Abstract
The elastic structures of many muscles include both an extramuscular free tendon as well as a sheet-like aponeurosis. An important distinguishing feature of aponeuroses is that these tendinous structures function as the attachment and insertion surfaces of muscle fascicles and therefore surround a substantial portion of the muscle belly. As a result, aponeuroses must expand both parallel (longitudinal) and perpendicular (transverse) to a muscle's line of action when contracting muscles bulge to maintain a constant volume. In this study, we use biplanar high-speed fluoroscopy to track the strain patterns of the turkey lateral gastrocnemius aponeurosis during active and passive force production in situ. We find that the behaviour of the aponeurosis during passive force production is consistent with uniaxial loading, as aponeuroses stretch only in the longitudinal direction. By contrast, our results show that aponeuroses are stretched in both longitudinal and transverse directions during active force production and that transverse strains are on average 4 times greater than longitudinal strains. Biaxial loading of aponeuroses appears to effectively modulate longitudinal stiffness, as we find the measured stiffness in the longitudinal direction varies in proportion to transverse strain. We conclude that biaxial strain during active force production distinguishes aponeuroses from free tendons and may function to dynamically modulate stiffness along the axis of muscle force production. It is likely that consideration of strains measured only in the longitudinal direction result in an underestimation of aponeurosis stiffness as well as its capacity for elastic energy storage.
The role of tendons in the storage and recovery of elastic energy is well established for a number of modes of terrestrial locomotion, including hopping (Biewener & Baudinette, 1995; Biewener et al. 1998), jumping (Roberts & Marsh, 2003; Henry et al. 2005), running (Cavagna et al. 1977; Alexander, 1984; Roberts et al. 1997) and walking (Cavagna et al. 1977; Ishikawa et al. 2006; Litchwark et al. 2007). During these diverse locomotor behaviours, forces exerted by contracting muscles stretch tendons, storing elastic energy that can be recovered upon unloading. The amount of energy stored in a tendon can be calculated as a function of the tendon's stiffness and the force applied (Zajac, 1989). As a result the mechanical behaviour of tendons can accurately be characterized as a spring in series with muscle.
This relatively simple mechanical behaviour is in part due to the simple structure and loading regimes of tendons. The extramuscular portion of a tendon (i.e. the free tendon) is rope-like, consisting of highly ordered bundles of collagen oriented primarily along the tendon's long axis (Jozsa et al. 1991). The actions of contracting muscles load free tendons only along this longitudinal axis. As a result of this simple loading regime, the uniaxial stiffness of tendons under tension can be used as a determinant of elastic energy storage (e.g. Bennett et al. 1986; Ker et al. 1986; Shadwick, 1990).
Free tendons are not the only spring-like elastic structures of muscle–tendon complexes. In many muscles, fascicles insert onto broad tendinous sheets (aponeuroses), which are in series with the free tendon. Similar to free tendons, collagen fascicles in aponeuroses are oriented longitudinally and are in line with the muscle's line of action (Scott & Loeb, 1995; Azizi et al. 2009). Based on such structural similarities, previous studies have considered the aponeurosis to be a mechanical extension of the free tendon, generally assuming that aponeuroses are loaded longitudinally during muscle force production (e.g. Brown et al. 1996).
Most studies of aponeurosis function have typically focused on the strain occurring along a single axis, oriented along the muscle's line of action and the free tendon's long axis. There is reason to expect, however, that unlike free tendons, aponeuroses may be loaded along more than one axis. Aponeuroses function as the attachment and insertion surfaces of muscle fascicles and therefore cover a substantial portion of the belly. The close association of aponeurosis and muscle fascicles has the potential to apply a loading regime that is more complex than that applied to the free tendon. When muscle fibres shorten they must expand orthogonal to the line of action in order to maintain a constant volume (Baskin & Paolini, 1967). The lateral expansion of a muscle during contraction could be associated with aponeurosis strain in directions orthogonal to the line of action (Scott & Loeb, 1995; van Donkelaar et al. 1999; Azizi et al. 2008). Therefore, aponeuroses may be more accurately characterized as sheet-like tendons that are loaded biaxially during muscle force production.
Biaxial loading in aponeuroses may have important functional implications. Previous studies have shown that the effective stiffness and strength of elastic structures increase significantly when the tissue is loaded biaxially (Lanir & Fung, 1974; Aspden, 1990). Therefore, loading of the aponeurosis in directions orthogonal to the muscle's line of action may function to dynamically increase tissue stiffness. Dynamic changes in stiffness may have important implications for the capacity of aponeuroses to store elastic energy during locomotion. However, the significance of biaxial loading patterns in aponeuroses remains largely unexplored.
In this study we use an in situ muscle preparation of the lateral gastrocnemius muscle of wild turkeys (Meleagris gallopavo) combined with high-speed biplanar fluoroscopy to characterize the strain patterns in the superficial aponeurosis in the longitudinal (along the muscle's line action) and transverse (orthogonal to the muscle's line of action) directions. We use this experimental protocol to quantify aponeurosis strains during active force production as the muscle undergoes a series of isotonic contractions across a range of forces. In addition, we characterize aponeurosis strains as the muscle is passively driven through a series of sinusoidal length changes. We hypothesize that if the orthogonal expansion of a contracting muscle is responsible for transverse loading in the aponeurosis, then passive length changes of the muscle–tendon will only stretch the tissue longitudinally while active force production will result in positive biaxial strains. Finally, we examine whether variation in transverse strains can effectively modulate longitudinal stiffness.
Methods
Animals
Adult, female wild turkeys (Meleagris gallopavo) were purchased from a breeder. Birds were housed at the Brown University animal care facility and provided with food and water ad libitum. All procedures were approved by the Brown University Institutional Animal Care and Use Committee.
In situ preparation
Four birds were deeply anaesthetized with inhaled isoflurane. A branch of the sciatic nerve was isolated and connected to a bipolar silver stimulating electrode. The electrode was constructed such that the bare silver wires were set in a small piece of plastic tubing and the nerve was placed inside the tubing in direct contact with the silver leads. Two 2 mm piezoelectric crystals (Sonometrics Corp., London, Ontario, Canada) were implanted along a superficial proximal fascicle of the lateral gastrocnemius muscle (Fig. 1A). These transducers were secured in place using a small drop of Vet-bond skin adhesive and used to measure fascicle lengths during each contraction. The leg was fixed to a rigid custom-made aluminium frame using an aluminium plate that was fastened to the femur with machine screws. The distal tendon of the muscle was isolated and the ossified portion of the tendon was clamped to the lever of the ergometer. The ossified tendon provided a secure, rigid and reliable connection with the ergometer. The ergometer was used to measure and control muscle force and track length changes in the whole muscle–tendon unit.
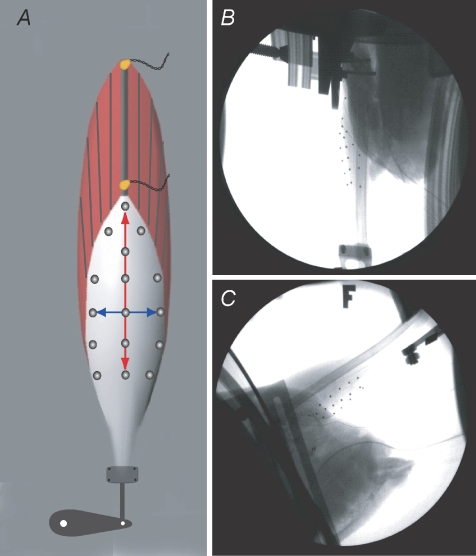
Figure 1. Methodological description of the in situ preparation used to quantify strain patterns in the lateral gastrocnemius aponeurosis of wild turkeys.
A, schematic diagram of the muscle preparation showing the location of the sonomicrometry crystals along a proximal muscle fascicle as well as the approximate position of the radio-opaque markers on the surface of the aponeurosis. The distal end of the tendon was attached to an ergometer, which controlled and measured muscle force and muscle–tendon length. B and C, single frames from videos captured using two high-speed fluoroscopes. The 3D positions of the surface markers were used to calculate the strain patterns in the aponeurosis during passive and active force production.
A thin layer of fascia was removed from the surface of the superficial aponeurosis and the area was cleaned prior to the placement of 15–20 radio-opaque steel markers. Markers (1 mm in diameter) were secured to the surface using cyanoacrylate and arranged such that the transverse and longitudinal length changes of the aponeurosis could be tracked (Fig. 1). The muscle was kept moist using physiological saline and muscle temperature was monitored and maintained between 36 and 39°C.
Prior to the tetanic isotonic contractions, the optimum stimulation voltage and fascicle length were determined. First, the muscle's twitch force was monitored as the stimulation voltage was increased by 1 V increments. The voltage that resulted in maximum twitch force was increased by 1 V and used to maximally stimulate the muscle. Second, a series of twitches at varying fascicle lengths were used to construct a length–tension relationship and determine the optimal operating length of the muscle. All tetanic contractions were started at lengths such that the shortening phase of the contraction encompassed the muscle's optimal length.
Muscles were stimulated supramaximally under isotonic conditions. Force was allowed to develop to a preset value and was maintained at a constant level using the ergometer (Fig. 2A). During the period of constant force, the muscle fascicles shortened at a nearly constant velocity (Fig. 2D). The muscle was allowed a minimum of 5 min between contractions in order to prevent fatigue. Each muscle was subject to approximately 10 contractions ranging in force from 10% to 100% of maximum isometric force. This protocol allowed us to quantify the strain patterns in the aponeurosis across the muscle's full range of shortening forces.
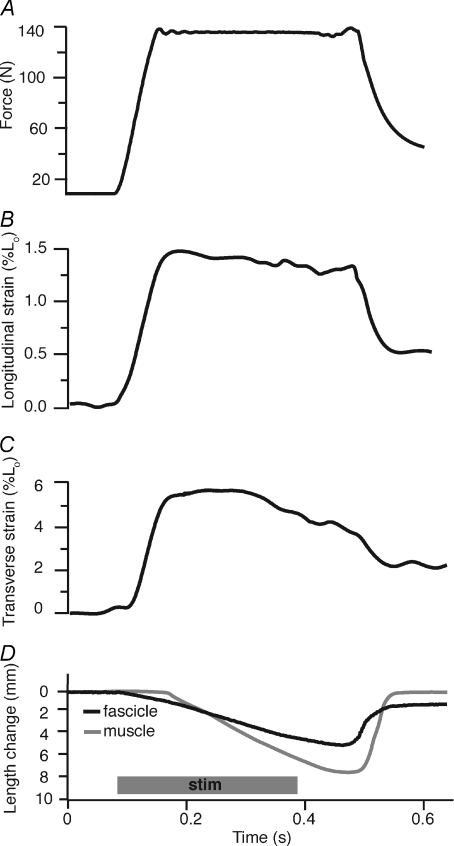
Figure 2. Time series data from a representative active isotonic contraction.
Force measured by the ergometer (A) is allowed to rise to preset level and is kept constant for most the contraction. During force production longitudinal (B) and transverse (C) aponeurosis strains are determined from marker positions, and muscle fascicle lengths (D, black line) and muscle–tendon lengths (D, grey line) are measured from sonomicrometry and the ergometer, respectively. Fascicle shortening in the early part of the contraction, before muscle–tendon movement, is associated with the stretch of aponeurosis in both the longitudinal and transverse directions. Positive strain values represent tissue extension. Note that longitudinal aponeurosis strain follows the force pattern. Also note that once aponeurosis length is constant the MTU shortens at a faster rate than the fascicle due to the architectural gearing (Azizi et al. 2008) present in this pinnate muscle.
Isotonic contractions were followed by a series of passive sinusoidal length changes applied to the muscle-tendon. These tests allowed us to compare the strain patterns of the aponeuroses during passive and active conditions. For all contractions, sonomicrometry and ergometer data were recorded at 4000 Hz using custom LabView software (v 7.1, National Instruments Corp., Austin, TX, USA). Following data collection birds were killed with an intravenous injection of pentobarbital sodium (1 ml) and the muscle was isolated for morphological measurements (Table 1).
Table 1.
Muscle and aponeurosis properties
| Aponeurosis dimensions |
||||
|---|---|---|---|---|
| Individual | Length | Width | LG mass | Po |
| 1 | 4.75 cm | 2.45 cm | 23.1 g | 287 N |
| 2 | 5.12 cm | 2.50 cm | 26.4 g | 346 N |
| 3 | 4.45 cm | 2.37 cm | 22.3 g | 321 N |
| 4 | 4.65 cm | 2.40 cm | 19.8 g | 234 N |
Po is the muscle's maximum isometric force.
Video fluoroscopy
Two C-arm fluoroscopes (OEC Model 9400) retrofitted with high-speed cameras (Photron Fastcam 1024, Photron Inc. San Diego, CA, USA) were used to image the aponeurosis during all contractions. The two C-arms were positioned at approximately 90 deg to each other and the in situ preparation was placed in the view of both C-arms such that all markers on the surface of the aponeurosis could be visualized (Fig. 1B and C). The contracting muscles were imaged at 70 kV and 3.0 MA and videos were recorded at 500 frames per second.
The 3D space being imaged was calibrated using an acrylic cube consisting of 64 equally spaced radio-opaque markers. The position of at least 20 of these markers was recorded in both camera views and used to derive the coefficients for direct linear transformation (DLT; Hedrick et al. 2002). DLT was then used to convert a pair of XY coordinates from each C-arm view to a single set of XYZ coordinates. To remove fluoroscope distortion we imaged a perforated steel sheet with uniform hole spacing after each experiment. A comparison of the fluoroscope image and the actual spacing of holes in the perforated sheet was used to calculate a transformation matrix needed to remove the distortion artifact from the digitized XY coordinates. The precision of this measurement and analysis system for tracking marker position has been determined to be ± 0.1 mm or better (Brainerd et al. 2007). All digitizing, DLT transformations and image undistortion protocols were performed using custom MATLAB (v. 7.0, The Mathworks, Inc., Natick, MA, USA) scripts.
Data analysis and statistics
Time series data collected from sonomicrometry and the ergometer during passive cycles and isotonic contractions were analysed using Igor Pro (v. 6.0; WaveMetrics, Inc., Lake Oswego, OR, USA). Muscle fascicle length changes (sonomicrometry), whole muscle–tendon length changes (ergometer), and muscle force (ergometer) were quantified for all contractions analysed. Digitized 3D coordinates of each radio-opaque marker were imported to Igor and synchronized with muscle force and length measurements. Only markers aligned along the longitudinal and transverse directions of the aponeurosis (Fig. 1) were used to calculate the length changes in each of these orthogonal directions during all contractions. Distance was measured between pairs of markers, and these distance values were summed for three to six markers oriented along the same axis. This approach minimized potential error in the strain measurements from possible changes in tissue curvature during a contraction. Length changes were converted to strain by normalizing to the distance between markers at rest prior to the contraction, which corresponded to approximately 5 N of passive force. In the aponeurosis, positive strain values correspond to lengthening. Peak strain was measured for each active isotonic contraction and used to examine the relationship between aponeurosis strain and muscle force. Longitudinal stiffness was calculated to compare aponeurosis behaviour during active and passive force production. Average longitudinal stiffness was quantified over the period of force development using force from the ergometer and longitudinal length changes from fluoroscope videos. Since many of the active contractions were associated with significantly higher forces than passive loading conditions, the formal statistical comparison was limited to active contractions where the muscle produced forces below 50 N. By comparing this subsample we were able to examine the effect of variation in transverse strains on longitudinal stiffness without the potentially confounding effect of variation in stiffness at different levels of force. A one-way ANOVA was used for this comparison.
Results
Active muscle force production was associated with measurable strains in both the longitudinal and transverse directions (Figs 2 and 3). As expected, aponeurosis length changes in the longitudinal direction occurred in direct proportion to muscle force. There was generally good agreement between changes in instantaneous force and longitudinal strain (Fig. 2). Peak longitudinal strain measured during isotonic contractions across a range of forces demonstrated a linear relationship (P < 0.0001) between strain and force (Fig. 3). This result is not surprising given that muscle force is oriented in the longitudinal direction and elastic structure in series with muscle will stretch in proportion to force.
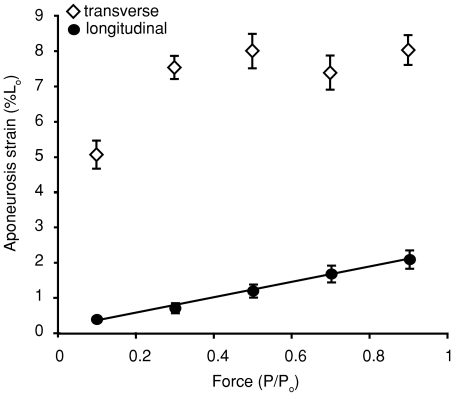
Figure 3. The relationship between active muscle force and peak aponeurosis strain during a muscle contraction.
Aponeurosis strain along the muscle's line of action (longitudinal) increases linearly with muscle force (P < 0.001). The data are fitted with a linear least-squares regression described by the equation y= 1.9x+ 0.17. Aponeurosis strain orthogonal to the muscle's line of action (transverse) increases with force across contractions at relatively low forces but reaches a plateau across the higher range of forces. Note that transverse strain is on average about four times greater than longitudinal. Data shown are pooled from four individuals. Error bars represent the standard error of the mean. Positive strain values represent tissue extension. Muscle force is plotted as the proportion of the maximum isometric force (Po).
The aponeurosis lengthened in the transverse direction during all active muscle contractions. Transverse strains were generally much larger than longitudinal strains (Figs 2 and 3). Unlike longitudinal strains, length changes in the transverse direction were not correlated to muscle force in a simple linear manner. For the range of isotonic contractions measured, strain increased with force over relatively low forces (Fig. 3). Above about 20%Po, transverse strains plateaued, with no further increase in strain with increasing contractile force (Fig. 3). We also observed that instantaneous length changes in the transverse directions were not as tightly coupled to muscle force as were longitudinal strains (Fig. 2).
The strain behaviour of the aponeurosis was measurably different during passive loading as compared with active loading. Most notably, the phase relationship between transverse strain and force was reversed. In active loading, the aponeurosis stretched in both the longitudinal and transverse directions during force development. During passive loading, increases in force were associated with positive (lengthening) strains in the longitudinal direction but negative (shortening) strains in the transverse (Fig. 4A–C). In all passive trials, the length patterns in the transverse and longitudinal direction were out of phase (Fig. 4B and C). The shortening observed in the transverse direction would be expected for a tissue loaded uniaxially.
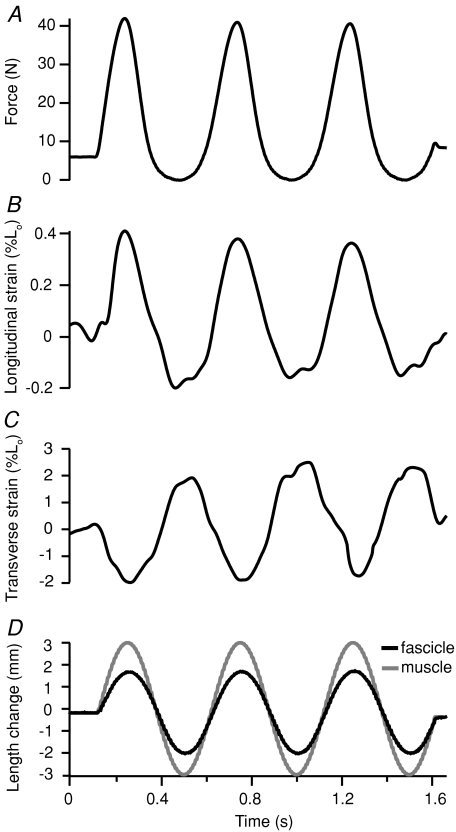
Figure 4. Representative time series data from sinusoidal length changes applied to the passive, unstimulated muscle–tendon.
During sinusoidal loading, increases in force (A) coincide with muscle fascicle (D, black lines) and muscle–tendon (D, grey lines) lengthening. Aponeurosis strain in the longitudinal direction (B) is in phase with force and muscle–tendon length; the aponeurosis lengthens as force and muscle–tendon length increase. Transverse aponeurosis strains (C) are out of phase, tending to decrease (shorten) as force and muscle–tendon length increases. Positive strain values represent lengthening and negative strain values represent shortening.
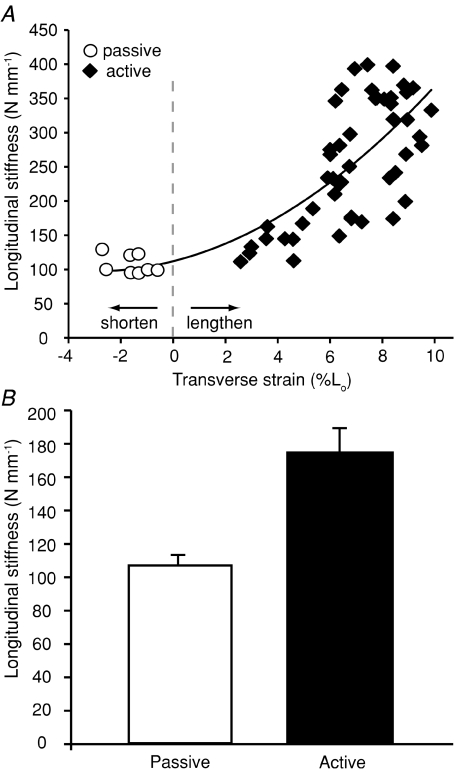
In order to determine how biaxial loading of the aponeurosis influenced its effective stiffness in the longitudinal direction, we measured longitudinal stiffness for contractions that varied in the degree to which the aponeurosis strained transversely (Fig. 5). There was a clear correlation between transverse strain measured at peak force and the stiffness measured over the same contraction. Generally longitudinal stiffness increased curvilinearly with increasing transverse strains (Fig. 5A). The influence of transverse loading on effective longitudinal stiffness was also apparent when measured values of longitudinal stiffness were compared for passive and active loading conditions. Measured longitudinal stiffness was significantly higher during active contractions, when the tissue was loaded biaxially, than during passive contractions (P= 0.004; Fig. 5B).
Figure 5. A comparison of measured longitudinal stiffness during passive and active force production.
A, data pooled for four individuals show longitudinal stiffness increases curvilinearly with increasing stretch of the aponeurosis in the transverse direction. Each data point represents a single contraction. The data are fitted with a second order polynomial (R2= 0.62). This pattern suggests that the biaxial loading of the aponeurosis during active contractions increases longitudinal stiffness. B, a comparison of average longitudinal stiffness during passive and active force production. The comparison shown is limited to active contractions occurring at forces comparable to passive tests (<50 N) in order to limit the confounding effect of large force variation. Stiffness differs significantly between passive and active conditions (P= 0.004). Error bars are the standard error of the mean.
Discussion
Our results show that as active muscle fibres develop force they stretch the aponeurosis in the longitudinal direction (along the muscle's line of action) (Figs 2 and 3). In addition, the shortening of fascicles results in an expansion of muscle width, which stretches the aponeurosis in the transverse direction (Figs 2 and 3). This biaxial strain pattern has been observed previously but its significance for the spring-like function of aponeuroses has not been appreciated. In isolated skin samples, biaxial loading increases tissue stiffness when compared to the uniaxial condition (Lanir & Fung, 1974). Theory indicates that this result may apply more generally to biomaterials (Aspden, 1990). Our results demonstrate this effect in the aponeurosis in situ, as longitudinal stiffness varied in proportion to the magnitude of transverse strain (Fig. 5). For example, measured stiffness during active contractions, when the aponeurosis was loaded biaxially, was 2–3 times greater than the measured stiffness during passive stretches, when the aponeurosis was subject only to longitudinal strains (Fig. 5). This observation suggests that the stiffness of the series elastic spring through which muscle contractile elements generate force is not fixed, but rather varies depending upon contractile conditions.
Several studies have observed aponeurosis behaviour that is difficult to reconcile with the idea that these structures act as fixed-stiffness springs. In a study of rat medial gastrocnemius, Zuurbier and colleagues (1994) found that the aponeurosis was at significantly shorter lengths when the muscle produced the same force in the active versus the passive condition (i.e. aponeurosis stiffness increased with activation). A study of the semitendinosus muscle of frogs reached similar conclusions (Lieber et al. 2000). The significant variation in the measured longitudinal stiffness of aponeuroses was attributed to the viscoelastic nature of tendinous tissues, since active contractions were conducted at higher velocities than passive stretches (Lieber et al. 2000). Our results suggest an alternative explanation for this behaviour. Active force production results in biaxial aponeurosis strains, which likely increase longitudinal stiffness compared to the passive state (Fig. 5B).
A classic study of the cat soleus found that the stiffness of the entire tendinous component (aponeurosis + free tendon) varied significantly as a function of force (Rack & Westbury, 1984). These changes in the longitudinal stiffness of the series elastic structures were primarily attributed to effects of the ‘toe’ region of tendon's stress–strain curve, where stiffness increases rapidly with increasing force, particularly at low force levels. We find that transverse strains increase substantially with force across low muscle forces (Fig. 3). It is therefore possible that the stiffness changes observed by Rack & Westbury (1984) could in part be explained by the observed variation in transverse aponeurosis strains at low forces (Fig. 3).
Implications for locomotor function
Variable stiffness in aponeuroses may have important consequences for musculoskeletal function. The spring-like behaviour of series elastic elements significantly affects the mechanics (Zajac, 1989), energetics (Cavagna et al. 1977; Alexander, 1984; Biewener & Roberts, 2000), and motor control (Rack & Westbury, 1984) of movement. Models of musculoskeletal function demonstrate that a tendon's capacity for energy storage and length change, and its tendency to influence muscle mechanics and energetics, is determined by its stiffness (Zajac, 1989; Ettema, 1996; Bojsen-Moller et al. 2005). In virtually all models, tendon behaviour is characterized by a single stiffness value (or a single function of tendon length). Our results for aponeurosis indicate an additional level of interaction between muscle and tendon behaviour, whereby the contractile conditions of the muscle influence the effective stiffness of the elastic element.
Biaxial loading and variable stiffness in aponeuroses may have implications for the energetics of locomotion. The capacity for energy storage and recovery in tendons is typically calculated from stiffness values for tendons measured in isolated preparations (Bennett et al. 1986; Shadwick, 1990). However, biaxial strains and variable stiffness in aponeuroses suggest that the in vivo behaviour of these elastic structures may depend on the context of the contraction and that the realized stiffness of the tissue may exceed that of isolated tissue preparations. Variable stiffness in aponeuroses may result in a capacity for energy storage and recovery that is greater than that assumed by most models of muscle–tendon systems.
The stiffness of the aponeurosis may also have a significant effect on fascicle length changes. Previous studies have observed variation in the length changes that occur in different regions of a muscle (Higham et al. 2008). It is possible that variable stiffness in the aponeurosis alters the compliance of elastic structures acting in series with fascicles in different regions of the muscle, which may result in regional variation in fascicle strains. Changes in the transverse strain of the aponeurosis also have the potential to change fascicle strains by altering the gear ratio with which fascicles operate (Azizi et al. 2008). Such changes in muscle shape have been shown to alter the fascicle strain needed for given amount of muscle–tendon strain (Azizi et al. 2008; Fig. 2D). Changes in the mechanical behaviour of the aponeurosis may therefore play an important role in determining the degree to which fascicle length changes are decoupled from that of the muscle–tendon.
The stiffness of tendinous structures can also impact the motor control strategy needed for various movements. The presence of a compliant series elastic element presents a challenge to the motor control system, as the stretch of tendons decouples the length of muscle fascicles from the desired joint position (Bernstein, 1967; Rack & Westbury, 1984). It has been suggested that muscles associated with fine positional control have stiffer tendons that undergo little length change, thereby simplifying a task's neural commands (Rack & Ross, 1984; Alexander & Ker, 1990; Ward et al. 2006). In muscles where tendons do stretch substantially, a constant stiffness in the series elastic structures is considered one way to simplify motor control (Monti et al. 2003). Our results for aponeuroses, however, suggest that many muscles operate in series with elastic elements that vary in their behaviour depending on contractile conditions (Fig. 5). Thus, the challenge presented to the motor control system by the presence of compliant tendons may be more significant than most studies assume.
Biaxial strains in aponeuroses
This study is the first to use biplanar fluoroscopy to characterize the biaxial strain patterns in aponeuroses. We find that during contractions at maximal force the aponeurosis stretches in the longitudinal direction by approximately 2.5% of its resting length (Fig. 3). This value falls within the range of values found in several previous studies, which have documented longitudinal aponeurosis strains ranging from 1% to 6% during near maximal force production (Huijing & Ettema, 1988; Ettema & Huijing, 1989; Scott & Loeb, 1995; van Donkelaar et al. 1999; Magnusson et al. 2001, 2003; Muramatsu et al. 2001, 2002; Arampatzis et al. 2006). However, several studies have found higher longitudinal strains in aponeuroses ranging from 7% to 12% (Lieber et al. 1991; Maganaris & Paul, 2000; Maganaris et al. 2001; Monti et al. 2003). It is unclear to what extent the relatively large range of maximum aponeurosis strains reported in the literature represent variation in aponeurosis properties between different muscles or in different species, versus variation due to methodological factors.
In this study we also find that transverse strains, those perpendicular to the muscle's line of action, are significantly higher than in the longitudinal direction and are approximately 8% during forceful contractions (Fig. 3). These results are consistent with several previous studies, which have also documented relatively high transverse strains (Scott & Loeb, 1995; van Donkelaar et al. 1999; Maganaris et al. 2001). These investigators suggest that the expansion of the aponeurosis in the transverse direction likely results from the need of contracting muscle fascicles to expand in directions perpendicular to the primary direction of force production in order to maintain a constant volume. Our results agree with this interpretation. The presence of large transverse strains driven by transverse muscle expansion distinguishes the mechanical behaviour of aponeuroses from that of free tendons.
Not all studies of aponeurosis mechanical behaviour have observed biaxial strains. A recent study of the rat soleus aponeurosis found that transverse strains were so small as to fall below the resolution of the imaging system (Monti et al. 2003). The focus of this study was primarily longitudinal strains, and it is possible that the spacing of implanted markers resulted in small, undetectable absolute length changes in the transverse direction. It is also possible that not all aponeuroses share the same mechanical behaviour and that variation in transverse strains is present in different systems.
Recent measurements of the material properties of isolated turkey lateral gastrocnemius aponeurosis demonstrate that the aponeurosis behaves as an efficient spring in both the longitudinal and transverse orientations (Azizi et al. 2009). The aponeurosis functions with relatively high resilience (elastic efficiency) in both the longitudinal and transverse directions (Azizi et al. 2009), which suggests minimal energy loss during the observed biaxial loading. However, the elastic modulus of the aponeurosis is about 7 times greater in the longitudinal than the transverse direction when isolated tissues are tested under uniaxial conditions (Azizi et al. 2009). These differences in stiffness are to be expected, as collagen fascicles are primarily oriented longitudinally along the muscle's line of action (Azizi et al. 2009). Despite the relative compliance of the aponeurosis in the transverse direction, the large strains measured in the present study suggest that the elastic energy associated with transverse strains may not be insignificant. If we use the elastic modulus from uniaxial materials tests as well as the dimensions of the aponeurosis we would estimate that the strain energy in the transverse direction may be as high as one-third that of the longitudinal direction during forceful contractions. However, such estimates must be interpreted cautiously given the biaxial loading of the tissue and the likely interaction between longitudinal and transverse directions. Nonetheless, our results suggest that consideration of elastic strain energy associated only with longitudinal strains may not fully reflect the function of aponeuroses as locomotor springs.
The structures responsible for the elastic properties of aponeuroses in the transverse direction remain poorly understood. Previous studies have described the presence of obliquely or even transversely oriented fibrils within tendon fascicles (Jozsa et al. 1991; Provenzano & Vanderby, 2006). In addition, tendons are covered by the epitenon, which consists of a network of fibrils some of which are transversely oriented and fuse to the primary fascicles (Gotoh, 1997; Kannus, 2000). Although these transversely oriented structures are modest in comparison to the primary longitudinal array of collagen fascicles, they may be sufficient to explain the relatively low elastic modulus in the transverse direction of aponeuroses.
Conclusions
The results of this study show that unlike free tendons, aponeuroses can behave as biaxially loaded, variable stiffness springs. We suggest that such biaxial loading during active force production increases aponeurosis stiffness dynamically, and potentially increases the energy that can be stored for a given strain. These results also suggest that the stiffness and potential for elastic energy storage may be significantly underestimated when only uniaxial strains are considered. Future studies of the biaxial material properties and in vivo strain patterns of aponeuroses will reveal the degree to which such changes in aponeurosis stiffness are tuned to enhance mechanical function during locomotion.
Acknowledgments
The authors would like to thank Dr Beth Brainerd and Dr Dave Baier for technical assistance during the data collection and analysis phase of the project. We thank Greg Halenda for help during data collection. We thank Dr Greg Sawicki for stimulating discussions and commenting on earlier versions of the manuscript. This research was conducted at Brown University. This work was supported by NIH grants F32AR 054246 to E.A. and AR055295 to T.J.R., NSF grant 0642428 to T.J.R., and the W. M. Keck Foundation.
Author contributions
E.A.: conception and design, collection, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published. T.J.R.: conception and design, collection, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published.
References
- Alexander RM. Elastic energy stores in running vertebrates. Am Zool. 1984;24:85–94. [Google Scholar]
- Alexander RM, Ker RF. The architecture of leg muscles. In: Winters JL, Woo SL, editors. Multiple Muscle Systems. New York: Springer; 1990. pp. 568–577. [Google Scholar]
- Arampatzis A, De Monte G, Karamanidis K, Morey-Klapsing G, Stafilidis S, Bruggemann GP. Influence of the muscle-tendon unit's mechanical and morphological properties on running economy. J Exp Biol. 2006;209:3345–3357. doi: 10.1242/jeb.02340. [DOI] [PubMed] [Google Scholar]
- Aspden RM. Constraining the lateral dimensions of uniaxially loaded materials increases the calculated strength and stiffness – Application to muscle and bone. J Mater Sci Mater M. 1990;1:100–104. [Google Scholar]
- Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci U S A. 2008;105:1745–1750. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Halenda GM, Roberts TJ. Mechanical properties of the gastrocnemius aponeurosis in wild turkeys. Integr Comp Biol. 2009;006:1–8. doi: 10.1093/icb/icp006. ICP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin RJ, Paolini PJ. Volume change and pressure development in muscle during contraction. Am J Physiol. 1967;213:1025–1030. doi: 10.1152/ajplegacy.1967.213.4.1025. [DOI] [PubMed] [Google Scholar]
- Bennett MB, Ker RF, Dimery NJ, Alexander RM. Mechanical-properties of various mammalian tendons. J Zool. 1986;209:537–548. [Google Scholar]
- Bernstein NA. Co-ordination and Regulation of Movements. New York: Pergamon Press; 1967. [Google Scholar]
- Biewener AA, Baudinette RV. In vivo muscle force and elastic energy-storage during steady-speed hopping of tammar wallabies (Macropus eugenii) J Exp Biol. 1995;198:1829–1841. doi: 10.1242/jeb.198.9.1829. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Konieczynski DD, Baudinette RV. In vivo muscle force-length behaviour during steady-speed hopping in tammar wallabies. J Exp Biol. 1998;201:1681–1694. doi: 10.1242/jeb.201.11.1681. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev. 2000;28:99–107. [PubMed] [Google Scholar]
- Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol. 2005;99:986–994. doi: 10.1152/japplphysiol.01305.2004. [DOI] [PubMed] [Google Scholar]
- Brainerd EL, Gatesy SM, Baier DB, Hedrick TL. Accurate 3D reconstruction of skeletal morphology and movement with CTX imaging. J Morphol. 2007;268:1053. [Google Scholar]
- Brown IE, Scott SH, Loeb GE. Mechanics of feline soleus. 2. Design and validation of a mathematical model. J Muscle Res Cell Motil. 1996;17:221–233. doi: 10.1007/BF00124244. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol Regul Integr Comp Physiol. 1977;233:R243–261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- Donkelaar CCV, Willems PJB, Muijtjens AMM, Drost MR. Skeletal muscle transverse strain during isometric contraction at different lengths. J Biomech. 1999;32:755–762. doi: 10.1016/s0021-9290(99)00073-1. [DOI] [PubMed] [Google Scholar]
- Ettema GJC, Huijing PA. Properties of the tendinous structures and series elastic component of EDL muscle tendon complex of the rat. J Biomech. 1989;22:1209–1215. doi: 10.1016/0021-9290(89)90223-6. [DOI] [PubMed] [Google Scholar]
- Ettema GJC. Mechanical efficiency and efficiency of storage and release of series elastic energy in skeletal muscle during stretch-shorten cycles. J Exp Biol. 1996;199:1983–1997. doi: 10.1242/jeb.199.9.1983. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Murashige N, Yamashita K. Ultrastructural observations on the tendon sheath of the rat tail. J Electron Microsc. 1997;46:247–252. doi: 10.1093/oxfordjournals.jmicro.a023516. [DOI] [PubMed] [Google Scholar]
- Hedrick TL, Tobalske BW, Biewener AA. Estimates of circulation and gait change based on a three-dimensional kinematic analysis of flight in cockatiels (Nymphicus hollandicus) and ringed turtle-doves (Streptopelia risotia) J Exp Biol. 2002;205:1389–1409. doi: 10.1242/jeb.205.10.1389. [DOI] [PubMed] [Google Scholar]
- Henry HT, Ellerby DJ, Marsh RL. Performance of guinea fowl Numida meleagris during jumping requires storage and release of elastic energy. J Exp Biol. 2005;208:3293–3302. doi: 10.1242/jeb.01764. [DOI] [PubMed] [Google Scholar]
- Higham TE, Biewener AA, Wakeling JM. Functional diversification within and between muscle synergists during locomotion. Biol Lett. 2008;4:41–44. doi: 10.1098/rsbl.2007.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijing PA, Ettema GJC. Length – force characteristics of aponeurosis in passive muscle and during isometric and slow dynamic contractions of rat gastrocnemius muscle. Acta Morphol Neerlando-Scand. 1988;26:51–62. [PubMed] [Google Scholar]
- Ishikawa M, Dousset E, Avela J, Kyrolainen H, Kallio J, Linnamo V, Kuitunen S, Nicol C, Komi PV. Changes in the soleus muscle architecture after exhausting stretch-shortening cycle exercise in humans. Eur J Appl Physiol. 2006;97:298–306. doi: 10.1007/s00421-006-0180-2. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Balint JB, Reffy A. Three-dimensional ultrastructure of human tendons. Acta Anatomica. 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- Ker RF, Dimery NJ, Alexander RM. The role of tendon elasticity in hopping in a wallaby (Macropus rufogriseus) J Zool. 1986;208:417–428. [Google Scholar]
- Lanir Y, Fung YC. Two-dimensional mechanical properties of rabbit skin. II. Experimental results. J Biomech. 1974;7:171–182. doi: 10.1016/0021-9290(74)90058-x. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Bougoulias K, Wilson AM. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech. 2007;40:157–164. doi: 10.1016/j.jbiomech.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Leonard ME, Brown CG, Trestik CL. Frog semitendinosis tendon load-strain and stress-strain properties during passive loading. Am J Physiol Cell Physiol. 1991;261:C86–C92. doi: 10.1152/ajpcell.1991.261.1.C86. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Leonard ME, Brown-Maupin CG. Effects of muscle contraction on the load-strain properties of frog aponeurosis and tendon. Cells Tissues Organs. 2000;166:48–54. doi: 10.1159/000016708. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Kawakami Y, Fukunaga T. Changes in aponeurotic dimensions upon muscle shortening: in vivo observations in man. J Anat. 2001;199:449–456. doi: 10.1046/j.1469-7580.2001.19940449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendinous tissue stretch upon maximum muscle force generation. J Biomech. 2000;33:1453–1459. doi: 10.1016/s0021-9290(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Rosager S, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol. 2001;531:277–288. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Monti RJ, Roy RR, Zhong H, Edgerton VR. Mechanical properties of rat soleus aponeurosis and tendon during variable recruitment in situ. J Exp Biol. 2003;206:3437–3445. doi: 10.1242/jeb.00550. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muraoka T, Kawakami Y, Fukunaga T. Superficial aponeurosis of human gastrocnemius is elongated during contraction: implications for modeling muscle-tendon unit. J Biomech. 2002;35:217–223. doi: 10.1016/s0021-9290(01)00196-8. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muraoka T, Takeshita D, Kawakami Y, Hirano Y, Fukunaga T. Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J Appl Physiol. 2001;90:1671–1678. doi: 10.1152/jappl.2001.90.5.1671. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Vanderby R. Collagen fibril morphology and organization: Implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Rack PMH, Ross HF. The tendon of flexor pollicis longus: its effects on the muscular control of force and position at the human thumb. J Physiol. 1984;351:99–110. doi: 10.1113/jphysiol.1984.sp015235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack PMH, Westbury DR. Elastic properties of the cat soleus tendon and their functional importance. J Physiol. 1984;347:479–495. doi: 10.1113/jphysiol.1984.sp015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL. Probing the limits to muscle-powered accelerations: lessons from jumping bullfrogs. J Exp Biol. 2003;206:2567–2580. doi: 10.1242/jeb.00452. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: The economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. Mechanical properties of aponeurosis and tendon of the cat soleus muscle during whole muscle isometric contractions. J Morphol. 1995;224:73–86. doi: 10.1002/jmor.1052240109. [DOI] [PubMed] [Google Scholar]
- Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol. 1990;68:1033–1040. doi: 10.1152/jappl.1990.68.3.1033. [DOI] [PubMed] [Google Scholar]
- Ward SR, Loren GJ, Lundberg S, Lieber RL. High stiffness of human digital flexor tendons is suited for precise finger positional control. J Neurophysiol. 2006;96:2815–2818. doi: 10.1152/jn.00284.2006. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- Zuurbier CJ, Everard AJ, Vanderwees P, Huijing PA. Length-force characteristics of the aponeurosis in the passive and active muscle condition and in the isolated condition. J Biomech. 1994;27:445–453. doi: 10.1016/0021-9290(94)90020-5. [DOI] [PubMed] [Google Scholar]