Abstract
This study characterizes the effects of shivering thermogenesis on metabolic fuel selection in Wistar rats. Because lipids account for most of the heat produced, we have investigated: (1) whether the rate of appearance of non-esterified fatty acids (Ra NEFAs) is stimulated by shivering, (2) whether mono-unsaturated (oleate) and saturated fatty acids (palmitate) are affected similarly, and (3) whether the partitioning between fatty acid oxidation and re-esterification is altered by cold exposure. Fuel oxidation was measured by indirect calorimetry and fatty acid mobilization by continuous infusion of 9,10-[3H]oleate and 1-[14C]palmitate. During steady-state cold exposure, results show that total heat production is unequally shared by the oxidation of lipids (52% of metabolic rate), carbohydrates (35%) and proteins (13%), and that the same fuel selection pattern is observed at all shivering intensities. All previous research shows that mammals stimulate Ra NEFA to support exercise or shivering. In contrast, results reveal that the Ra NEFA of the rat remains constant during cold exposure (∼55 μmol kg−1 min−1). No preferential use of mono-unsaturated over saturated fatty acids could be demonstrated. The rat decreases its rate of fatty acid re-esterification from 48.4 ± 6.4 to 19.6 ± 6.3 μmol kg−1 min−1 to provide energy to shivering muscles. This study is the first to show that mammals do not only increase fatty acid availability for oxidation by stimulating Ra NEFA. Reallocation of fatty acids from re-esterification to oxidation is a novel, alternative strategy used by the rat to support shivering.
The physiological response of endotherms to cold exposure has been well characterized, particularly for mammals (Young, 1988; Jacobs et al. 1994; Gautier, 2000). In rodents, the relative importance of shivering and non-shivering thermogenesis is clearly established (Fuller et al. 1975; Banet et al. 1978; Gautier et al. 1991), but most research has focused on the role of brown adipose tissue (Himms-Hagen, 1996; Cannon & Nedergaard, 2004). Comparatively little information is available on shivering, and it is limited to changes in the rates of heat exchange, oxygen consumption, breathing, and electromyographic activity (Fuller et al. 1975; Oufara et al. 1987; Gautier, 2000). The effects of shivering on metabolic fuel selection have only been rarely investigated in model animals (Smith & Davidson, 1982; Adán et al. 1995). Yet, there is an important need for greater evaluation of these models to strengthen the understanding of common mechanisms of thermogenesis in endotherms.
For adult humans, the relative contribution of lipids, carbohydrates (CHOs) and proteins to total heat production depends on the intensity of shivering and on the size of glycogen reserves. During low-intensity shivering, the thermogenic response of this ∼70 kg furless organism is mainly supported by lipid oxidation. Carbohydrates become dominant as shivering intensifies, but preference for lipids persists if glycogen stores are depleted (Haman et al. 2002, 2004a,b,c, 2005). An adequate supply of lipids to shivering muscles is accomplished by stimulating fatty acid mobilization from adipose reserves (Vallerand et al. 1999). A 120 g shorebird, the ruff sandpiper, is the only small endotherm whose fuel selection pattern has been measured during cold exposure. Over 80% of its heat production comes from lipid oxidation at all shivering intensities (Vaillancourt et al. 2005). However, this highly aerobic migratory bird is also known for its record capacity to process lipids (Vaillancourt & Weber, 2007) and may not exhibit the typical fuel selection pattern of a small animal with large surface to volume ratio.
Here, our primary goal was to characterize the effects of shivering intensity on fuel selection in the rat: a small endotherm with average aerobic capacity and no extraordinary tolerance to cold. With its small body size (∼200 times smaller than humans), large surface to volume ratio (∼4 times higher than humans), and negligible thermogenic contribution from brown adipose tissue (animals acclimated to 27°C; Himms-Hagen, 1996; Cannon & Nedergaard, 2004), it is unclear what fuel mixture is used by the rat to support shivering. Determining at what rate and in what proportion different oxidative fuels are used for thermogenesis struck us as essential information to characterize the energetics of shivering in this rodent model. Results reveal that carbohydrates only play an important role at the onset of cold exposure, but that prolonged shivering is mainly supported by lipids. Therefore, additional goals were to determine: (1) whether the mobilization of fatty acids from lipid reserves is stimulated by shivering, (2) whether the fluxes of mono-unsaturated (oleate) and saturated fatty acids (palmitate) are affected similarly because preference for mono-unsaturates has usually been observed in nature (Leyton et al. 1987; Raclot & Groscolas, 1993, 1995; Sidell et al. 1995), and (3) whether the partitioning between fatty acid oxidation and re-esterification is altered by cold exposure. We hypothesized that the rate of appearance of fatty acids would be stimulated by shivering, that this effect would be stronger for oleate than palmitate, and that total fatty acid flux would be preferentially directed towards oxidation rather than re-esterification.
Methods
Animals
All experimental protocols complied with The Journal of Physiology policies and regulations (Drummond, 2009) and they were approved by the Animal Care Committee of the University of Ottawa in accordance with the requirements of the Canadian Council on Animal Care. Male Wistar rats (384 ± 14 g; n= 16) were obtained from Charles River (St-Hyacinthe, QC, Canada) and housed in groups of three or four. Some animals were used exclusively for non-invasive measurements (indirect calorimetry) and others were catheterized to monitor fatty acid kinetics (indirect calorimetry + continuous tracer infusion). All individuals were kept under 12 h light:12 h dark photoperiod, at 60% humidity and 27 ± 1°C, to minimize brown adipose tissue (Himms-Hagen, 1996; Cannon & Nedergaard, 2004). They had access to water and rodent chow ad libitum (Ralston Purina, Woodstock, Ontario, Canada; 57% carbohydrates, 5% fat and 18% proteins) and were acclimated to these conditions for more than 2 weeks before measurements. At the end of experiments, the animals were killed by an overdose of anaesthetic.
Indirect calorimetry and cold exposure
Food was withheld for 1 h before measurements. This duration was selected because subsequent monitoring of metabolism lasted 5 additional hours (without food), a significant fasting period for a 300–400 g animal. It ensured that the rats had normal glycogen reserves at the onset of shivering. Rates of oxygen consumption ( ) and carbon dioxide production (
) and carbon dioxide production (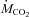 ) were measured with an Oxymax system (Columbus Instruments, Columbus, OH, USA) connected to a modified respirometer supplied with room air at 3.6–5.0 l min−1 (Vaillancourt et al. 2005). Oxygen and CO2 concentrations were measured in the inflow and outflow air after removing water vapour with calcium sulphate (Drierite). All analysers were calibrated with known gas mixtures before and after each experiment.
) were measured with an Oxymax system (Columbus Instruments, Columbus, OH, USA) connected to a modified respirometer supplied with room air at 3.6–5.0 l min−1 (Vaillancourt et al. 2005). Oxygen and CO2 concentrations were measured in the inflow and outflow air after removing water vapour with calcium sulphate (Drierite). All analysers were calibrated with known gas mixtures before and after each experiment. 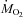 and
and 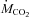 were corrected for dry gas under standard temperature and pressure (STPD) (see Weber & O’Connor, 2000). Each animal was measured at three temperatures (15, 10 and 5°C) in random order, with a minimum of 3 days between measurements. For each experiment, the animal was kept for 75 min at 27°C, before a 20–30 min cooling period to reach 15, 10 or 5°C, followed by a 3 h period of shivering at constant temperature.
were corrected for dry gas under standard temperature and pressure (STPD) (see Weber & O’Connor, 2000). Each animal was measured at three temperatures (15, 10 and 5°C) in random order, with a minimum of 3 days between measurements. For each experiment, the animal was kept for 75 min at 27°C, before a 20–30 min cooling period to reach 15, 10 or 5°C, followed by a 3 h period of shivering at constant temperature.
Surgical procedures
Catheters were prepared with 20 cm segments of polyethylene tubing (PE-50; Intramedic, Clay Adams, Becton Dickinson, Rutherford, NJ, USA) and sterilized with ethylene oxide. They were surgically placed in the right jugular vein and left carotid artery under 2.5% halothane anaesthesia, 2 days before measuring fatty acid kinetics by continuous tracer infusion. They were inserted 15 mm into the vessels, sutured in place, and exteriorized between the scapulas. They were filled with heparinized saline (20 U ml−1) and penicillin G (125 000 U ml−1), and flushed daily with saline. The heparinized saline used to fill the catheters between measurements was carefully withdrawn before flushing. Buprenorphine was administered as an analgesic (0.04 mg kg−1, subcutaneously) on the morning and afternoon of the day of surgery and 1 day post-surgery.
Fatty acid kinetics
Continuous tracer infusion was performed for 1 h at 27°C (baseline) and 2 h at 5°C (shivering) to measure the effects of cold exposure. The infusate was freshly prepared for each experiment by mixing 4.44 MBq of 9,10-[3H]oleate (specific activity: 370 GBq mmol−1) and 4.44 MBq of 1-[14C]palmitate (2.07 GBq mmol−1) (Amersham, Oakville, Ontario, Canada) in sterile saline containing delipidated rat albumin. Labelled fatty acids were administered at 1 ml h−1 with a calibrated syringe pump (Harvard Apparatus, South Natick, MA, USA) through the venous catheter. Mean infusion rates were 30.9 ± 1.7 kBq kg−1 min−1 for oleate and 36.0 ± 2.45 kBq kg−1 min−1 for palmitate (n= 7). These conditions ensured that isotopic steady state was reached in less than 45 min (McClelland et al. 1999, 2001). Fatty acids (labelled + unlabelled) were infused at rates only accounting for < 0.001% of the endogenous rate of appearance of oleate (Ra oleate = rate of endogenous oleate naturally released in the circulation) and < 0.15% of Ra palmitate (or trace amounts). Blood samples (0.5 ml each) were drawn from the arterial catheter 55 and 60 min after starting the infusion to measure baseline fatty acid kinetics at 27°C, and every 30 min after the onset of environmental cooling to quantify the effects of shivering. Plasma was separated immediately after sampling and kept at −20°C until analyses.
Plasma analyses
Heptadecanoate (17:0; 0.30 mg ml−1; a fatty acid not found in animals) was added to plasma as internal standard for subsequent analysis of non-esterified fatty acids by gas chromatography. Total plasma lipids were extracted twice in chloroform:methanol (2:1 v/v) (Folch et al. 1957). The aqueous phase was discarded. The organic phase containing the lipids was dried at 70°C under N2 and resuspended in hexane:isopropanol (3:2 v/v). Neutral lipids (NLs), non-esterified fatty acids (NEFAs) and phospholipids (PLs) were separated by sequential elution from Supelclean solid-phase extraction tubes (LC-NH2, Sigma, St Louis, MO, USA). NLs were eluted with chloroform:isopropanol (2:1 v/v), NEFAs with isopropyl ether:acetic acid (98:2 v/v) and PLs with methanol. After methylation (NEFAs) or acid transesterification with acetyl chloride in methanol (NLs and PLs) (Abdul-Malak et al. 1989), the fatty acid composition of each fraction was analysed by gas chromatography. Individual fatty acid methyl esters were separated on a Hewlett-Packard 5890 series II (with a HP 7673 autosampler) equipped with a flame-ionization detector and a 30 m fused silica column (Supelco 2330, Sigma) (McClelland et al. 1999). Exact retention times of individual fatty acids were determined with pure standards (Sigma).
Calculations and statistics
Rates of CHOs and lipid oxidation were calculated from 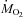 and
and 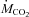 using the equations of Frayn (Frayn, 1983), assuming a rate of nitrogen excretion of 0.641 mg N min−1 kg−1 (an average value for Wistar rats eating 18% proteins; Younes et al. 1995; Daenzer et al. 2001). Ra oleate and Ra palmitate were calculated with the steady-state equation of Steele (Steele, 1959). The rate of appearance of non-esterified fatty acids (Ra NEFA) was calculated by dividing Ra oleate by the fractional contribution of oleate to total NEFAs because oleate was the most abundant NEFA in plasma. The rate of fatty acid re-esterification was calculated by subtracting the rate of fatty acid oxidation from Ra NEFA (both expressed in μmol fatty acids kg−1 min−1). The relative rate of fatty acid re-esterification (% total fatty acids released undergoing re-esterification) was calculated as the absolute rate of fatty acid re-esterification divided by Ra NEFA. Statistical comparisons were performed using one- or two-way repeated measures analysis of variance (RM-ANOVA) or Friedman repeated measures ANOVA on ranks when assumptions of normality or homoscedasticity were not met. When significant overall changes were detected, the Bonferroni post hoc test was used to determine which means were different from baseline. All percentages were transformed to the arcsine of their square root before analysis. Statistical threshold was set at P < 0.05 and all values presented are means ± standard error of the mean (s.e.m.).
using the equations of Frayn (Frayn, 1983), assuming a rate of nitrogen excretion of 0.641 mg N min−1 kg−1 (an average value for Wistar rats eating 18% proteins; Younes et al. 1995; Daenzer et al. 2001). Ra oleate and Ra palmitate were calculated with the steady-state equation of Steele (Steele, 1959). The rate of appearance of non-esterified fatty acids (Ra NEFA) was calculated by dividing Ra oleate by the fractional contribution of oleate to total NEFAs because oleate was the most abundant NEFA in plasma. The rate of fatty acid re-esterification was calculated by subtracting the rate of fatty acid oxidation from Ra NEFA (both expressed in μmol fatty acids kg−1 min−1). The relative rate of fatty acid re-esterification (% total fatty acids released undergoing re-esterification) was calculated as the absolute rate of fatty acid re-esterification divided by Ra NEFA. Statistical comparisons were performed using one- or two-way repeated measures analysis of variance (RM-ANOVA) or Friedman repeated measures ANOVA on ranks when assumptions of normality or homoscedasticity were not met. When significant overall changes were detected, the Bonferroni post hoc test was used to determine which means were different from baseline. All percentages were transformed to the arcsine of their square root before analysis. Statistical threshold was set at P < 0.05 and all values presented are means ± standard error of the mean (s.e.m.).
Results
Rates of gas exchange and fuel oxidation
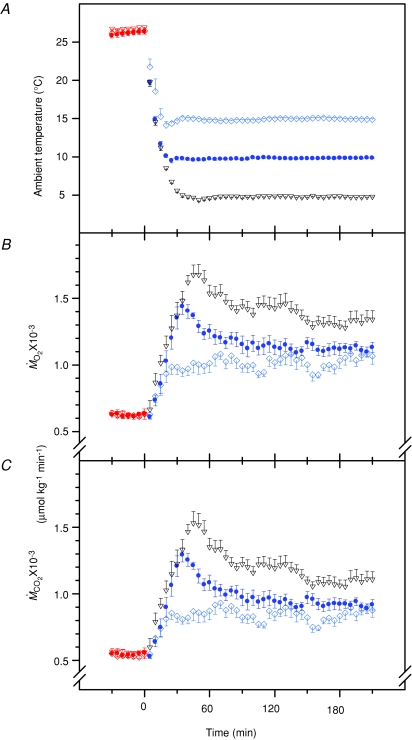
Changes in environmental temperature and their effects on the rates of oxygen consumption (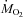 ) and carbon dioxide production (
) and carbon dioxide production (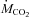 ) are presented in Fig. 1. Temperature decreased from 27°C (thermoneutral baseline) to 15, 10 or 5°C in 20–30 min and stayed at these levels for 3 h (Fig. 1A). Baseline metabolic rate was 625 ± 10 μmol O2 kg−1 min−1. Cold exposure caused an increase in
) are presented in Fig. 1. Temperature decreased from 27°C (thermoneutral baseline) to 15, 10 or 5°C in 20–30 min and stayed at these levels for 3 h (Fig. 1A). Baseline metabolic rate was 625 ± 10 μmol O2 kg−1 min−1. Cold exposure caused an increase in 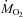 proportional to shivering intensity (P < 0.001; Fig. 1B). Metabolic rate reached its highest value of 1677 ± 73 μmol O2 kg−1 min−1 after 45 min of shivering at 5°C (Fig. 1B).
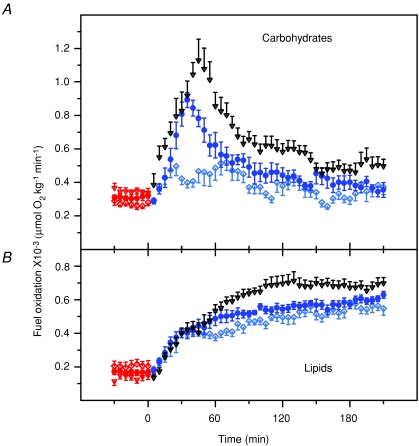
proportional to shivering intensity (P < 0.001; Fig. 1B). Metabolic rate reached its highest value of 1677 ± 73 μmol O2 kg−1 min−1 after 45 min of shivering at 5°C (Fig. 1B). 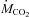 was 546 ± 8 μmol CO2 kg−1 min−1 under thermoneutral conditions and increased in proportion to shivering intensity to reach a maximum of 1530 ± 88 μmol CO2 kg−1 min−1 after 45 min at 5°C (P < 0.001; Fig. 1C). The effects of cold exposure on the rates of CHOs and lipid oxidation are shown in Fig. 2. Oxidation rates of both fuels were stimulated by shivering (P < 0.001). CHOs oxidation showed a rapid increase during the first 45 min of shivering before declining progressively until the end of the experiments (Fig. 2A). The effect of cold exposure on CHOs oxidation was proportional to shivering intensity and a maximal rate of 1128 ± 126 μmol O2 kg−1 min−1 was reached after 45 min at 5°C (P < 0.001). Lipid oxidation showed a progressive increase from baseline throughout the experiments (Fig. 2B). The effect of cold exposure on lipid oxidation was proportional to shivering intensity and a maximal rate of 712 ± 54 μmol O2 kg−1 min−1 was reached after 130 min at 5°C (P < 0.001). Steady-state values for rates of gas exchange and fuel oxidation at thermoneutrality and during the last 30 min of cold exposure are summarized in Table 1.
was 546 ± 8 μmol CO2 kg−1 min−1 under thermoneutral conditions and increased in proportion to shivering intensity to reach a maximum of 1530 ± 88 μmol CO2 kg−1 min−1 after 45 min at 5°C (P < 0.001; Fig. 1C). The effects of cold exposure on the rates of CHOs and lipid oxidation are shown in Fig. 2. Oxidation rates of both fuels were stimulated by shivering (P < 0.001). CHOs oxidation showed a rapid increase during the first 45 min of shivering before declining progressively until the end of the experiments (Fig. 2A). The effect of cold exposure on CHOs oxidation was proportional to shivering intensity and a maximal rate of 1128 ± 126 μmol O2 kg−1 min−1 was reached after 45 min at 5°C (P < 0.001). Lipid oxidation showed a progressive increase from baseline throughout the experiments (Fig. 2B). The effect of cold exposure on lipid oxidation was proportional to shivering intensity and a maximal rate of 712 ± 54 μmol O2 kg−1 min−1 was reached after 130 min at 5°C (P < 0.001). Steady-state values for rates of gas exchange and fuel oxidation at thermoneutrality and during the last 30 min of cold exposure are summarized in Table 1.
Figure 1.
Changes in ambient temperature (A) and rates of oxygen consumption (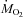 ) (B) and carbon dioxide production (
) (B) and carbon dioxide production (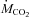 ) (C) of male Wistar rats before (27°C, thermoneutral control) and during cold exposure (15°C, ◊; 10°C, •; 5°C, ▿). Values are means ±s.e.m. (n= 9).
) (C) of male Wistar rats before (27°C, thermoneutral control) and during cold exposure (15°C, ◊; 10°C, •; 5°C, ▿). Values are means ±s.e.m. (n= 9).
Figure 2.
Rates of oxygen consumption (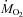 ) accounted for by carbohydrate (A) and lipid (B) oxidation in male Wistar rats before (27°C, thermoneutral control) and during cold exposure (15°C, ◊; 10°C, •; 5°C, ▿). Values are means ±s.e.m. (n= 9).
) accounted for by carbohydrate (A) and lipid (B) oxidation in male Wistar rats before (27°C, thermoneutral control) and during cold exposure (15°C, ◊; 10°C, •; 5°C, ▿). Values are means ±s.e.m. (n= 9).
Table 1.
Oxygen consumption (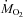 ), carbon dioxide production (
), carbon dioxide production (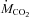 ), respiratory exchange ratio (RER), and rates of carbohydrate and lipid oxidation in rats before (27°C control) and during the last 30 min of a 3 h exposure to 15, 10 and 5°C
), respiratory exchange ratio (RER), and rates of carbohydrate and lipid oxidation in rats before (27°C control) and during the last 30 min of a 3 h exposure to 15, 10 and 5°C
| Shivering |
||||
|---|---|---|---|---|
| Parameters | Control 27°C | 15°C | 10°C | 5°C |
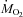 (μmol O2 kg−1 min−1) (μmol O2 kg−1 min−1) |
625 ± 10a | 1068 ± 26b | 1121 ± 21b | 1326 ± 61c |
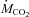 (μmol CO2 kg−1 min−1) (μmol CO2 kg−1 min−1) |
546 ± 8a | 876 ± 25b | 915 ± 19b | 1098 ± 57c |
| RER | 0.875 ± 0.003a | 0.818 ± 0.008b | 0.815 ± 0.004b | 0.826 ± 0.007b |
| Carbohydrate oxidation (μmol O2 kg−1 min−1) | 308 ± 6a | 365 ± 32b | 370 ± 20b | 501 ± 54c |
| Lipid oxidation (μmol O2 kg−1 min−1) | 172 ± 8a | 557 ± 30b | 605 ± 18c | 679 ± 31d |
Values are means ±s.e.m. (n= 9). Values not sharing a common superscript are significantly different (P < 0.05).
Effects of shivering on fuel selection
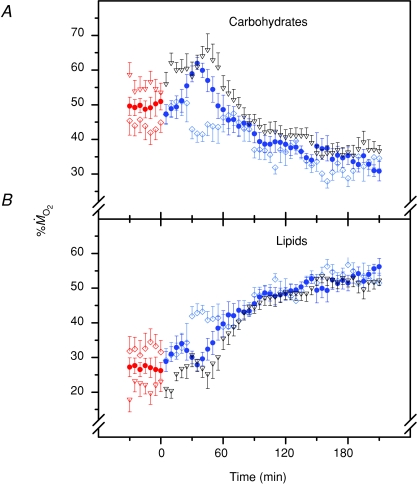
The time course of changes in the relative contributions of CHOs and lipids to 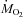 during cold exposure are presented in Fig. 3. At 27°C, CHOs were the dominant fuel, accounting for 49 ± 2% of
during cold exposure are presented in Fig. 3. At 27°C, CHOs were the dominant fuel, accounting for 49 ± 2% of 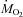 , whereas lipids provided 27 ± 2% of
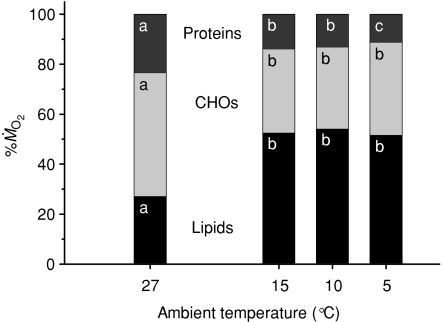
, whereas lipids provided 27 ± 2% of 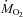 . Overall, shivering caused a large decrease in the relative contribution of carbohydrates (Fig. 3A; P < 0.001) and a large increase in the relative contribution of lipids (Fig. 3B; P < 0.001). Steady-state patterns of fuel selection at thermoneutrality and during the last 30 min of cold exposure are summarized in Fig. 4. The relative contribution of CHOs decreased from 49 to 34% of
. Overall, shivering caused a large decrease in the relative contribution of carbohydrates (Fig. 3A; P < 0.001) and a large increase in the relative contribution of lipids (Fig. 3B; P < 0.001). Steady-state patterns of fuel selection at thermoneutrality and during the last 30 min of cold exposure are summarized in Fig. 4. The relative contribution of CHOs decreased from 49 to 34% of 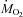 (P < 0.001). This decline was compensated by an increase in the relative contribution of lipids from 27 to 53% of
(P < 0.001). This decline was compensated by an increase in the relative contribution of lipids from 27 to 53% of 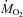 (P < 0.001). However, these changes in relative rates of CHOs and lipid oxidation remained independent of shivering intensity (P > 0.05).
(P < 0.001). However, these changes in relative rates of CHOs and lipid oxidation remained independent of shivering intensity (P > 0.05).
Figure 3.
Relative contributions of carbohydrates (A) and lipids (B) to total energy expenditure in male Wistar rats before (27°C, thermoneutral control) and during cold exposure (15°C, ◊; 10°C, •; 5°C, ▿). Values are means ±s.e.m. (n= 9).
Figure 4.
Relative contributions of carbohydrates (CHOs), lipids and proteins to total energy expenditure in male Wistar rats before (27°C, thermoneutral control) and during cold exposure (15, 10 and 5°C). Values are means for the last 30 min at each temperature (n= 9). Values not sharing a common superscript are significantly different (P < 0.05).
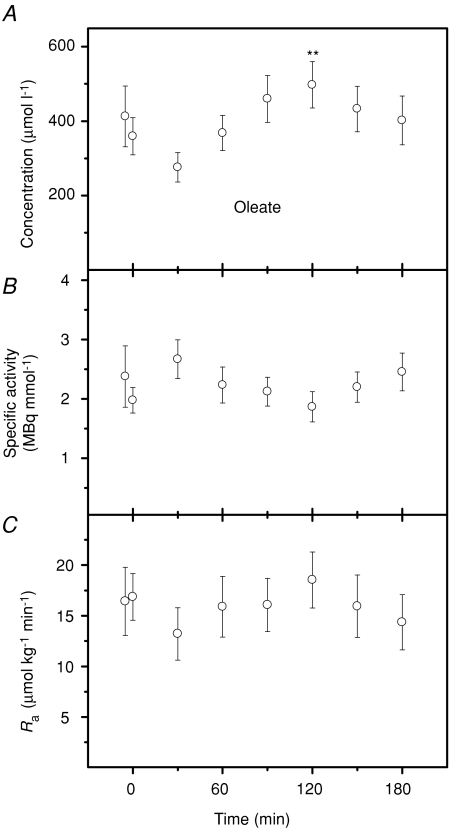
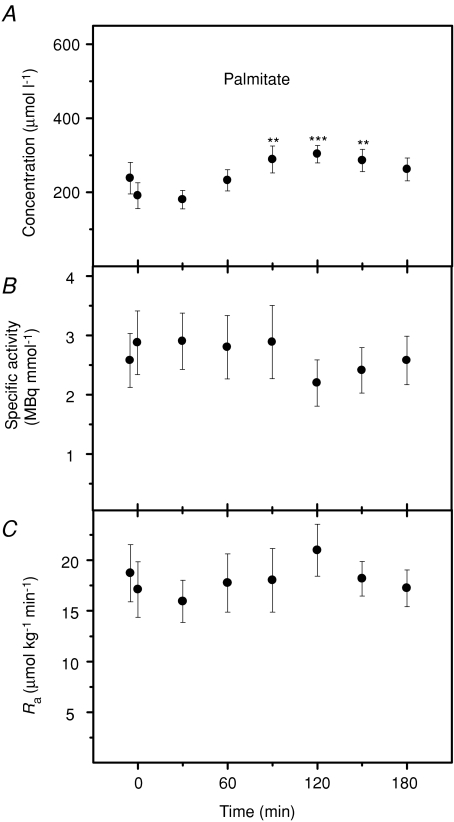
Oleate and palmitate kinetics
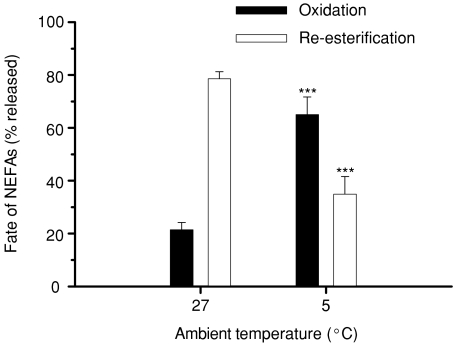
The kinetics of oleate and palmitate were measured by continuous tracer infusion during prolonged exposure to 5°C. Changes in concentration, specific activity and rate of appearance are presented in Fig. 5 (oleate) and Fig. 6 (palmitate). Both fatty acids showed transient increases in concentration during shivering (P < 0.01): to 498 ± 62 μmol l−1 for oleate and 303 ± 24 μmol l−1 for palmitate (Figs 5A and 6A). Plasma specific activities of oleate and palmitate were not affected by cold exposure (P > 0.05; Figs 5B and 6B). Rates of appearance (Ra) of oleate and palmitate remained at baseline levels throughout cold exposure (P > 0.05; Figs 5C and 6C). Mean rates of appearance were 15.9 ± 2.7 μmol kg−1 min−1 for oleate and 18.0 ± 2.1 μmol kg−1 min−1 for palmitate. Steady-state values for oleate and palmitate at thermoneutrality and during shivering are summarized in Table 2, where rate of appearance of total NEFAs and rate of fatty acid re-esterification have also been calculated. Shivering had no effect on Ra NEFA, but significantly decreased the rate of re-esterification (Table 2). Changes in the relative partitioning of Ra NEFA between oxidation and re-esterification are presented in Fig. 7. Re-esterification was dominant under thermoneutral conditions, whereas most fatty acids were oxidized during cold exposure. Shivering caused a large increase in relative oxidation (from 21 to 65%; P < 0.001) and a large decrease in relative re-esterification (from 79 to 35%; P < 0.001).
Figure 5.
Plasma oleate concentration (A), specific activity (B) and rate of appearance (Ra) (C) of male Wistar rats before (27°C) and during cold exposure (5°C). Asterisks indicate differences from thermoneutral values (**P < 0.01). Values are means ±s.e.m. (n= 7).
Figure 6.
Plasma palmitate concentration (A), specific activity (B) and rate of appearance (Ra) (C) of male Wistar rats before (27°C) and during cold exposure (5°C). Asterisks indicate differences from thermoneutral values (**P < 0.01, ***P < 0.001). Values are means ±s.e.m. (n= 7).
Table 2.
Concentrations and rates of appearance of oleate, palmitate and total NEFAs in the plasma of adult Wistar rats at thermoneutrality (27°C control) and during shivering (5°C)
| Parameters | Control 27°C | Shivering 5°C |
|---|---|---|
| Oleate concentration (μmol l−1) | 360 ± 50 | 402 ± 65 |
| % oleate in plasma NEFAs | 27.6 ± 1.2 | 27.4 ± 1.2 |
| Ra oleate (μmol kg−1 min−1) | 16.9 ± 2.3 | 14.3 ± 2.7 |
| Palmitate concentration (μmol l−1) | 191 ± 35 | 262 ± 31** |
| % palmitate in plasma NEFAs | 14.8 ± 1.0 | 18.9 ± 1.6* |
| Ra palmitate (μmol kg−1 min−1) | 17.1 ± 2.7 | 17.2 ± 1.8 |
| NEFA concentration (μmol l−1) | 1319 ± 190 | 1441 ± 202 |
| Ra NEFA (μmol kg−1 min−1) | 60.5 ± 6.3 | 50.6 ± 6.5 |
| Rate of FA re-esterification (μmol kg−1 min−1) | 48.4 ± 6.4 | 19.6 ± 6.3*** |
The rate of fatty acid re-esterification is also indicated. Values are means ±s.e.m. for steady-state values during the last 30 min at each temperature (n= 7). Ra, rate of appearance; NEFAs, non-esterified fatty acids; FA, fatty acid; *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 7.
Metabolic fate of non-esterified fatty acids released from lipid reserves (oxidation, filled bars; re-esterification, open bars) in rats before (27°C, thermoneutral control) and during cold exposure (5°C). Asterisks indicate differences from thermoneutral values (***P < 0.001). Values are means ±s.e.m. (n= 7).
Discussion
This study characterizes the effects of cold exposure on metabolic fuel selection in the rat. It shows that prolonged shivering is primarily supported by lipid oxidation (> 50%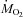 ) and that the relative importance of the different fuels is independent of shivering intensity (Figs 3 and 4; Table 1). Even though the use of carbohydrates is strongly stimulated at the onset of shivering, this substrate stops playing a dominant role after 1 h in the cold (Figs 2 and 3). The results provide no evidence supporting the preferential use of mono-unsaturated over saturated fatty acids by shivering muscles (Figs 5 and 6; Table 2). Unlike humans, rats do not stimulate fatty acid mobilization from lipid reserves during cold exposure (Table 2; Figs 5C and 6C). Their alternative strategy is to fuel shivering muscles by re-directing fatty acids normally committed for re-esterification towards oxidation (Fig. 7).
) and that the relative importance of the different fuels is independent of shivering intensity (Figs 3 and 4; Table 1). Even though the use of carbohydrates is strongly stimulated at the onset of shivering, this substrate stops playing a dominant role after 1 h in the cold (Figs 2 and 3). The results provide no evidence supporting the preferential use of mono-unsaturated over saturated fatty acids by shivering muscles (Figs 5 and 6; Table 2). Unlike humans, rats do not stimulate fatty acid mobilization from lipid reserves during cold exposure (Table 2; Figs 5C and 6C). Their alternative strategy is to fuel shivering muscles by re-directing fatty acids normally committed for re-esterification towards oxidation (Fig. 7).
Fuel selection during shivering
After 2 h of cold exposure, the fuel selection pattern of the rat reaches a steady state where total heat production is mainly supported by lipids, although CHOs and proteins still play significant roles (Figs 3 and 4). The most striking effect of prolonged shivering is a 295% increase in lipid oxidation, whereas CHOs oxidation is only stimulated by 63% (Table 1; Fig. 2). Consequently, shivering affects the fuel selection pattern of rats by increasing the relative use of lipids from 28 to 52% of 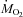 , and by decreasing the contribution of CHOs (from 49 to 35%) and proteins (from 23 to 13%) (Figs 3 and 4). The relative importance of the different fuels is the same at all shivering intensities, with lipids retaining dominance even at the lowest temperature (Fig. 4). Carbohydrates are only responsible for most of the heat produced during the first hour of cold exposure (5°C and 10°C in Fig. 3A). At 5°C, the onset of shivering is characterized by a 265% increase in CHOs oxidation followed by a progressive decrease over the next few hours (Fig. 2A). This decrease could be linked to changes in fibre recruitment over shivering time (Haman et al. 2004b). In addition, it may be caused by limited CHOs reserves that must be spared to be able to prolong shivering (Table 1; Fig. 3A). To explore this possibility, we have calculated for how long shivering could be sustained before depleting glycogen reserves. Assuming that a 384 g rat has 161 g muscle (Arola et al. 1979) and a 22 g liver (Konijnenberg et al. 2004), a total of 1.94 g of glycogen is available for heat production (with 5.5 mg glycogen (g muscle)−1 and 48 mg glycogen (g liver)−1 in fed animals (Friman et al. 1991)). At the highest rate of CHOs oxidation measured in this study (1128 μmol O2 kg−1 min−1; Fig. 2A), glycogen stores would be depleted in 2.5 h. Therefore, the large increase in lipid oxidation observed throughout cold exposure may be necessary to delay the depletion of CHOs and to continue shivering. At the lower rates of CHOs oxidation reached at steady state (Table 1), glycogen reserves would last between 5.6 and 7.7 h depending on ambient temperature.
, and by decreasing the contribution of CHOs (from 49 to 35%) and proteins (from 23 to 13%) (Figs 3 and 4). The relative importance of the different fuels is the same at all shivering intensities, with lipids retaining dominance even at the lowest temperature (Fig. 4). Carbohydrates are only responsible for most of the heat produced during the first hour of cold exposure (5°C and 10°C in Fig. 3A). At 5°C, the onset of shivering is characterized by a 265% increase in CHOs oxidation followed by a progressive decrease over the next few hours (Fig. 2A). This decrease could be linked to changes in fibre recruitment over shivering time (Haman et al. 2004b). In addition, it may be caused by limited CHOs reserves that must be spared to be able to prolong shivering (Table 1; Fig. 3A). To explore this possibility, we have calculated for how long shivering could be sustained before depleting glycogen reserves. Assuming that a 384 g rat has 161 g muscle (Arola et al. 1979) and a 22 g liver (Konijnenberg et al. 2004), a total of 1.94 g of glycogen is available for heat production (with 5.5 mg glycogen (g muscle)−1 and 48 mg glycogen (g liver)−1 in fed animals (Friman et al. 1991)). At the highest rate of CHOs oxidation measured in this study (1128 μmol O2 kg−1 min−1; Fig. 2A), glycogen stores would be depleted in 2.5 h. Therefore, the large increase in lipid oxidation observed throughout cold exposure may be necessary to delay the depletion of CHOs and to continue shivering. At the lower rates of CHOs oxidation reached at steady state (Table 1), glycogen reserves would last between 5.6 and 7.7 h depending on ambient temperature.
The effects of shivering on fuel selection have been measured in rats, humans and one species of bird, but meaningful comparisons between species are complicated by differences in body size and thermal insulation that affect metabolic rate (Vaillancourt et al. 2005; Weber & Haman, 2005; Vaillancourt & Weber, 2007). To standardize cold stress between studies, we have only compared fuel selection patterns among shivering animals showing the same relative increase in mass-specific resting metabolic rate (factorial increase in RMR). Data from wild sandpipers suggest that birds and mammals have very different fuel selection patterns. Comparing ruff sandpipers and rats shivering at 1.7 RMR (birds exposed to 5°C, Vaillancourt et al. 2005, vs. rats exposed to 15°C, Fig. 4) shows that lipids play a much more important thermogenic role in the birds (82% lipids, 12% CHOs and 6% proteins). In contrast, comparing rats and humans shivering at ∼2.2 RMR reveals that the fuel selection patterns of these two mammalian species are virtually identical (51% lipids, 38% CHOs and 11% proteins) (Fig. 4 and Haman et al. 2002). Therefore, the 27°C-acclimated Wistar rat may be a valuable experimental model to gain insights on the regulation of fuel metabolism in shivering humans, at least up to 2.2 RMR.
Supplying fatty acids to support shivering
The observed dominance of lipid-based thermogenesis led us to investigate how rats provide fatty acids to shivering muscles. Animals always mobilize fatty acids from triacylglycerol reserves at higher rates than strictly necessary for energy metabolism and, therefore, a significant fraction of total NEFAs released is re-esterified. Under resting, thermoneutral conditions, a large percentage of the fatty acids appearing in the circulation undergoes re-esterification (62% of Ra NEFA in humans, Wolfe et al. 1990; 80% in rabbits, Reidy & Weber, 2002; and 39–78% in rats, Kalderon et al. 2000; McClelland et al. 2001 and Fig. 7 of this study). When the overall need for fatty acids increases, adequate regulation of Ra NEFA is essential to provide enough fuel for oxidation and to maintain sufficient rates of re-esterification for triacylglycerol and phospholipid turnover. During shivering or exercise, this could potentially be achieved by stimulating Ra NEFA, by reducing re-esterification, or through a combination of both. All previous studies show that stimulating Ra NEFA is the strategy used by mammals to support endurance exercise or shivering. Prolonged exercise causes significant stimulation of Ra NEFA for all mammalian species measured to date and the increase is proportional to body size (1.2-fold in rats, McClelland et al. 2001; 1.4-fold in goats, Weber et al. 1996; 1.8-fold in dogs, Weber et al. 1996; and 2- to 6-fold in humans, Wolfe et al. 1990; Friedlander et al. 1999). In exercising mammals, adjustments in Ra NEFA are therefore responsible for maintaining an ‘excess’ supply of fatty acids for re-esterification. The only previous cold-exposure study dealing with the partitioning of total fatty acids between oxidation and re-esterification was performed in humans. It reports that shivering causes a 3-fold increase in Ra NEFA without modifying the proportion of fatty acids allocated to re-esterification (∼51%) (Vallerand et al. 1999). The present study is the first to show that mammals can reduce re-esterification to increase fatty acid availability for oxidation. Shivering rats support thermogenesis by redirecting fatty acids normally allocated to re-esterification towards oxidation, and this alternative strategy is illustrated in Fig. 7. In the rat, shivering causes a large decrease in percentage re-esterification (79% to 35%) to allow the necessary increase in percentage oxidation (21% to 65%).
Preferential mobilization (Jezierska et al. 1982; Raclot & Groscolas, 1993, 1995) and oxidation of mono-unsaturated over saturated fatty acids (Leyton et al. 1987; Sidell, 1991; Sidell et al. 1995) has been widely observed in nature. Oleate is often the most abundant fatty acid present in the lipid reserves of birds, mammals and fish (Blem, 1990; Florant et al. 1990; Lund & Sidell, 1992). Therefore, we anticipated that the fluxes of oleate and palmitate would not be affected similarly by cold exposure in rats. Contrary to expectation, our results provide no evidence for the preferential use of mono-unsaturated fatty acids because the fluxes of oleate and palmitate are not stimulated by prolonged shivering (Figs 5 and 6). However, it is premature to eliminate the possibility that oleate could also be a preferred oxidative fuel in shivering rats because direct measurements of oleate and palmitate oxidation are not available. To resolve this issue, the technical challenge of quantifying rates of oxidation for individual fatty acids will have to be overcome.
Conclusions
These results demonstrate that lipids dominate the fuel selection pattern of the rat during steady-state cold exposure, with shivering being unequally supported by the oxidation of lipids (52% of 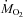 ), carbohydrates (35%) and proteins (13%). The same relative use of the different oxidative fuels is observed at all shivering intensities. Even though CHOs oxidation is strongly stimulated at the onset of cold exposure, its thermogenic contribution is rapidly reduced to spare limited glycogen reserves and prolong shivering. No preferential use of mono-unsaturated over saturated fatty acids by shivering muscles was observed, but direct measurements of palmitate and oleate oxidation are needed to eliminate this possibility. This study is the first to show that increased fatty acid supply to fuel energy metabolism can also be achieved by reducing re-esterification, not only by stimulating Ra NEFA as previously reported for other mammals. The novel strategy used by rats to fuel shivering muscles is to reallocate fatty acids from re-esterification to oxidation.
), carbohydrates (35%) and proteins (13%). The same relative use of the different oxidative fuels is observed at all shivering intensities. Even though CHOs oxidation is strongly stimulated at the onset of cold exposure, its thermogenic contribution is rapidly reduced to spare limited glycogen reserves and prolong shivering. No preferential use of mono-unsaturated over saturated fatty acids by shivering muscles was observed, but direct measurements of palmitate and oleate oxidation are needed to eliminate this possibility. This study is the first to show that increased fatty acid supply to fuel energy metabolism can also be achieved by reducing re-esterification, not only by stimulating Ra NEFA as previously reported for other mammals. The novel strategy used by rats to fuel shivering muscles is to reallocate fatty acids from re-esterification to oxidation.
Acknowledgments
We wish to thank Sylvie Émond, Bill Fletcher, Jennifer Keyte and Kim Yates (Animal Care and Veterinary Services, University of Ottawa). E.V. is the recipient of an Alexander Graham Bell Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the study was funded by an NSERC Discovery Grant to J.M.W.
Glossary
Abbreviations
- CHOs
carbohydrates
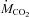
rate of carbon dioxide production
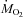
rate of oxygen consumption
- NEFAs
non-esterified fatty acids
- NLs
neutral lipids
- PLs
phospholipids
- Ra
rate of appearance
- RMR
resting metabolic rate
Author contributions
All authors were equally involved in experimental design, data analysis and writing. E.V. carried out the experiments with some help from J.-M.W. This research was performed in the biology department of the University of Ottawa.
References
- Abdul-Malak N, Brichon G, Meister R, Zwingelstein G. Environmental temperature and metabolism of molecular species of phosphatidylcholine in the tissues of the rainbow trout. Lipids. 1989;24:318–324. [Google Scholar]
- Adán C, Ardévol A, Remesar X, Alemany M, Fernández-López JA. Hindleg muscle energy and substrate balances in cold-exposed rats. J Exp Biol. 1995;198:1243–1251. doi: 10.1242/jeb.198.6.1243. [DOI] [PubMed] [Google Scholar]
- Arola L, Herrera E, Alemany M. A method for the estimation of striated muscle mass in small laboratory animals. Rev Esp Fisiol. 1979;35:215–218. [PubMed] [Google Scholar]
- Banet M, Hensel H, Liebermann H. The central control of shivering and non-shivering thermogenesis in the rat. J Physiol. 1978;283:569–584. doi: 10.1113/jphysiol.1978.sp012520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blem CR. Avian energy storage. Curr Ornithol. 1990;7:59–113. [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Daenzer M, Petzke KJ, Bequette BJ, Metges CC. Whole-body nitrogen and splanchnic amino acid metabolism differ in rats fed mixed diets containing casein or its corresponding amino acid mixture. J Nutr. 2001;131:1965–1972. doi: 10.1093/jn/131.7.1965. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florant GL, Nuttle LC, Mullinex DE, Rintoul DA. Plasma and white adipose tissue lipid composition in marmots. Am J Physiol Regul Integr Comp Physiol. 1990;258:R1123–R1131. doi: 10.1152/ajpregu.1990.258.5.R1123. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Casazza GA, Horning MA, Usaj A, Brooks GA. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J Appl Physiol. 1999;86:2097–2105. doi: 10.1152/jappl.1999.86.6.2097. [DOI] [PubMed] [Google Scholar]
- Friman G, Ilbäck N-G, Crawford DJ, Neufeld HA. Metabolic responses to swimming exercise in Streptococcus pneumoniae infected rats. Med Sci Sports Exerc. 1991;23:415–421. [PubMed] [Google Scholar]
- Fuller CA, Horwitz BA, Horowitz JM. Shivering and nonshivering thermogenic responses of cold-exposed rats to hypothalamic warming. Am J Physiol. 1975;228:1519–1524. doi: 10.1152/ajplegacy.1975.228.5.1519. [DOI] [PubMed] [Google Scholar]
- Gautier H. Body temperature regulation in the rat. J Therm Biol. 2000;25:273–279. doi: 10.1016/s0306-4565(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Gautier H, Bonora M, Ben M, Barek S, Sinclair JD. Effects of hypoxia and cold acclimation on thermoregulation in the rat. J Appl Physiol. 1991;71:1355–1363. doi: 10.1152/jappl.1991.71.4.1355. [DOI] [PubMed] [Google Scholar]
- Haman F, Legault SR, Rakobowchuk M, Ducharme MB, Weber J-M. Effects of carbohydrate availability on sustained shivering: II. Relating muscle recruitment to fuel selection. J Appl Physiol. 2004a;96:41–49. doi: 10.1152/japplphysiol.00428.2003. [DOI] [PubMed] [Google Scholar]
- Haman F, Legault SR, Weber J-M. Fuel selection during intense shivering in humans: EMG pattern reflects carbohydrate oxidation. J Physiol. 2004b;556:305–313. doi: 10.1113/jphysiol.2003.055152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haman F, Péronnet F, Kenny G, Doucet E, Massicotte D, Lavoie C, Weber J-M. Effects of carbohydrate availability on sustained shivering: I. Oxidation of plasma glucose, muscle glycogen and proteins. J Appl Physiol. 2004c;96:32–40. doi: 10.1152/japplphysiol.00427.2003. [DOI] [PubMed] [Google Scholar]
- Haman F, Péronnet F, Kenny G, Massicotte D, Lavoie C, Scott C, Weber J-M. Effect of cold exposure on fuel utilization in humans: Plasma glucose, muscle glycogen and lipids. J Appl Physiol. 2002;93:77–84. doi: 10.1152/japplphysiol.00773.2001. [DOI] [PubMed] [Google Scholar]
- Haman F, Péronnet F, Kenny GP, Massicotte D, Lavoie C, Weber J-M. Partitioning oxidative fuels during cold exposure: Muscle glycogen becomes dominant as shivering intensifies. J Physiol. 2005;566:247–256. doi: 10.1113/jphysiol.2005.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J. Neural and hormonal responses to prolonged cold exposure. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology. II. New York: Oxford University Press; 1996. pp. 439–480. section 4. [Google Scholar]
- Jacobs I, Martineau L, Vallerand AL. Thermoregulatory thermogenesis in humans during cold stress. In: Holloszy JO, editor. Exercise Sport Science Review. Baltimore: Williams and Wilkins; 1994. pp. 221–250. [PubMed] [Google Scholar]
- Jezierska B, Hazel JR, Gerking SD. Lipid mobilization during starvation in the rainbow trout Salmo gairdneri Richardson, with attention to fatty acids. J Fish Biol. 1982;21:681–692. [Google Scholar]
- Kalderon B, Mayorek N, Berry E, Zevit N, Bar-Tana J. Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab. 2000;279:E221–E227. doi: 10.1152/ajpendo.2000.279.1.E221. [DOI] [PubMed] [Google Scholar]
- Konijnenberg MW, Bijster M, Krenning EP, de Jong M. A stylized computational model of the rat for organ dosimetry in support of preclinical evaluations of peptide receptor radionuclide therapy with 90Y, 111In, or 177Lu. J Nucl Med. 2004;45:1260–1269. [PubMed] [Google Scholar]
- Leyton J, Drury PJ, Crawford MA. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br J Nutr. 1987;57:383–393. doi: 10.1079/bjn19870046. [DOI] [PubMed] [Google Scholar]
- Lund ED, Sidell BD. Neutral lipid compositions of Antarctic fish tissues may reflect use of fatty acyl substrates by catabolic systems. Mar Biol. 1992;112:377–382. [Google Scholar]
- McClelland GB, Hochachka PW, Reidy S, Weber J-M. High-altitude acclimation increases the triacylglycerol/fatty acid cycle at rest and during exercise. Am J Physiol Endocrinol Metab. 2001;281:E537–E544. doi: 10.1152/ajpendo.2001.281.3.E537. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Hochachka PW, Weber J-M. Effect of high-altitude acclimation on NEFA turnover and lipid utilization during exercise in rats. Am J Physiol Endocrinol Metab. 1999;277:E1095–E1102. doi: 10.1152/ajpendo.1999.277.6.E1095. [DOI] [PubMed] [Google Scholar]
- Oufara S, Barré H, Rouanet J-L, Chatonnet J. Adaptation to extreme ambient temperatures in cold-acclimated gerbils and mice. Am J Physiol Regul Integr Comp Physiol. 1987;253:R39–R45. doi: 10.1152/ajpregu.1987.253.1.R39. [DOI] [PubMed] [Google Scholar]
- Raclot T, Groscolas R. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J Lipid Res. 1993;34:1515–1526. [PubMed] [Google Scholar]
- Raclot T, Groscolas R. Selective mobilization of adipose tissue fatty acids during energy depletion in the rat. J Lipid Res. 1995;36:2164–2173. [PubMed] [Google Scholar]
- Reidy SP, Weber J-M. Accelerated substrate cycling: a new energy-wasting role for leptin in vivo. Am J Physiol Endocrinol Metab. 2002;282:E312–E317. doi: 10.1152/ajpendo.00037.2001. [DOI] [PubMed] [Google Scholar]
- Sidell BD. Physiological roles of high lipid content in tissues of Antarctic fish species. In: di Prisco G, Maresca B, Tota B, editors. Biology of Antarctic Fish. New York: Springer Verlag; 1991. pp. 220–231. [Google Scholar]
- Sidell BD, Crockett EL, Driedzic WR. Antarctic fish tissues preferentially catabolize monoenoic fatty acids. J Exp Zool. 1995;271:73–81. [Google Scholar]
- Smith OLK, Davidson SB. Shivering thermogenesis and glucose uptake by muscles of normal or diabetic rats. Am J Physiol Regul Integr Comp Physiol. 1982;242:R109–R115. doi: 10.1152/ajpregu.1982.242.1.R109. [DOI] [PubMed] [Google Scholar]
- Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Med Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Vaillancourt E, Prud’Homme S, Haman F, Guglielmo CG, Weber J-M. Energetics of a long-distance migrant shorebird (Philomachus pugnax) during cold exposure and running. J Exp Biol. 2005;208:317–325. doi: 10.1242/jeb.01397. [DOI] [PubMed] [Google Scholar]
- Vaillancourt E, Weber J-M. Lipid mobilization of long-distance migrant birds in vivo: the high lipolytic rate of ruff sandpipers is not stimulated during shivering. J Exp Biol. 2007;210:1161–1169. doi: 10.1242/jeb.003012. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Zamecnik J, Jones PJH, Jacobs I. Cold stress increases lipolysis, FFA Ra and TG/FFA cycling in humans. Aviat Space Environ Med. 1999;70:42–50. [PubMed] [Google Scholar]
- Weber J-M, Brichon G, Zwingelstein G, McClelland G, Saucedo C, Weibel ER, Taylor CR. Design of the oxygen and substrate pathways: IV. Partitioning energy provision from fatty acids. J Exp Biol. 1996;199:1667–1674. doi: 10.1242/jeb.199.8.1667. [DOI] [PubMed] [Google Scholar]
- Weber J-M, Haman F. Fuel selection in shivering humans. Acta Physiol Scand. 2005;184:319–329. doi: 10.1111/j.1365-201X.2005.01465.x. [DOI] [PubMed] [Google Scholar]
- Weber J-M, O’Connor T. Energy metabolism of the Virginia opossum during fasting and exercise. J Exp Biol. 2000;203:1365–1371. doi: 10.1242/jeb.203.8.1365. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Klein S, Carraro F, Weber J-M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol Endocrinol Metab. 1990;258:E382–E389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- Younes H, Demigné C, Behr S, Rémésy C. Resistant starch exerts a lowering effect on plasma urea by enhancing urea N transfer into the large intestine. Nutr Res. 1995;15:1199–1210. [Google Scholar]
- Young AJ. Human adaptation to cold. In: Pandolf KB, Sawka MN, Gonzalez RR, editors. Human Performance Physiology and Environmental Medicine at Terrestrial Extremes. Traverse City, MI, USA: Cooper Publishing Group; 1988. pp. 401–434. [Google Scholar]