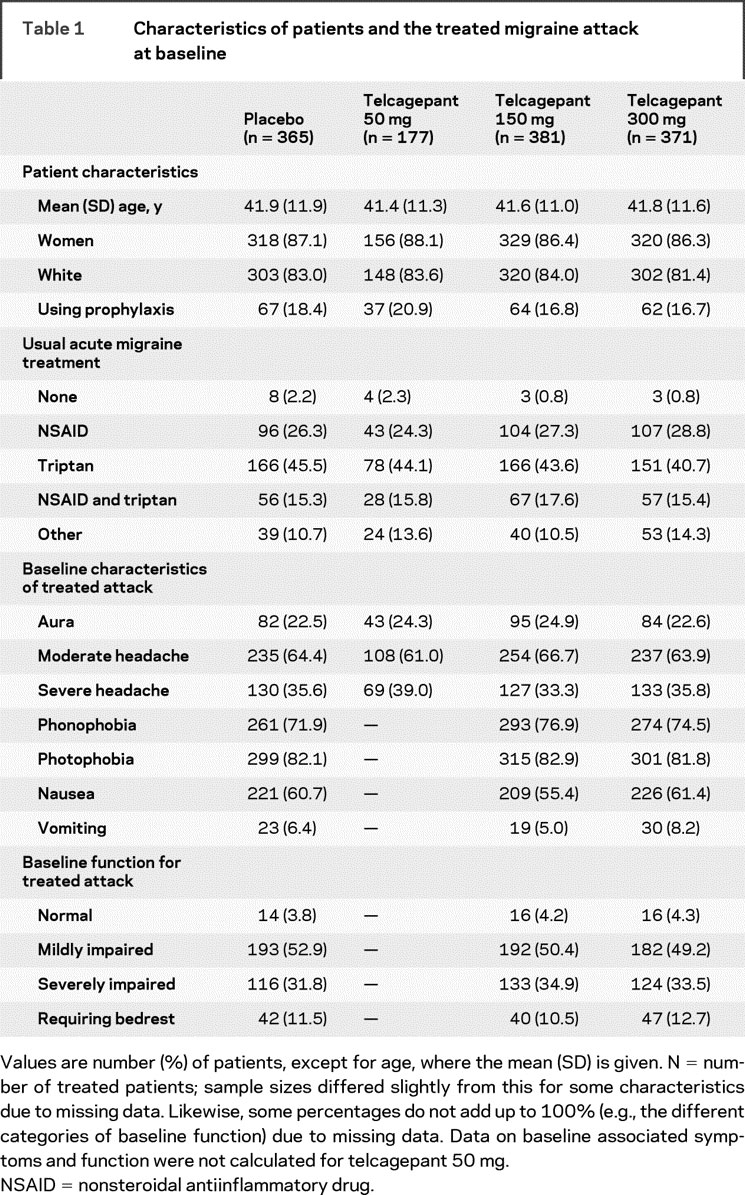
The trial profile is shown in the figure. A total of 1,294 patients were treated. Of these, 562 were from European sites, 169 were from Central and South American sites, and 563 were from sites in the United States. Characteristics of the patients taking treatment and baseline characteristics of their treated migraine attacks are summarized in table 1. The treatment groups had generally similar demographic profiles and baseline attack characteristics.
Figure Trial profile
*Patients had the option of taking a blinded second dose of study drug if the headache was still moderate or severe at 2 hours or for headache recurrence within 48 hours. †One fewer than the number initially treated because a patient randomized to telcagepant 300 mg took her sister’s medication instead (the sister was also in the study and was assigned to placebo). ‡One more than the number initially treated because of the patient randomized to telcagepant 300 mg who took placebo.
Table 1 Characteristics of patients and the treated migraine attack at baseline
Efficacy.
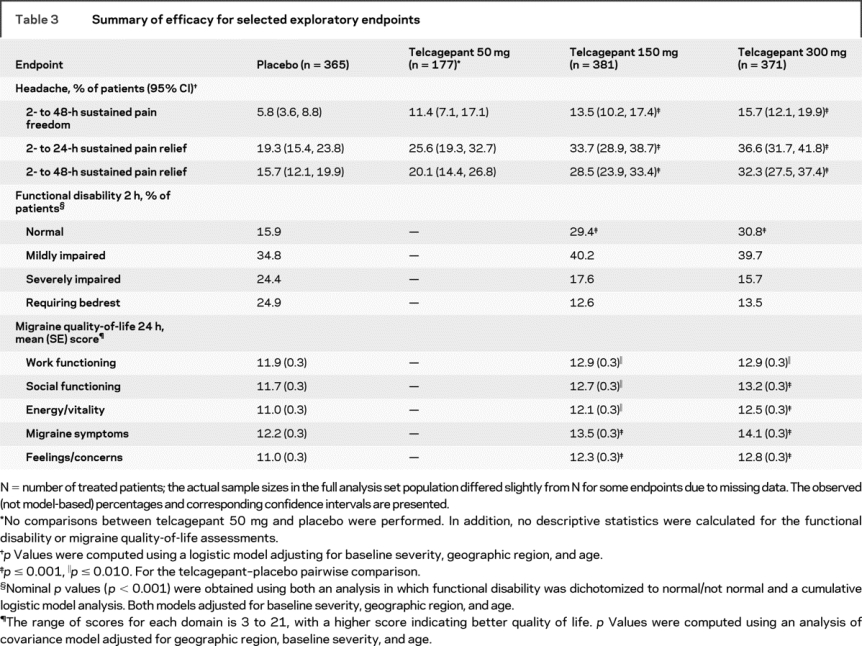
The percentages of responders in each treatment group are shown in table 2 for each of the primary and secondary endpoints. Based on the closed testing procedure and the logistic regression model, both telcagepant 300 mg and 150 mg were superior to placebo on all five co-primary 2-hour endpoints as well as on the key secondary endpoint of 2- to 24-hour sustained pain freedom (table e-1). Although formal statistical testing was not performed for telcagepant 50 mg vs placebo, visual inspection of the observed response proportions suggests that the efficacy of the 50-mg dose was intermediate between placebo and the 2 higher telcagepant doses for all primary and secondary endpoints. A similar pattern of results was observed for the exploratory measures, including 2- to 24-hour sustained pain relief, 2- to 48-hour sustained pain relief and pain freedom, functional disability, and quality of life assessments (table 3).
Table 2 Summary of efficacy for primary and secondary endpoints: Percentage of patients (95% CI)
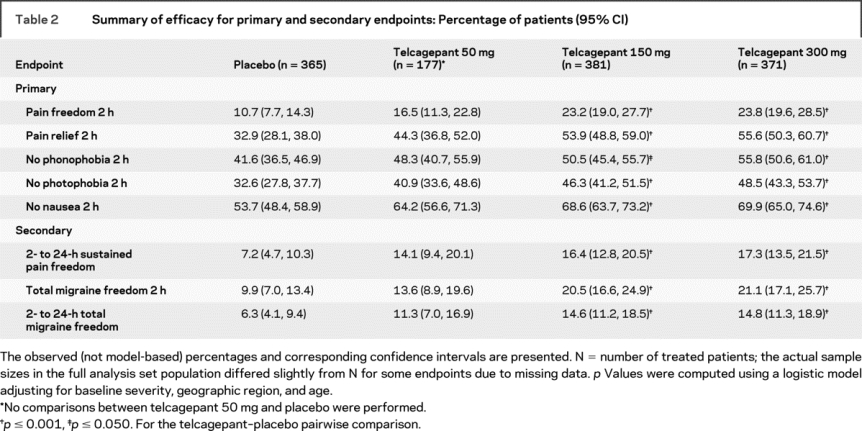
Table 3 Summary of efficacy for selected exploratory endpoints
Telcagepant 300 mg and 150 mg appeared to have similar efficacy, although telcagepant 300 mg tended to be numerically slightly more effective on most measures. Both telcagepant 300 mg and 150 mg appeared numerically more effective than telcagepant 50 mg. The results of the dose-response trend analysis for pain freedom at 2 hours indicated a dose-response relationship over the 0 to 300 mg dose range (p < 0.001) and the 0 to 150 mg dose range (p < 0.01), but not for the 0 to 50 mg dose range (p = 0.06). For pain relief at 2 hours, the dose-response trend analysis indicated a dose-response relationship over the 0 to 300 mg dose range (p < 0.001), the 0 to 150 mg dose range (p < 0.01), and the 0 to 50 mg dose range (p = 0.01).
Tolerability and safety.
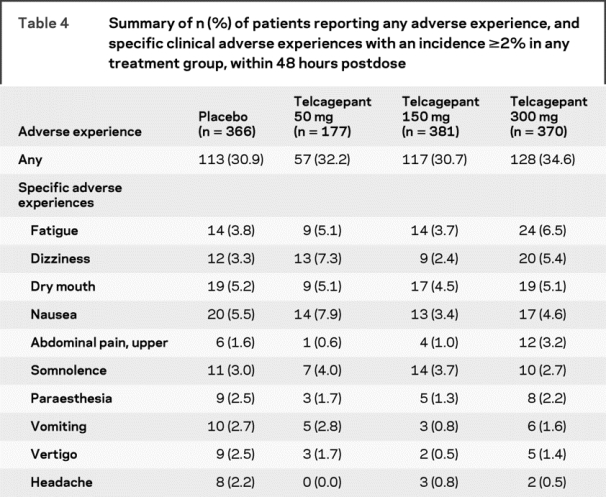
Telcagepant was generally well-tolerated in the acute treatment of migraine. Adverse experiences occurring up to 48 hours after dosing are summarized in table 4. The percentage of patients with adverse experiences was slightly higher for telcagepant 300 mg compared with placebo, although statistical analyses were not performed. Telcagepant 150 mg and 50 mg had adverse experience rates that were similar to placebo. Among the most common specific adverse experiences (incidence ≥2% in at least one of the treatment groups), the following showed a numerical increase compared to placebo for at least one dose of telcagepant: fatigue, dizziness, nausea, upper abdominal pain, somnolence, and vomiting. For telcagepant 300 mg, the most common specific adverse experiences showing a numerical increase compared to placebo were fatigue, dizziness, and upper abdominal pain. The majority of adverse experiences occurred during the first 48 hours after dosing and the tolerability profile in the analysis looking at adverse experiences occurring up to 14 days after dosing (table e-2) was very similar to that described above for adverse experiences occurring within 48 hours. No patients died during the study. There were 2 serious adverse experiences: 1 in a patient who received placebo (closed head injury) and 1 in a patient who received telcagepant 150 mg (hypertension). The serious adverse experience of hypertension occurred at the posttreatment clinic visit 4 days after administration of telcagepant 150 mg. The patient experienced a migraine attack and blood pressure elevation (170/106). The patient took eletriptan and experienced pain resolution after 2 hours but blood pressure remained elevated (180/120) and she was admitted for observation. The patient had a previous medical history of hypertension which was diagnosed 3 years before but was not active at the time of study enrollment. Neither serious adverse experience was considered to be related to study drug by the investigator (determination made while blinded to treatment).
Table 4 Summary of n (%) of patients reporting any adverse experience, and specific clinical adverse experiences with an incidence ≥2% in any treatment group, within 48 hours postdose
Laboratory abnormalities during the study were uncommon and no clinically relevant differences were seen between treatment groups. Other assessments, including the percentage of patients who exceeded predefined levels of change on laboratory parameters, vital sign measurements, ECG measurements, and physical examinations, indicated no clinically meaningful differences between treatment groups.
DISCUSSION
This large phase 3 study supports the hypothesis that CGRP plays an important role in migraine pathogenesis and that oral CGRP receptor antagonists such as telcagepant are effective in treating migraine symptoms of headache, photophobia, phonophobia, and nausea, with tolerability similar to placebo. In patients who treated a single migraine attack, doses of 150 or 300 mg of telcagepant were significantly more effective than placebo on all primary and secondary endpoints. Telcagepant 300 mg and 150 mg appeared to have similar efficacy, although telcagepant 300 mg tended to be slightly more effective on most measures. The 50 mg dose was not statistically tested but demonstrated numerical effects that were intermediate between placebo and telcagepant 150 mg and 300 mg.
The results of the present study are in general agreement with the previous phase 3 trial comparing 150 and 300 mg telcagepant with placebo or 5 mg zolmitriptan.14 Efficacy rates for 2-hour pain freedom following telcagepant 300 mg were comparable in the 2 studies (23.8% in the current study and 26.9% in the previous study). The efficacy of 150 mg appeared more similar to 300 mg in the present study than in the previous study, but no statistical testing between these 2 telcagepant doses was performed in either study.
In agreement with the phase 2 study13 and the previous phase 3 study,14 telcagepant was well-tolerated with an adverse experience profile similar to that of placebo. Specific adverse experiences which were slightly more common for telcagepant 300 mg than placebo in this study were fatigue, dizziness, and upper abdominal pain. No clinically relevant changes in laboratory measures, including transaminase levels, were seen although these were assessed up to 7 days or more after dosing.
The strength of the present study is the large sample size of patients who typify the migraine study population with baseline characteristics well-balanced across treatment groups. Another strength is the wide range of primary and secondary outcome parameters which were prespecified in the analysis and selected exploratory endpoints which included functional disability and quality of life outcomes. Functional outcomes clearly showed decreased impairment and improved quality of life scores with telcagepant over placebo.
Although telcagepant is not a direct vasoconstrictor and in principle is therefore safe for use in patients with known vascular disease, such patients were excluded from the current trial. The exclusion was made under guidance from the Food and Drug Administration pending the availability of phase 1 safety data in patients with cardiovascular disease. A dedicated phase 3 outpatient study in patients with both migraine and stable cardiovascular disease is ongoing to help guide the appropriate use of telcagepant in this population (NCT00662818). Additional studies to investigate the long-term efficacy, tolerability, and consistency of response for telcagepant are in progress (NCT00443209 and NCT00483704).
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by James Kost, Xiaoyin Fan, and Christopher Assaid from Merck Research Laboratories.
DISCLOSURE
This study was funded by Merck Research Laboratories. The funding organization was involved in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Dr. Connor is an employee of, and owns stock and holds stock options in, Merck & Co., Inc. Dr. Shapiro serves/has served on scientific advisory boards for, and received honoraria and funding for travel from, Merck & Co, Inc., MAP Pharmaceuticals, and NuPathe, and has received research support from Merck & Co, Inc. and the NIH [NHLBI R01HL71944 (coinvestigator)]. Dr. Diener has received honoraria for participation in clinical trials, contribution to advisory boards, or lectures from Addex Pharma, Allergan, Almirall, AstraZeneca, Bayer Vital, Berlin Chemie, Coherex Medical, CoLucid, Böhringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Grünenthal, Janssen-Cilag, Lilly, La Roche, 3M Medica, Minster, MSD, Novartis, Johnson & Johnson, Pierre Fabre, Pfizer, Schaper and Brümmer, SanofiAventis, and Weber & Weber; and has received research support from Allergan, Almirall, AstraZeneca, Bayer, GSK, Janssen-Cilag, and Pfizer. Headache research at the Department of Neurology in Essen is supported by the German Research Council (DFG), the German Ministry of Education and Research (BMBF), and the European Union. Dr. Lucas has served on scientific advisory boards for GlaxoSmithKline, Merck & Co. Inc., and Bayer (formerly Berlex); has received honoraria from Allergan, GlaxoSmithKline, Merck & Co. Inc., EMD Serono, Biogen Idec, Pfizer, Endo, OMP, Teva Neuroscience, the National Multiple Sclerosis Society, and Headache Cooperative of the Pacific; has served on the speaker’s bureaus of GlaxoSmithKline, Merck & Co. Inc., EMD Serono, Biogen Idec, and Pfizer; and has received research support from GlaxoSmithKline, Merck & Co. Inc., Allergan, Nupathe, MAP, Biogen Idec, Sanofi-Aventis, and AGA. Dr. Kost, Dr. Fan, Dr. Fei, Dr. Assaid, Dr. Lines, and Dr. Ho are employees of, and own stock and hold stock options in, Merck & Co., Inc.
Supplementary Material
APPENDIX
The investigators who participated in the study are as follows: Austria: Werner Poewe, University Klinik fur Neurologie Innsbruck; Susanne Groblschegg, ClinPharm International GmbH, Wien; Christian Wober, AKH Wien–Neurologie, Wien. Colombia: Carlos Santiago Uribe, Fundacion Centro de Investigacion Clinica CIC, Medellin, Antioquia; Nhora Patricia Ruiz, Fundacion Cardiovascular de Colombia, Bucaramanga, Santander; Jimmy Crump, Consultorio Dr. J. Crump, Barranquilla, Atlantico; Marcela De la Ossa, IPS CAS CAFAM Floresta, Bogota, Cundinamarca; Ignacio Salgado, Clinica del Country, Centro de Investigacion, Bogota, Cundinamarca. France: Dominique Valade, Hopital Lariboisiere–Unite, Paris; Vincent Cahagne, Hôpital Pontchaillou, Rennes; Gerard Mick, Centre Hospitalier–Reseau, Voiron; Claire Le Jeunne, Hôpital Hotel Dieu, Paris; Gilles Geraud, CHU Toulouse–Hôpital de Rangueil, Toulouse cedex 9; Virginie Dousset, Hôpital Pellegrin, Bordeaux cedex; Christian Lucas, CHRU Hôpital Roger Salengro, Lille cedex; Michel Lanteri-Minet, CHU Pasteur, Nice. Germany: Bettina Bergtholdt, emovis GmbH, Berlin; Hans Christoph Diener, Neurologische Uni-Klinik, Essen; Uwe Reuter, Charite Universitatsmedizin Berlin; Stefan Evers, Westf. Wilhelms-Universitat Munster; Hartmut Goebel, Neurolog-Verhaltensmed, Kiel; Jan Peter Jansen, Schmerzzentrum Berlin; Arne May, Universitatsklinikum Eppendorf, Hamburg; Wolfgang Molt, Praxis fur Neurologie und Stuttgart; Harald Braun, Schmerz- und Palliativzentrum, Wiesbaden; Claudio Padovan, Praxis Claudio Padovan, Munchen; Volker Pfaffenrath, Praxis Dr. Pfaffenrath, Munchen; Joachim Drews, Praxis Dr. Drews, Munchen; Gunther, Schumann, Praxis fur Neurologie und Psychiatrie, Bochum; Karl Otto Sigel, Praxis Dr. Sigel, Unterhaching; Hans Detlev Stahl, ClinPharm International GmbH & Co, Leipzig. Israel: Amnon Mosek, Sourasky Medical Center, Tel Aviv; Rachel Hering, Meir Medical Center, Sfar Saba; Yaron River, Bnzi Zion Medical Center, Haifa; Arieh Kuritzky, Rabin Medical Center, Petah Tikva; Gal Ifergan, Soroka Medical Center, Beer Sheva. Mexico: Rafael Villalobos, Hospital Neustra Senora de la Salud, San Luis Potosi; Guillermo Garcia Ramos, Medica Sur Cif-Biotec, Mexico; Ildefonso Rodriguez, Centro Medico del Potosi, S.L.P.; Alejandro Marfil, Hospital Universitario de Monterrey, Nuevo Leon. Norway: Aud Nome Dueland, Sandvika Nevrosenter AS, Sandvika; Anne Christine Poole, Sjolyst Medisinske Senter, Oslo; Vidar Moldegard, Gjovik Iegesenter, Gjovik; Lars Jacob Stovner, St. Olavs Hospital HF, Trondheim, Marit Gronning, Haukeland universitetssykehus HF, Bergen; Paal Dag Norheim, Forus Akutten AS, Sandnes; Tadeusz Tomala, Svelvik Legesenter, Svelvik. United States: Fares J. Arguello, Radiant Research, Inc., Salt Lake City, UT; John E. Castaldo, Lehigh Valley Hospital, Allentown, PA; Angel R. Chinea, Centro Neurologico, Guaynabo, PR; Gregory V. Collins, Charlotte Clinical Research, Charlotte, NC; Stephen E. Daniela, SCIREX Corporation, Austin, TX; Matthew G. Davis, Rochester Clinical Research, Inc., Rochester, NY; Joel D. Eade, Internal Medicine Associates, Campbellsville, KY: Eric Eross/Troy Anderson, Dedicated Clinical Research, Litchfield Park, AZ; Mildred V. Farmer, Meridien Research, St. Petersburg, FL; Brian J. Feaver, R/D Clinical Research, Inc., Lake Jackson, TX; Stuart W. Fox, The Neuro Science Center of Northern New Jersey, Morristown; Larry I. Gilderman, University Clinical Research, Inc., Pembroke Pines, FL; Daniel B. Groblewski, Jacksonville Center for Clinical Research, Jacksonville, FL; Daniel E. Grosz, Pharmacology Research Institute, Encino, CA; Paul A. Hartley, Preferred Primary Care Physicians Associates, Uniontown, PA; Bruce D. Kohrman, Miami Research Associates, South Miami, FL; Richard A. Drause, ClinSearch, Chattanooga, TN; David B. Kudrow, CA Medical Clinic for Headache, Santa Monica, CA; Robert S. Kunkel, Health Research Associates, Cleveland, OH; Sylvia Lucas, University of Washington, Seattle; Dennis C. McCluskey, Radiant Research, Mogadore, OH; Francis E. McGee, Richmond, VA; Laszlo L. Mechtler, Dent Neurologic Institute, Amherst, NY; Alberto Odio, Alta California Medical Group, Inc., Simi Valley, CA; Terry L. Poling, Heartland Research Associates, LLC, Wichita, KS; Antoinette A. Pragalos, Community Research, Cincinnati, OH; Neil Pugach, Brighton Research Group, LLC, Virginia Beach, VA; James R. Shoemaker, Ormond Medical Arts Pharmaceutical Research Center, Ormond Beach, FL; Larry S. Seidman, Philadelphia Clinical Research, LLC, Philadelphia, PA; Robert E. Shapiro, Fletcher Allen Health Care, Burlington, VT; Thomas Shiovitz, California Neuroscience Research, Sherman Oaks; Michael K. Sowell, University of Louisville Kentucky Neuroscience Research; Stuart R. Stark, The Innovative Clinical Research Center, Alexandria, VA; Michael W. Warren, Oyster Point Family Health Center, Lancaster, PA; John Gregory Willis, Georgetown Medical Center Clinic, Georgetown, TX; Alberto R. Yataco, International Research Center, Towson, MD.
Address correspondence and reprint requests to Dr. Tony Ho, Merck Research Laboratories, UG 4C-18, PO Box 1000, North Wales, PA 19454-1099 tony_ho@merck.com
Supplemental data at www.neurology.org
Disclosure: Author disclosures are provided at the end of the article.
Presented at the European Headache and Migraine Trust International Congress, London, UK, September 4–7, 2008.
Received January 13, 2009. Accepted in final form June 29, 2009.
REFERENCES
- 1.Diener HC, Limmroth V. Advances in pharmacological treatment of migraine. Exp Opin Invest Drugs 2001;10:1831–1845. [DOI] [PubMed] [Google Scholar]
- 2.Dodick D, Lipton RB, Martin V, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT1B/1D agonists) in the acute treatment of migraine. Headache 2004;44:414–425. [DOI] [PubMed] [Google Scholar]
- 3.Arulmani U, MaassenVanDenBrink A, Villalon CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharm 2004;500:315–330. [DOI] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine attacks. Ann Neurol 1990;28:183–187. [DOI] [PubMed] [Google Scholar]
- 5.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993;33:48–56. [DOI] [PubMed] [Google Scholar]
- 6.Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol 2005;58:561–563. [DOI] [PubMed] [Google Scholar]
- 7.Gallai V, Sarchielli P, Floridi A, et al. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both ictally and interictally. Cephalalgia 1995;15:384–390. [DOI] [PubMed] [Google Scholar]
- 8.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 2002;22:54–61. [DOI] [PubMed] [Google Scholar]
- 9.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 2004;84:903–934. [DOI] [PubMed] [Google Scholar]
- 10.Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilation. Journal of Clinical Pharmacology and Therapeutics 2005;77:202–213. [DOI] [PubMed] [Google Scholar]
- 11.Petersen KA, Birk S, Lassen LH, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers Cephalalgia 2005;25:139–147. [DOI] [PubMed] [Google Scholar]
- 12.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 2004;350:1104–1110. [DOI] [PubMed] [Google Scholar]
- 13.Ho TW, Mannix LK, Fan X, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine Neurology 2008;70:1304–1312. [DOI] [PubMed] [Google Scholar]
- 14.Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 2008;372:2115–2123. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24:9–160. [DOI] [PubMed] [Google Scholar]
- 16.Santanello NC, Hartmaier SL, Epstein RS, Silberstein SD. Validation of a new quality of life questionnaire for acute migraine headache. Headache 1995;35:330–337. [DOI] [PubMed] [Google Scholar]
- 17.Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika 1976;63:655–660. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.