Abstract
A world-wide series of epidemiological and experimental studies have demonstrated that there is an association between being small at birth, accelerated growth in early postnatal life and the emergence of insulin resistance in adult life. The aim of this study was to investigate why accelerated growth occurs in postnatal life after in utero growth restriction. Samples of quadriceps muscle were collected at ∼140 days gestation (term ∼150 days gestation) from normally grown fetal lambs (Control, n= 7) and from growth restricted fetal lambs (placentally restricted: PR, n= 8) and from Control (n= 14) and PR (n= 9) lambs at 21 days after birth. The abundance of the insulin and IGF1 receptor protein was higher in the quadriceps muscle of the PR fetus, but there was a lower abundance of the insulin signalling molecule PKCζ, and GLUT4 protein in the PR group. At 21 days of postnatal age, insulin receptor abundance remained higher in the muscle of the PR lamb, and there was also an up-regulation of the insulin signalling molecules, PI3Kinase p85, Akt1 and Akt2 and of the GLUT4 protein in the PR group. Fetal growth restriction therefore results in an increased abundance of the insulin receptor in skeletal muscle, which persists after birth when it is associated with an upregulation of insulin signalling molecules and the glucose transporter, GLUT4. These data provide evidence that the origins of the accelerated growth experienced by the small baby after birth lie in the adaptive response of the growth restricted fetus to its low placental substrate supply.
A world-wide series of epidemiological, clinical and experimental studies have demonstrated that there is an association between growing slowly before birth, accelerated growth in early postnatal life and the emergence of insulin resistance, type 2 diabetes and obesity in later life (Barker, 1992; McCance et al. 1994; Lithell et al. 1996; Leger et al. 1997; Bavdekar et al. 1999; Jaquet et al. 2000; McMillen et al. 2005). Infants who are small for gestational age have low circulating insulin (Economides et al. 1989, 1991) and insulin-like growth factor-1 (IGF1) (Enzi et al. 1981) concentrations at birth, and then undergo a period of accelerated postnatal growth during the first few years of life (Fitzhardinge & Steven, 1972; Albertsson-Wikland et al. 1993). This period of accelerated early growth is initially associated with an increased insulin sensitivity, and precedes the subsequent emergence of insulin resistance (Curhan et al. 1996; Whincup et al. 1997; Bavdekar et al. 1999; Eriksson et al. 2001; Levy-Marchal & Jaquet, 2004). Experimental studies using a range of rodent models of intrauterine growth restriction (IUGR), including uteroplacental ligation (Siebel et al. 2008), global maternal nutrient restriction (Holemans et al. 1996) and maternal low protein diets (Ozanne et al. 1996, 2005; Ozanne, 2001) report that prenatal growth restraint is associated with an early phase of enhanced insulin sensitivity, followed by the later emergence of insulin resistance and type 2 diabetes. These animal models have provided important insights into the mechanisms which control the transition from a period of enhanced insulin sensitivity to insulin resistance.
Given that muscle represents the major site of postprandial glucose disposal, it is not surprising that changes in the functional characteristics of muscle fibres during the perinatal period are important in the programming of insulin resistance and type 2 diabetes. The offspring of rats fed a low protein diet during pregnancy have improved glucose tolerance at 3 months of age (Ozanne et al. 1996). Importantly, isolated muscle strips from these low-protein animals exhibit enhanced basal and insulin-stimulated glucose uptake, which is associated with a twofold increase in the abundance of insulin receptors in muscle membranes (Ozanne et al. 1996). By 15 months of age, however, there is a decrease in insulin sensitivity in skeletal muscle from the group exposed to the low-protein diet in utero (Ozanne et al. 2003). This impaired insulin action is associated with changes in the expression of the insulin receptor, and a decrease in the abundance of signalling molecules downstream of the insulin receptor, including the zeta (ζ)-isoform of protein kinase C, an isoform that is positively involved in GLUT4-mediated glucose transport (Ozanne et al. 2003), and the p85α regulatory subunit of phosphoinositide 3-kinase (Ozanne et al. 2005).
Rodent studies have also provided evidence that a reduced capacity for mitochondrial biogenesis and oxidative metabolism in growth restricted offspring may contribute to the impaired insulin sensitivity in the adult. In the uterine artery ligation model, the expression of mitochondrial proteins is lower in IUGR offspring at 3 weeks of postnatal age, suggesting that these offspring have a reduced capacity for oxidative metabolism (Lane et al. 2001). Furthermore, the mRNA expression and protein abundance of the master regulator of mitochondrial biogenesis, peroxisome proliferator activated receptor-γ co-activator-1α (PGC-1α), is decreased in male, but not female, IUGR offspring (Lane et al. 2003). In males, the reduced expression of PGC-1α was associated with a reduced expression of downstream target genes involved in cellular oxidative metabolism, including mitochondrial transcription factor A (mtTFA) and cytochrome oxidase (COX) III mRNA (Lane et al. 2003; Wadley et al. 2008). It would therefore appear that IUGR may programme mitochondrial function in a sexually dimorphic manner, and that a reduced oxidative capacity, which is known to be associated with impaired insulin sensitivity, may contribute to the development of insulin resistance in IUGR offspring.
Whilst these animal models provide insights into the mechanisms which govern the transition from the period of enhanced insulin sensitivity in early life to later insulin resistance, there are no studies which have determined the mechanisms which contribute to the transition from reduced growth in utero to enhanced insulin sensitivity in postnatal life, or which have adequately explained why it is that poor growth in utero should result in increased insulin sensitivity and accelerated growth after birth.
In the present study, we have utilised an experimental model of fetal growth restriction in the sheep. This model allows the collection of muscle samples from growth restricted animals before birth and during the period of accelerated growth in early postnatal life. The quadriceps muscle was selected because it has been shown that reduced size at birth is predictive of reduced individual and summed skeletal muscle mass, including the quadriceps muscle. Furthermore in placentally restricted lambs there is a positive relationship between the mass of the vastus lateralis and the postnatal growth rate (De Blasio et al. 2007). The quadriceps also represents a large muscle group in the postnatal sheep and hence makes a significant contribution to glucose metabolism. The aim of the present study was therefore to determine the impact of intrauterine growth restriction in the sheep on mRNA expression and protein abundance of insulin signalling molecules and activity of oxidative and glycolytic enzymes in quadriceps muscle before birth and in early postnatal life.
Methods
Animals and surgery
All procedures were approved by The University of Adelaide Animal Ethics Committee. Thirty-eight singleton bearing Merino ewes were used in this study. For all surgical procedures general anaesthesia was induced by intravenous injection of sodium thiopentone (1.25 g i.v., Pentothal, Rhone Merieux, Pinkenba, Qld, Australia) and maintained with 2.5–4% halothane (Fluothane, ICI, Melbourne, Vic, Australia) in oxygen. The analgesic xylazine (0.02 mg kg−1) was administered by intramuscular injection to all ewes in the immediate post-operative period.
Seventeen non-pregnant ewes underwent surgery to remove the majority of endometrial caruncles from the uterus, leaving 3–8 caruncles in each horn in order to induce experimental restriction of placental and fetal growth (placental restriction group; PR) (Edwards et al. 1999; Morrison et al. 2007).
From around 109 days (d) gestation all ewes were housed in individual pens in rooms with a 12 h light–dark cycle and a daily temperature of ∼20°C. Each pregnant ewe was supplied with a diet which consisted of 1 kg lucerne chaff (85% dry matter, metabolisable energy (ME) content = 8.3 MJ kg−1) and 500 g concentrated pellets containing: straw, cereal, hay, clover, barley, oats, lupins, almond shells, oat husks and limestone (90% dry matter, ME content = 8.0 MJ kg−1; Johnsons and Sons, Kapunda, SA, Australia). The diet was calculated to provide 100% of the energy requirements for the maintenance of a pregnant ewe bearing a singleton fetus, as specified by the Ministry of Agriculture, Fisheries and Food, UK (Aldermann et al. 1975). After giving birth to her lamb, the ewe was fed 1 kg of lucerne chaff and 1 kg of concentrated pellets at 09.00–11.00 h each day in order to meet the additional energy demands of lactation(Aldermann et al. 1975). If the ewe consumed all of her morning feed ration before 15.00 h, then an additional 1 kg of lucerne chaff was provided to the ewe on that day. After birth, each ewe and her lamb were housed in an individual pen in an indoor housing facility which was maintained at a constant ambient temperature of ∼20°C and a 12 h light–dark cycle.
Fetal study
Between 109 and 124 d of gestation (term ∼150 d) surgery was performed on control (n= 7) and carunclectomised (n= 8) pregnant ewes as previously described (Edwards et al. 1999; Morrison et al. 2007) for the insertion of vascular catheters in a fetal and maternal jugular vein, fetal carotid artery and amniotic cavity. All ewes and fetuses received antibiotics (procaine penicillin (250 mg ml−1), dihydrostreptomycin (250 mg ml−1), and procaine hydrochloride (20 mg ml−1); Penstrep Illium; Troy Laboratories, Smithfield, NSW, Australia) at the time of surgery. Animals were allowed to recover from surgery for at least 4 d. Fetal arterial blood samples (3.5 ml) were collected between 08.00 and 11.00 h throughout late gestation, for the measurement of arterial blood gas status (ABL 520 blood gas analyser; Radiometer, Copenhagen, Denmark).
Ewes were killed between 140 and 145 d of pregnancy with a lethal overdose (∼30 mg/kg) of sodium pentobarbitone delivered intravenously (Virbac Pty Ltd, Peakhurst, NSW, Australia). Fetuses were delivered by hysterectomy, weighed and killed by decapitation. Samples of quadriceps muscle were dissected from Control (n= 7; 5 males, 2 females) and PR fetuses (n= 8; 6 males, 2 females), frozen in liquid nitrogen and stored at −80°C.
Lamb study
Control (n= 14) and PR (n= 9) ewes lambed and were housed with their lambs throughout the experimental period. After birth, Control (n= 14, 8 males, 6 females) and PR (n= 9, 5 males, 4 females) lambs were weighed and crown-rump length measured daily between 10.00 and 14.00 h. Venous blood samples were collected in chilled tubes after approximately 60 min of non-suckling on alternate days between 09.00 h and 13.00 h, beginning on the day of birth (day 1). All blood samples were centrifuged at 1500 g for 10 min, and plasma was stored at −20°C. On postnatal day 21, lambs were killed with a lethal overdose (∼30 mg/kg) of sodium pentobarbitone delivered by intravenous injection (Virbac Pty Ltd, Peakhurst, NSW, Australia) and samples of quadriceps muscle were dissected, frozen in liquid nitrogen and stored at −80°C.
Plasma glucose and insulin assays
Plasma glucose was measured by an in vitro enzymatic colorimetric method (COBAS MIRA automated analysis system, Roche Diagnostica, Basel, Switzerland). The sensitivity of the assay was 0.01 mmol l−1. Plasma insulin concentrations were measured using a radioimmunoassay (Linco Research, Inc., MO, USA) previously validated for sheep plasma (Muhlhausler et al. 2006). The sensitivity of the assay was 0.1 ng ml−1. The intra- and inter-assay CV for both assays were each <10%.
Isolation of RNA, production of cDNA and qRT-PCR analysis
RNA was extracted from 100 mg quadriceps muscle (Trizol reagent, Invitrogen Australia Pty Limited, Australia) from all fetuses and lambs. RNA was purified using the RNeasy Mini Kit (Qiagen, Basel, Switzerland). The quality and concentration of the RNA were determined by measuring absorbance at 260 and 280 nm, and RNA integrity confirmed by agarose gel electrophoresis. cDNA was synthesised using the purified RNA (∼2 μg) and Superscript 3 reverse transcriptase (Invitrogen Australia Pty Limited, Mount Waverley, Australia) with random hexamers.
The relative expression of mRNA transcripts of the insulin receptors A and B (IRA and IRB), glucose transporter-1 (GLUT1), glucose transporter-4 (GLUT4), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), peroxisome proliferator-activated receptor-α (PPARα) mRNA transcripts and the housekeeper gene acidic ribosomal protein large subunit P0 (ARP-P0) (Muhlhausler et al. 2007) were measured by quantitative real time reverse transcription-PCR (qRT-PCR) using the Sybr Green system in an ABI Prism 7500 Sequence Detection System (PE Applied Biosystems, Foster City, CA, USA). The primer sequences used were (IRA, F: 5′CATCCCCAGGCCTTCGA3′, R: 5′ACGGCTGCTGTCACATTCC3′ (Y16093); IRB, F: 5′CATCCCCAGAAAATCATCTTCAG3′, R: 5′CAAGGGCTCTGCGTTTCCT3′ (Y16092); GLUT1, F: 5′TAACCGCAACGAGGAGAACC3′ (U89029); GLUT4, F: 5′GTGGCCATCTTTGGCTTCGTG3′, R: 5′CGGCTGA-ATCTGGTCAAAC3′(AY949177).
Each amplicon was sequenced to ensure the authenticity of the DNA product and qRT-PCR melt curve analysis performed to demonstrate amplicon homogeneity. Each qRT-PCR reaction well contained: 6 μl Sybr Green Master Mix (PE Applied Biosystems, Foster City, CA), 1 μl primer, 2.0 μl molecular grade H2O and 1.0 μl of cDNA (50 ng ul−1). Controls containing no reverse transcriptase were also used. The cycling conditions consisted of 40 cycles of 95°C for 15 min and 60°C for 1 min. At the end of each run dissociation melt curves were obtained.
The abundance of each mRNA transcript was measured and expression relative to ARP-P0 calculated using the comparative threshold cycle (Ct) method (Q-gene qRT-PCR analysis software), which provides a quantitative measure of the relative abundance of a specific transcript in different tissues by the comparative threshold cycle (Ct) method, which takes into account any differences in the amplification efficiencies of the target and reference genes. The Ct value was taken as the lowest statistically significant (>10 standard deviation (s.d.)) increase in fluorescence above the background signal in an amplification reaction.
Insulin signalling protein analysis
The abundance of protein for the insulin signalling molecules and subunits of the mitochondrial respiratory chain were determined using western blotting as described in detail elsewhere (Forhead et al. 2008). Briefly, tissue samples (50 mg) were homogenised in lysis buffer, and centrifuged at 15 000 g at 4°C for 5 min to remove lipid and insoluble material. Protein content of the clarified extracts was determined by a modification of the Lowry method. Equal volumes of protein (10 μg and 15 μg for insulin signalling and mitochondrial respiratory chain proteins, respectively) were subjected to SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membrane (Millipore, MA, USA), blocked overnight and then incubated with primary antibody against: insulin receptor β subunit, IGF1 receptor, PI3 kinase p85, Akt1, Akt2, Ser473 phosphoAKT, protein kinase Cζ and GLUT4 (Forhead et al. 2008) or with an antisera cocktail that cross-reacts with several subunits of the mitochondrial respiratory chain (MS601, Mitosciences, Eugene, OR, USA) which was validated for use in sheep tissues. Membranes were washed and bound antibody detected using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents according to the manufacturer's instructions (Amersham/GE Healthcare, Little Chalfont, UK). Image Quant software (GE Healthcare) was used to quantify specific bands. To monitor the linearity of the density measurements, 10 μg and 20 μg of the same protein sample was loaded onto each gel to confirm that the chemiluminescent signal changed in a linear manner for all experiments. Prior to western blotting analysis samples (20 μg protein) were subjected to SDS-PAGE and gels stained with coomassie blue. This revealed that there were no differences in abundance of the major proteins present in samples between the different experimental groups.
Measurement of enzyme activity and mitochondrial proteins
Muscle samples (50 mg) were homogenized 1 : 19 (w/v) in 50 mmol l−1 Tris-HCl, 1 mmol l−1 EDTA, and 0.1% Triton X-100, pH 7.2, using a Polytron (Kinematica, Littau-Lucerne, Switzerland) and subjected to three freeze–thaw cycles. The activities of cytochrome c oxidase (CCO), citrate synthase (CS), succinate dehydrogenase (SDH), β-hydroxyacyl-CoA dehydrogenase (βHAD), hexokinase (HK), phosphofructokinase (PFK) and lactate dehydrogenase (LDH) were determined using a Spectra Max 250 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) at 37°C as described in detail elsewhere (MacArthur et al. 2008). Briefly, cytochrome c oxidase (CCO) activity was measured in a reaction mixture containing 100 mm KH2PO4/K2HPO4 and 0.1 mm cytochrome c reduced with sodium hydrosulfite (Na2S2O4), pH 7.0. The rate of change in absorbance was monitored at 550 nm (e−19.1 mmol ml−1 cm−1). Citrate synthase activity was measured in a reaction mixture containing 100 mm Tris-HCl, 1 mm MgCl2, 1 mm EDTA, 0.2 mm dithio-bis(2-nitrobenzoic acid), 0.3 mm acetyl CoA and 0.5 mm oxaloacetate, pH 8.2. The rate of change in absorbance was monitored at 412 nm (e−13.6 mmol ml−1 cm−1). Succinate dehydrogenase (SDH) activity of extracts was measured spectrophotometrically by assessing the rate of 2,6-dichlorophenol-indophenol (DCIP) reduction at 600 nm (e−21 mmol ml−1 cm−1). SDH assays were conducted in 50 mm KH2PO4/K2HPO4 buffer, pH 7.0, 20 mm succinate, 2.5 mm antimycin A, 2.5 mm KCN, 0.45 mm phenazine methosulphate and 0.12 mm DCIP. For β-hydroxyacyl CoA dehydrogenase (βHAD), the reaction mixture contained 50 mm imidazole, 0.15 mm NADH and 0.1 mm acetoacetyl CoA, pH 7.4. Lactate dehydrogenase (LDH) assays were conducted in 10 mm KH2PO4/K2HPO4 buffer, 0.2 mm NADH and 2 mm pyruvate, pH 7.2. Phosphofructokinase activity was measured in a reaction mixture containing 50 mm imidazole, 6 mm MgCl2, 60 mm KCl, 5 mm ATP, 0.4 mm NADH, excess aldolase (1 U), excess triosephosphate isomerase (50 U), excess α-glycerophosphate dehydrogenase (8 U) and 5 mm fructose-6-phosphate, pH 7.4. βHAD, LDH and PFK activities were determined by monitoring the rate of NADH oxidation at 340 nm (e−6.22 mmol ml−1 cm−1). Hexokinase assays were conducted in 50 mm imidazole, pH 7.4, 9 mm ATP, 9 mm MgCl2, 0.6 mm NADP, excess levels of glucose-6-phosphate dehydrogenase (4 U) and 5 mm glucose, by assessing the rate of NADP reduction at 340 nm (e−6.22 mmol ml−1 cm−1). All assays were conducted in duplicate, and reaction rates were linear for >2 min.
Statistical analysis and calculations
Data for protein expression/phosphorylation are presented as percentage of mean control value ±s.e.m. All other values are presented as the mean ±s.e.m. P < 0.05 was considered statistically significant.
Fetal study
The effects of PR on fetal size, mRNA and protein expression and enzyme activities were determined using a Student's unpaired t-test. Relationships between variables were determined using linear regression and partial correlation analyses.
Lamb study
The classification of IUGR was determined using a frequency distribution curve of birth weights of control singleton lambs from a separate cohort (n= 45) studied in this laboratory over the preceding 5 years. Newborn lambs in the current study were classified as IUGR when their birth weight was greater than 2 s.d. below the mean of this cohort, equivalent to the 3rd percentile (IUGR: < 4.3 kg,) or normally grown if their birth weight was within 2 s.d. of the mean (Control: 4.5–6.7 kg). Two lambs in the PR group were excluded from the study due to the fact that their weight did not fall below the 4.3 kg cut-off point for IUGR. No control fetuses were excluded.
Daily growth rate (%) was calculated as body weight gained per day as a percentage increase from the previous day's body weight ((body weightn/body weightn−1× 100) − 100)%.
The effects of placental restriction and sex on mRNA and protein levels and enzyme activity were determined using two-way analysis of variance (ANOVA). In the presence of an interaction between the effects of placental restriction and sex, the effects of PR were determined separately in male and female lambs. Relationships between variables were determined using linear regression and partial correlation analyses. All analyses were performed using SPSS for Windows v. 16 (SPSS Inc, Chicago, IL, USA).
Results
Placental and fetal growth restriction, fetal hypoxaemia and hypoglycaemia
Mean gestational arterial 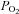 was lower in PR fetuses compared to controls (Control, 24.57 ± 2.2 mmHg; PR, 14.39 ± 0.8 mmHg; P < 0.0001). PR fetuses also had significantly lower plasma glucose concentrations during late gestation (Control, 1.01 ± 0.03 mmol l−1; PR, 0.68 ± 0.07 mmol l−1; P < 0.01). Fetal weight was lower in PR fetuses at 141–144 d gestation (Control, 5.14 ± 0.2 kg; PR, 2.69 ± 0.3 kg; P < 0.0001). The protein content of quadriceps muscle was also lower in the PR group (Control, 57.5 ± 2.6 mg (g wet weight)−1; PR, 48.5 ± 1.0 mg (g wet weight)−1, P < 0.01).
was lower in PR fetuses compared to controls (Control, 24.57 ± 2.2 mmHg; PR, 14.39 ± 0.8 mmHg; P < 0.0001). PR fetuses also had significantly lower plasma glucose concentrations during late gestation (Control, 1.01 ± 0.03 mmol l−1; PR, 0.68 ± 0.07 mmol l−1; P < 0.01). Fetal weight was lower in PR fetuses at 141–144 d gestation (Control, 5.14 ± 0.2 kg; PR, 2.69 ± 0.3 kg; P < 0.0001). The protein content of quadriceps muscle was also lower in the PR group (Control, 57.5 ± 2.6 mg (g wet weight)−1; PR, 48.5 ± 1.0 mg (g wet weight)−1, P < 0.01).
Placental restriction and the expression of the insulin and IGF1 receptor and post-receptor signalling proteins in fetal muscle
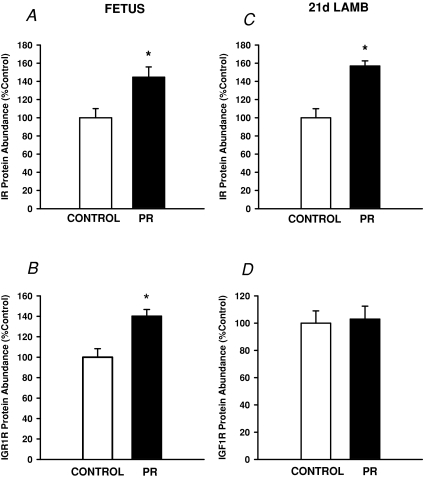
The mRNA expression of both the A and B subunits of the IR in quadriceps muscle was not different between Control and PR fetuses (IRA Control, 0.038 ± 0.006; PR, 0.055 ± 0.01; IRB Control, 0.041 ± 0.02; PR, 0.017 ± 0.003). The protein abundance of the IR and IGF1R in quadriceps muscle were each higher (P < 0.01) in PR fetuses compared to Controls (Fig. 1A and B).
Figure 1. The abundance of protein for the insulin receptor (IR, A and C) and IGF1 receptor (IGF1R, B and D) in quadriceps muscle in the Control (open bars) and PR (filled bars) fetus (A and B) and 21 d lamb (C and D).
Protein abundance is expressed as a percentage of the mean value of the Control group. *Significant effect of PR on protein abundance (P < 0.05).
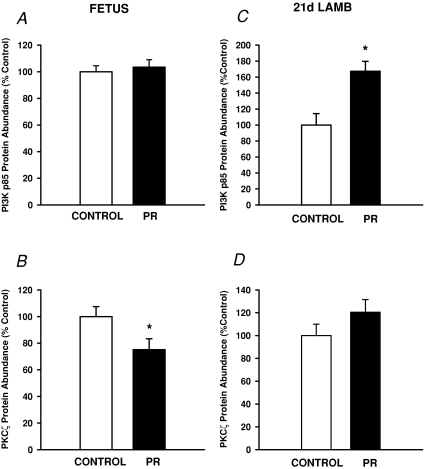
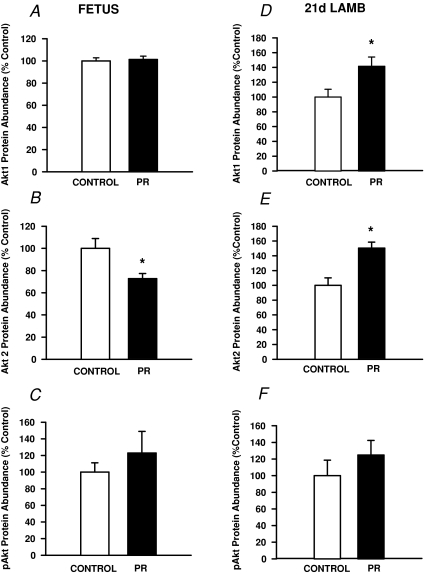
There was no effect of placental restriction on the abundance of protein for the PI3Kp85α subunit, Akt1 or pAkt within the fetal quadriceps muscle (Figs 2A and 3A and C). The abundance of Akt2 (P < 0.02) and PKCζ (P < 0.05) was lower in PR compared to Control fetuses (Figs 2B and 3B).
Figure 2. The abundance of protein for PI3K (A and C) and PKCζ (B and D) in quadriceps muscle in the Control (open bars) and PR (filled bars) fetus (A and B) and 21 d lamb (C and D).
Protein abundance is expressed as a percentage of the mean value of the Control group. *Significant effect of PR on protein abundance (P < 0.05).
Figure 3. The abundance of protein for Akt1 (A and D), Akt2 (B and E) and pAkt (C and F) in quadriceps muscle in the Control (open bars) and PR (filled bars) fetus (A, B and C) and 21 d lamb (D, E and F).
Protein abundance is expressed as a percentage of the mean value of the Control group. *Significant effect of PR on protein abundance (P < 0.05).
Placental restriction and expression of glucose transporters, PGC-1α and PPARα in fetal muscle
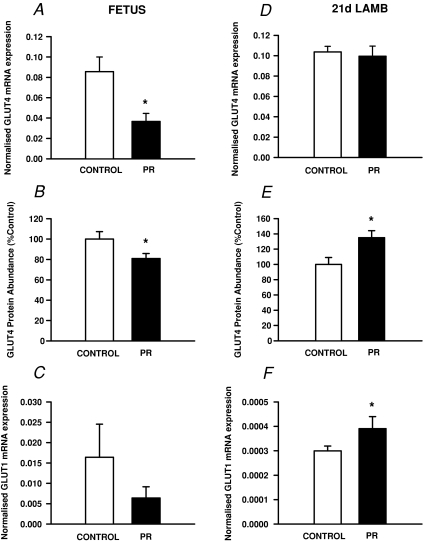
The expression of GLUT4 mRNA and protein was each lower (P < 0.05) in the quadriceps muscle of PR fetuses (Fig. 4A and B). There was no difference in the expression of GLUT1 mRNA between Control and PR fetuses (Fig. 4C). In PR fetuses, but not in Control fetuses, GLUT1 mRNA expression was inversely related to fetal body weight at ∼140 d gestation (r2= 0.65; P < 0.01).
Figure 4. The relative expression of GLUT4 mRNA (A and D), abundance of GLUT4 protein (B and E) and expression of GLUT1 mRNA (C and F) in quadriceps muscle in the Control (open bars) and PR (filled bars) fetus (A, B and C) and 21 d lamb (D, E and F).
The expression of GLUT1 and GLUT4 mRNA was normalised to the housekeeper gene ARP-P0. Protein abundance is expressed as a percentage of the mean value of the Control group. *Significant effect of PR on normalised mRNA expression or protein abundance (P < 0.05).
Placental restriction resulted in a decrease in PGC-1α mRNA (Control, 0.032 ± 0.005; PR, 0.019 ± 0.004, P < 0.05) and PPARα mRNA (Control, 0.35 ± 0.04; PR, 0.20 ± 0.05, P < 0.05) expression in quadriceps muscle.
Placental restriction and the activity of mitochondrial and glycolytic enzymes in fetal muscle
Citrate synthase (CS) (P= 0.08) and succinate dehydrogenase activity (SDH) (P < 0.09) tended to be lower in PR fetuses compared to Controls (Table 1), and the expression of CS (r2= 0.62; P < 0.01, n= 15) and SDH (r2= 0.57; P < 0.01, n= 15) was each directly related to fetal body weight. There was no effect of placental restriction on the activity of β-HAD, CCO, HK, PFK or LDH in fetal quadriceps muscle (Table 1).
Table 1.
The effect of placental restriction on activity of glycolytic and oxidative enzymes and on the protein abundance of subunits from respiratory complexes I–IV in fetal quadriceps muscle at ∼140 d gestation
| Control (n= 7) | PR (n= 8) | |
|---|---|---|
| Cytochrome c oxidase (μmol min−1 (g wet wt)−1) | 4.91 ± 0.54 | 4.43 ± 0.74 |
| Citrate synthase (μmol min−1 (g wet wt)−1) | 3.13 ± 0.31 | 2.43 ± 0.21# |
| Succinate dehydrogenase (μmol min−1 (g wet wt)−1) | 0.29 ± 0.03 | 0.22 ± 0.02# |
| β-Hydroxyacyl CoA dehydrogenase (μmol min−1 (g wet wt)−1) | 0.46 ± 0.04 | 0.40 ± 0.03 |
| Hexokinase (μmol min−1 (g wet wt)−1) | 0.19 ± 0.01 | 0.20 ± 0.02 |
| Phosphofructokinase (μmol min−1 (g wet wt)−1) | 6.8 ± 1.19 | 7.0 ± 0.76 |
| Lactate dehydrogenase (μmol min−1 (g wet wt)−1) | 45.7 ± 3.1 | 42.1 ± 3.5 |
| Mitochondrial complex I abundance (a.u.) | 140.9 ± 19.1 | 92.2 ± 15.1 |
| Mitochondrial complex II abundance (a.u.) | 32.3 ± 5.8 | 24.1 ± 4.4 |
| Mitochondrial complex III abundance (a.u.) | 88.7 ± 3.5 | 71.7 ± 4.4* |
| Mitochondrial complex IV abundance (a.u.) | 77.4 ± 4.6 | 65.1± 2.9* |
#P < 0.09, *P < 0.05 compared to Control fetuses. a.u., arbitrary units.
There was a significant reduction in the abundance of protein subunits from Complex III and Complex IV (P < 0.05, Table 1). There was no difference, however, in the abundance of protein subunits from Complex I (P= 0.07) or Complex II in the muscle of PR fetuses compared to Controls (Table 1).
Placental restriction, postnatal growth and expression of the insulin receptor and post insulin signalling proteins in muscle of the postnatal lamb
PR lambs were lighter at birth (Control, 5.86 ± 0.12 kg; PR, 3.76 ± 0.17, P < 0.001). The fractional growth rate during the first 3 weeks of postnatal life was higher in PR lambs compared to Controls (Control, 4.26 ± 0.14%; PR, 5.26 ± 0.16%, P < 0.01). The body weight of PR lambs was, however, still lower than Control lambs at 21 d of age (Control, 13.22 ± 0.17 kg; PR, 10.00 ± 0.40 kg, P < 0.01). Plasma glucose (Control, 5.83 ± 0.19 mmol l−1; PR, 5.50 ± 0.11 mmol l−1) and insulin (Control, 2.08 ± 0.16 ng ml−1; PR, 1.77 ± 0.29 ng ml−1) concentrations during the first 3 weeks of postnatal life were not different between Control and PR lambs.
There was no difference in the protein content of the muscle of Control and PR lambs at 21 d of age (Control, 78.2 ± 3.1 mg (g wet weight)−1; PR, 83.7 ± 5.3 mg (g wet weight)−1). There was also no effect of placental restriction or sex on IRA or IRB mRNA expression in the quadriceps muscle at 21 d (IRA Control, 0.086 ± 0.005, PR, 0.095 ± 0.009; IRB Control, 0.034 ± 0.003, PR, 0.035 ± 0.005). The abundance of IR protein was higher (P < 0.001), however, in the muscle of both male and female lambs in PR lambs at 21 d (Fig. 1C). There was no effect of placental restriction on the abundance of IGF1R protein in the muscle of either male or female lambs (Fig. 1D).
The abundance of PI3K p85α subunit (Fig. 2C), Akt1 and Akt2 (Fig. 3D and E) in skeletal muscle was higher (P < 0.02) in PR male and female lambs compared to their Control counterparts at 21 d of postnatal age. There was no effect of placental restriction on the abundance of pAkt (Fig. 3F) or PKCζ (Fig. 2D) in lamb quadriceps muscle.
Placental restriction and expression of glucose transporters, PGC-1α and PPARα in lamb muscle
There was no difference in the expression of GLUT4 mRNA in the muscle between the PR and Control lambs (Fig. 4D). The abundance of GLUT4 protein, however, was higher (P < 0.02) in the quadriceps muscle of the PR lambs compared to Controls at 21 d in both males and females (Fig. 4E). The expression of GLUT1 mRNA was also higher (P < 0.05) in muscle of PR lambs, in both males and females (Fig. 4F).
PGC-1α mRNA expression was significantly higher in the skeletal muscle of male (Control, 0.031 ± 0.004; PR, 0.063 ± 0.009, P < 0.05), but not female, PR lambs compared to Controls. The expression of PPARα mRNA was not different, however, between either males or females or between PR and Control lambs.
Placental restriction and the activity of mitochondrial and glycolytic enzymes in lamb muscle
The activity of hexokinase in skeletal muscle was increased in both male and female lambs in the PR group (Control, 0.19 ± 0.02; PR, 0.26 ± 0.02, P < 0.05). There was no effect of PR or sex on the activity of any other mitochondrial enzymes at 21 d (Table 2). There was also no effect of placental restriction on the abundance of any of the subunits from complexes I–IV in the postnatal lamb (Table 2).
Table 2.
There was no effect of placental restriction on the activity per mg wet weight of mitochondrial enzymes, and abundance of protein subunits from mitochondrial respiratory complexes I–IV in lamb quadriceps muscle at 21 d postnatal age
| Control (n= 14) | PR (n= 9) | |
|---|---|---|
| Cytochrome c oxidase (μmol min−1 (g wet wt)−1) | 8.2 ± 0.4 | 7.6 ± 0.6 |
| Citrate synthase (μmol min−1 (g wet wt)−1) | 6.9 ± 0.3 | 7.2 ± 0.6 |
| Succinate dehydrogenase (μmol min−1 (g wet wt)−1) | 0.32 ± 0.02 | 0.27 ± 0.03 |
| β-Hydroxyacyl CoA dehydrogenase (μmol min−1 (g wet wt)−1) | 0.63 ± 0.02 | 0.67 ± 0.06 |
| Phosphofructokinase (μmol min−1 (g wet wt)−1) | 40.6 ± 3.2 | 36.9 ± 4.9 |
| Lactate dehydrogenase (μmol min−1 (g wet wt)−1) | 145.5 ± 7.1 | 133.3 ± 13.2 |
| Complex I abundance (a.u.) | 181.3 ± 31.6 | 182.4 ± 11.0 |
| Complex II abundance (a.u.) | 38.1 ± 9.5 | 47.8 ± 6.3 |
| Complex III abundance (a.u.) | 107.6 ± 6.3 | 112.2 ± 5.3 |
| Complex IV abundance (a.u.) | 101.8 ± 4.9 | 92.4 ± 3.8 |
a.u., arbitrary units.
Discussion
The present study has provided new insights into the molecular mechanisms underlying the transition from growth restriction in utero to postnatal catch-up growth. We found that whilst the abundance of insulin and IGF1 receptors were increased in the quadriceps muscle of growth restricted fetus, this was accompanied by a decrease in the abundance of the insulin signalling molecule, PKCζ, and of the insulin responsive glucose transporter, GLUT4. Interestingly, however, the increase in the abundance of the insulin receptor in the IUGR lamb persisted after birth and was then associated with an up-regulation of Akt1 and 2 and GLUT4 protein. These data provide the first evidence for the in utero origins of the increased insulin sensitivity in skeletal muscle which drives accelerated growth after birth.
Placental restriction and insulin signalling in fetal skeletal muscle
Consistent with previous studies using the placentally restricted (PR) sheep model, PR fetuses in the current study were chronically hypoxaemic, hypoglycaemic and growth restricted (Simonetta et al. 1997; Duffield et al. 2008). A key finding of the present study was that fetal substrate deprivation was associated with an increased abundance of insulin and IGF1 receptor (IGF1R) protein in the quadriceps muscle before birth. It has consistently been reported that plasma concentrations of both insulin and IGF1 are reduced in growth-restricted fetuses (Kind et al. 1995; Cetin et al. 2001; Duffield et al. 2008). Previous studies have shown that insulin and IGF1 receptor abundance in muscle is increased proportionally in response to a decrease in availability of signalling hormone (Duckworth et al. 1998; Flati et al. 2008), which suggests that increases in IR and IGF1 receptor abundance in the growth restricted fetus may be a response to the low circulating concentrations of these hormones.
The increase in the abundance of IR protein in the PR fetus did not appear to lead to increased insulin action (as assessed by Akt phosphorylation), at least under basal conditions. We found that there was a downregulation of Akt2, PKCζ and the insulin-responsive glucose transporter, GLUT4, in the muscle of the growth restricted fetuses. The IRS–PI3k–Akt pathway stimulates GLUT4 translocation and regulates fusion of GLUT4 containing vesicles with the plasma membrane (Kanzaki, 2006), whilst atypical PKC isoforms lambda and zeta (aPKCλ/ζ) play a role as downstream targets for the IRS–PI3K signalling pathway (Kanzaki, 2006). Therefore, while growth restricted fetuses had an increased abundance of insulin receptors, they also had reduced abundance of signalling molecules responsible for the translocation of GLUT4, and lower GLUT4 protein content.
Recent studies have reported that there was no difference in insulin sensitivity between placentally restricted and control fetal sheep in late gestation as measured by hyperinsulinaemic euglycaemic clamp (Owens et al. 2007). This suggests, therefore, that the reduced abundance of signalling proteins downstream of the insulin receptor in the growth restricted fetus is most likely due to the low level of activation of the receptor by insulin. A decrease in the insulin-mediated glucose uptake and protein synthesis in skeletal muscle would be an important adaptive response, enabling this tissue to maintain basal metabolic activity in the low insulin, low glucose environment of the growth restricted fetus.
Placental restriction and insulin signalling in skeletal muscle of the postnatal lamb
As expected, the IUGR lambs in the present study were smaller and thinner at birth and had a higher daily fractional growth rate in the first 2 weeks of life compared with control lambs. Importantly, there was a greater abundance of insulin receptor protein in the skeletal muscle of the IUGR lamb at 21 d of postnatal age. Furthermore, and in direct contrast to the growth-restricted fetus, we found that by 21 d of postnatal life, there was an increase in protein expression of Akt1 and Akt2 and increased abundance of the insulin-sensitive glucose transporter, GLUT4, and the insulin-independent glucose transporter, GLUT1, in the skeletal muscle of growth restricted lambs. Therefore, we have shown that in growth restricted lambs, there is an increase in the proteins required for the promotion of GLUT4-containing vesicle translocation and fusion with the cellular membrane. This suite of molecular changes is consistent with an increase in both basal, i.e. insulin-independent, and insulin-stimulated glucose uptake in the skeletal muscle of the postnatal lambs undergoing accelerated growth (De Blasio et al. 2006).
We found that the protein abundance, but not mRNA expression, of the insulin receptor was increased in the IUGR postnatal lamb. This suggests that the stable increase in insulin receptor protein is likely to be a consequence of reduced protein turnover or production, rather than increased gene transcription. One possibility is that reduced clearance of the insulin receptor within the skeletal muscle of the growth restricted fetus occurs as a consequence of the low insulin concentrations before birth, and that this reduced clearance rate persists into postnatal life and results in an enhanced capacity for insulin signalling.
Experimental studies using a range of rodent models of intrauterine growth restriction (IUGR) have shown that IUGR is associated with an early phase of enhanced insulin sensitivity, followed by the later emergence of insulin resistance and type 2 diabetes (Ozanne et al. 1996; Ozanne, 2001; Ozanne et al. 2005). It has also been shown that young healthy men who had a low birth weight have reduced skeletal muscle expression of the PI3K p85α regulatory subunit, the p110β catalytic subunit and PKCζ and GLUT4, changes which have been shown to precede the development of whole body insulin resistance and glucose intolerance (Ozanne et al. 2005). In a recent follow-up study, in the same cohort of LBW men there was a marked insulin-mediated up-regulation of the PI3K p85α and p110β subunits and reduced PKCζ and pAkt responses, both of which are required for insulin-stimulated glucose uptake (Jensen et al. 2008). Interestingly increased expression of p85α has been implicated in insulin resistance as it has been proposed that excess p85α may act to sequester IRS-1 and PI3K enzymatic activity into inert cellular foci incapable of PI-3,4,5-trisphosphate (PIP3) generation (Luo et al. 2005).
The data from the current study highlight that IUGR results in changes in the insulin signalling pathway which are present in fetal life and which may be the antecedents of the initial phase of enhanced insulin sensitivity which is associated with accelerated growth in early postnatal life and which precedes the later emergence of insulin resistance.
Placental restriction and protein synthesis in fetal and lamb skeletal muscle
In the current study we found that growth-restricted fetuses were not only smaller in late gestation, but also had a lower skeletal muscle protein content. We found that fetal growth restriction resulted in a significant decrease in the abundance of Akt2 within the skeletal muscle. The Akt signalling pathway activates the mTOR complex which controls protein synthesis (Kimball, 2007). The reduced availability of Akt2 in the skeletal muscle of growth restricted fetuses may therefore reduce the capacity for insulin action through this pathway and explain the reduction in muscle protein content. By 21 d of postnatal age, however, we found that the abundance of both Akt1 and Akt2 proteins was significantly increased in the skeletal muscle of IUGR lambs and that myocellular protein content in IUGR lambs was restored to levels present in control lambs, suggesting a rapid recovery of muscle protein synthesis in the early postnatal period. It would be important to determine whether in the growth restricted lamb insulin action results in an increased pAkt response in order to determine whether enhanced activation of the Akt signalling proteins represents the molecular basis for the increased rate of muscle protein synthesis in IUGR offspring in the immediate postnatal period.
Growth restriction and mitochondrial biogenesis in skeletal muscle
Mitochondria play a central role in the regulation of cellular energy metabolism, and impaired mitochondrial function in skeletal muscle has been associated with the onset of insulin resistance both in animal models and in human subjects (Højlund et al. 2008). Whilst there is clear evidence from previous studies that fetal growth restriction is associated with an increased incidence of insulin resistance in later life (Ozanne, 2001; Ozanne & Hales, 2002), there are few studies which have investigated the impact of IUGR on markers of mitochondrial biogenesis and activity of mitochondrial enzymes before or after birth (Peterside et al. 2003; Selak et al. 2003).
In the present study, we found that the mRNA expression of PGC-1α, a key transcriptional activator of mitochondrial biogenesis, was lower in skeletal muscle of growth restricted fetuses. Furthermore, abundance of two respiratory complexes of the electron transport chain were lower, and the activity of citrate synthase and succinate dehydrogenase tended to be lower, in skeletal muscle in growth restricted fetuses. Citrate synthase catalyses the first step in the Krebs cycle, whilst succinate dehydrogenase plays a vital role in both the Krebs cycle and the electron transport chain. Correct functioning of both these enzymes is therefore essential for the complete oxidation of glucose. These changes may therefore represent an adaptation which reduces the capacity of the muscle for oxidative phosphorylation, and thus slows muscle growth, when there is a decrease in fetal substrate supply. These results are somewhat different from those in the rodent, in which fetal growth restriction induced by uterine artery ligation in late gestation was associated with increased abundance of PGC-1α mRNA and protein in the skeletal muscle at birth and at 21 d of age (Lane et al. 2003). Other studies have also shown, however, that these animals have reduced PGC-1α mRNA and protein and cytochrome oxidase III and IV mRNA at 6 months of age (Wadley et al. 2008), and that this precedes the development of insulin resistance and glucose intolerance in this animal model of fetal growth restriction (Siebel et al. 2008).
In addition to its role in mitochondrial biogenesis, PGC-1α is also known to activate intracellular fatty acid oxidation through the activation of PPARα, and the abundance of PPARα mRNA in skeletal muscle was also significantly lower in growth restricted fetuses. Whilst experimental restriction of placental growth has been shown to decrease plasma glucose concentrations, it is not clear whether plasma concentrations of free fatty acids are also lower, and therefore the potential implication of placental restriction on fetal fatty acid metabolism is unknown.
At 21 d of postnatal age, expression of PGC-1α mRNA was higher in skeletal muscle of growth restricted male, but not female, lambs. It is interesting that the response of PGC-1α in skeletal muscle was the only change in this study which was sex specific. This is consistent with previous work in rats, in which increases in PGC-1α mRNA and protein in rat pups exposed to uterine artery ligation in late gestation were more marked in male offspring compared to females (Lane et al. 2003).
Interestingly, there appeared to be no effect of fetal growth restriction on the abundance of respiratory complexes or activity of key oxidative (SDH, CS, CCO, βHAD) and glycolytic enzymes (PFK, LDH) at 21 d of postnatal age, with the exception of a significant increase in the activity of the glycolytic enzyme hexokinase in both growth restricted male and female lambs. The principal role of hexokinase is to convert glucose to glucose-6-phosphate, which is trapped within the cell and used as a substrate for glycolysis (Arai et al. 1995). The increase in hexokinase activity may therefore result in greater efficiency of the muscle cell to retain glucose and facilitate its metabolic conversion.
Summary
It is well established that an accelerated growth and increased peripheral insulin sensitivity are present in the early postnatal period following fetal growth restriction, but to date there have been no studies which have determined the mechanisms which explain the transition from growth restriction in utero to enhanced insulin sensitivity and catch-up growth in the early postnatal period. The present study has provided important and novel insights into the molecular mechanisms underlying the transition from growth restriction in utero to postnatal catch-up growth which commonly occurs in growth-restricted infants after birth.
We have shown that fetal growth restriction is associated with an up-regulation of insulin receptors before birth. Importantly, the increase in the abundance of the insulin receptor persists in the IUGR postnatal lamb and is then associated with an up-regulation of Akt1 and 2 and GLUT4 protein. The presence of higher insulin receptor abundance when the fetus emerges into the postnatal environment where nutrient supply is no longer constrained may explain the accelerated assimilation of nutrients into skeletal muscle, and accelerated growth rate in these infants. The findings of this study therefore suggest that catch-up growth has its origins in the fetal adaptive response to a low substrate and insulin environment. A greater understanding of the mechanism that determines the programmed increase in insulin receptor abundance in the IUGR offspring may contribute to a better understanding of the mechanisms which result in the subsequent emergence of insulin resistance and metabolic disease in adult life in growth restricted infants.
Acknowledgments
This work was funded by the National Health and Medical Research Council of Australia (NHMRC) (I.C.McM.) and a Channel 7 Children's Health Research Grant (B.S.M.). B.S.M. is supported by a Peter Doherty Post Doctoral Fellowship (NHMRC) and N.T. and J.M. are supported by Career Development Awards (NHMRC). S.O. is funded by the British Heart Foundation. J.M. is also a Research Fellow of the National Heart Foundation of Australia. We are grateful to Laura O’Carroll and Anne Jurisevic for their expert assistance with animal surgery and experimental protocols.
Glossary
Abbreviations
- Akt1
protein kinase B
- βHAD
β-hydroxyacyl-CoA dehydrogenase
- CCO
cytochrome c oxidase
- CS
citrate synthase
- GLUT1
glucose transporter type 1 (insulin independent)
- GLUT4
glucose transporter type 4 (insulin dependent)
- HK
hexokinase
- IGF1R
insulin like growth factor 1 receptor
- IR
insulin receptor
- IRA
insulin receptor type A
- IRB
insulin receptor type B
- IUGR
intrauterine growth restriction
- LDH
lactate dehydrogenase
- p-Akt
phosphorylated Akt
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- PFK
phosphofructokinase
- PI3Kp85α
phosphoinositide 3-kinase p85α subunit
- PKCξ
phosphokinase C ξ isoform
- PPARα
peroxisome proliferator-activated receptor-α
- PR
placental restriction
- SDH
succinate dehydrogenase
Author contributions
All authors contributed to the conception and design or analysis and interpretation of data. All authors have actively participated in the process of drafting the article or revising it critically for important intellectual content and have given final approval of the version to be published.
References
- Albertsson-Wikland K, Wennergren G, Wennergren M, Vilbergsson G, Rosberg S. Longitudinal follow-up of growth in children born small for gestational age. Acta Paediatr. 1993;82:438–443. doi: 10.1111/j.1651-2227.1993.tb12718.x. [DOI] [PubMed] [Google Scholar]
- Aldermann GA, Morgan DE, Harvard A, Edwards RE, Todd JR. Ministry of Agriculture, Fisheries and Food: Technical Bulletin 33. London: Her Majesty's Stationery Office; 1975. Energy allowances and feeding systems for ruminants; pp. 34–36. [Google Scholar]
- Arai T, Washizu T, Sagara M, Sako T, Nigi H, Matsumoto H, Sasaki M, Tomoda I. D-Glucose transport and glycolytic enzyme activities in erythrocytes of dogs, pigs, cats, horses, cattle and sheep. Res Vet Sci. 1995;58:195–196. doi: 10.1016/0034-5288(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Fetal and Infant Origins of Adult Disease. London: British Medical Journal Publishing Group; 1992. [Google Scholar]
- Bavdekar A, Yajnik C, Fall C, Bapat S, Pandit A, Deshpande V, Bhave S, Kellingray S, Joglekar C. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes. 1999;48:2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- Cetin I, Radaelli T, Taricco E, Giovannini N, Alvino G, Pardi G. The endocrine and metabolic profile of the growth-retarded fetus. J Pediatr Endocrinol Metab. 2001;14:1497–1505. [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth and adiposity in the young lamb. Endocrinology. 2006;148:1350–1358. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol. 2007;292:R875–886. doi: 10.1152/ajpregu.00430.2006. [DOI] [PubMed] [Google Scholar]
- Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- Duffield JA, Vuocolo T, Tellam R, Yuen BS, Muhlhausler BS, McMillen IC. Placental restriction of fetal growth decreases IGF1 and leptin mRNA expression in the perirenal adipose tissue of late gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1413–1419. doi: 10.1152/ajpregu.00787.2007. [DOI] [PubMed] [Google Scholar]
- Economides DL, Nicolaides KH, Campbell S. Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J Perinatal Med. 1991;19:97–105. doi: 10.1515/jpme.1991.19.1-2.97. [DOI] [PubMed] [Google Scholar]
- Economides DL, Proudler A, Nicolaides KH. Plasma insulin in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;160:1091–1094. doi: 10.1016/0002-9378(89)90167-1. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Simonetta G, Owens JA, Robinson JS, McMillen IC. Restriction of placental and fetal growth in sheep alters fetal blood pressure responses to angiotensin II and captopril. J Physiol. 1999;515:897–904. doi: 10.1111/j.1469-7793.1999.897ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi G, Zanardo V, Caretta F, Inelmen EM, Rubaltelli F. Intrauterine growth and adipose tissue development. Am J Clin Nutr. 1981;34:1785–1790. doi: 10.1093/ajcn/34.9.1785. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25:735–740. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- Fitzhardinge PM, Steven EM. The small-for-date infant. I. Later growth patterns. Pediatrics. 1972;49:671–681. [PubMed] [Google Scholar]
- Flati V, Pasini E, D’Antona G, Speca S, Toniato E, Martinotti S. Intracellular mechanisms of metabolism regulation: the role of signalling via the mammalian target of rapamycin pathway and other routes. Am J Cardiol. 2008;101:16E–21E. doi: 10.1016/j.amjcard.2008.02.075. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Lamb CA, Franko KL, O’Connor DM, Wooding FBP, Cripps RL, Ozanne S, Blache D, Shen QW, Du M, Fowden AL. Role of leptin in the regulation of growth and carbohydrate metabolism in the ovine fetus during late gestation. J Physiol. 2008;586:2393–2403. doi: 10.1113/jphysiol.2007.149237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Højlund K, Mogensen M, Sahlin K, Beck-Nielsen H. Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinol Metabol Clin North Am. 2008;37:713–731. doi: 10.1016/j.ecl.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Holemans K, Verhaeghe J, Dequeker J, Van Assche FA. Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J Soc Gynecol Investig. 1996;3:71–77. doi: 10.1016/1071-5576(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 2000;85:1401–1406. doi: 10.1210/jcem.85.4.6544. [DOI] [PubMed] [Google Scholar]
- Jensen CB, Martin-Gronert MS, Storgaard H, Madsbad S, Vaag A, Ozanne SE. Altered PI3-kinase/Akt signalling in skeletal muscle of young men with low birth weight. PLoS ONE. 2008;3:e3738. doi: 10.1371/journal.pone.0003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M. Insulin receptor signals regulating GLUT4 translocation and actin dynamics. Endocr J. 2006;53:267–293. doi: 10.1507/endocrj.kr-65. [DOI] [PubMed] [Google Scholar]
- Kimball SR. The role of nutrition in stimulating muscle protein accretion at the molecular level. Biochem Soc Trans. 2007;35:1298–1301. doi: 10.1042/BST0351298. [DOI] [PubMed] [Google Scholar]
- Kind KL, Owens JA, Robinson JS, Quinn KJ, Grant PA, Walton PE, Gilmour RS, Owens PC. Effect of restriction of placental growth on expression of IGFs in fetal sheep: relationship to fetal growth, circulating IGFs and binding proteins. J Endocrinol. 1995;146:23–34. doi: 10.1677/joe.0.1460023. [DOI] [PubMed] [Google Scholar]
- Lane RH, Kelley DE, Ritov VH, Tsirka AE, Gruetzmacher EM. Altered expression and function of mitochondrial β-oxidation enzymes in juvenile intrauterine-growth-retarded rat skeletal muscle. Pediatr Res. 2001;50:83–90. doi: 10.1203/00006450-200107000-00016. [DOI] [PubMed] [Google Scholar]
- Lane RH, Maclennan NK, Daood MJ, Hsu JL, Janke SM, Pham TD, Puri AR, Watchko JF. IUGR alters postnatal rat skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1 gene expression in a fibre specific manner. Pediatr Res. 2003;53:994–1000. doi: 10.1203/01.PDR.0000064583.40495.51. [DOI] [PubMed] [Google Scholar]
- Leger J, Limoni C, Czernichow P. Prediction of the outcome of growth at 2 years of age in neonates with intra-uterine growth retardation. Early Hum Dev. 1997;48:211–223. doi: 10.1016/s0378-3782(96)01855-5. [DOI] [PubMed] [Google Scholar]
- Levy-Marchal C, Jaquet D. Long-term metabolic consequences of being born small for gestational age. Pediatr Diabetes. 2004;5:147–153. doi: 10.1111/j.1399-543X.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell U-B, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ. 1996;312:406–410. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. The p85 regulatory subunit of phosphoinositide 3-kinase down-regulates IRS-1 signalling via the formation of a sequestration complex. J Cell Biol. 2005;170:455–464. doi: 10.1083/jcb.200503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Seto JT, Chan S, Quinlan KGR, Raftery JM, Turner N, Nicholson MD, Kee AJ, Hardeman EC, Gunning PW, Cooney GJ, Head SI, Yang N, North KN. An Actn3 knockout mouse provides mechanistic insights into the association between α-actinin-3 deficiency and human athletic performance. Hum Mol Genet. 2008;17:1076–1086. doi: 10.1093/hmg/ddm380. [DOI] [PubMed] [Google Scholar]
- McCance DR, Pettitt DJ, Hanson RL, Jacobsson LTH, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994;308:942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565:9–17. doi: 10.1113/jphysiol.2004.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R306–313. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Adam PA, Findlay CL, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 2006;20:1257–1259. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ (PPARγ), adiponectin and leptin mRNA expression in adipose tissue before birth. Endocrinology. 2007;148:878–885. doi: 10.1210/en.2006-1115. [DOI] [PubMed] [Google Scholar]
- Owens JA, Gatford KL, De Blasio MJ, Edwards LJ, McMillen IC, Fowden AL. Restriction of placental growth in sheep impairs insulin secretion but not sensitivity before birth. J Physiol. 2007;584:935–949. doi: 10.1113/jphysiol.2007.142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE. Metabolic programming in animals: Type 2 diabetes. Br Med Bull. 2001;60:143–152. doi: 10.1093/bmb/60.1.143. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN. Early programming of glucose-insulin metabolism. Trends Endocrinol Metab. 2002;13:368–373. doi: 10.1016/s1043-2760(02)00666-5. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia. 2005;48:547–552. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, Hartil K, Petry CJ, Buckley AJ, Mosthaf-Seedorf L. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003;177:235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N, Smith GD. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol Endocrinol Metab. 1996;271:E1128–1134. doi: 10.1152/ajpendo.1996.271.6.E1128. [DOI] [PubMed] [Google Scholar]
- Peterside IE, Selak MA, Simmons RA. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E1258–1266. doi: 10.1152/ajpendo.00437.2002. [DOI] [PubMed] [Google Scholar]
- Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E130–137. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- Siebel AL, Mibus A, De Blasio MJ, Westcott KT, Morris MJ, Prior L, Owens JA, Wlodek ME. Improved lactational nutrition and postnatal growth ameliorates impairment of glucose tolerance by uteroplacental insufficiency in male rat offspring. Endocrinology. 2008;149:3067–3076. doi: 10.1210/en.2008-0128. [DOI] [PubMed] [Google Scholar]
- Simonetta G, Rourke AK, Owens JA, Robinson JS, McMillen IC. Impact of placental restriction on the development of the sympathoadrenal system. Paediatric Research. 1997;42:805–811. doi: 10.1203/00006450-199712000-00015. [DOI] [PubMed] [Google Scholar]
- Wadley GD, Siebel AL, Cooney GJ, McConell GK, Wlodek ME, Owens JA. Uteroplacental insufficiency and reducing litter size alters skeletal muscle mitochondrial biogenesis in a sex-specific manner in the adult rat. Am J Physiol Endocrinol Metab. 2008;294:E861–869. doi: 10.1152/ajpendo.00037.2008. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Cook DG, Adshead F, Taylor SJ, Walker M, Papacosta O, Alberti KG. Childhood size is more strongly related than size at birth to glucose and insulin levels in 10–11-year-old children. Diabetologia. 1997;40:319–326. doi: 10.1007/s001250050681. [DOI] [PubMed] [Google Scholar]