Channelopathies are mendelian disorders arising from mutations in genes that encode ion channel subunits.1 Mutations in SCN4A, which encodes the α-subunit of the muscle sodium channel Nav1.4, underlie paramyotonia congenita (PMC),2 while mutations in KCNA1, which encodes the potassium channel subunit Kv1.1, cause episodic ataxia type 1 (EA1) and isolated myokymia.3 We describe the clinical, genetic, and functional expression studies in an unusual patient who harbors mutations in both SCN4A and KCNA1 and report that sodium channel dysfunction dominates the phenotype.
Case history.
A 34-year-old woman had a lifelong history of muscle cramps and weakness affecting upper and lower limbs. Symptoms were exacerbated by exercise and cold weather, which induced both muscle stiffness and difficulty releasing her grip. Her father, paternal uncle, and second son are similarly affected. Examination demonstrated weakness of intrinsic hand muscles (Medical Research Council 4/5) with paradoxical myotonia of hand grip and eye closure.
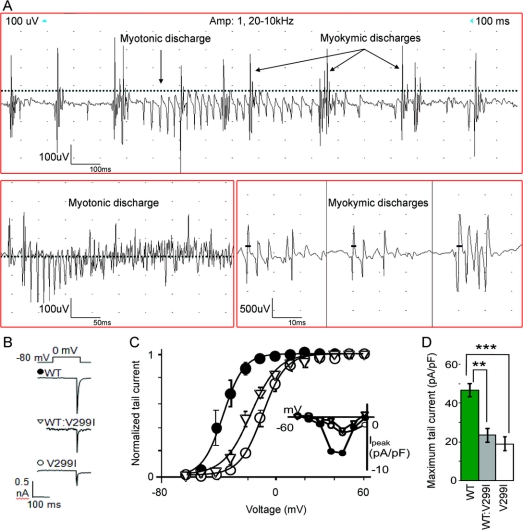
Neurophysiology confirmed a diagnosis of PMC, with a cooling test showing a rapid decrement in amplitude and area, which did not recover with rewarming. A McManis test was positive, showing a >40% decrement in compound muscle action potential (CMAP) during exercise with recovery during the prolonged rest period. Unexpectedly, needle EMG demonstrated sustained doublets, triplets, and multiplets interspersed with the myotonic discharges in the right abductor digiti minimi, biceps, tibialis anterior, and thoracic paraspinals (figure A). Because generalized myokymic discharges are not a recognized feature of PMC, this prompted further direct questioning about her symptoms. She reported that since the age of 16, she had experienced brief attacks of slurred speech and unsteadiness lasting seconds to minutes. Emotional stress was a trigger for these episodes, which the patient dismissed as “panic attacks.” Reexamination on a separate occasion confirmed fine lateral movements of her fingers suggestive of myokymia. This raised the possibility of a clinical diagnosis of EA1 and PMC, with the latter dominating the phenotype.
Figure Clinical neurophysiology and functional studies on the V299I mutation identified in the patient
(A) Myokymic discharges intermixed with myotonic discharges. (B) Representative current traces of HEK cells expressing Kv1.1 WT, WT:V299I, and V299I obtained by applying a voltage step from a holding potential of −80 mV to 0 mV. Low potassium internal solution (in mM: KCl, 2.5; NaCl, 140; HEPES, 10; and EGTA, 10, pH = 7.3) was used to generate tail currents for assessing current density (see e-Methods). (C) Normalized voltage dependence of activation for cell expressing WT, mutant V299I, and WT: V299I (n = 5 for each). Tail currents were sampled 1 msec after return to −80 mV. Error bars are SEM. Inset: Peak potassium currents from representative cells expressing WT, V299I, and WT: V299I. (D) Maximum tail current density measured at +60 mV for WT, V299I, and WT: V299I. Error bars are SEM.
Genetic results.
Sequencing SCN4A revealed the functionally characterized point mutation T1313M, confirming the diagnosis of PMC. T1313M segregated with affected members in her family. Sequencing KCNA1 identified a heterozygous G to A change at nucleotide position 895, which predicts a valine to isoleucine substitution at the highly conserved codon 299 in the fourth transmembrane segment of the alpha subunit. The V299I mutation has not been reported previously and was absent in a large panel of over 200 control chromosomes. The mutation was absent in the patient’s father. It was not possible to obtain DNA from the mother but she did not have EA1 clinically.
Electrophysiology.
The V229I mutation conferred a significant positive shift in the V1/2Max of the voltage dependence of activation of Kv1.1 channels (figure, B and C) when expressed alone (V299I: +26.9 mV, p < 0.0001) and also when coexpressed with WT channels (coexpression: +18.8 mV p < 0.0001; WT alone: −35.3 ± 0.07, n = 5). Cells expressing mutant V299I channels also had markedly reduced maximal potassium current density, as measured from tail currents, compared with cells expressing WT (V299I: 19.3 ± 3.5 pA/pF, n = 5; WT: 47.2 ± 3.4 pA/pF, n = 5, p = 0.0005) (e-Methods on the Neurology® Web site at www.neurology.org). When V299I and WT subunits were coexpressed, the current density was similar to V299I alone (figure, D), and significantly lower than WT alone (23.6 ± 3.9 pA/pF, n = 5, p = 0.002). These findings are consistent with a dominant-negative effect of the mutation.
Discussion.
This patient has 2 genetically confirmed diagnoses: PMC and EA1. Although some EA1 mutations cause subtle effects on Kv1.1 function,4 the new mutation identified here caused moderately severe loss of function. We have previously reported an imperfect correlation between the decrease in current density and clinical severity of different EA1 mutations.5 In this patient, despite quite a severe loss of Kv1.1 function, the attacks were mild and the PMC symptoms dominated the clinical picture.
EA1 patients do not always complain of unsteadiness or imbalance but rather dizziness or anxiety. It is important to recognize the varied nature of patient descriptions in order to avoid missing the diagnosis of EA1. The coexistence of two ion channelopathies in this patient raises the possibility of interplay. Interaction between ion channel genes has been studied by combining two epilepsy-associated channel mutations in mice, which resulted in negation of the effects of each.6 Here, one might predict that the myokymia would trigger muscle myotonia through repetitive firing and exacerbate the muscle symptoms. However, the PMC phenotype was no worse than in other patients with the T1313M mutation alone.7 The mechanisms of possible interactions between these 2 mutant channels are likely to be complex. In this double mutant case, PMC was the dominant clinical phenotype.
Supplementary Material
Supplemental data at www.neurology.org
Supported by the Medical Research Council UK through an MRC Centre grant (G0601943) and by the National Center for Research Resources (grant 5U54 RR019498-05) through the NIH–Consortium for Clinical Investigation of Neurologic Channelopathies. S.R. holds a Wellcome Trust training fellowship. This work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme.
Disclosure: Dr. Rajakulendran receives research support from a Wellcome Trust Research Training Fellowship. Dr. Tomlinson receives research support from the British Medical Association (Vera Down Fellowship), the Charity Aid Foundation (Patrick Berthoud Fellowship), and the UK Brain Research Trust. Dr. Kullmann receives research support from the Wellcome Trust, Action Medical Research, the Myasthenia Gravis Association, and the Medical Research Council, and serves as an Associate Editor for Brain and a clinical editor for the Journal of Physiology. Dr. Schorge receives salary funding from a charitable contribution from the Worshipful Company of Pewterers. Dr. Hanna receives research support from the Medical Research Council and serves as Deputy Editor of the Journal of Neurology, Neurosurgery & Psychiatry. Drs. Tan, Matthews, Labrum, and Sud report no disclosures.
Received January 14, 2009. Accepted in final form April 24, 2009.
Address correspondence and reprint requests to Prof. M.G. Hanna, Medical Research Council Centre for Neuromuscular Diseases, Department of Molecular Neuroscience, Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London, WC1N 3BG, UK; m.hanna@ion.ucl.ac.uk
&NA;
- 1.Hanna MG. Genetic neurological channelopathies. Nat Clin Pract Neurol 2006;2:252–263. Review. [DOI] [PubMed]
- 2.Ptacek LJ, George AL, Jr., Barchi RL, et al. Mutations in an S4 segment of the adult skeletal muscle sodium channel cause paramyotonia congenita. Neuron 1992;8:891–897. [DOI] [PubMed] [Google Scholar]
- 3.Browne DL, Gancher ST, Nutt JG, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet 1994;8:136–140. [DOI] [PubMed] [Google Scholar]
- 4.Rajakulendran S, Schorge S, Kullmann DM, Hanna MG. Episodic ataxia type 1: a neuronal potassium channelopathy. NeuroRx 2007;4:258–266. Review. [DOI] [PubMed]
- 5.Eunson LH, Rea R, Zuberi SM, et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol 2000;48:647–656. [PubMed] [Google Scholar]
- 6.Glasscock E, Qian J, Yoo JW, Noebels JL. Masking epilepsy by combining two epilepsy genes. Nat Neurosci 2007;10:1554–1558. [DOI] [PubMed] [Google Scholar]
- 7.Matthews E, Tan SV, Fialho D, et al. What causes paramyotonia in the United Kingdom? Common and new SCN4A mutations revealed. Neurology 2008;70:50–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.