Abstract
The target of rapamycin (TOR) protein is a conserved regulator of ribosome biogenesis, an important process for cell growth and proliferation. However, how TOR is involved remains poorly understood. In this study, we find that rapamycin and nutrient starvation, conditions inhibiting TOR, lead to significant nucleolar size reduction in both yeast and mammalian cells. In yeast, this morphological change is accompanied by release of RNA polymerase I (Pol I) from the nucleolus and inhibition of ribosomal DNA (rDNA) transcription. We also present evidence that TOR regulates association of Rpd3–Sin3 histone deacetylase (HDAC) with rDNA chromatin, leading to site-specific deacetylation of histone H4. Moreover, histone H4 hypoacetylation mutations cause nucleolar size reduction and Pol I delocalization, while rpd3Δ and histone H4 hyperacetylation mutations block the nucleolar changes as a result of TOR inhibition. Taken together, our results suggest a chromatin-mediated mechanism by which TOR modulates nucleolar structure, RNA Pol I localization and rRNA gene expression in response to nutrient availability.
Keywords: histone deacetylase/nucleolus/rDNA/RNA polymerase I/target of rapamycin
Introduction
Control of cell growth and proliferation requires that cells rapidly change their protein biosynthetic capacity in response to nutrients and mitogens as well as stressful conditions. Modulation of protein synthesis involves coordinated changes in both the rate of translational initiation and the abundance of ribosomes. Ribosome concentration fluctuates in response to growth conditions (Venema and Tollervey, 1999; Warner, 1999; Leary and Huang, 2001; Fatica and Tollervey, 2002; Moss and Stefanovsky, 2002; Peculis, 2002; Grummt, 2003). When cells are growing under favorable conditions, a high ribosome concentration is needed to meet the demand for protein synthesis. However, ribosome biogenesis is a high energy- and nutrient-consuming process. In yeast, for instance, several hundred genes participate in the production of ribosomes, involving all three RNA polymerases and accounting for a major portion of the total cellular biosynthetic output (Venema and Tollervey, 1999; Warner, 1999; Leary and Huang, 2001; Fatica and Tollervey, 2002; Moss and Stefanovsky, 2002; Peculis, 2002; Grummt, 2003). Ribosomal DNA (rDNA) transcription is an important initial step for ribosome biogenesis, producing rRNA products that represent 60% of total yeast transcripts during normal growth. To conserve resources, cells must limit the production of new ribosomes during nutrient starvation. On the other hand, deregulation of ribosome biogenesis has been implicated in uncontrolled growth and tumorigenesis in mammalian cells (Ruggero and Pandolfi, 2003).
The nucleolus is the site of rDNA transcription by RNA polymerase I (Pol I), rRNA maturation and assembly of ribosomes (Scheer and Hock, 1999). There are ∼150 tandem copies of rDNA genes in yeast. In addition to the components of Pol I and rDNAs, the nucleolus contains many other nucleolar proteins and small nucleolar RNAs (snoRNAs) required for ribosome biogenesis. The nucleolus occupies a discrete subnuclear region. In budding yeast, the nucleolus can be seen by indirect immunofluorescence (IF) microscopy with antibodies against nucleolar proteins such as fibrillarin. Additionally, it can be detected by fluorescence in situ hybridization (FISH) with rDNA probes or by electron density images captured by electron microscopy (EM). The yeast nucleolus normally appears as a single crescent shaped region, occupying about one third of the nucleus along the nuclear envelope. In contrast, mammalian nucleoli typically appear as several large, discrete foci per nucleus. There is also increasing evidence suggesting that the nucleolus is involved in other cellular processes such as longevity, mitotic entry and tumor surveillance (Guarente and Kenyon, 2000; Olson et al., 2000; Visintin and Amon, 2000).
Rapamycin is an antibiotic clinically used for organ transplantation and restenosis prevention. Rapamycin analogs (CCI779 and RAD001) are also undergoing cancer clinical trials. Rapamycin is a highly specific inhibitor of TOR, the target of rapamycin, protein. Mutations at a conserved serine residue of the FKBP12-rapamycin-binding domain, disrupt the binding of rapamycin to TOR and confer dominant rapamycin resistance (Zheng et al., 1995). TOR is a phosphatidylinositol kinase-related kinase highly conserved in all eukaryotes (Hunter, 1995; Keith and Schreiber, 1995). Tor1 and Tor2 are the yeast targets of rapamycin. Like their orthologs in higher eukaryotes, Tor1/2 are key mediators of nutrient signal transduction to control cell growth (Dennis et al., 1999; Kuruvilla and Schreiber, 1999; Schmelzle and Hall, 2000; Raught et al., 2001; Rohde et al., 2001; Abraham, 2002). Though Tor1/2 redundantly regulate cell growth in a rapamycin-sensitive manner, Tor2 also has a unique function that regulates actin polarization in a rapamycin-insensitive manner (Zheng et al., 1995; Helliwell et al., 1998). While TOR proteins are well known translational regulators (Dennis et al., 1999; Kuruvilla and Schreiber, 1999; Schmelzle and Hall, 2000; Raught et al., 2001; Rohde et al., 2001; Abraham, 2002), they are implicated in several other cellular processes as well. For example, Tor1/2 physically interact with the GATA-type transcription factor Gln3 (Bertram et al., 2000). Nutrient starvation or inhibition of TOR by rapamycin causes rapid dephosphorylation and nuclear accumulation of Gln3 (Beck and Hall, 1999; Bertram et al., 2000), leading to expression of many starvation-responsive genes (Cardenas et al., 1999; Hardwick et al., 1999; Bertram et al., 2000; Shamji et al., 2000; Chan et al., 2001).
TOR has recently been shown as a conserved regulator of rDNA transcription. Rapamycin treatment rapidly inhibits rDNA transcription in both mammalian and yeast cells (Mahajan, 1994; Leicht et al., 1996; Zaragoza et al., 1998; Powers and Walter, 1999). The finding that TOR positively regulates both biosynthesis and activity of the translation machinery underscores the central role of TOR in cell growth control. In this study, we show that rapamycin treatment and nutrient starvation, conditions that inhibit TOR signaling, cause a rapid increase in the binding of Rpd3 to rDNA chromatin, leading to deacetylation of histone H4 at K5,12 in the same chromatin region. Mutational analysis indicates that Rpd3-dependent H4 deacetylation is a critical step in the regulation of nucleolar structure, Pol I localization and rDNA transcription. Taken together, our data suggest a chromatin-mediated mechanism of controlling nucleolar structure and function by TOR in response to nutrient conditions.
Results
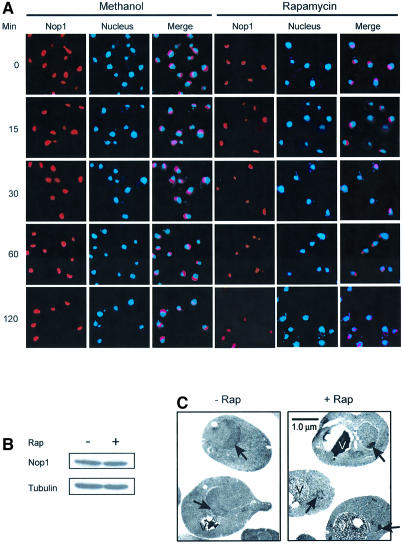
The nucleolus is a dynamic structure that can be altered by stress or mutations. We have investigated a possible role of TOR in nucleolar structural organization by IF staining of Nop1, the yeast fibrillarin. Exponentially growing yeast cells have a large crescent shaped nucleolus localized at the periphery of the nucleus (Figure 1A). When rapamycin was added, however, nucleolar size was rapidly reduced (Figure 1A). In addition, the nucleoli underwent dramatic morphological change, becoming granular in shape (Figure 1A). Nop1 protein level remained the same during rapamycin treatment (Figure 1B), suggesting that rapamycin treatment leads to nucleolar reorganization. This conclusion was further supported by EM analysis. EM pictures showed that rapamycin changed the nucleolus from a crescent shape to a much smaller granular structure with higher electron density (Figure 1C). A small percentage of rapamycin-treated cells also showed dense fibrillar structures (Figure 1C). Additionally, the vacuoles were dramatically enlarged by rapamycin as observed previously (Figure 1C and Chan et al., 2001). In contrast, no significant morphological change was observed with the nucleus as judged by DAPI staining and EM (Figure 1; data not shown).
Fig. 1. TOR regulates nucleolar structures. (A) Rapamycin causes rapid reorganization of nucleolar structures. Exponentially growing wild-type cells (FM391) were treated with rapamycin for different times. The drug carrier methanol was used as a control. Nucleolar structures are visualized by IF with a Nop1 antibody (red). The yeast nuclei were stained with DAPI (blue). (B) Rapamycin does not affect Nop1 protein level. Exponentially growing yeast cells (FM391) were treated with rapamycin for 1 h. Nop1 protein was determined by western blot. Tubulin was used as a loading control. (C) EM analysis of nucleolar structures before and after rapamycin treatment. Exponentially growing yeast cells (FM391) were treated with rapamycin for 1 h and used for EM analysis. The arrowhead indicates the yeast nucleolus. V, vacuole. (D) The effect of rapamycin on nucleolar structure is due to inhibition of TOR. The yeast strain carrying a wild-type TOR1 (SZy998) or a dominant rapamycin-resistant TOR1 allele (TOR1-RR) (SZy997) was treated with rapamycin for 1 h. The morphology and structure of the yeast nucleolus was examined by IF with a Nop1 antibody. (E) Inhibition of protein synthesis is insufficient to cause nucleolar reorganization. Wild-type yeast cells (FM391) were treated with cycloheximide (CHX) for 1 h. Nucleolar structure was analyzed as for (D). (F) Inhibition of rDNA transcription is not sufficient to change nucleolar morphology. Wild-type and rrn3-1 temperature-sensitive mutant (YCC95) strains were switched from permissive temperature (25°C) to restrictive temperature (38°C) for 1 h. Nucleolar structure was determined as for (D).
The rapamycin effect was due to inhibition of TOR, since rapamycin failed to elicit significant nucleolar change in the presence of a dominant rapamycin-resistant TOR allele (TOR1-RR; Figure 1D). The effect of rapamycin on nucleolar structure was independent of yeast genetic background since several other strains (e.g. W303) showed essentially the same response to rapamycin (data not shown). TOR is required for protein synthesis and rDNA transcription, and rapamycin treatment inhibits ribosome biogenesis and protein synthesis. A simple explanation for rapamycin-induced nucleolar morphology change is inhibition of TOR signal transduction to rDNA transcription. One caveat in this interpretation is that nucleolar reorganization is caused by an indirect effect of protein synthesis/ribosome biogenesis inhibition. To address this issue, we incubated yeast with cycloheximide, a general inhibitor of protein synthesis. Treatment with cycloheximide at a concentration that blocks protein synthesis (data not shown and Choi et al., 2000), did not significantly affect nucleolar morphology (Figure 1E). Since Rrn3 is an RNA Pol I transcription factor essential for rDNA transcription, we used the rrn3-1/syc1-8 temperature-sensitive mutant (Cadwell et al., 1997) to inhibit rDNA transcription and ribosome biogenesis (Cadwell et al., 1997). However, this mutation failed to affect nucleolar structure at the restrictive temperature (38°C; Figure 1F). Taken together, these observations demonstrate that inhibition of protein synthesis and ribosome biogenesis is insufficient to result in nucleolar reorganization, suggesting that TOR signaling has a direct role in nucleolar structure regulation.
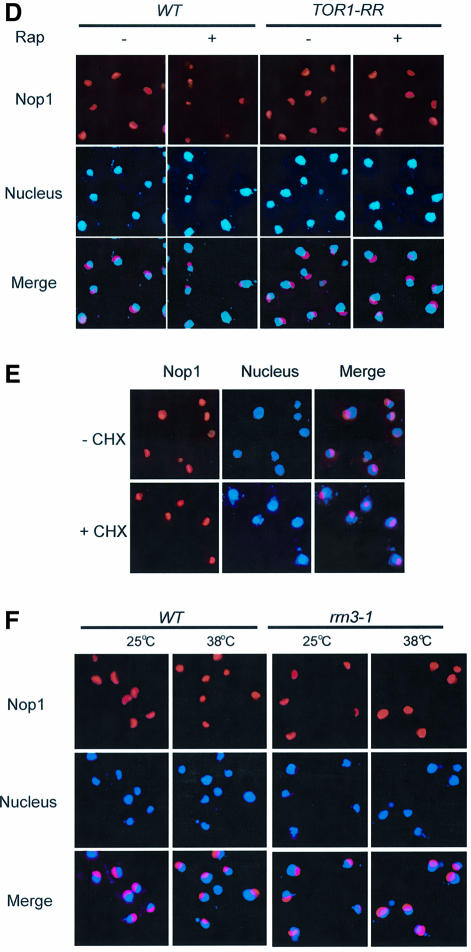
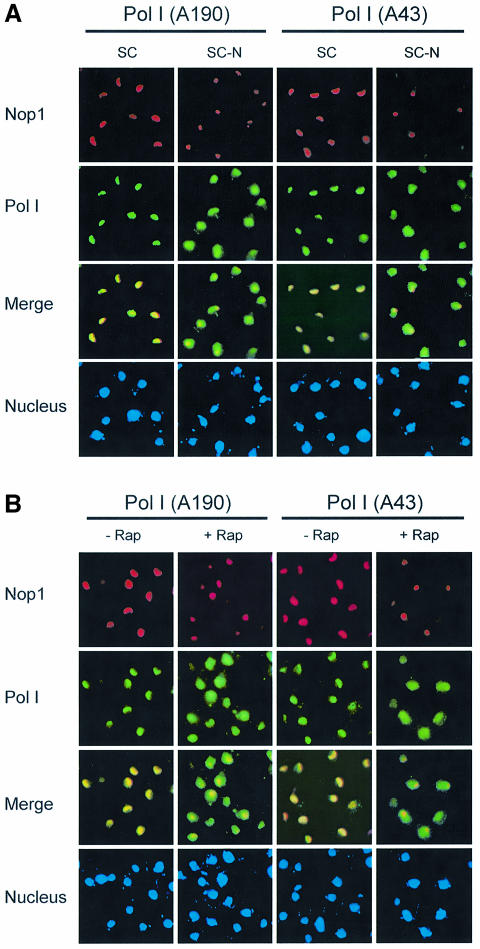
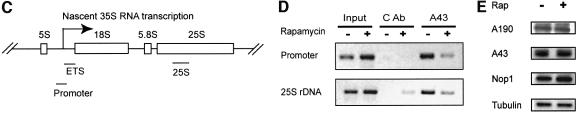
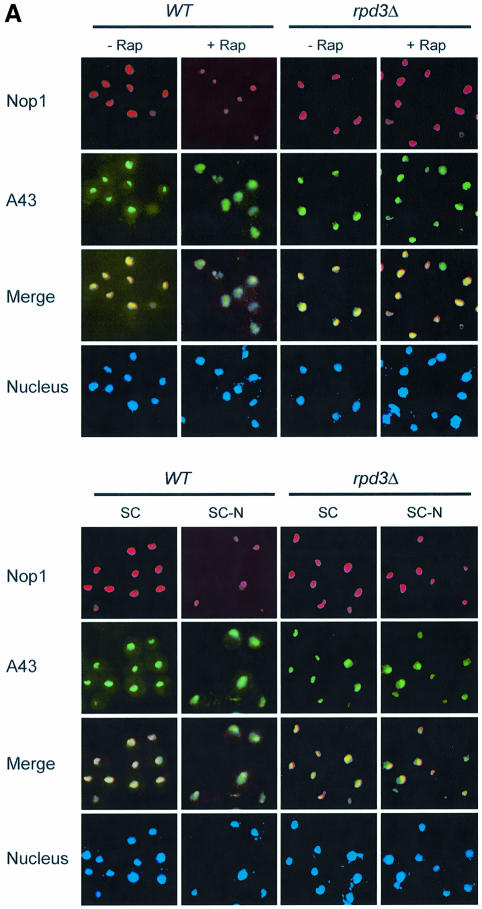
Since TOR is a nutrient sensor, we investigated the effect of starvation on nucleolar structure. We found that nitrogen deprivation caused a rapid reduction of nucleolar size comparable to rapamycin treatment (Figure 2A; data not shown). The fact that nutrient starvation phenocopies rapamycin treatment suggests that TOR mediates nutrient signal transduction to regulate nucleolar structure. To investigate the possible mechanism of TOR regulation of rDNA transcription, we examined RNA Pol I localization by IF with antibodies specific for the Pol I A43 and A190 subunits. Under normal nutrient conditions, both A43 and A190 are localized in the nucleolus as indicated by the crescent shapes of their IF images, which overlapped with that of Nop1 (Figure 2A). When cells were starved of nitrogen, however, both A43 and A190 became diffusely distributed throughout the nucleus (Figure 2A). Essentially the same result was obtained with rapamycin (Figure 2B). A43 delocalization from the nucleolus became obvious within 20 min of rapamycin treatment or nitrogen starvation (data not shown). In agreement with the IF results, rapamycin caused A43 to dissociate from the rRNA promoter and coding regions as determined by chromatin immunoprecipitation (ChIP) assay (Figure 2C and D). Since rapamycin did not affect the protein levels of A43 and A190 (Figure 2E), the decreased A43 binding to rDNA was not due to reduced A43 protein level. Therefore, rapamycin and nutrient starvation cause rapid delocalization of RNA Pol I from the nucleolus, suggesting a possible mode of regulation for rDNA transcription by TOR.
Fig. 2. Nutrient starvation and rapamycin cause RNA Pol I delocalization from the nucleolus. (A) Nitrogen starvation causes nucleolar reorganization and RNA Pol I delocalization from the nucleolus. Wild-type yeast (FM391) in SC medium was switched to SC minus nitrogen (SC –N) for 1 h. Localization of A190 and A43 was examined by IF with antibodies specific for A190 and A43, respectively. (B) Rapamycin causes RNA Pol I delocalization from the nucleolus. Exponentially growing wild-type yeast cells (FM391) were treated with rapamycin for 1 h. Localization of A190 and A43 was determined as for (A). (C) The structural organization of a yeast rDNA unit and the primers used for ChIP assays and northern blot. ETS, externally transcribed spacer. (D) Rapamycin causes dissociation of A43 from rDNA chromatin. Wild-type yeast (FM391) was treated with rapamycin for 1 h. RNA Pol I association with rDNA chromatin was determined by ChIP with an A43 antibody and by PCR with rDNA primer pairs. CAb, control antibody. (E) Short-term rapamycin treatment does not affect A43 and A190 protein levels. Wild-type yeast (FM391) was treated with rapamycin for 1 h. The levels of A190, A43, Nop1 and Tub1 were determined by western blot.
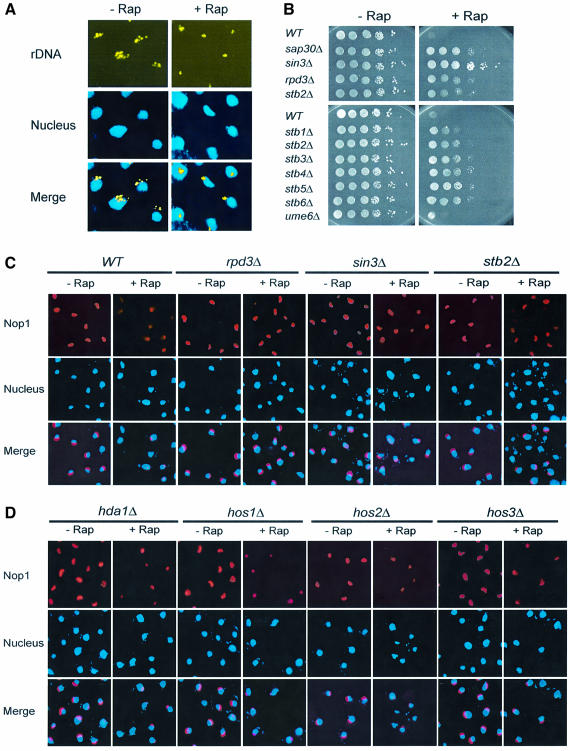
As a complementary study to the nucleolar structural analysis, we used FISH to determine the rDNA chromatin structure. In this experiment, yeast cells were hybridized with a digoxigenin (DIG)-labeled rDNA probe and a DIG-specific antibody. In exponentially growing cells, rDNA showed punctate patterns, with discrete foci organized in crescent shapes at nuclear periphery (Figure 3A). When treated with rapamycin, however, rDNA became much more condensed, usually with one focus per nucleus (Figure 3A). This observation suggests that rDNA chromatin undergoes dramatic remodeling and becomes condensed in the presence of rapamycin. The similarity between nucleolar reorganization and rDNA chromatin condensation raised the possibility that the two events were closely linked. Acetylation/deacetylation at the N-terminal lysine residues of histone H4 regulates chromatin remodeling (Jenuwein and Allis, 2001; Berger, 2002; Schreiber and Bernstein, 2002). Rpd3–Sin3 histone deacetylase (HDAC) is known to be important for such regulation in yeast (Kasten et al., 1997). While acetylated H3/4 are associated with open chromatin forms, chromatin with deacetylated H3/4 is more condensed. Intriguingly, we have recently isolated several members of Rpd3–Sin3 HDAC, including Rpd3, Sin3 and Sap30, by the partial rapamycin resistance phenotype of their deletion mutants during a genomic rapamycin-sensitivity screen (Figure 3B and Chan et al., 2000). In addition, deletion of STB1/2/3/4/5/6, whose gene products are associated with Rpd3 (Rundlett et al., 1996; Kadosh and Struhl, 1997), also conferred partial rapamycin resistance (Figure 3B). In contrast, deletion of UME6, a sporulation-specific transcription factor that recruits Rpd3 to the promoters of meiotic and metabolic genes (Rundlett et al., 1998), did not affect rapamycin sensitivity (Figure 3B). Therefore, Rpd3–Sin3 HDAC appears to be involved in TOR signaling, possibly regulating nucleolar structure. In support of this hypothesis, rpd3Δ and sin3Δ mutations blocked the effect of rapamycin (Figure 3C). In addition, deletion of SAP30 and STB genes produced similar phenotypes (Figure 3C; data not shown). Rpd3 is one of five distinct HDAC enzymes encoded by the yeast genome: Hda1, Hos1/2/3 and Rpd3 (Kurdistani and Grunstein, 2003). Unlike RPD3, however, deletion of other HDAC genes did not affect rapamycin-induced nucleolar reorganization (Figure 3D). Therefore, Rpd3–Sin3 HDAC, but not other HDACs is necessary for nucleolar reorganization.
Fig. 3. Rpd3–Sin3 HDAC is required for nucleolar reorganization. (A) Rapamycin causes rDNA chromatin condensation as judged by FISH of rDNA. Exponentially growing yeast cells (FM391) were treated with rapamycin for 1 h and subjected to FISH analysis. A pseudo yellow color was generated for rDNA for merging with DAPI images. (B) Deletion of the Rpd3–Sin3 HDAC components confers partial rapamycin resistance. Cultures of wild-type and mutant strains (FM391 and derivatives, see Table I) were serially diluted (10-fold), spotted onto SC plates without or with 25 nM rapamycin and incubated for 2 (–Rap) and 5 days (+Rap), respectively. (C) Deletion of the Rpd3–Sin3 HDAC components blocks rapamycin-induced nucleolar reorganization. Wild-type or mutant strains (FM391 and derivatives, see Table I) were treated with rapamycin for 1 h. Nucleolar structures were examined by IF with a Nop1 antibody. (D) Hda1 and Hos1/2/3 are not required for nucleolar reorganization. Wild-type and mutant strains (FM391 and derivatives, see Table I) were treated with rapamycin for 1 h. Nucleolar structures were examined as for (C).
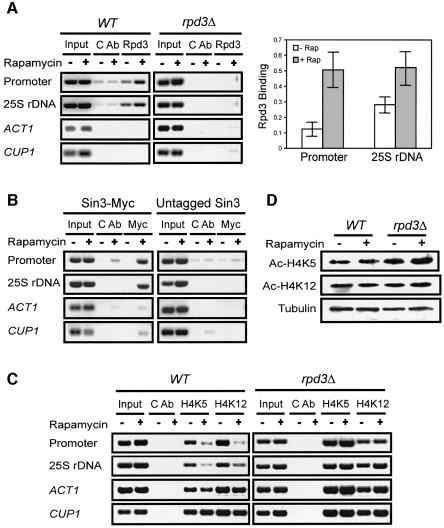
We next investigated whether TOR regulates the recruitment of Rpd3 and Sin3 to rDNA chromatin using the ChIP assay. Rpd3 was co-immunoprecipitated with rDNA by the Rpd3 antibody in the wild-type strain, but not by a control antibody or by the Rpd3 antibody in the rpd3Δ strain, demonstrating the specificity of the ChIP assay (Figure 4A). Rapamycin caused increased Rpd3 binding to rDNA at both the rDNA promoter (4.1-fold) and coding region (1.9-fold; Figure 4A). Similarly, rapamycin enhanced Sin3 binding to rDNA (Figure 4B). In contrast, the binding of Rpd3 and Sin3 to the ACT1 and CUP1 regions was essentially undetectable (Figure 4A and B). The rapamycin-induced binding of Rpd3–Sin3 HDAC to rDNA chromatin further suggests that TOR regulates histone deacetylation. K5, 8, 12 and 16 of histone H4 are acetylated (Jenuwein and Allis, 2001; Berger, 2002; Schreiber and Bernstein, 2002) and Rpd3 is responsible for K5,12 deacetylation (Rundlett et al., 1996, 1998; Kadosh and Struhl, 1998). We next examined the H4 acetylation state at these sites before and after rapamycin treatment. In this study, we performed the ChIP assay with antibodies specific for acetylated H4 K5 and K12. Indeed, rapamycin caused a significant decrease in acetylation at K5/12 at rDNA chromatin, but not ACT1 and CUP1 chromatin regions (Figure 4C). This decrease in H4 acetylation was blocked by the rpd3Δ mutation (Figure 4C). In contrast, the acetylation level of total cellular histone H4 K5/12 remained relatively constant before and after rapamycin treatment (Figure 4D). Taken together, these results show that rapamycin promotes the association of Rpd3–Sin3 HDAC with rDNA chromatin, leading to site-specific histone H4 K5/12 deacetylation.
Fig. 4. Rapamycin promotes the association of Rpd3–Sin3 HDAC with rDNA chromatin and causes concomitant histone H4 deacetylation. (A) Rapamycin treatment promotes Rpd3 association with rDNA. Left panel: wild-type (FM392) and rpd3Δ mutant (FM392 rpd3Δ) cells were treated with rapamycin for 1 h. Rpd3 association with rDNA chromatin was determined by ChIP with an Rpd3 antibody. ACT1 and CUP1 were used as controls. CAb, control antibody. Right panel: quantification of Rpd3 binding to rDNA chromatin (three independent experiments). (B) Rapamycin treatment stimulates Sin3 association with rDNA. The Sin3-Myc (TLY446) cells were treated with rapamycin for 1 h. Sin3 association with rDNA was determined by ChIP with a Myc-specific monoclonal antibody (9E10). ACT1 and CUP1 were used as controls. CAb, control antibody. (C) Rapamycin causes histone H4 deacetylation at rDNA chromatin. Wild-type (FM392) and rpd3Δ mutant (FM392 rpd3Δ) cells were treated with rapamycin for 1 h. Histone H4 acetylation at rDNA chromatin was determined by ChIP with an acetylated H4 K5 or K12 antibody. ACT1 and CUP1 were used as controls. CAb, control antibody. (D) Rapamycin has little effect on overall histone H4 K5/12 acetylation. Wild-type (FM392) and rpd3Δ mutant (FM392 rpd3Δ) cells were treated with rapamycin for 1 h. Total acetylated histone H4 was examined by western blot with acetylated histone H4 K5/12 antibodies. Tubulin was used as a loading control.
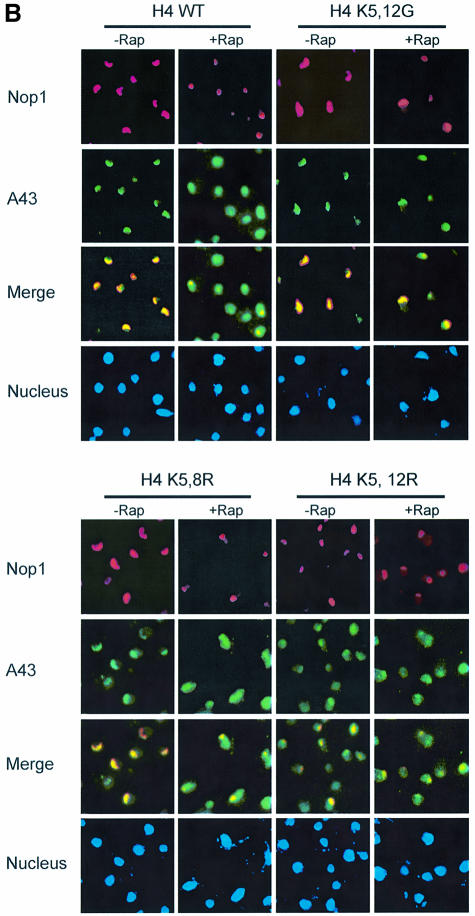
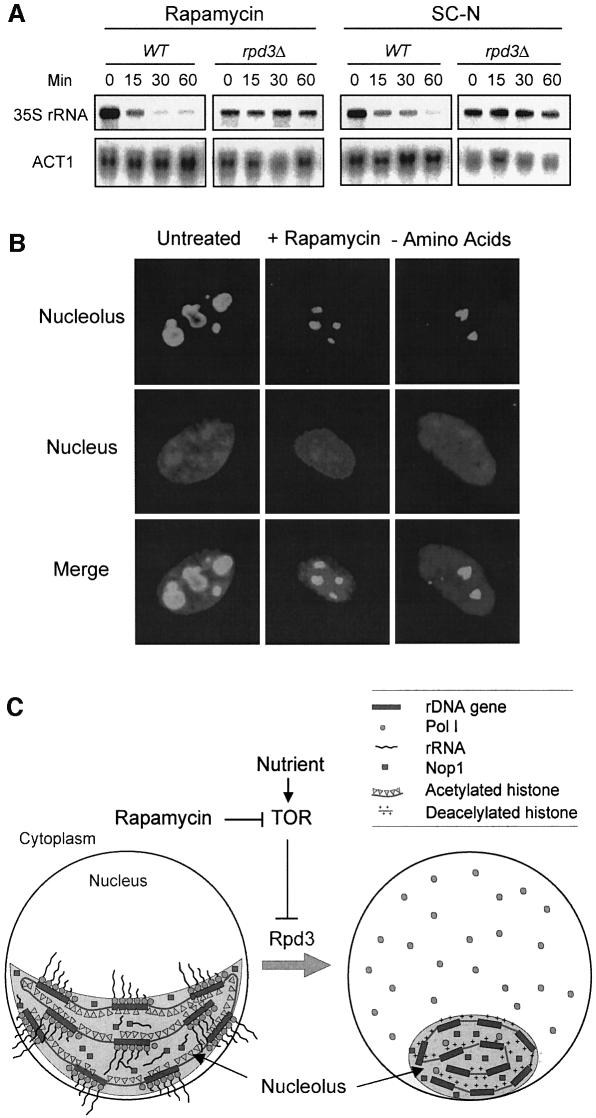
Because Rpd3 was required for nucleolar structural regulation, we wondered whether it also plays a role in Pol I localization. We found that the rpd3Δ mutation blocked the ability of rapamycin or nitrogen starvation to induce A43 delocalization (Figure 5A). Histone H4 lysine mutants have been generated that mimic the acetylated (K→Q or G) or deacetylated (K→R) state (Megee et al., 1990; Ma et al., 1998). Like the rpd3Δ mutant, the K5,12G and H4 K5,8,12,16Q mutants blocked rapamycin-induced nucleolar reorganization and RNA Pol I delocalization (Figure 5B; data not shown). In contrast, the H4 K5,12R mutant, but not the K5,8R mutant, elicited nucleolar reorganization and Pol I A43 delocalization in the absence of rapamycin (Figure 5B). Therefore, histone H4 deacetylation at K5,12 alone can cause nucleolar reorganization and Pol I delocalization. We further investigated the effect of H4 acetylation/deacetylation on rDNA transcription. In this experiment, nascent rRNA transcripts were measured by northern blot using a 32P-labeled DNA probe specific for the 5′ externally transcribed spacer (ETS) sequence. The ETS sequence is rapidly removed from the pre-rRNA transcripts (35S) during rRNA processing and therefore can be used to measure the rate of rDNA transcription (Sandmeier et al., 2002). Rapamycin treatment and nitrogen starvation rapidly inhibited rDNA transcription in the wild-type strain (Figure 6A). This inhibition, however, was blocked by the rpd3Δ mutation (Figure 6A). Taken together, these data suggest that Rpd3-mediated histone H4 deacetylation at rDNA chromatin is a critical step in the control of nucleolar reorganization, RNA polymerase I localization and rDNA transcription by TOR.
Fig. 5. Rpd3 and H4 deacetylation are required for regulation of nucleolar organization and RNA Pol I localization. (A) Rpd3 is required for nucleolar reorganization and RNA Pol I delocalization. Wild-type (FM392) and mutant (FM392 rpd3Δ) cells were under rapamycin treatment (top panel) or nitrogen starvation for 1 h (bottom panel). Nucleolar structures were detected by IF with a Nop1 antibody (red). Localization of A43 was examined by IF with an A43-specific antibody (green). (B) The effect of histone H4 lysine mutations on nucleolar organization and RNA Pol I localization. Wild-type H4 (MAY200) and H4 K5,12G (MAY512G) (top panel) and H4 K5,8R (MSY613) and H4 K5,12R (MSY641) (bottom panel) cells were treated with rapamycin for 1 h. The nucleolar structure and the localization of A43 was detected as for (A).
Fig. 6. Rpd3 is required for inhibition of rDNA transcription by rapamycin and nutrient starvation. (A) Rpd3 is required for inhibition of rDNA transcription by rapamycin and nitrogen starvation. Wild-type (FM392) and mutant (FM392 rpd3Δ) cells were treated with rapamycin or switched to SC minus nitrogen (SC –N). Aliquots of cells were withdrawn at different times. The nascent rRNA transcript (35S) was detected by northern blot with 32P-labeled ETS probe. (B) Rapamycin and amino acid starvation cause a decrease of the nucleolar size in mammalian cells. REFs were treated with rapamycin or under amino acid starvation for 24 h. Nucleolar structures were analyzed by IF with a nucleophosmin antibody. The nucleus was stained with DAPI. (C) A model for the regulation of nucleolar structure and function by TOR. See Discussion for details.
Discussion
Based on the results presented here, we propose a mechanistic model for how TOR regulates nucleolar structure and function (Figure 6C). In this model, TOR regulates accessibility of Rpd3–Sin3 HDAC complex to the rDNA chromatin domain in response to nutrient availability. During nutrient starvation or rapamycin treatment, conditions that inhibit TOR, Rpd3–Sin3 HDAC becomes associated with rDNA chromatin, thereby causing histone H4 deacetylation, rDNA chromatin condensation and reduced nucleolar size. This chain of events further leads to RNA Pol I delocalization from the nucleolus and inhibition of rDNA transcription. Inhibition of rDNA transcription may be the result of both rDNA chromatin condensation and RNA Pol I delocalization. A smaller nucleolus could further provide spatial hindrance to rDNA transcription and ribosome biogenesis. In light of a recent finding that TOR regulates ribosomal protein (RP) gene expression by histone acetylation/deacetylation (Rohde and Cardenas, 2003), it appears that acetylation/deacetylation is broadly used by TOR in ribosome biogenesis. In addition, we have recently shown that silent information regulator 3 (Sir3) mediates TOR signaling to subtelomeric gene expression (Ai et al., 2002). These observations indicate that TOR uses multiple chromatin-dependent mechanisms to control gene expression.
We find that rapamycin induces Rpd3 association with rDNA chromatin and decreases acetylation content in histone H4 at this chromatin region in an Rpd3-dependent manner (Figure 4). Histone H4 hyperacetylation mutants (K5,8,12,16Q and K5,12G) block rapamycin-induced nucleolar structure reorganization and Pol I delocalization (Figure 5B; data not shown). Conversely, the H4 hypoacetylation (K5,12R) mutation, but not the K5,8R mutation causes nucleolar reorganization and RNA Pol I delocalization in the absence of rapamycin (Figure 5B). Therefore, K5 and K12 appear to be the key regulatory sites, which is consistent with the recent findings that Rpd3 is responsible for histone H4 deacetylation at K5/12 (Rundlett et al., 1996, 1998; Kadosh and Struhl, 1998). These observations are also in agreement with a recent report that histone H4 is a component of the upstream activating factor for rDNA transcription and is required for high level rDNA transcription (Keener et al., 1997). Since TOR regulates rDNA transcription in both yeast and mammals (Mahajan, 1994; Leicht et al., 1996; Zaragoza et al., 1998; Powers and Walter, 1999), we investigated the effect of rapamycin and nutrient starvation on nucleolar morphology in primary mammalian cells such as rat embryo fibroblasts (REFs) using nucleophosmin as a marker. We found that rapamycin and amino acid starvation drastically reduced the nucleolar size in these cells (Figure 6B). Additionally, treatment of NIH 3T3 cells with trichostatin A, an inhibitor of mammalian Sin3 (mSin3) HDAC, causes decreased H4 acetylation at the rDNA region and increased in rDNA transcription (Hirschler-Laszkiewicz et al., 2001; Zhou et al., 2002). These studies suggest a conserved mechanism in the control of nucleolar structure and rDNA transcription.
The finding that inhibition of TOR results in Pol I delocalization from the nucleolus provides a possible mechanistic explanation for how TOR controls rDNA transcription. In addition, rDNA chromatin condensation may create an additional barrier to RNA Pol I since condensed chromatin restricts transcriptional activity. This mode of regulation potentially offers rapid and reversible control of rDNA transcription in response to changing nutrient conditions. A recent study shows that the rpd3Δ mutation itself is insufficient to sustain active rDNA transcription during extended stationary phase, an apparent long-term starvation condition. Inhibition of rDNA transcription occurs despite the fact that rDNA chromatin shows an open conformation as seen by Psoralen cross-linking analysis (Sandmeier et al., 2002). This observation suggests that an alternative mechanism(s) is used to cope with long-term starvation. A recent DNA microarray study of diauxic shift shows that the mRNA levels of four RNA Pol I subunits, Rpa12, 43, 49 and 190, are decreased by 2 to 4-fold, suggesting that inhibition of Pol I expression is a likely mechanism under such conditions (DeRisi et al., 1997).
Materials and methods
Yeast strains, plasmids, and antibodies
Strains used in this study are listed in Table I. Different yeast strains were obtained from the following sources: FM391/392 and derivatives (M.Johnston); rrn3-1/syc1-8 (J.Carbon; Cadwell et al., 1997), HHT2-HHF2 (MAY200) and hht2-Δ430 hhf2-K5,12G (MAY512G) (M.Grunstein; Ma et al., 1998); HHF1 (MSY157), hhf1-10 (MSY535), hhf1-14 (MSY613) and hhf1-15 (MSY641) (M.Smith; Megee et al., 1990). Antibodies were obtained from the following sources: Nop1 antibody (EnCor Biotechnology, Inc); FITC- and Texas Red-conjugated secondary antibodies (Molecular Probes, Inc); Mouse anti-DIG antibody (Roche Molecular Biochemicals Diagnostics); acetylated histone H4 penta and K5 antibodies (Upstate Biotechnology); acetylated histone H4 K12 antibody (Cell Signaling Technology); A43 and A190 antibodies (C.Carles and M.Riva; Peyroche et al., 2000); Rpd3 antibody (A.Mitchell; Lamb and Mitchell, 2001); Myc-specific monoclonal antibody (mAb) 9E10 (Harlan Laboratories); tubulin antibody (Sigma) and B23 nucleophosmin mAb (Zymed).
Table I. Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| FM391 | MATa his1Δ leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| FM392 | MATα hisΔ1 leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| MAY200 | MATa ade2-101 his3-Δ201 lys2-801 trp1-Δ901 ura3-52 (hht1 hhf1)::LEU2 (hht2 hhf2)::HIS3plus pRM200 (CENT4 ARS1 TRP1 HHT2-HHF2) and pRM102 (CENT4 ARS1 URA3pGAL10-HHT2 pGAL1-HHF2) | Ma et al. (1998) |
| MAY512G | MATa ade2-101 his3-Δ201 lys2-801 trp1-Δ901 ura3-52 (hht1 hhf1)::LEU2 (hht2 hhf2)::HIS3plus pK512G (CENT4 ARS1 TRP1 hht2-Δ430, hhf2-K5, 12G) | Ma et al. (1998) |
| MSY157 | MATα ura3-52 ade2-101 lys2-801 (hht1 hhf1)Δ | Megee et al. (1990) |
| MSY535 | MATα ura3-52 lys2-Δ201 leu2-3 112 (hht1 hhf1)Δ pMS386[LEU2 HHT1 hhf1-10] | Megee et al. (1990) |
| MSY613 | MATα ura3-52 lys2-Δ201 leu2-3 112 (hht1 hhf1)Δ(hht2 hhf2) pMS386[LEU2 HHT1 hhf1-14] | Megee et al. (1990) |
| MSY641 | MATα ura3-52 lys2-Δ201 leu2-3 112 (hht1 hhf1)Δ (hht2 hhf2) pMS386[LEU2 HHT1 hhf1-15 | Megee et al. (1990) |
| SZy996 | FM391 tor1Δ::KanMX | Research Genetics |
| SZy997 | SZy996 [pYDF80-TOR1S1792I] | This study |
| SZy998 | SZy996 [pYDF81-TOR1] | This study |
| SZy1032 | FM391 rpd3Δ::KanMX | Research Genetics |
| SZy1033 | FM392 rpd3Δ::KanMX | Research Genetics |
| SZy1034 | FM391 sin3Δ::KanMX | Research Genetics |
| SZy1035 | FM391 stb1Δ::KanMX | Research Genetics |
| SZy1036 | FM391 stb2Δ::KanMX | Research Genetics |
| SZy1037 | FM391 stb3Δ::KanMX | Research Genetics |
| SZy1038 | FM391 stb4Δ::KanMX | Research Genetics |
| SZy1039 | FM391 stb5Δ::KanMX | Research Genetics |
| SZy1040 | FM391 stb6Δ::KanMX | Research Genetics |
| SZy1041 | FM392 stb2Δ::KanMX | Research Genetics |
| SZy1042 | FM391 sap30Δ::KanMX | Research Genetics |
| SZy1043 | FM391 ume6Δ::KanMX | Research Genetics |
| SZy1044 | FM391 hos1Δ::KanMX | Research Genetics |
| SZy1045 | FM391 hos2Δ::KanMX | Research Genetics |
| SZy1046 | FM391 hos3Δ::KanMX | Research Genetics |
| SZy1047 | FM391 hda1Δ::KanMX | Research Genetics |
| TLY2 | MATa rme1Δ5::LEU2 ura3 trp1::hisG leu2::hisG lys2 ho::LYS2 arg6 | Lamb and Mitchell (2001) |
| TLY446 | MATa rme1Δ5::LEU2 ura3 trp1::hisG leu2::hisG lys2 ho::LYS2 arg6 SIN3-MYC | Lamb and Mitchell (2001) |
| YCC95 | MATα ade5 his7-2 leu2-3 leu2-112 trp1-289amber ura3-52 syc1-8 | Cadwell et al. (1997) |
| W303 | MATa ura3-1 leu2-3,-112 his3-11,-15 trp1-1 ade2-1 can1-100 | Research Genetics |
Indirect immunofluorescence (IF) and fluorescence in situ hybridization (FISH)
Yeast IF was performed as described before (Burke et al., 2000; Bertram et al., 2002). For rapamycin treatment, exponentially growing cells in yeast extracts–peptone–dextrose (OD600 = 0.2) at 30°C were treated with 200 nM rapamycin or the drug carrier (methanol). For nutrient starvation, exponentially growing cells in synthetic complete (SC) medium were shifted to SC minus nitrogen (SC –N) or SC medium. Yeast nuclei were stained with 50 ng/ml DAPI. Mammalian IF was carried out as described before (Choi et al., 2002). FISH analysis was carried out as described (Guacci et al., 1994). A 25S rDNA probe was labeled with DIG using the BIONICK Labeling System (Invitrogen) as described (Gotta et al., 1997). Fluorescent samples were analyzed using an Olympus microscope equipped with a digital camera.
Electron microscopy (EM)
Yeast cells were fixed with glutaraldehyde (2%) and formaldehyde (2%) in a phosphate–magnesium buffer (40 mM K2HPO4, 0.5 mM MgCl2 pH 6.5), digested with glusulase (PerkinElmer Life Sciences) and zymolyase 20-T (Seikagaku) in a phosphate–citrate buffer (170 mM KH2PO4 and 30 mM sodium citrate pH 5.8) at 30°C for 2 h, and embedded as described before (Byers and Goetsch, 1991). EM images were captured by a JEOL 100CX electron microscope (Department of Cell Biology, Washington University).
Cell extracts and western blot analysis
Exponentially growing cells were lysed with glass beads by vortexing in disruption buffer [50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40, protease inhibitor cocktail (Roche)]. Protein samples (5–10 µg) were separated on SDS–polyacrylamide gels and then transferred to PVDF membranes. The primary antibodies were used with the following dilutions: Nop1, 1:500; tubulin, 1:200; Histone1:1000; A43 and A190, 1:1000.
Chromatin immunoprecipitation (ChIP)
Exponentially growing cells (OD600 = 0.5) were treated with rapamycin (200 nM) or the drug carrier methanol for 1 h and fixed in 1% formaldehyde for 30 min at 30°C. The ChIP assay were performed as described before (Lieb et al., 2001). 1 mg cell extracts were used for each ChIP experiment. Primer pairs used for PCR are as follows: the 35S rDNA promoter region, 5′-GTTTTGGTTTCGGTTGTGAA-3′ and 5′-GAAGTACCTCCCAACTACTT-3′; the 25S rDNA coding region, 5′-AGGACGTCATAGAGGGTGAGAATC-3′ and 5′-TTGACTTACGTC GCAGTCCTCAGT-3′; ACT1, 5′-CCAATTGCTCGAGAGATTTC-3′ and 5′-CGTGATAAGTGATAGTGATATTC-3′; and CUP1, 5′-TCTTT TCCGCTGAACCGTTCCAGCA-3′ and 5′-GGCATTGGCACTCATG ACCTTCAT-3′.
Northern blot
Total RNA was prepared by the phenol-freezing extraction method (Schmitt et al., 1990), separated on a 1.2% formaldehyde agarose gel, transferred to a Magnagraph nylon membrane and hybridized with a 32P-labeled DNA probe specific for the 5′-ETS region of 35S rRNA. ACT1 was used as a loading control.
Acknowledgments
Acknowledgements
We thank J.Carbon, C.Carles, M.Grunstein, M.Johnston, A.Mitchell, M.Reva and M.Smith for generously providing antibodies and strains, and M.Levy for EM analysis. This work was supported by grants from National Institutes of Health (RO1CA77668, RO1CA99004 and RO1GM62817) and the Department of Defense to X.F.Z.
References
- Abraham R.T. (2002) Identification of TOR signaling complexes: more TORC for the cell growth engine. Cell, 111, 9–12. [DOI] [PubMed] [Google Scholar]
- Ai W., Bertram,P.G., Tsang,C.K., Chan,T.F. and Zheng,X.F. (2002) Regulation of subtelomeric silencing during stress response. Mol. Cell, 10, 1295–1305. [DOI] [PubMed] [Google Scholar]
- Beck T. and Hall,M.N. (1999) The TOR signaling pathway controls nuclear localization of nutrient-regulated transcriptional factors. Nature, 402, 689–692. [DOI] [PubMed] [Google Scholar]
- Berger S.L. (2002) Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev., 12, 142–148. [DOI] [PubMed] [Google Scholar]
- Bertram P.G., Choi,J., Carvalho,J., Ai,W.D., Zeng,C.B., Chan,T.F. and Zheng,X.F.S. (2000) Tripartite regulation of Gln3p by TOR, Ure2p and phosphatases. J. Biol. Chem., 275, 35727–35733. [DOI] [PubMed] [Google Scholar]
- Bertram P.G., Choi,J.H., Carvalho,J., Chan,T.-F., Ai,W. and Zheng,X.F.S. (2002) Convergence of TOR-nitrogen and Snf1-glucose signaling pathways onto Gln3. Mol. Cell. Biol., 22, 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Dawson,D. and Stearns,T. (2000) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Byers B. and Goetsch,L. (1991) Preparation of yeast cells for thin-section electron microscopy. Methods Enzymol., 194, 602–606. [DOI] [PubMed] [Google Scholar]
- Cadwell C., Yoon,H.J., Zebarjadian,Y. and Carbon,J. (1997) The yeast nucleolar protein Cbf5p is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3. Mol. Cell. Biol., 17, 6175–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas M., Cutler,N., Lorenz,M., Di Como,C. and Heitman,J. (1999) The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev., 13, 3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T.F., Carvalho,J., Riles,L. and Zheng,X.F.S. (2000) A chemical genomics approach toward understanding the global functions of TOR. Proc. Natl Acad. Sci. USA, 97, 13227–13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T.-F., Bertram,P.G., Ai,W. and Zheng,X.F.S. (2001) Regulation of APG14 expression by the GATA-type transcription factor Gln3p. J. Biol. Chem., 276, 6463–6467. [DOI] [PubMed] [Google Scholar]
- Choi J., Adames,N., Chan,T.F., Zeng,C.B., Cooper,J. and Zheng,X.F.S. (2000) TOR signaling regulates the stability and functions of microtubules. Curr. Biol., 10, 861–864. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Bertram,P.G., Drenan,R., Carvalho,J., Zhou,H.H. and Zheng,X.F.S. (2002) The FKBP12-rapamycin-associated protein (FRAP) is a CLIP-170 kinase. EMBO Rep., 3, 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P.B., Fumagalli,S. and Thomas,G. (1999) Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr. Opin. Genet. Dev., 9, 49–54. [DOI] [PubMed] [Google Scholar]
- DeRisi J.L., Iyer,V.R. and Brown,P.O. (1997) Exploring the metabolic and genetic control of gene expression on a genomic scale. Science, 278, 680–686. [DOI] [PubMed] [Google Scholar]
- Fatica A. and Tollervey,D. (2002) Making ribosomes. Curr. Opin. Cell Biol., 14, 313–318. [DOI] [PubMed] [Google Scholar]
- Gotta M., Strahl-Bolsinger,S., Renauld,H., Laroche,T., Kennedy,B.K., Grunstein,M. and Gasser,S.M. (1997) Localization of Sir2p: the nucleolus as a compartment for silent information regulators. EMBO J., 16, 3243–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I. (2003) Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev., 17, 1691–1702. [DOI] [PubMed] [Google Scholar]
- Guacci V., Hogan,E. and Koshland,D. (1994) Chromosome condensation and sister chromatid pairing in budding yeast. J. Cell Biol., 125, 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. and Kenyon,C. (2000) Genetic pathways that regulate ageing in model organisms. Nature, 408, 255–262. [DOI] [PubMed] [Google Scholar]
- Hardwick J.S., Kuruvilla,F., Tong,J.K., Shamji,A.F. and Schreiber,S.L. (1999) Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc. Natl Acad. Sci. USA, 96, 14866–14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S.B., Schmidt,A., Ohya,Y. and Hall,M.N. (1998) The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol., 8, 1211–1214. [DOI] [PubMed] [Google Scholar]
- Hirschler-Laszkiewicz I., Cavanaugh,A., Hu,Q., Catania,J., Avantaggiati,M.L. and Rothblum,L.I. (2001) The role of acetylation in rDNA transcription. Nucleic Acids Res., 29, 4114–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (1995) When a lipid kinase is not a lipid kinase? When a lipid kinase is a protein kinase? Cell, 83, 1–4. [DOI] [PubMed] [Google Scholar]
- Jenuwein T. and Allis,C.D. (2001) Translating the histone code. Science, 293, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Kadosh D. and Struhl,K. (1997) Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell, 89, 365–371. [DOI] [PubMed] [Google Scholar]
- Kadosh D. and Struhl,K. (1998) Targeted recruitment of the Sin3–Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol. Cell. Biol., 18, 5121–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten M.M., Dorland,S. and Stillman,D.J. (1997) A large protein complex containing the yeast Sin3p and Rpd3p transcriptional regulators. Mol. Cell. Biol., 17, 4852–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener J., Dodd,J.A., Lalo,D. and Nomura,M. (1997) Histones H3 and H4 are components of upstream activation factor required for the high-level transcription of yeast rDNA by RNA polymerase?I. Proc. Natl Acad. Sci. USA, 94, 13458–13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith C.T. and Schreiber,S.L. (1995) PIK-related kinases: DNA repair, recombination and cell cycle checkpoints. Science, 270, 50–51. [DOI] [PubMed] [Google Scholar]
- Kurdistani S.K. and Grunstein,M. (2003) Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell Biol., 4, 276–284. [DOI] [PubMed] [Google Scholar]
- Kuruvilla F. and Schreiber,S.L. (1999) The PIK-related kinases intercept conventional signaling pathways. Chem. Biol., 6, R129–R136. [DOI] [PubMed] [Google Scholar]
- Lamb T.M. and Mitchell,A.P. (2001) Coupling of Saccharomyces cerevisiae early meiotic gene expression to DNA replication depends upon RPD3 and SIN3. Genetics, 157, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary D.J. and Huang,S. (2001) Regulation of ribosome biogenesis within the nucleolus. FEBS Lett., 509, 145–150. [DOI] [PubMed] [Google Scholar]
- Leicht M., Simm,A., Bertsch,G. and Hoppe,J. (1996) Okadaic acid induces cellular hypertrophy in AKR-2B fibroblasts: involvement of the p70S6 kinase in the onset of protein and rRNA synthesis. Cell Growth Differ., 7, 1199–1209. [PubMed] [Google Scholar]
- Lieb J.D., Liu,X., Botstein,D. and Brown,P.O. (2001) Promoter-specific binding of Rap1 revealed by genome-wide maps of protein–DNA association. Nat. Genet., 28, 327–334. [DOI] [PubMed] [Google Scholar]
- Ma X.J., Wu,J., Altheim,B.A., Schultz,M.C. and Grunstein,M. (1998) Deposition-related sites K5/K12 in histone H4 are not required for nucleosome deposition in yeast. Proc. Natl Acad. Sci. USA, 95, 6693–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan P. (1994) Modulation of transcription of rRNA genes by rapamycin. Int. J. Immunopharmacol., 16, 711–721. [DOI] [PubMed] [Google Scholar]
- Megee P.C., Morgan,B.A., Mittman,B.A. and Smith,M.M. (1990) Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science, 247, 841–845. [DOI] [PubMed] [Google Scholar]
- Moss T. and Stefanovsky,V.Y. (2002) At the center of eukaryotic life. Cell, 109, 545–548. [DOI] [PubMed] [Google Scholar]
- Olson M.O., Dundr,M. and Szebeni,A. (2000) The nucleolus: an old factory with unexpected capabilities. Trends Cell Biol., 10, 189–196. [DOI] [PubMed] [Google Scholar]
- Peculis B.A. (2002) Ribosome biogenesis: ribosomal RNA synthesis as a package deal. Curr. Biol., 12, R623–R624. [DOI] [PubMed] [Google Scholar]
- Peyroche G., Milkereit,P., Bischler,N., Tschochner,H., Schultz,P., Sentenac,A., Carles,C. and Riva,M. (2000) The recruitment of RNA polymerase I on rDNA is mediated by the interaction of the A43 subunit with Rrn3. EMBO J., 19, 5473–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T. and Walter,P. (1999) Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae. Mol. Biol. Cell, 10, 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B., Gingras,A.-C. and Sonenberg,N. (2001) The target of rapamycin (TOR) protein. Proc. Natl Acad. Sci. USA, 98, 7037–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde J.R. and Cardenas,M.E. (2003) The Tor pathway regulates gene expression by linking nutrient sensing to histone acetylation. Mol. Cell. Biol., 23, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde J., Heitman,J. and Cardenas,M. (2001) The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem., 276, 7027–7036. [DOI] [PubMed] [Google Scholar]
- Ruggero D. and Pandolfi,P.P. (2003) Does the ribosome translate cancer? Nat. Rev. Cancer, 3, 179–192. [DOI] [PubMed] [Google Scholar]
- Rundlett S.E., Carmen,A.A., Kobayashi,R., Bavykin,S., Turner,B.M. and Grunstein,M. (1996) HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc. Natl Acad. Sci. USA, 93, 14503–14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundlett S.E., Carmen,A.A., Suka,N., Turner,B.M. and Grunstein,M. (1998) Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature, 392, 831–835. [DOI] [PubMed] [Google Scholar]
- Sandmeier J.J., French,S., Osheim,Y., Cheung,W.L., Gallo,C.M., Beyer,A.L. and Smith,J.S. (2002) RPD3 is required for the inactivation of yeast ribosomal DNA genes in stationary phase. EMBO J., 21, 4959–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U. and Hock,R. (1999) Structure and function of the nucleolus. Curr. Opin. Cell Biol., 11, 385–390. [DOI] [PubMed] [Google Scholar]
- Schmelzle T. and Hall,M.N. (2000) TOR, a central controller of cell growth. Cell, 103, 253. [DOI] [PubMed] [Google Scholar]
- Schmitt M.E., Brown,T.A. and Trumpower,B.L. (1990) A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res., 18, 3091–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S.L. and Bernstein,B.E. (2002) Signaling network model of chromatin. Cell, 111, 771–778. [DOI] [PubMed] [Google Scholar]
- Shamji A., Kuruvilla,F. and Schreiber,S. (2000) Partitioning the transcriptional program induced by rapamycin among the effectors of the Tor proteins. Curr. Biol., 10, 1574–1581. [DOI] [PubMed] [Google Scholar]
- Venema J. and Tollervey,D. (1999) Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet., 33, 261–311. [DOI] [PubMed] [Google Scholar]
- Visintin R. and Amon,A. (2000) The nucleolus: the magician’s hat for cell cycle tricks. Curr. Opin. Cell Biol., 12, 372–377. [DOI] [PubMed] [Google Scholar]
- Warner J.R. (1999) The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci., 24, 437–440. [DOI] [PubMed] [Google Scholar]
- Zaragoza D., Ghavidel,A., Heitman,J. and Schultz,M.C. (1998) Rapamycin induces the G0 program of transcriptional repression in yeast by interfering with the TOR signaling pathway. Mol. Cell. Biol., 18, 4463–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.F., Florentino,D., Chen,J., Crabtree,G.R. and Schreiber,S.L. (1995) TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell, 82, 121–130. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Santoro,R. and Grummt,I. (2002) The chromatin remodeling complex NoRC targets HDAC1 to the ribosomal gene promoter and represses RNA polymerase I transcription. EMBO J., 21, 4632–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]