Abstract
Aims
We evaluated the ability of a dual-species community of oral bacteria to produce the universal signalling molecule, autoinducer-2 (AI-2), in saliva-fed biofilms.
Methods and Results
Streptococcus oralis 34, S. oralis 34 luxS mutant and Actinomyces naeslundii T14V were grown as single- and dual-species biofilms within sorbarods fed with 25% human saliva. AI-2 concentration in biofilm effluents was determined by the Vibrio harveyi BB170 bioluminescence assay. After homogenizing the sorbarods to release biofilm cells, cell numbers were determined by fluorometric analysis of fluorescent antibody-labelled cells. After 48 h, dual-species biofilm communities of interdigitated S. oralis 34 and A. naeslundii T14V contained 3.2 × 109 cells: fivefold more than single-species biofilms. However, these 48-h dual-species biofilms exhibited the lowest concentration ratio of AI-2 to cell density.
Conclusions
Oral bacteria produce AI-2 in saliva-fed biofilms. The decrease of more than 10-fold in concentration ratio seen between 1 and 48 h in S. oralis 34–A. naeslundii T14V biofilms suggests that peak production of AI-2 occurs early and is followed by a very low steady-state level.
Significance and Impact of the Study
High oral bacterial biofilm densities may be achieved by inter-species AI-2 signalling. We propose that low concentrations of AI-2 contribute to the establishment of oral commensal biofilm communities.
Keywords: Actinomyces naeslundii, autoinducer-2, cell-cell signalling, communication, dual-species biofilm, oral bacteria, Streptococcus oralis.
Introduction
The oral cavity contains greater than 700 phylotypes of bacteria (Aas et al. 2005). Many of these oral species coaggregate with each other (Kolenbrander et al. 2002) and it is likely that these intergeneric interactions facilitate an ordered and reproducible successional process of biofilm development (Li et al. 2004; Diaz et al. 2006). Coaggregation is mediated by highly specific and complementary cell-surface-associated adhesins and receptors that bring species into intimate contact (McIntire et al. 1978; Kolenbrander 1995). This process is believed to contribute to the juxtaposition of synergistic species (Kolenbrander et al. 2006). Close proximity, as a consequence of coaggregation, can facilitate efficient communication by the production and detection of metabolites (Egland et al. 2004) and cell–cell signalling molecules such as autoinducer-2 (AI-2; Surette et al. 1999; Kolenbrander et al. 2002).
AI-2 is formed from the spontaneous rearrangement of 4,5-dihydroxy-2,3-pentanedione (DPD; Duerre et al. 1971; Semmelhack et al. 2005), which is a product of the LuxS enzyme in the catabolism of S-ribosylhomocysteine. AI-2 is an umbrella designation and consists of a family of inter-convertible furanosyl molecules (Semmelhack et al. 2005), which are produced by bacteria from a taxonomically diverse range of species (Sun et al. 2004). Because AI-2 is produced by such a broad range of species and can induce the bioluminescence of Vibrio harveyi, AI-2 has been proposed to be a universal inter-species bacterial signalling molecule (Bassler et al. 1997). Research to support this hypothesis includes AI-2-mediated changes in gene expression within V. harveyi, Vibrio cholerae and Escherichia coli (Xavier and Bassler 2005b; Kendall et al. 2007). Further, AI-2 production by bacteria indigenous to the human oral cavity has been reported for 19 species belonging to 12 genera of oral bacteria (Fong et al. 2001; Frias et al. 2001; Blehert et al. 2003; McNab et al. 2003; Yoshida et al. 2005; James et al. 2006a,b). The luxS gene encodes LuxS and has been disrupted in six of these species where changes in biofilm-forming ability and cellular characteristics have been observed. Thus, AI-2-based signalling has been proposed to mediate inter-species communication between oral bacteria as well as biofilm community development within the human oral cavity (Kolenbrander et al. 2006).
AI-2 produced by oral bacteria can be difficult to detect and quantify. Some of the conditions that affect AI-2 detection in other systems include: (i) AI-2 can be sequestered or degraded by enteric bacteria (Xavier and Bassler 2005a; Xavier et al. 2007), and (ii) AI-2 forms spontaneously inter-convertible molecular structures that have distinct receptor-binding specificity (Miller et al. 2004; Semmelhack et al. 2005). In addition, AI-2 may occur at concentrations that are below the threshold for detection by a bioluminescence assay (Rickard et al. 2006) that is sensitive to subtle changes in experimental conditions (DeKeersmaecker and Vanderleyden 2003; Vilchez et al. 2007). Another complication is that within most natural environments, including the human oral cavity, bacteria predominantly exist in biofilms (Hall-Stoodley et al. 2004), where cells are in close proximity with one another. In biofilms, they can interact with each other and create a localized environment that is distinct from the surrounding fluid phase. Until now, a model system to detect concentrations of AI-2 in a biofilm had not been developed. Indeed, within the human oral cavity, the production of AI-2 by bacteria in biofilms is presumed but has yet to be demonstrated (Kolenbrander et al. 2006).
Using saliva-fed flowcells, Palmer et al. (2001) demonstrated that mono-species biofilms of the AI-2-producing oral bacteria Streptococcus oralis 34 and Actinomyces naeslundii T14V did not grow, but together the pair exhibited ‘luxuriant inter-digitated growth’. Further, a luxS mutant of S. oralis 34 was subsequently constructed (Rickard et al. 2006) that did not produce AI-2 and did not form mutualistic interactions with A. naeslundii T14V. Chemical complementation, via the addition of chemically synthesized AI-2 to saliva at a concentration of 80–800 pmol l−1, re-established mutualism between the S. oralis 34 luxS mutant and A. naeslundii T14V (Rickard et al. 2006). The lower threshold for detection of AI-2 by the V. harveyi bioluminescence assay is c. 100 nmol l−1, which is 10 to 100 times higher than the concentrations of AI-2 that mediated mutualism in the flowcell system. This conclusion was supported by our inability to detect AI-2 in the flowcell effluent, although it was readily detected in human saliva when a nanomolar concentration of synthetic AI-2 was added to saliva. Furthermore, it was hypothesized that coaggregation brought the two species into intimate contact and that the summed AI-2 concentration mediated mutualism between the pair (Rickard et al. 2006).
The inability to detect AI-2 was likely because of the relatively low cell numbers in the flowcells and a resultant low concentration of AI-2. Thus, the aim of this work was: (i) to develop a naturally relevant biofilm where the AI-2 produced by a mutualistic pair of oral species could be detected and (ii) to develop a combination of fluorescence- and bioluminescence-based techniques for obtaining a concentration ratio reflecting the concentration of AI-2 (nanomol l−1) in the biofilm and the cell density of the biofilm (cell number ml−1).
Materials and methods
Strains and culture conditions
Actinomyces naeslundii T14V, S. oralis 34 and S. oralis 34 luxS mutant were used as inocula for the formation of single- and dual-species biofilms. Prior to biofilm studies, all strains were grown in liquid CAMG medium, which contained tryptone, yeast extract, Tween 80 and glucose (0.2%) buffered to pH 7.5 with K2HPO4 (Cisar et al. 1979), under a CO2-enriched atmosphere (5%) at 37°C. For biofilm studies, batch cultures were harvested by centrifugation (3500 g for 7 min) and washed twice with 25% sterile human saliva (1 ml saliva per wash). The cell density was normalized to 5 × 108 cells ml−1 in 25% saliva, as determined by spectrophotometric measurement at 600 nm and by direct cell counts with a Petroff-Hausser cell counter (C.A. Hauser and Son, Philadelphia, PA, USA). A 1-ml inoculum was used for each sorbarod (see next).
Preparation of 25% saliva
Saliva was collected from a minimum of six healthy adults, who were nonsmokers and had not been subjected to antibiotics within 3 months prior to donating. Saliva was pooled, and treated with 2.5 mmol l−1 dithiothreitol (Sigma, St. Louis, MO, USA) for 10 min to reduce salivary protein aggregation. The treated saliva was then centrifuged at 18 000 g for 20 min and the supernatant was diluted 1: 4 with distilled water to produce 25% saliva. The diluted saliva was filtered through a 0.22-μm-pore size Supor Mach low-protein-binding filter (Nalge Nunc, Rochester, NY, USA). When not used immediately, the filtered saliva was stored at −20°C for a maximum of 2 months. Prior to use, saliva was thawed and re-filtered to remove precipitate that formed during the freeze-thaw process.
Sorbarod biofilms
Sorbarod devices (Hodgson et al. 1995) were used to support the growth of single- and dual-species biofilms of S. oralis 34, S. oralis 34 luxS mutant and A. naeslundii T14V under conditions relevant to the human oral cavity. Sorbarod filters (paper-wrapped sheaf of tightly packed fibres; cylinder 10 mm diameter; 20 mm length) were a gift from Prof. Peter Gilbert (University of Manchester, Manchester, UK). Sorbarods were autoclaved, inserted into 3 ml Monoject™ syringes (Sherwood Medical, St. Louis, MO, USA) and sealed with the plunger gasket. Sorbarod devices were then connected to silicon tubing (5 mm internal diameter) and an upstream peristaltic pump (Minipuls III, Gilson, Middleton, WI, USA). Sorbarods were fed 25% saliva at a flow rate of 50 μl min−1 at a temperature of 37°C for 20 min to condition the sorbarods for colonization of oral bacteria.
For the study of mono-species biofilms, saliva-conditioned sorbarods were inoculated with 1 ml that contained 5 × 108 exponential-phase S. oralis 34, S. oralis 34 luxS mutant, or A. naeslundii T14V cells in 25% saliva. Inoculated sorbarods were allowed to stand for 20 min to allow adherence of bacteria to the sorbarod fibres, and then flow was resumed. Dual-species biofilms were developed by inoculating with 0.5 ml containing 1 × 108 streptococcal cells and then, after 20 min, the sorbarod was inoculated with 0.5 ml containing 5 × 108 actinomyces cells.
Determination of planktonic and biofilm streptococcal and actinomyces cell numbers
To relate fluorescence to cell numbers, cultures of streptococci or actinomyces were labelled with antibody that had been conjugated with Alexa Fluor® fluorescent dye. Anti-S. oralis 34 immunoglobulin G (IgG) was labelled with Alexa Fluor® 488 and anti-A. naeslundii T14V IgG was labelled with Alexa Fluor® 633 using the manufacturer’s instructions (Invitrogen, Eugene, OR, USA). Batch cultures of cells were washed in phosphate-buffered saline (PBS) plus 1% bovine serum albumin (BSA) and 5 μg ml−1 of antibody was added to the cell suspension. After 20 min, the cells were washed in PBS and serially diluted. Direct counts of different concentrations of the labelled cells were obtained with a Petroff–Hauser counting chamber and the corresponding fluorescence in the samples was determined in a Wallac 1420 VICTOR 3™ fluorimeter (PerkinElmer, Boston, MA, USA). A linear standard curve was constructed by plotting fluorescence against cell number.
To determine the numbers of cells in a sorbarod biofilm, an entire sorbarod was taken from its device and homogenized with a tissue-tearor™ (Biospec Products, Oklahoma City, OK, USA) in 5 ml of ice-cold PBS plus 1% BSA. Microscopic inspection of the homogenate indicated that the vast majority of the biofilm cells were no longer in association with the disrupted sorbarod fibres and were free-floating in suspension. To 1 ml of the suspension, 5 μg ml−1 of Alexa Fluor®-conjugated anti-S. oralis 34 or anti-A. naeslundii T14V antibody (Palmer et al. 2001; Rickard et al. 2006) was added and incubated at room temperature for 20 min. The labelled cells were then washed in PBS and 100-μl aliquots were dispensed to triplicate wells of a 96-well plate and the fluorescence intensity was determined in a Wallac 1420 VICTOR 3™ set to fluorescence mode. Cell number was obtained from the standard curve.
Determination of AI-2 concentration in sorbarod effluent
Results from previous studies of S. oralis 34 and A. naeslundii T14V demonstrated that AI-2 was produced by each organism when grown in commercial media in batch culture (Rickard et al. 2006). To determine if cells of each species produce AI-2 when they are members of biofilms in saliva, effluent was collected from the sorbarod biofilms. The collected effluent was passed through a 0.22-μm MCE filter (Fisher Scientific, Suwanee, GA, USA) and the cell-free spent saliva was subjected to a modified AI-2 bioluminescence bioassay (Surette and Bassler 1998; Rickard et al. 2006). Briefly, cells of V. harveyi BB170 were grown at 30°C in batch cultures of autoinducer bioassay (AB) medium (Surette and Bassler 1998). After 14 h of growth, V. harveyi BB170 cultures were diluted 1: 500 in fresh AB medium. The diluted BB170 culture was dispensed at 90 μl per well of a 96-well plate and stored at −70°C. Subsequently, 10 μl of cell-free effluent saliva was added to 90 μl of thawed, diluted BB170 culture in a 96-well plate. Bioluminescence relative to sterile, 25% saliva was calculated as fold induction (Blehert et al. 2003) during incubation and sampling at a constant temperature of 30°C and controlled agitation within a Wallac 1420 VICTOR 3™ set to luminescence mode.
To convert the amount of AI-2 from fold induction to molar concentrations, nmol l−1 to mmol l−1 quantities of chemically synthesized DPD were compared against the induced amount of bioluminescence of the V. harveyi BB170 reporter. By comparing fold induction with DPD concentration (80 nmol l−1 to 800 nmol l−1 DPD) a direct positive relationship between the two was determined (R2 = 0.9938).
Confocal laser scanning microscopy (CLSM) image analysis
Sections of developed biofilms were excised by cutting the sorbarod sheaf longitudinally using a sterile scalpel and a section was transferred to a sterile Petri dish where it was labelled for 20 min with 5 μg ml−1 of anti-S. oralis 34 or anti-A. naeslundii T14V Alexa Fluor®-conjugated antibody. To visualize the sorbarod fibres, sorbarod sections were subsequently bathed in 10 ml of PBS (pH 7.2) containing 1 μg ml−1 of calcofluor white (Invitrogen) and then bathed in 10 ml of PBS (pH 7.2) to remove excess antibodies and calcofluor white. Sorbarod biofilms were subsequently examined using a TCS-SP2 confocal microscope (Leica, Exton, PA, USA). To visualize labelled S. oralis 34 and A. naeslundii T14V, 488- and 633-nm excitation wavelengths were used, respectively. Confocal images were obtained using a Leica HCX APO L 63×/0.9 dipping lens. Each biofilm was scanned at randomly selected positions. Z-series were generated by vertical optical sectioning at every position with the Z-slice thickness set to 1 μm. Image acquisition and analysis was made with the software Imaris ver. 3.3.1 (Bitplane AG, Zurich, Switzerland) and all images presented are maximum projections of the entire confocal image stack.
Results
Standard curve for calculation of AI-2 concentration in saliva and sorbarod biofilm effluent
Known quantities of DPD were added to 25% saliva and the corresponding bioluminescence of V. harveyi BB170 was determined. Similar to findings by Semmelhack et al. (2005) and DeKeersmaecker et al. (2005), the bioluminescence of V. harveyi BB170 in response to nmol l−1 to mmol l−1 concentrations of DPD was linear only between DPD concentrations of c. 0.08 and 0.8 μmol l−1 (Fig. 1a). Using this range, we applied a line of best-fit (Fig. 1b) to calculate the concentrations of AI-2 in effluent samples from sorbarod biofilms.
Figure 1.

(a) The relationship between 4,5-dihydroxy-2,3-pentanedione (DPD) concentration and fold induction of bioluminescence by Vibrio harveyi BB170. Values below a fold induction of 2 (dotted horizontal line) were considered to be background. Data points with circles around them were used for regression analysis (b) to allow for the calculation of DPD (autoinducer-2) concentrations based upon fold induction values (R2 = 0.9938).
Correlation of fluorescence and cell number
To determine the number of biofilm cells within a sorbarod, we developed a fluorescence-based assay, which allowed for the calculation of each cell type in the sorbarod biofilm. Streptococcal and actinomyces cells were labelled with Alexa-Fluor®-488- and Alexa-Fluor®-633-conjugated antibodies, respectively, and the fluorescence of different concentrations of cells was measured. Fluorescence in a sample was correlated to cell numbers per millilitre in the sample after counting the cells with a Petroff–Hauser counting chamber (Fig. 2). A direct relationship between S. oralis 34, S. oralis 34 luxS mutant or A. naeslundii T14V cell numbers and fluorescence was determined for cell densities between 1 × 107 and 2 × 109 cells ml−1.
Figure 2.
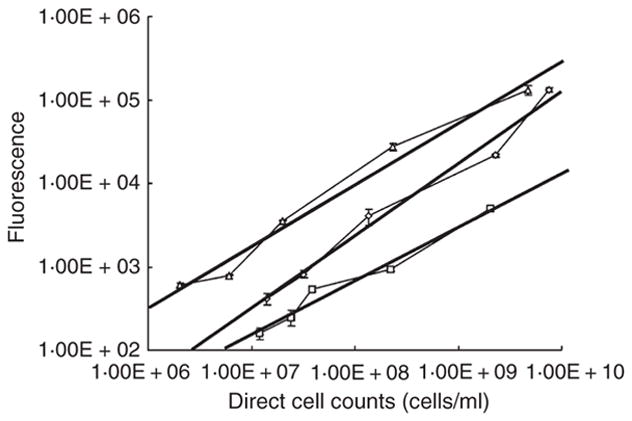
The relationship between fluorescence intensity and cell numbers (per ml) of Streptococcus oralis 34 (triangles), S. oralis 34 luxS mutant (diamonds) and Actinomyces naeslundii T14V (squares). R2 values are >0.95 for each dataset.
Production of AI-2 in single-species biofilms
Streptococcus oralis 34, S. oralis 34 luxS mutant and A. naeslundii T14V formed single-species biofilms in flowing 25% saliva. For each strain, cell numbers doubled twice before levelling-off within 24 h after inoculation (Fig. 3). In addition, changes in AI-2 concentration were also detected in the effluent over the 48-h sampling period. Compared with A. naeslundii T14V, AI-2 concentrations in the effluent from S. oralis 34 biofilms were consistently twofold lower at each time point during biofilm development (Fig. 3). No AI-2 could be detected in the saliva effluent from S. oralis 34 luxS mutant single-species biofilms (data not shown). The concentration ratio, an indication of the AI-2 concentration surrounding the cells in the biofilm, was calculated by dividing the concentration of AI-2 (nmol l−1) by the cell density in the sorbarod. Concentration ratios were calculated to be between 0.55 and 1.8 for S. oralis 34 and 0.35 and 0.77 for A. naeslundii T14V (Fig. 3). Single-species biofilms of S. oralis 34 and A. naeslundii T14V shared a common characteristic in that the concentration ratios steadily decreased by c. 54–67% as the biofilms developed.
Figure 3.
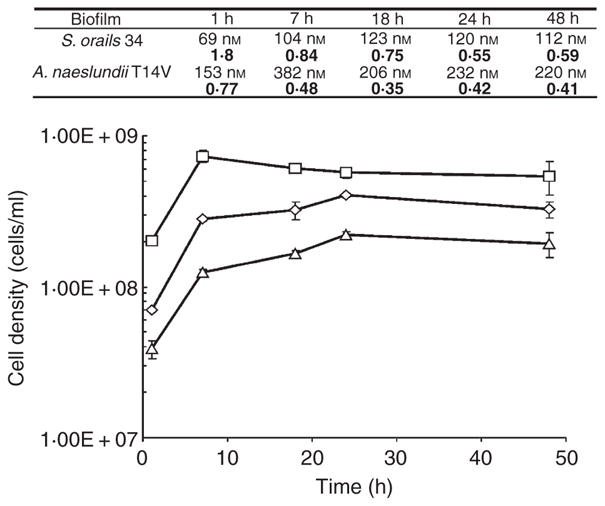
Changes in cell numbers of single-species biofilms of Streptococcus oralis 34 (triangles), S. oralis 34 luxS mutant (diamonds) and Actinomyces naeslundii T14V (squares). Associated table shows autoinducer-2 (AI-2) concentrations from the effluent of single-species biofilms of S. oralis 34 and A. naeslundii T14V at each time point. The upper value is the calculated (determined using the data shown in Fig. 1b) concentration of AI-2 produced by the biofilm and the lower (bold) value is the concentration ratio (nanomoles of AI-2 l−1 divided by cell numbers ml−1). AI-2 concentration within the effluent from S. oralis 34 luxS mutant biofilms was below the threshold for detection and quantification.
Development of dual-species biofilms and production of AI-2
In contrast to the development of single-species biofilms, cell numbers in the biofilm containing S. oralis 34 and A. naeslundii T14V increased at least four doublings and up to 40-fold for A. naeslundii T14V, indicating a mutualistic relationship (Fig. 4). Whereas the numbers of cells in wild-type dual-species biofilm levelled-off by 24 h, the numbers of A. naeslundii T14V in dual-species biofilms containing the S. oralis 34 luxS mutant decreased during the 18–48 h period. Similar to single-species biofilms, the concentration ratios decreased as the biofilm developed (Fig. 4); however, the decrease in concentration ratio was more pronounced than for single-species biofilms. The concentration ratio from dual-species biofilms containing S. oralis 34 and A. naeslundii T14V decreased by 94% during 48 h of development, but 86% of this decrease occurred in the first 7 h. The concentration ratio from dual-species biofilms containing the S. oralis 34 luxS mutant and A. naeslundii T14V decreased by 74% during 48 h of development, and, again, most of the decrease occurred in the first 7 h. These decreases resulted in concentration ratios that were up to 15-fold less than observed in single-species biofilms after 48 h of biofilm development (Figs 3 and 4).
Figure 4.

Changes in cell numbers of dual-species biofilms of Streptococcus oralis 34 (filled triangles) and Actinomyces naeslundii T14V (filled squares) and S. oralis 34 luxS mutant (open triangles) and A. naeslundii T14V (open squares). Associated table shows autoinducer-2 (AI-2) concentrations from the effluent at each time point; upper value is the calculated concentration of AI-2 produced by the dual-species biofilm and the lower (bold) value is the concentration ratio (nanomoles of AI-2 l−1 divided by the combined cell numbers of both species ml−1).
CLSM of dual-species biofilms
CLSM was employed to study the microbial community composition and architecture of the single- and dual-species biofilms after 48 h of growth. The addition of Alexa-Fluor®-conjugated antibodies and calcofluor white allowed for the labelling of bacteria and sorbarod fibres, respectively. Sorbarod fibres were densely packed and varied in size from 10 to >50 μm in width. Scattered single-species microcolonies and fibre-attached cells of S. oralis 34, S. oralis 34 luxS mutant and A. naeslundii T14V were visible on sorbarod fibres (data not shown). Dual-species biofilms containing S. oralis 34 and A. naeslundii T14V, consisted of many cells associated with the sorbarod fibres (Fig. 5a,b), as compared with the biofilms of S. oralis 34 luxS mutant and A. naeslundii T14V (Fig. 5c,d). Further, inter-digitated biofilm clusters of S. oralis 34 and A. naeslundii T14V were observed (Fig. 5a,b). Similar tightly bound inter-digitated clusters of S. oralis 34 and A. naeslundii T14V have been reported in saliva-fed and saliva-conditioned glass flowcells (Palmer et al. 2001). Such inter-digitated biofilm structures are consistent with mutualism and improved growth and/or attachment of communities in flowing environments. Thus, mutualism may account for higher numbers of cells in the wild-type dual-species biofilm, as visualized by CLSM (Fig. 5) and as confirmed by fluorometric measurements (Fig. 4). Taken together, we have shown that AI-2 is produced by oral bacteria in biofilms relevant to natural systems.
Figure 5.
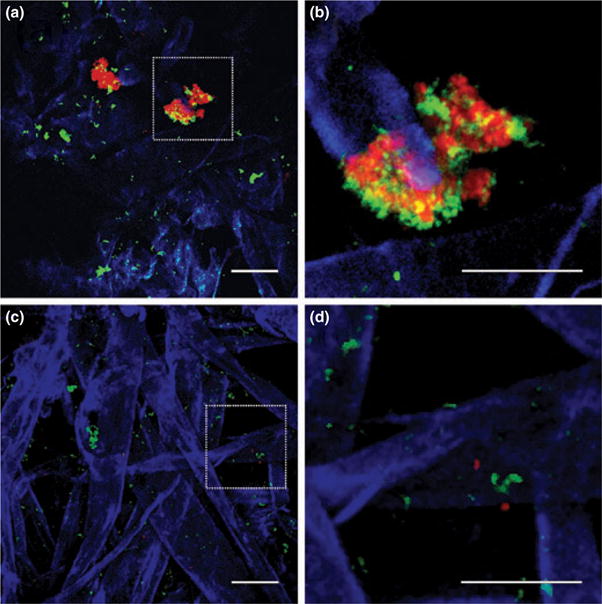
Confocal laser scanning micrographs of 48-h-old sorbarods showing the presence of Streptococcus oralis 34 (green) and Actinomyces naeslundii T14V (red) cells attached to cellulose acetate sorbarod fibres (blue). (a) Dual-species biofilm of S. oralis 34 and A. naeslundii T14V, (b) a magnified section showing inter-digitated growth of the pair, (c) a dual-species biofilm of S. oralis 34 luxS mutant and A. naeslundii T14V and (d) a magnified section showing the absence of inter-digitated growth. Bar represents 30 μm.
Discussion
While hypothesized to occur in biofilms composed of oral bacteria (Kolenbrander et al. 2006), AI-2 has not been detected in saliva-fed flow systems. We propose that the inability to detect AI-2 in flowcells previously is because of the low cell numbers in those flowcells, which supported c. 1 × 107 cells ml−1 in a growth chamber volume of only 250 μl. As we have shown here, when cell densities equal 1 × 109 cells ml−1 in the sorbarod containing vastly more surface area available for biofilm growth, the amount of biofilm-produced AI-2 is in the nanomolar range: the concentration needed to detect AI-2 by the standard V. harveyi bioluminescence assay.
The concentration ratio (nanomoles of AI-2 l−1 divided by cell numbers ml−1) is an estimate of the production of AI-2 in the sorbarod. It does not take into account possible binding by AI-2 receptors on the streptococci and actinomyces or possible degradation of AI-2 and thus it is at best an estimate. Nevertheless, the concentration ratios for dual-species S. oralis 34–A. naeslundii T14V biofilms decreased from 0.67 to 0.039 in effluent samples during 48 h. By comparison, the concentration ratios for dual-species S. oralis 34 luxS mutant–A. naeslundii T14V biofilms decreased from 0.37 to 0.092. These numbers reflect a twofold higher initial amount of AI-2 in the wild-type dual-species biofilm followed by a twofold lower amount at 18–48 h as compared with the dual-species S. oralis 34 luxS mutant–A. naeslundii T14V biofilm. These data suggest that the initial concentration ratio is more important than the later concentration ratios, as both biofilms exhibited rapid decreases in the first 7 h. Thus, it may be essential to produce a high threshold level of AI-2 initially in order to sustain a biofilm composed of both species. The wild-type biofilm sustained growth of more than 1 × 109 cells of each species, whereas the number of actinomyces decreased to 1.7 × 108 cells in the biofilm with the streptococcal luxS mutant. In addition, the wild-type biofilm exhibited communities of interdigitated species, whereas the biofilm containing the streptococcal luxS mutant and actinomyces exhibited communities of single species (Fig. 5).
This work supports the hypothesis that S. oralis 34 and A. naeslundii T14V produce low concentrations of AI-2 in saliva-fed biofilms relevant to a natural oral environment. Elegant work by Shao et al. (2007) and James et al. (2006b) demonstrated that a ribose-binding-receptor (RbsB) and AI-2 binding receptor (LsrB) on the surface of the oral bacterium Aggregatibacter actinomycetemcomitans mediates uptake of AI-2. Further, disruption of the rbsB or lsrB reduced the ability of A. actinomycetemcomitans to form biofilm. A search of the EMBL DNA sequence database indicates that similar genes to rbsB are present in Streptococcus agalactiae 2603V/R, Streptococcus agalactiae A909 and Streptococcus sanguinis SK36. Additionally, interrogation of the public TIGR genome databases indicated that rbsB homologues are present in Streptococcus mitis NCTC 12261, which is a close relative of S. oralis 34, suggesting that rbsB is likely to be in the S. oralis 34 genome. We were unable to find an rbsB homologue in the two Actinomyces species genomes (A. naeslundii MG1, TIGR, Rockville, MD and Actinomyces odontolyticus ATCC 17982, Washington University, Saint Louis, MO). Thus, endeavours to generate an rbsB mutant of S. oralis 34 and further investigate the roles of coaggregation and AI-2 in communication between S. oralis 34 and A. naeslundii T14V are currently underway.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health. The authors thank R.J. Palmer, Jr., R. Levine, J. Kolenbrander and J. Thompson for suggestions. The DPD was a kind gift from B. Bassler and M. Semmelhack.
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blehert DS, Palmer RJ, Jr, Xavier JB, Almeida JS, Kolenbrander PE. Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions. J Bacteriol. 2003;185:4851–4860. doi: 10.1128/JB.185.16.4851-4860.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar JO, Kolenbrander PE, McIntire FC. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979;24:742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeersmaecker SC, Vanderleyden J. Constraints on detection of autoinducer-2 (AI-2) signalling molecules using Vibrio harveyi as a reporter. Microbiology. 2003;149:1953–1956. doi: 10.1099/mic.0.C0117-0. [DOI] [PubMed] [Google Scholar]
- DeKeersmaecker SC, Varszegi C, van Boxel N, Habel LW, Metzger K, Daniels R, Marchal K, De Vos D, et al. Chemical synthesis of (S)-4,5-dihydroxy-2,3-pentanedione, a bacterial signal molecule precursor, and validation of its activity in Salmonella typhimurium. J Biol Chem. 2005;280:19563–19568. doi: 10.1074/jbc.M412660200. [DOI] [PubMed] [Google Scholar]
- Diaz PI, Chalmers NI, Rickard AH, Kong C, Milburn CL, Palmer RJ, Jr, Kolenbrander PE. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol. 2006;72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerre JA, Baker DJ, Salisbury L. Structure elucidation of a carbohydrate derived from S-ribosylhomocysteine by enzymatic cleavage. Fed Proc. 1971;30:1067. [Google Scholar]
- Egland PG, Palmer RJ, Jr, Kolenbrander PE. Interspecies communication in Streptococcus gordonii–Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci USA. 2004;101:16917–16922. doi: 10.1073/pnas.0407457101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong KP, Chung WO, Lamont RJ, Demuth DR. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect Immun. 2001;69:7625–7634. doi: 10.1128/IAI.69.12.7625-7634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69:3431–3434. doi: 10.1128/IAI.69.5.3431-3434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Hodgson AE, Nelson SM, Brown MR, Gilbert P. A simple in vitro model for growth control of bacterial biofilms. J Appl Bacteriol. 1995;79:87–93. doi: 10.1111/j.1365-2672.1995.tb03128.x. [DOI] [PubMed] [Google Scholar]
- James CE, Hasegawa Y, Park Y, Yeung V, Tribble GD, Kuboniwa M, Demuth DR, Lamont RJ. LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect Immun. 2006a;74:3834–3844. doi: 10.1128/IAI.01768-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D, Shao H, Lamont RJ, Demuth DR. The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals. Infect Immun. 2006b;74:4021–4029. doi: 10.1128/IAI.01741-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall MM, Rasko DA, Sperandio V. Global effects of the cell-to-cell signaling molecules autoinducer-2, autoinducer-3, and epinephrine in a luxS mutant of enterohemorrhagic Escherichia coli. Infect Immun. 2007;75:4875–4884. doi: 10.1128/IAI.00550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE. Coaggregations among oral bacteria. Methods Enzymol. 1995;253:385–397. doi: 10.1016/s0076-6879(95)53033-9. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontology 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Li J, Helmerhorst EJ, Leone CW, Troxler RF, Yaskell T, Haffajee AD, Socransky SS, Oppenheim FG. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol. 2004;97:1311–1318. doi: 10.1111/j.1365-2672.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- McIntire FC, Vatter AE, Baros J, Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978;21:978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J Bacteriol. 2003;185:274–284. doi: 10.1128/JB.185.1.274-284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Palmer RJ, Jr, Kazmerzak K, Hansen MC, Kolenbrander PE. Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infect Immun. 2001;69:5794–5804. doi: 10.1128/IAI.69.9.5794-5804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard AH, Palmer RJ, Jr, Blehert DS, Campagna SR, Semmelhack MF, Egland PG, Bassler BL, Kolenbrander PE. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol. 2006;60:1446–1456. doi: 10.1111/j.1365-2958.2006.05202.x. [DOI] [PubMed] [Google Scholar]
- Semmelhack MF, Campagna SR, Federle MJ, Bassler BL. An expeditious synthesis of DPD and boron binding studies. Org Lett. 2005;7:569–572. doi: 10.1021/ol047695j. [DOI] [PubMed] [Google Scholar]
- Shao H, Lamont RJ, Demuth DR. Autoinducer-2 is required for biofilm growth of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infect Immun. 2007;75:4211–4218. doi: 10.1128/IAI.00402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Daniel R, Wagner-Dobler I, Zeng AP. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol Biol. 2004;4:36. doi: 10.1186/1471-2148-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette MG, Bassler BL. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sci USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez R, Lemme A, Thiel V, Schulz S, Sztajer H, Wagner-Dobler I. Analysing traces of autoinducer-2 requires standardization of the Vibrio harveyi bioassay. Anal Bioanal Chem. 2007;387:489–496. doi: 10.1007/s00216-006-0824-4. [DOI] [PubMed] [Google Scholar]
- Xavier KB, Bassler BL. Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. J Bacteriol. 2005a;187:238–248. doi: 10.1128/JB.187.1.238-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell–cell communication. Nature. 2005b;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier KB, Miller ST, Lu W, Kim JH, Rabinowitz J, Pelczer I, Semmelhack MF, Bassler BL. Phosphorylation and processing of the quorum-sensing molecule autoinducer-2 in enteric bacteria. ACS Chem Biol. 2007;2:128–136. doi: 10.1021/cb600444h. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Ansai T, Takehara T, Kuramitsu HK. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl Environ Microbiol. 2005;71:2372–2380. doi: 10.1128/AEM.71.5.2372-2380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


