Abstract
In non-human primate models of AIDS, attenuated lentiviruses provide the most reliable protection from challenge with pathogenic virus but the extent to which the vaccine virus replicates after challenge is unclear. At 7 and 14 days after vaginal challenge with pathogenic SIVmac239, plasma SIVenv RNA levels were significantly lower in female macaques immunized 6 months earlier with live, attenuated SHIV89.6. compared to unimmunized control animals. In 2 SHIV-immunized, unprotected macaques SIV replication produced high-level plasma viremia with dissemination of challenge virus to all tissues on day 14 after challenge. In protected, SHIV-immunized monkeys, SIV replication was controlled in all tissues, from the day of challenge through 14 days post-challenge. Further, in CD8+ T cell-depleted SHIV-immunized animals, SIV replication and dissemination were more rapid than in control animals. These findings suggest that replication of a pathogenic AIDS virus can be controlled at the site of mucosal inoculation by live-attenuated lentivirus immunization.
Keywords: attenuated SHIV, dissemination, sexual transmission, HIV
Introduction
HIV is a sexually transmitted disease and a vaccine that can stop HIV transmission offers the best opportunity to stop the AIDS pandemic. In the simian immunodeficiency virus mac (SIVmac) rhesus macaque model of HIV infection, attenuated lentivirus vaccines have consistently provided the most effective protection against systemic and mucosal challenge with pathogenic SIV (Abel et al., 2003; Abel et al., 2004; Almond et al., 1995; Miller et al., 1997). The degree of protection conferred by an attenuated lentivirus vaccine is related to the ability of the vaccine virus to replicate in the host (Lohman et al., 1994) and highly attenuated viruses do not replicate sufficiently to generate a protective immune response (Ruprecht, 1999) (Busch et al., 2005). Although reversion to virulence and integration-based carcinogenesis preclude the use of attenuated lentivirus vaccines in humans, identifying the mechanisms by which attenuated lentiviruses confer protection will be useful for developing other vaccine approaches.
The route of immunization with nonpathogenic SHIV 89.6 does not alter the level of protection, as either vaginal, intranasal or intravenous inoculation of SHIV 89.6 produces protection from uncontrolled virus replication after vaginal SIV challenge in about 60% of the immunized animals (Abel et al., 2003). The similar level of protection following mucosal and systemic inoculation of SHIV is likely due to SIV-specific T cell responses in the vagina that are primed and maintained by vaccine virus replication in the genital tract during the 6−8 months of systemic SHIV infection, even after IV inoculation (Genescà et al., 2008b). Thus in this model, the immunizing virus establishes a persistent, disseminated infection and antiviral immune responses are distributed to all tissues.
We recently established a correlation between protection from uncontrolled viral replication after vaginal SIV challenge and the presence of CD8+ T cell responses in the vagina (Genesca, McChesney, and Miller, 2009). The SHIV-induced, antiviral CD8+ T-cell response is characterized by cytolytic T-cells in the vagina of 60% of immunized animals, but these antiviral T cell responses are much more inconsistent in cervix and other tissues (Genescà et al., 2008a). CD8+ T-cell depletion at the time of SIV challenge abrogates the protective effect of SHIV immunization confirming the critical role of CD8+ T cells in the observed protection (Genescà et al., 2008b). Unexpectedly, after SIV challenge, the protective antiviral T-cell response does not significantly expand in blood or tissues, except in the vagina (Genescà et al., 2008b).
While the nature of immunologic responses to infection with attenuated SHIV is being elucidated, characterizing the viral populations present in blood and tissues after SIV challenge has not been undertaken. In fact, in other attenuated lentivirus vaccine models it is unclear if “vaccine failure” is due to replication of the vaccine virus, the challenge virus, or both (Almond et al., 1995; Berry et al., 2008; Kwofie et al., 2002; Mackay et al., 2004; Reynolds et al., 2008; Rose et al., 1995; Shibata et al., 1997; Silverstein et al., 2000b; Ui et al., 1999; Wakrim et al., 1996). Recombination between vaccine and challenge virus (Gundlach et al., 2000; Reynolds et al., 2008), persistence of vaccine virus after challenge (Mackay et al., 2004; Silverstein et al., 2000a) or persistence of both vaccine and challenge virus (Khatissian et al., 2001) have been reported. The assays commonly used to assess levels of viral replication do not distinguish between vaccine and challenge virus. Further, the relative contribution of the vaccine and challenge viruses to vaccine failure, the extent of SIV dissemination and the sites of SIV replication after challenge of SHIV89.6 immunized monkeys are not known. As infection of CD4+ T cells by an “attenuated” virus vaccine could deplete target cells in tissues interfering with challenge virus replication, it is critical to understand the extent to which vaccine virus contributes to uncontrolled virus replication after pathogenic virus challenge. Thus, to better understand the anatomic sites of vaccine and challenge virus replication, we sought to determine the extent of SIV dissemination and replication in tissues of SHIV-immunized animals after challenge.
Materials and Methods
Animals
These studies have been described in detail in previous publications characterizing the relationship between anti-SIV T-cell responses and plasma vRNA levels (Genescà et al., 2008a; Genescà et al., 2008b). Briefly, the female rhesus macaques (Macaca mulatta) used in these studies were housed at the California National Primate Research Center in accordance with the American Association for Accreditation of Laboratory Animal Care standards. The experiments were approved by the Institutional Animal Care and Use Committee of the University of California, Davis. All animals were negative for antibodies to HIV-2, SIV, type-D retrovirus, and simian T cell lymphotropic virus type 1 at the time the study was initiated.
A total of 35 females were intravenously infected with live, virulence attenuated SHIV89.6 for six to eight months, as previously described (Abel et al., 2003). After the 6−8 month immunization period, at the day 0 time-point, nine of these animals were necropsied and a detailed study of the SIV-specific T cells responses has been published (Genescà et al., 2008a). The remaining immunized macaques (n=26), were challenged with pathogenic SIVmac239 by intravaginal inoculation, as described (Abel et al., 2003; Miller et al., 1997), and necropsied at 3 (n=3), 7 (n=6) or 14 days (n=12) p.c.. Furthermore , 5 immunized macaques were infused intravenously with a depleting monoclonal antibody (MAb) directed against CD8α(cM-T807; 50mg/kg body weight; Centocor, Malvern, Pa.) on the day of challenge as previously described (Genescà et al., 2008a), and necropsied at 14 days p.c.. Twenty-one naive female macaques were vaginally challenged with pathogenic SIVmac239 and necropsied at 3 (n=3), 7 (n=9) and 14 days (n=9) p.c. as unimmunized, control animals.
Animals were randomly assigned to the immunized or unimmunized groups and they were distributed as evenly as possible for the necropsy dates according to their major histocompatibility complex (MHC) class I genotype and country of origin (Genescà et al., 2008a).
Intravaginal SIVmac239 inoculation
The pathogenic SIVmac239 stock used in these studies was produced in rhesus PBMC as previously described (Miller et al., 1997) and contained approximately 105 TCID50 /ml. The virus challenge consisted of 2 intravaginal inoculations (separated by 4 hours) with 1 ml of the undiluted SIVmac239 stock.
Tissue collection and sample preparation
Genital tract tissues (cervix, vagina) and genital lymph nodes (obturator, inguinal and iliac lymph nodes), gut tissues and associated LNs (colon and mesenteric lymph nodes), distal lymphoid tissues (axillary lymph nodes and spleen) and blood were collected at the time of necropsy and analyzed for vRNA levels. Tissues were stored in RNAlater (Ambion, Austin, TX) and kept at −20°C until preparation of RNA. Tissue samples were homogenized with a Power Homogenizer (Power-Gen, Fisher Scientific) according to the manufacturers protocol. Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) following the manufacturers suggested protocol. RNA samples were DNase treated with DNA-free (Ambion) for 1 hr at 37°C. cDNA was prepared using random hexamer primers (Amersham-Pharmacia Biotech, Inc) and SuperScript III Reverse Transcriptase (RT) (Invitrogen).
Virological analysis
RT-PCR was used to detect and quantify SIVgag, SIVenv and HIVenv RNA levels. Samples were tested in replicates of 11 reactions carried out in 96-well optical plates (Applied Biosystems, Foster City, CA) in a 25 μl reaction volume containing 5 μl cDNA and 20 μl Mastermix (Applied Biosystems) using the ABI 7900 robotic thermalcycler. All sequences were amplified for 2 min at 50°C and 10 min at 95°C followed by 50 cycles of 15 s at 95°C and 1 min at 60°C. The following primer pairs and probes were used: SIVgag forward primer 1, 5’−3’ GGG AGA TGG GCG TGA GAA A, reverse primer, CGT TGG GTC GTA GCC TAA TTT T and probe TCA TCT GCT TTC TTC CCT GAC AAG ACG GA; SIVenv forward primer GAG ACT AGT TCT TGT ATA GCC CAGGAT AA, reverse primer CTT TTT AAC CCT GTC ATG TTG AAT TTA C, and probe CAT TTG CTC TTG TTC CAA GCC TGT GCA; HIVenv forward primer AGG CCT GTC CAA AGG TAT CCT T, reverse primer ATC CAT TGA ATG TCT TAT TGT TAC ACT TTA G, and probe TCG CAA ACC CAG CCG GGA CA. The copy numbers of SIVgag, SIVenv and HIVenv were calculated based on standard curves for viral gene plasmids spanning a concentration range from 0.1 to 108 copies. The lower detection limit of the assay was 10 copies. The results were analyzed with SDS 7900 system software version 2.3 (Applied Biosystems). The results for each sample are reported as log10 vRNA copies per μg of tissue RNA.
To evaluate the specificity and determine the background of the PCR assays for each viral gene, RNA isolated from 11 individual tissues from 6 rhesus macaques that had never been exposed to SIV were subjected to amplification. There was no amplification of SIVgag, or HIVenv from any of these tissue samples. Thus 50 SIVgag or HIVenv copies/ug tissue RNA (or 1.7 log10 SIVgag or HIVenv copies/ug tissue RNA) was used as the cutoff for determining if a tissue sample from an SIV-inoculated monkey was positive. However, SIVenv was amplified from most tissue samples of the uninfected animals. Among these negative tissues, the colon had the highest average copy number. The mean SIVenv RNA level in the SIV negative colon samples plus 2 standard deviations was 132 SIVenv copies/ug tissue RNA. Thus 132 SIVenv copies/ug tissue RNA (or 2.1 log10 SIVenv copies/ug tissue RNA) was used as the cutoff for determining if a tissue sample from an SIV inoculated animal was positive for SIVenv RNA.
Enumeration of SIV-infected cells in tissue sections
The number of SIV RNA+ cells in 6-μm-thick sections of paraffin-embedded tissues was determined by in situ hybridization (ISH) using eight radio-labeled (S35) anti-sense riboprobes (0.7 to 1.5 kb) spanning the whole SIVmac239 genome as previously described (Hu, Gardner, and Miller, 2000; Hu et al., 1998). It should be noted that it was not possible to distinguish between cells infected with the vaccine virus, SHIV89.6, and cells infected with the challenge virus, SIVmac239. Negative controls for ISH were slides with SIV-negative tissue sections and slides with SIV-positive tissue sections but hybridized to SIV sense probes. To quantify the number of SIV-infected cells, the slides were counterstained with hematoxylin (Vector) after the ISH labeling. The slides were viewed on a Axiophot microscope and photographed with an AxioCam camera (Carl Zeiss). SIV-positive cells were counted in randomly chosen fields, and the frequency of SIV-positive cells as the number of SIV RNA positive cells per ten low power fields (10x objective) in sections of cervix and vagina or SIV RNA+ cells /mm2 in axillary LN sections was calculated (not shown).
Statistical Analysis
GraphPad Prism version 5 for Apple OSX10.4 (GraphPad Software, San Diego California USA) and Macintosh G5 computers (Apple Inc., Cupertino CA) were used for all analyses. The p values for comparisons of mean tissue and plasma vRNA levels in each animal group were evaluated by unpaired one-tailed T test. Differences in the mean vRNA levels in tissues among the different animal groups were tested with a one-way ENV and Bonferroni's multiple comparison post-hoc test. Analysis of vRNA levels among all tissues at each time point was done using a one-way ENV with Dunn's multiple comparison post test.
Results
The relative contribution of the vaccine and challenge viruses to plasma viremia after vaginal SIVmac239 challenge
The overall design and challenge outcome of this study based on plasma vRNA levels have been recently reported (Genescà et al., 2008a). Briefly, 26 animals previously infected with SHIV89.6 for 6−8 months were challenged vaginally with SIVmac239 along with 21 unvaccinated control animals that received only challenge virus. The SHIV immunized animals were necropsied at the time of challenge (n=9), 3 days p.c. (n=3), 7 days p.c. (n=6), and 14 days p.c. (n=12), while the unvaccinated control animals were necropsied at 3 days p.c. (n=3), 7 days p.c. (n=9), and 14 days p.c. (n=9). We have previously shown that approximately 60% SHIV-immunized rhesus macaques maintain plasma vRNA levels below 104 copies/ml and normal CD4+ T numbers in blood for 6 months after intravaginal challenge with SIV (Abel et al., 2003; Busch et al., 2005; Genesca et al., 2007). These animals are defined as “protected”. However in SHIV-immunized rhesus macaques that have plasma vRNA levels above 104 copies/ml at any time during the 6-month observation period after vaginal SIVmac239 challenge, CD4+ T cell numbers steadily decline and these animals are defined as “unprotected” (Abel et al., 2003; Busch et al., 2005; Genesca et al., 2007).
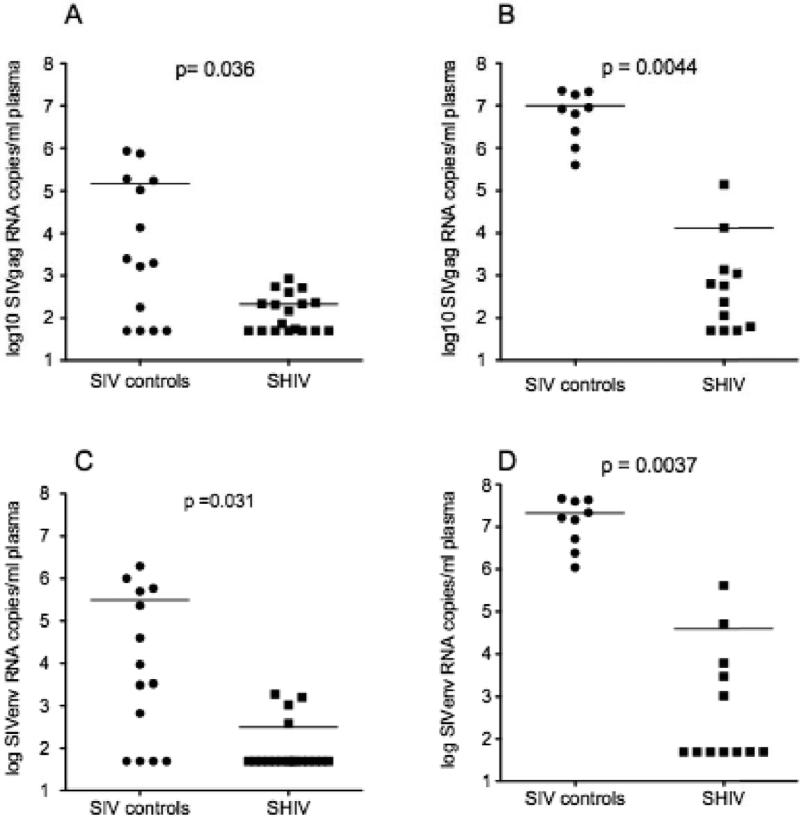
As previously reported using the SIVpol-specific bDNA assay to measure viral load (Genescà et al., 2008a), the mean plasma gag vRNA level of the unimmunized control animals was significantly higher than the mean plasma gag vRNA level of the SHIV immunized animals at both 7 and 14 days p.c. using an SIV gag RT-PCR assay (Fig. 1a and 1b). Of note, 2 of the SHIV-immunized animals had plasma SIV gag RNA levels > 104 copies/ml at 14 days PI, thus these 2 animals are defined as unprotected and would be expected to have steadily declining numbers of CD4+ T cells (Abel et al., 2003; Busch et al., 2005; Genesca et al., 2007).
FIG. 1. Plasma vRNA levels in naive control and SHIV-immunized monkeys monkeys after vaginal challenge with SIVmac239.
Plasma SIVgag concentration (copies/ml) at (a) 7 days and (b) 14 days p.c.. Plasma SIVenv concentration (copies/ml) at (c) 7 days and (d) 14 days p.c.. Each symbol represents the result for an individual animal. The horizontal line indicates the mean plasma vRNA level in each animal group. The p values were calculated using a unpaired one-tailed T test.
To determine if the vaccine or challenge virus was the source of the plasma vRNA, plasma SIVgag and SIVenv vRNA levels were measured by RT-PCR (Fig. 1). At 7 days p.c. SIVenv RNA was detected in plasma of 10 of 18 SHIV-naïve control animals (ranging from 102- >105 vRNA copies/ml), but SIVenv RNA was detected in only 4 of 18 SHIV-immunized monkeys (less than 103 vRNA copies/ml). The difference in the mean plasma SIVenv RNA level of the 2 groups was statistically significant (Fig. 1c and 1d). By day 14 p.c. all 9 of the control animals had SIVenv vRNA in plasma (105−108 vRNA copies/ml), but only 5 of 12 SHIV-immunized monkeys were plasma SIVenv RNA+ (103−106 vRNA copies/ml), and the mean plasma SIVenv RNA levels of the 2 groups were significantly different (Fig. 1c and 1d). As with SIVgag RNA, the plasma SIV env RNA levels in the 2 “unprotected” SHIV-immunized animals were > 104 copies/ml at 14 days PI.
SIV gag and SIVenv RNA levels in the tissues of naïve control animals after vaginal SIV challenge
The RT-PCR assay was used to quantify the number of SIVgag copies in a tissue sample and results are reported using vRNA copies/ug tissue RNA. We classified samples with < 104 vRNA copies /ug tissue RNA to have low levels of vRNA; samples with 104−106 vRNA copies /ug tissue RNA to have moderate levels of vRNA; and samples with >106 vRNA copies /ug tissue RNA to have high levels of vRNA.
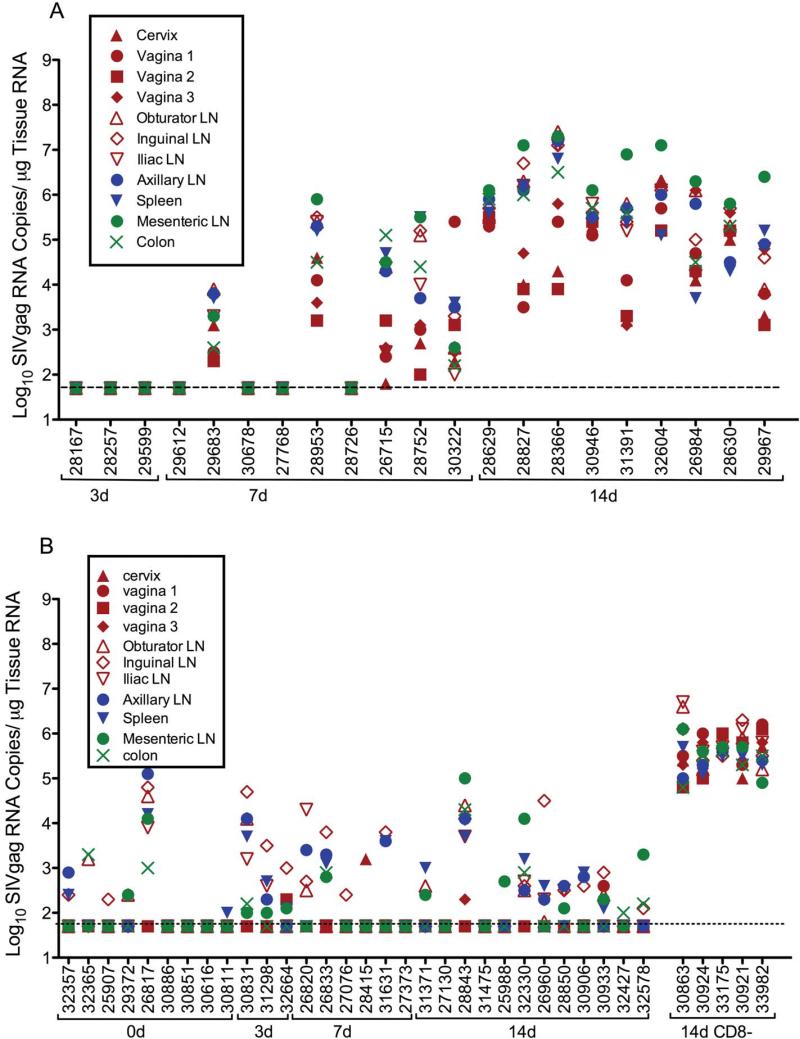
SIV gag RNA was not detected in any tissue sample collected from the 3 naive control animals necropsied at 3 days p.c., (fig. 2). At 7 days p.c. SIV gag RNA was detected in at least one tissue collected from 7 of 9 control animals (Fig 2). Of these 7 vRNA+ animals, moderate SIVgag RNA levels were found in multiple tissues of 5 animals while 2 animals had only a low level of SIV gag RNA in one tissue. By day 14 after vaginal SIVmac239 inoculation, moderate to high levels of SIV gag vRNA were detected in the tissues of all 9 unimmunized control animals necropsied (Fig 2).
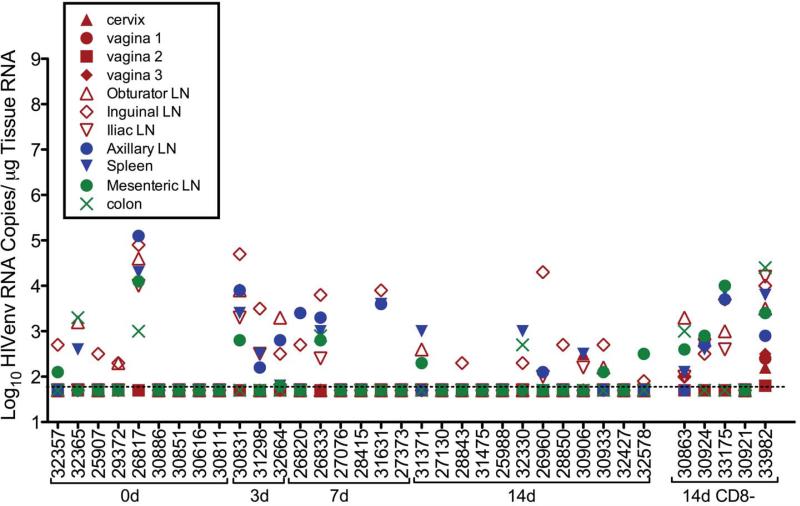
FIG. 2. SIVgag RNA levels in tissues of naïve control and SHIV immunized monkeys after vaginal SIVmac239challenge.
(a) naïve control animals (SIVgag RNA copies/μg tissue RNA) and (b) SHIV-immunized animals (SIVgag RNA copies/μg tissue RNA); CD8+ lymphocyte-depleted, SHIV-immunized animals are indicated by a solid horizontal line under the animal numbers at the far right of the x-axis. Anatomic sites are denoted as follows: genital tract (closed red symbols), genital lymph nodes (open red symbols), peripheral lymphoid tissues (closed blue symbols), and intestinal tract/mesenteric LN (green symbols). The vRNA levels for the indicated tissues are displayed vertically above the animal numbers along the x-axis. The animals are grouped by the timing of necropsy after vaginal SIVmac239 challenge, as indicated by the brackets under the animal numbers. The horizontal dotted line indicates the limit of quantification for this assay and represents the cutoff value for designating positive samples.
Among the 7 SIVgag RNA+ control animals at day 7 PC after vaginal SIV challenge, the genital lymph nodes had the highest SIVgag RNA levels in 2 animals, and in the remaining 5 animals the tissue that had the highest SIV gag RNA levels was unique to each animal (Fig 2). At 7 days p.c., the lowest SIVgag RNA levels in the control animals as a group were in the genital tract (cervix, vagina) (Fig 2). Among the 9 control animals necropised at day 14 p.c., the highest levels of SIVgag RNA were seen in the colon and mesenteric lymph nodes draining the intestinal tract, with intermediate vRNA levels in the systemic lymphoid tissues (axillary lymph node and spleen), and the lowest vRNA levels were in the genital tract and the genital lymph nodes (cervix, vagina, obturator lymph node, inguinal lymph node, and iliac lymph node) (fig. 2).
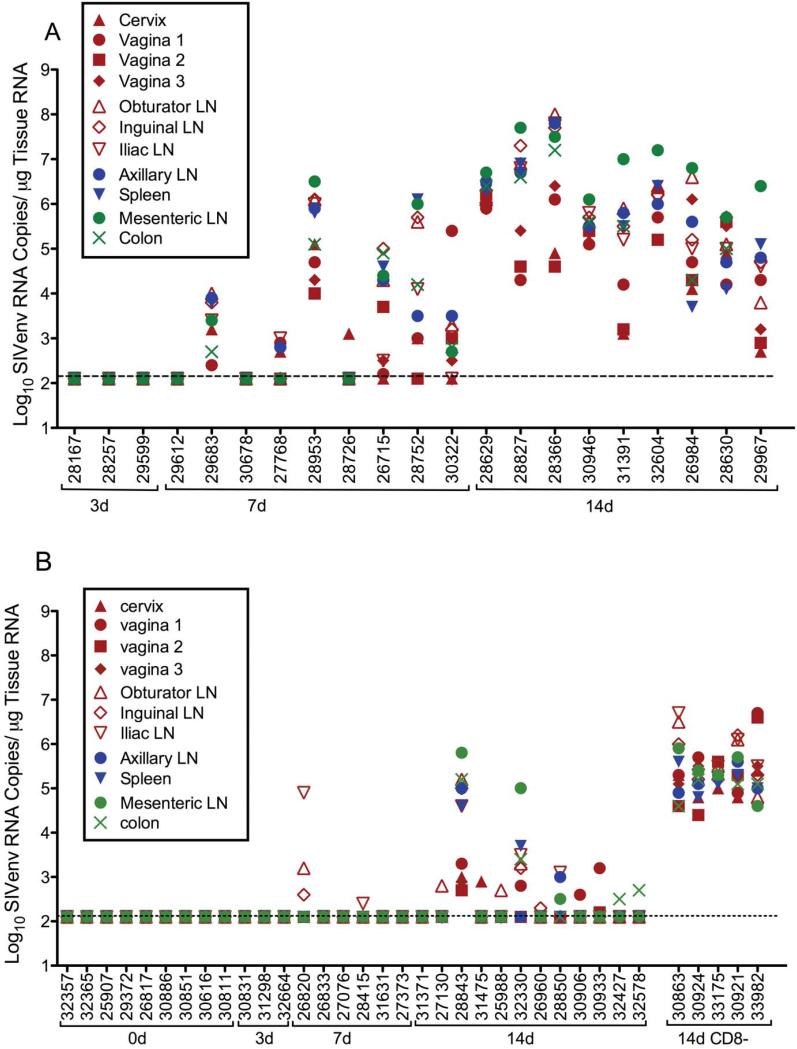
In the unimmunized control animals, the SIVenv RNA levels in every tissue sample were essentially identical to the SIVgag RNA levels in the matched sample (Fig 3). Tissue samples from animals necropsied at 3 days p.c. were uniformly negative for SIVenv. By 7 days p.c. SIVenv RNA was detected in multiple tissue samples from 7 of 9 unimmunized control animals (Fig 3). Of these 7 SIVenv RNA+ control animals, low to moderate SIVenv RNA levels were found in multiple tissues of 5 animals and 2 animals had low levels of SIVenv RNA in multiple tissues. At 7 days p.c., the lowest SIVenv RNA levels in the SIV control animals were generally in the genital tract (cervix, vagina) (fig. 3). By day 14 after vaginal SIVmac239 inoculation, moderate to high of SIVenv RNA levels (SIV copies/ug tissue RNA) were detected in all tissue samples collected from all 9 naïve control animals. At day 14 p.c., the highest SIVenv RNA levels were in the mesenteric lymph nodes of 8 animals and the lowest SIVenv RNA levels were in the genital tract (cervix, vagina,) (fig. 3).
FIG. 3. SIVenv RNA levels in tissues of SHIV-immunized monkeys and naïve monkeys after vaginal SIVmac239challenge.
(a) naïve control animals (SIVenv RNA copies/μg tissue RNA and (b) SHIV-immunized animals (SIVenv RNA copies/μg tissue RNA; CD8+ lymphocyte depleted SHIV-immunized animals are indicated by a solid horizontal line under the animal numbers at the far right of the x-axis. The figure organization is similar to Fig. 2.
SIVgag and SIVenv RNA levels in tissues of SHIV-immunized animals before, and after, vaginal SIV challenge
Prior to SIV challenge, low levels of SIVpol RNA representing residual SHIV replication were detected using a bDNA assay in tissues of 6 of 9 SHIV-immunized animals and the colon was most consistently SIV RNA positive tissue in these animals (Genescà et al., 2008b). After vaginal SIV challenge, the anatomic distribution of SIVgag RNA in tissues of SHIV-immunized animals was very distinct from the distribution of SIVgag RNA in tissues of the SIV control animals (Fig 2). In the SHIV-immunized animals that had SIVgag RNA in tissues at days 3 and 7 p.c., the tissues with the highest levels of SIVgag RNA were consistently in the vagina, cervix or genital lymph nodes (Fig 2). Ten of 12 SHIV immunized animals necropsied at day 14 p.c. controlled virus replication, and 8 of these animals had detectable SIVgag RNA in tissues. In these 8 animals, the highest levels of SIVgag RNA were in the mesenteric LN of 2 of animals, in the spleen of 2 animals and in the inguinal LN, colon or axillary LN of the remaining 4 animals (Fig 2). However in the two SHIV-immunized animals (28843, 32330) with relatively uncontrolled viral replication, the levels of SIVgag RNA were highest in the colon and mesenteric lymph nodes at day 14 p.c. (Fig 2).
After vaginal SIV challenge, the anatomic distribution of SIVenv RNA and SIVgag RNA in tissues of SHIV-immunized animals was similar (Fig 3), and very distinct from the distribution of SIVenv RNA in tissues of the SIV control animals. Thus, at days 3 and 7 p.c, the genital lymph nodes, vagina/cervix or colon had the highest levels of SIVenv (SIVenv RNA copies/ug tissue RNA) in the 7 SHIV-immunized animals that had SIVenv RNA in tissues (Fig 3). In 8 of the 10 SHIV immunized animals necropsied at day 14 that controlled virus replication (Fig 3) the highest levels of SIVenv RNA were found in the genital LN, the vagina or cervix. In the 2 remaining protected animals the highest levels of SIVenv RNA were in the mesenteric LN (Fig 5c). Similarly, in the two unprotected, SHIV-immunized animals (28843, 32330) with relatively uncontrolled viral replication at day 14 p.c., the SIVenv levels were highest in the mesenteric lymph nodes (Fig 3).
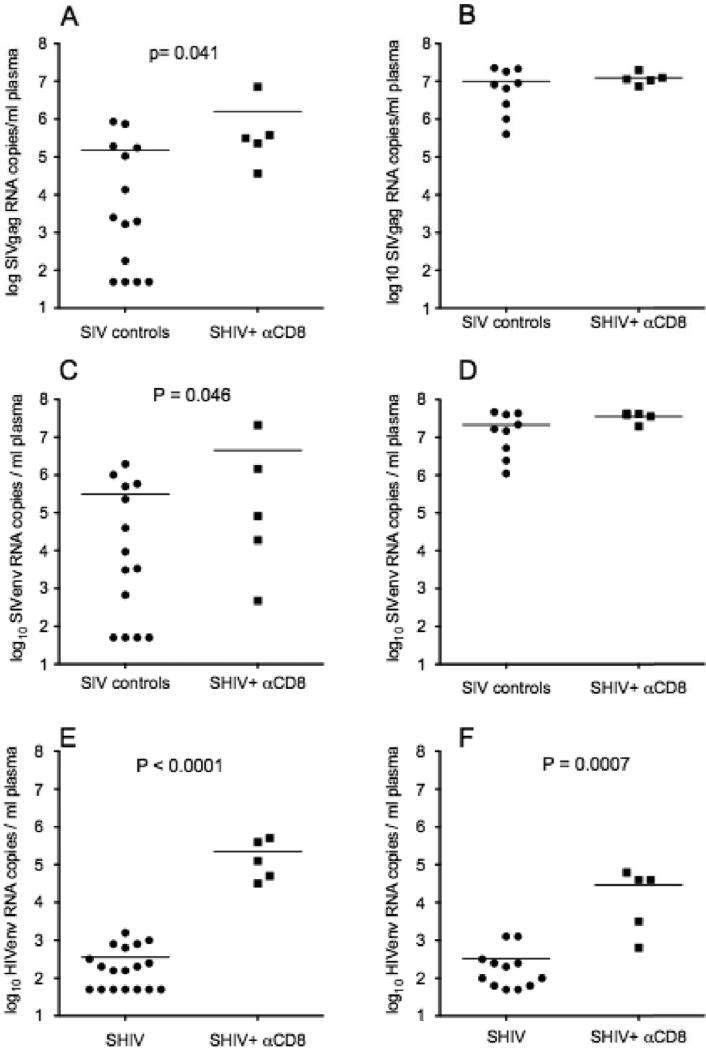
Fig. 5. Plasma vRNA levels in SHIV-immunized monkeys, SHIV-immunized, CD8+ lymphocyte-depleted monkeys and naive control monkeys after vaginal challenge with SIVmac239.
Comparison of plasma SIVgag concentration (copies/ml) at (a) 7 days and (b) 14 days p.c.; and plasma SIVenv concentration (copies/ml) at (c) 7 days and (d) 14 days p.c. in naïve control and CD8+ T cell-depleted SHIV-immunized animals. Comparison of plasma HIVenv concentration (copies/ml) in SHIV-immunized and CD8+ T cell-depleted, SHIV-immunized animals at (e) 7 days and (f) 14 days p.c.. Each symbol represents the result for an individual animal. The horizontal line indicates the mean plasma vRNA level in each animal group. P values were generated with an unpaired one-tailed T test.
SHIV-immunized animals have no significant increase in tissue SIVgag and SIVenv RNA levels after SIV challenge
To assess the kinetics of viral replication in tissues of control and SHIV-immunized animals, the mean SIVgag and SIVenv RNA levels in the tissues of each immunized and control animal was calculated and the group means were compared. As expected, the mean SIVgag RNA level in tissues of the unimmunized control animals was significantly higher on day 14 p.c. compared to day 7p.c. (p < 0.0001, one-tailed T-test, data not shown). As with SIVgag RNA, the mean level of SIVenv RNA in tissues of the unimmunized control animals was significantly higher on day 14 p.c. compared to day 7p.c. (p < 0.0001, one-tailed T-test, data not shown).
In marked contrast the mean SIVgag RNA levels in the tissues of the SHIV immunized animals decreased, although not significantly, from day 7 p.c. compared to day 14 p.c. (data not shown). Thus the immunized animals controlled viral replication in all tissues after vaginal SIV challenge. Further, the mean tissue SIVenv RNA level of the SHIV immunized animals did not increase from day 7 p.c. to day 14 p.c. (data not shown). Thus although the 2 unprotected, SHIV-immunized animals were included in the analysis, the SHIV-immunized animals controlled SIV replication in all tissues after vaginal SIV challenge.
SHIV-immunized animals have significantly lower total body SIVgag and SIVenv RNA levels after vaginal SIV challenge than control animals
To assess the level of protection in SHIV-immunized animals, the mean SIVgag and SIVenv RNA levels in the tissues of each immunized and control animal was calculated and the group means were compared. At day 7 p.c. there was a trend toward higher mean vRNA level in every tissue of the control animals compared to SHIV-immunized animals (data not shown) and at day 14 p.c. the mean SIVgag RNA level in every tissue of the control animals was significantly higher compared to SHIV-immunized animals (p<0.0001, One-way Anova, data not shown). Further when the mean SIVgag RNA in all of an animal's tissue samples was calculated to produce a total-body SIVgag RNA level for each animal, we found that although not significantly lower, by day 7 p.c. the mean total body SIVgag RNA level of the vaccinated animal tissues tended to be lower (p=0.065, one-tailed T-test) than the mean total body SIVgag RNA level of control animals (data not shown). By day 14 p.c., the virus was fully disseminated in the control animals and the mean total body SIVgag RNA level of the SHIV immunized animals was significantly lower than the mean total body SIVgag RNA level in the SIV control animals (p<0.0001, one-tailed T-test, data not shown).
Like SIVgag, at day 7 p.c. there was a trend toward higher mean SIVenv RNA level in every tissue of the control animals compared to SHIV-immunized animals (data not shown) and at day 14 p.c. the mean SIVenv RNA level in every tissue of the control animals was significantly higher compared to SHIV-immunized animals (p<0.0001, one-way Anova, data not shown). Further when the mean SIVenv RNA in an animal's tissue samples was calculated to produce a total body SIVenv RNA level for each animal, we found that by day 7 p.c. the mean total body SIVenv RNA level of the vaccinated animals was significantly lower (p=0.037) than the mean total body SIVenv RNA level of control animals (data not shown). By day 14 p.c., the virus was fully disseminated in the control animals and the mean total body SIVenv RNA level of the SHIV immunized animals was significantly lower than the mean total body SIVenv RNA level in the SIV control animals (p<0.0001, one-tailed T-test, data not shown).
HIVenv RNA levels in tissues of SHIV-immunized animals before, and after, after vaginal SIV challenge
To determine the level of SHIV virus replication in the tissues of the SHIV-immunized animals, HIVenv RNA levels were measured by RT-PCR. As expected, HIVenv RNA was undetectable in all tissue samples collected from the unimmunized control animals at all time points (data not shown). On the day of challenge, HIVenv RNA was detected in the tissues of 5 of 9 SHIV-immunized animals with high HIVenv RNA in the tissue samples of 1 of the 9 SHIV immunized animals, and low to moderate HIVenv RNA levels in tissues of 4 of the 9 immunized animals (Fig 4). At 3 days p.c., 3 of 3 SHIV-immunized animals had low to moderate HIVenv RNA levels in most tissues (Fig 4). At 7 days p.c., moderate to low HIVenv RNA levels were detected in all 6 animals, and at 14 days p.c. 10 of 12 animals had moderate to low HIVenv RNA levels in at least one tissue (fig. 4). In most of the HIVenv RNA+ animals necropsied from the day 0 to day 14 p.c, the lymph nodes had the highest HIVenv RNA levels among the tissues tested, but there were no significant differences in HIVenv RNA levels among the tissues.
FIG. 4. HIVenv RNA levels in tissues of SHIV-immunized monkeys after vaginal SIVmac239challenge.
(a) SHIV-immunized animals (HIVenv RNA copies/μg tissue RNA; CD8+ lymphocyte-depleted SHIV-immunized animals are indicated by a solid horizontal line under the animal numbers at the far right of the x-axis. The figure organization is similar to Fig. 2.
Relative levels of SIVgag, SIVenv and HIVenv RNA in blood of SHIV-immunized, CD8+ T-cell depleted animals after vaginal SIV challenge
The protective effect of SHIV-immunization was eliminated in 5 animals after pharmacological depletion of CD8+ T-cells (Fig. 5). Thus, the mean plasma SIV gag RNA level of the SHIV-immunized, CD8+ T-cell-depleted animals at both 7 and 14 days p.c was significantly higher than the mean SIVgag RNA level of the SHIV-immunized animals (data not shown). In fact at 7 days p.c., the mean plasma SIVgag RNA level of the SHIV-immunized, CD8+ T-cell-depleted animals was significantly higher than the mean plasma SIVgag RNA level of the naïve control animals (Fig. 5A). SIVenv RNA was readily detectable in the plasma of all the SHIV-immunized CD8+ T cell depleted animals at days 7 and 14 p.c. (Fig. 5 C and D). At day 7, all 5 of the CD8+ depleted animals had SIVenv RNA in plasma compared to only 10 of 14 naïve control animals. Further, at 7 days p.c. the mean plasma SIVenv RNA level in the CD8+ T-cell depleted animals was significantly higher than the mean plasma SIVenv RNA level of the naïve control animals (Fig. 5C). By day 14 p.c. plasma SIV gag and SIVenv RNA levels in naïve control and SHIV-immunized CD8+ T cell depleted animals were similar. All of 5 of the CD8+ depleted animals had HIVenv RNA in plasma at both 7 and 14 days p.c. (Fig. 5 E&F). Further, at both time points the mean plasma HIVenv RNA levels in the CD8+ T-cell depleted animals was significantly higher than in the T cell intact SHIV-immunized animals. The high plasma vRNA levels at 7 days p.c. demonstrate that, compared to un-immunized control animals, SIV replication and dissemination was significantly enhanced in SHIV-immunized animals that were depleted of CD8+ T cells on the day of SIV challenge.
Relative levels of SIVgag, SIVenv and HIVenv RNA in tissues of SHIV-immunized CD8+ T-cell depleted animals after vaginal SIV challenge
All 5 CD8+ T cell depleted animals had uncontrolled viral replication and high levels of SIVgag RNA in all tissues samples collected at day 14 p.c. (fig 2). There were similar levels of SIVenv RNA in the tissue samples from these animals (fig.3). Low to moderate levels of HIVenv RNA were detected in tissues from 4 of 5 CD8+ depleted animals, although these levels of HIVenv RNA were considerably lower than the SIVgag RNA levels measured in the same samples (fig. 4).
Strikingly, the anatomic pattern of vRNA in the tissues of SHIV-immunized, CD8+ T-cell depleted animals was unlike that in tissues of the T cell-intact, SHIV immunized animals that were necropsied on day 14 after challenge. Thus, 3 of the 5 SHIV-immunized CD8+ T-cell depleted animals had very high levels of SIVgag and SIVenv RNA (>106 vRNA copies/ug tissue RNA) in the reproductive tract and the other 2 SHIV-immunized CD8+ T-cell depleted animals had very high levels of SIVgag and SIVenv RNA (>106 vRNA copies/ug tissue RNA) in the genital lymph nodes (inguinal, axillary and iliac lymph nodes) (fig. 2c, 2d and fig. 3c and 3d).
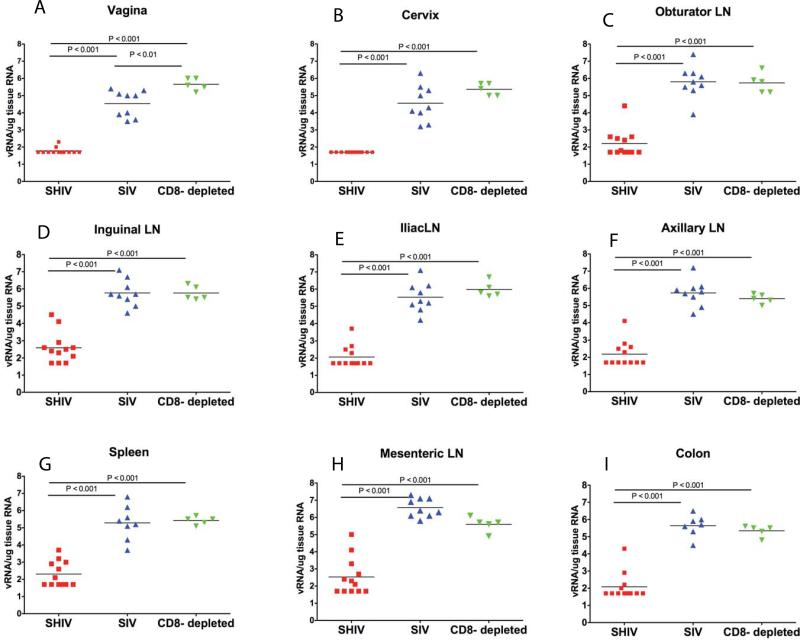
Comparison of SIV replication in tissues of study animals at 14 days p.c.
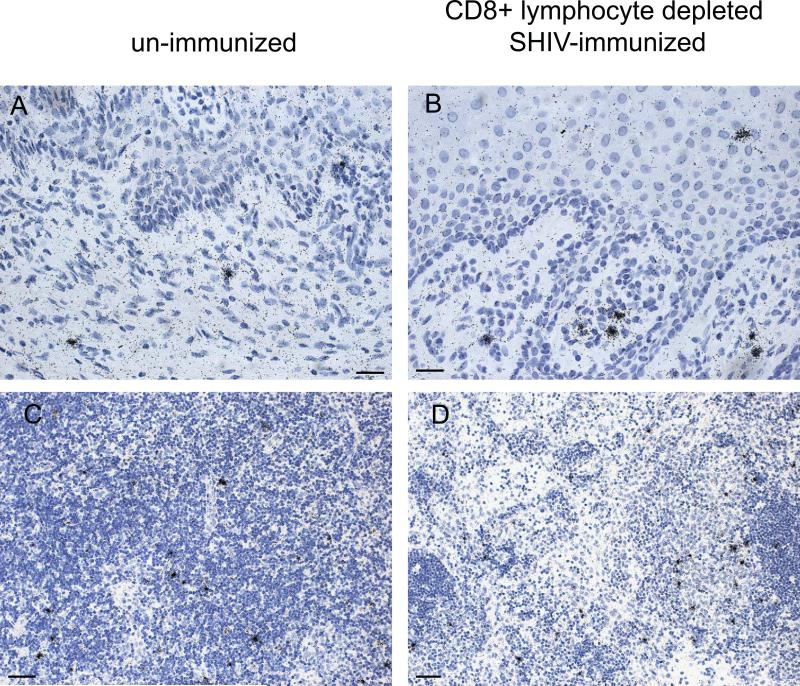
The relative levels of viral replication (mean number of SIVgag RNA copies/ ug tissue RNA) between tissues of SIV control animals, SHIV-immunized animals and SHIV-immunized CD8+ T cell depleted animals at day 14 p.c. were compared using a one way ENV with Bonferroni's multiple comparison test (fig 6). In the genital tract (vagina and cervix), the portal of virus entry and initial site of SIV replication after vaginal SIV inoculation, the SHIV-immunized animals had significantly lower levels of SIV gag than the control animals and the CD8+ T cell depleted SHIV-immunized animals (fig 6). Strikingly, the CD8+ T cell depleted SHIV-immunized animals had significantly higher levels of SIV gag RNA in the vagina than the unimmunized control monkeys (fig 6a). The Taqman RT-PCR results were confirmed by ISH labeling of SIV RNA+ cells in tissue sections (fig. 7) and quantitative image analysis. Thus, there were significantly more SIV RNA+ cells in histologic sections of vagina (p = 0.003, unpaired 2-tailed T test) but not the axillary LN, of the SHIV-immunized CD8 T cell depleted animals compared to the unimmunized control animals (fig.7). This result demonstrates that the amount of vRNA in the vaginal mucosa of the T cell depleted monkeys was higher than in the control animals.
Fig 6. Comparison of SIVgag RNA copies/ug tissue RNA in tissues at 14 days p.c. among SHIV-immunized, SHIV-immunized CD8 depleted, and naïve control monkeys.
To determine the extent to which the relative levels of virus replication in specific tissues were altered by the SHIV immunization, SIVgag vRNA copies/ug tissue RNA are compared in the cervix, vagina, obturator LN, inguinal LN, iliac LN, axillary LN, spleen, mesenteric LN, and colon. Statistical differences calculated by one-way ENV with Bonferroni's multiple comparison test.
Fig 7. Comparison of the density of SIV RNA+ cells in the genital tract and axillary lymph node of naïve control and SHIV-immunized CD8+ T cell depleted animals by in-situ hybridization.
Panels a and c: tissues from control SIV infected animal at 14 days p.c.. Panels b and d: tissues from SHIV-immunized CD8+ T cell depleted animal at 14 days p.c.. Panels a and b, vagina, bars indicate 25 um; Panels c and d, axillary LN, bars indicate 50 um. Hematoxylin counterstain.
Among the tissues that become infected as virus disseminates from the portal of entry, the lymph nodes that drain the genital tract (iliac, inguinal and obturator lymph nodes) become infected by lymphatic dissemination and they are the secondary sites of SIV replication after vaginal SIV inoculation. The level of virus replication (SIVgag RNA copies/ug tissue RNA) in the genital lymph nodes of the SHIV-immunized animals was significantly lower than in the matched samples from control animals and CD8+ T cell depleted immunized animals (fig. 6C-E). Further, among the tissues that become infected by hematogenous dissemination from the genital lymph nodes and are tertiary sites of SIV replication after vaginal SIV inoculation, there were significantly lower levels of vRNA in systemic lymphoid tissues (spleen, axillary lymph nodes) and gut-associated lymphoid tissues (mesenteric lymph nodes and colon) of the SHIV-immunized animals than in the same tissues of the control animals and the CD8+ T cell depleted SHIV-immunized animals (fig. 6 F-I). The levels of virus replication in tissues initially infected by lymphatic or blood borne SIV dissemination from the genital tract were similar in the CD8+ T cell depleted immunized animals and unimmunized control monkeys (fig 7).
Discussion
We have previously determined that the protection from uncontrolled SIV replication that is induced in 60% of rhesus macaques by SHIV89.6 immunization is due to the presence of CD8+ T cells in the vaginal mucosa of the protected animals (Genescà et al., 2008a; Genescà et al., 2008b). Further in SHIV-immunized animals that are monitored for 6 months after SIV challenge, the uncontrolled viral replication that characterizes the unprotected animals does not become apparent until plasma vRNA levels rise 2−3 months after vaginal SIV challenge (Abel et al., 2003; Busch et al., 2005; Genesca et al., 2007; Miller et al., 1997). This strongly suggests that ongoing SIV replication occurs in tissues of SHIV-immunized, unprotected animals even when little plasma vRNA is detected. The goal of the present study was to define the extent of attenuated SHIV89.6 (vaccine virus) and pathogenic SIV (challenge virus) replication in the tissues of rhesus macaques immunized with SHIV89.6 for 6−8 months and then challenged vaginally with SIVmac239. Based on both SIVgag and SIVenv RNA levels, it was straightforward to distinguish the immunized animal group from the control animal group at 7days p.c., because mean plasma and tissue vRNA levels in the immunized animals were significantly lower than in the naïve control animals. Thus there is control of viral replication in all tissues of the SHIV-immunized animals at day 7 after vaginal SIV challenge. Control of virus replication across all of the anatomic sites suggests that either the infection was contained near the site of vaginal inoculation by SIV-specific CD8+ T cells or that virus replication was efficiently controlled by systemic SIV-specific CD8+ T cell responses after the virus disseminated to all tissues. The former explanation seems more likely because as we recently reported (Genescà et al., 2008a), after challenge, SIV-specific CD8+ T cell responses in the vagina expanded but there was no detectable expansion of T cell responses in any other anatomic site. As expansion of SIV-specific T cells is presumably antigen-driven, the combined results of the virologic and immunologic analyses are most consistent with the conclusion that there is little systemic dissemination and replication of SIV in the SHIV-immunized and protected animals.
The ability of SHIV89.6 to induce protective CD8+ T cell responses in the genital tract seems to be related to its pattern of persistent replication. Although SHIV89.6 did not replicate to high levels in any tissue at 6−8 months post-immunization, HIVenv RNA produced by SHIV89.6 replication was consistently found in the reproductive tract and draining lymph nodes on the day of challenge and at 7 and 14 days after vaginal SIV challenge. The persistence of SHIV replication likely explains the presence of antiviral T cells in the vaginal mucosa of the SHIV immunized animals on the day of challenge (Genescà et al., 2008b). However the low-level residual SHIV89.6 replication that was detected on the day of challenge in tissues of all the immunized animals did not increase after vaginal SIV challenge. This was also the case in the 2 unprotected, SHIV-immunized animals in which SIVgag and SIVenv RNA levels were relatively high in the plasma and tissues at 14 days p.c. while HIVenv RNA levels were in the same low range found in the SHIV-immunized animals necropsied on the day of SIV challenge. Thus SHIV89.6 replication did not significantly contribute to uncontrolled viral replication in the immunized but unprotected monkeys after vaginal SIVmac239 challenge.
In our previous studies approximately 60% of SHIV immunized animals control challenge virus replication so that plasma vRNA levels never exceed 104 copies/ml over a 6-month observation period. Although the current study involved only a 14-day observation period after vaginal SIV challenge, some of the SHIV-immunized animals controlled virus replication and some did not. It was not possible to distinguish protected from unprotected animals among the SHIV-immunized group at day 7 p.c.. However by 14 days p.c., SIV replication had increased dramatically in 2 of the 10 SHIV–immunized animals and these 2 animals (28843, 32330) were considered unprotected. This is the same proportion of animals that were unprotected at 14 days p.c. in our previous SHIV immunization SIV challenge studies (Abel et al., 2003). Further, although the levels of viral replication were reduced in these 2 un-protected SHIV-immunized animals, compared to unimmunized control animals the anatomic distribution of viral replication in these 2 animals was similar to the control animals.
Fourteen days after vaginal SIV challenge, the anatomic distribution of virus in tissues among the SHIV-immunized, protected animals was distinct from the distribution of virus in the unimmunized control animals that had relatively uncontrolled viral replication (Figs 2, 3 and 6). In the control animals, the highest levels of vRNA were in the mesenteric lymph node and colon, the lowest in the reproductive tract and draining lymph nodes with intermediate SIVenv levels in the systemic lymph nodes. At day 14 p.c. in the SHIV-immunized animals that controlled SIV replication, the highest levels of SIVenv RNA were found in the genital mucosa and genital lymph nodes. This result supports the conclusion that SIV dissemination was limited in the SHIV-immunized protected animals. In marked contrast, the relative levels of SIVenv RNA among the tissues of SHIV-immunized, unprotected animals and the un-immunized control animals were similar at day 14 p.c.. Thus, 14 days after vaginal SIV challenge, the SIVenv RNA levels were highest in the mesenteric LNs of the un-immunized control animals and the SHIV-immunized animals that did not control viral replication. The SIVenv RNA in the mesenteric lymph nodes is convincing evidence that SIV rapidly disseminates and replicates in the unprotected SHIV-immunized animals.
At both days 7 and 14 p.c., SIVenv RNA plasma levels were significantly higher in the CD8-depleted SHIV immunized animals compared to the CD8+ T cell intact SHIV-immunized animals. This result confirms our previous conclusion that CD8+ T cells are responsible for the control of challenge virus replication after vaginal SIV challenge (Genescà et al., 2008a). The fact that the CD8+ T cell depleted SHIV-immunized animals had a significantly higher mean plasma SIVgag RNA level (SIVgag copies/ml plasma) than the unimmunized control animals at 7 days p.c. indicates that after vaginal inoculation, SIV replication was enhanced in SHIV-immunized CD8+ T cell depleted monkeys compared to naïve control animals. Further at both day 7 and 14 p.c., plasma HIVenv RNA levels were significantly higher in the CD8+ T cell depleted SHIV-immunized animals compared to the HIVenv RNA levels in T cell intact SHIV-immunized animals, consistent with a loss of immune control of vaccine virus replication in the CD8-depleted, SHIV-immunized animals.
Depletion of the CD8+ T-cell population alters the anatomic distribution of viral replication. In the CD8+ T cell-depleted animals, the highest levels of SIV replication are in the genital tract and genital lymph nodes and in fact the vRNA levels in these tissues exceeded those found in the colon and mesenteric LN of control animals. This very high level of viral replication in the vagina and genital lymph nodes is unprecedented and suggests that in the absence of antiviral CD8+ T cells, SIV replication in the genital tract was enhanced by prior SHIV immunization. The potential to increase viral replication in SHIV-immunized animals is consistent with our previous report that SHIV-immunized animals have numerous SIV-specific CD4+ T cells in the vaginal mucosa on the day of challenge (Genescà et al., 2008b). It seems likely that these CD4+ T cells in the vaginal mucosa of the immunized animals provide more cellular targets compared to the naïve animals leading to enhanced local viral replication. Thus an increased number of infected target cells in the genital tract is the likely source of the high levels of plasma vRNA seen at 7 days p.c. in the CD8+ T cell depleted animals. As this effect was only seen in the genital tract, the SIV-specific CD4+ T cells that enhance virus replication in the genital mucosa were apparently not present, or did not enhance SIV replication, in the other lymphoid tissues. Thus, SHIV–immunized animals have the potential to support enhanced challenge virus replication in the genital tract and this potential is realized after SIV specific effector CD8+ T cells are eliminated by CD8+ T cell depletion. It is possible that the higher vRNA levels found in the plasma and tissues of the CD8-depleted animals was a result of the high levels of immune activation induced by the administration of the cM-T807 antibody. However a recent study suggests that the immune activation that occurs in response to CD8+ T cell depletion does not contribute to enhanced SIV replication (Okoye et al., 2009).
The results of the CD8+ T cell depletion experiment essentially eliminate a role for viral interference in the protection we observe in animals immunized with attenuated SHIV 89.6. If infection of CD4+ T cells by SHIV89.6 had depleted these critical target cells making them unavailable to support SIV replication, we would not expect to see enhanced viral replication in CD8+ T cell depleted animals relative to control animals at 7 days p.c. This result demonstrates that viral replication after challenge results from a balance between SIV replication in abundant target cells and efficient killing of those infected target cells by antiviral CD8+ T cells.
Our findings are similar in some respects to the results reported when macaques immunized with an attenuated SHIV are challenged with a closely related pathogenic SHIV; the persistence of the vaccine virus is a primary correlate of protection from challenge virus replication and disease progression (Mackay et al., 2004; Silverstein et al., 2000b). In our system, the attenuated SHIV virus persisted in tissues until the day of challenge in at least one tissue from 6 of 9 animals examined (Figs. 2 and 3) and (Genescà et al., 2008b) and SHIV vRNA remained at low but detectable levels after SIV challenge (fig 2). In fact, CD8+ T cells also control replication of the attenuated SHIV vaccine virus in our model, as demonstrated by the increased replication of the vaccine virus which contributed moderately to viral replication in CD8+ depleted monkeys after vaginal SIV challenge (fig. 6). However, our conclusion that the attenuated vaccine virus persists in tissues after pathogenic virus challenge differs from a report (Berry et al., 2008) that the vaccine virus is undetectable after pathogenic SIV challenge in macaques immunized with an attenuated molecular clone of SIVmac251. However, CD8+ T cells do not seem to play a role in protection in the macaques immunized with attenuated SIVmac251 (Stebbings et al., 1998).
This study establishes that in protected SHIV-immunized monkeys, control of SIV replication extended anatomically from the genital tract portal of entry to the gut associated and systemic lymphoid tissues, and temporally from the day of challenge through 14 days PC. Further, in unprotected, SHIV-immunized monkeys, the challenge virus is the source of the relatively high levels of vRNA in tissues and blood, with the highest vRNA levels in mesenteric lymph node and colon. However after CD8+ T cells were depleted, the SIV specific CD4+ T cell responses found in the genital tract of SHIV-immunized animals (Genescà et al., 2008b) apparently enhanced virus replication at the portal of entry. As T cell vaccines elicit both CD8+ and CD4+ T cell responses, the potential for enhanced viral replication, and perhaps enhanced susceptibility to infection as seen in the STEP trial, may always exist co-exist with protective immune responses in AIDS vaccine recipients. Thus even in animals immunized with a protective vaccine, a delicate balance exists between protective antiviral immunity and immune activation capable of driving HIV replication. This balance must be negotiated to develop an effective HIV vaccine.
Acknowledgements
This work was supported by Public Health Service grants U51RR00169 from the National Center for Research Resources and P01 AI066314 and R01 AI44480 from the National Institute of Allergy and Infectious Diseases and a gift from the James B. Pendleton Charitable Trust. The authors thank Drs. M.P. Busch, A. Haase, N. Miller and K. Reimann for helpful discussions; and the Primate Services Unit at the CNPRC, Tracy Rourke, Lili Guo and Roxana Colon for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller CJ. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77(5):3099–3118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel K, La Franco-Scheuch L, Rourke T, Ma ZM, De Silva V, Fallert B, Beckett L, Reinhart TA, Miller CJ. Gamma Interferon-Mediated Inflammation Is Associated with Lack of Protection from Intravaginal Simian Immunodeficiency Virus SIVmac239 Challenge in Simian-Human Immunodeficiency Virus 89.6-Immunized Rhesus Macaques. J Virol. 2004;78(2):841–854. doi: 10.1128/JVI.78.2.841-854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott EJ. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345(8961):1342–4. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- Berry N, Stebbings R, Ferguson D, Ham C, Alden J, Brown S, Jenkins A, Lines J, Duffy L, Davis L, Elsley W, Page M, Hull R, Stott J, Almond N. Resistance to superinfection by a vigorously replicating, uncloned stock of simian immunodeficiency virus (SIVmac251) stimulates replication of a live attenuated virus vaccine (SIVmacC8). J Gen Virol. 2008;89(9):2240–2251. doi: 10.1099/vir.0.2008/001693-0. [DOI] [PubMed] [Google Scholar]
- Busch M, Abel K, Li J, Piatak M, Jr., Lifson JD, Miller CJ. Efficacy of a SHIV 89.6 Proviral DNA Vaccine against Mucosal SIVmac239 Challenge. Vaccine. 2005;23:4036–4047. doi: 10.1016/j.vaccine.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Genesca M, Li J, Fritts L, Chohan P, Bost K, Rourke T, Blozis SA, McChesney MB, Miller CJ. Depo-Provera abrogates attenuated lentivirus-induced protection in male rhesus macaques challenged intravenously with pathogenic SIVmac239. J Med Primatol. 2007;36(4−5):266–275. doi: 10.1111/j.1600-0684.2007.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesca M, McChesney MB, Miller CJ. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J Intern Med. 2009;265(1):67–77. doi: 10.1111/j.1365-2796.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genescà M, S. PJ, H. JJ, Li J, Bost K, Lu D, McChesney MB, Miller CJ. With minimal systemic T cell expansion, CD8+ T cells mediate protection from vaginal SIV challenge in rhesus macaques immunized with attenuated SHIV89.6. J Virology. 2008a;82:11181–11196. doi: 10.1128/JVI.01433-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genescà M, Skinner PJ, Bost K, Lu D, Wang Y, Rourke TL, Haase AT, McChesney MB, Miller CJ. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 2008b;1:219–228. doi: 10.1038/mi.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach BR, Lewis MG, Sopper S, Schnell T, Sodroski J, Stahl-Hennig C, Uberla K. Evidence for recombination of live, attenuated immunodeficiency virus vaccine with challenge virus to a more virulent strain. J Virol. 2000;74(8):3537–3542. doi: 10.1128/jvi.74.8.3537-3542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Pope M, Brown C, O'Doherty U, Miller CJ. Immunophenotypic characterization of SIV-infected dendritic cells in the cervix, vagina and draining lymph nodes of rhesus macaques. Lab. Invest. 1998;78:435–451. [PubMed] [Google Scholar]
- Khatissian E, Monceaux V, Cumont MC, Kieny MP, Aubertin AM, Hurtrel B. Persistence of pathogenic challenge virus in macaques protected by simian immunodeficiency virus SIVmacDeltanef. J Virol. 2001;75:1507–1515. doi: 10.1128/JVI.75.3.1507-1515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwofie TB, Miura T, Ibuki K, Enose Y, Suzuki H, Ui M, Kuwata T, Hayami M. Characterization of simian and human immunodeficiency chimeric viruses re-isolated from vaccinated macaque monkeys after challenge infection. Archives of Virology. 2002;147(6):1091–1104. doi: 10.1007/s00705-002-0811-9. [DOI] [PubMed] [Google Scholar]
- Lohman BL, McChesney MB, Miller CJ, McGowan E, Joye SM, Van Rompay KKA, Reay E, Antipa L, Pedersen NC, Marthas ML. A partially attenuated simian immunodeficiency virus induces host immunity that correlates to resistance to pathogenic virus challenge. J. Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay GA, Liu Z, Singh DK, Smith MS, Mukherjee S, Sheffer D, Jia F, Adany I, Sun KH, Dhillon S, Zhuge W, Narayan O. Protection against late-onset AIDS in macaques prophylactically immunized with a live simian HIV vaccine was dependent on persistence of the vaccine virus. J Immunol. 2004;173:4100–4107. doi: 10.4049/jimmunol.173.6.4100. [DOI] [PubMed] [Google Scholar]
- Miller CJ, McChesney MB, Lu X, Dailey PJ, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71(3):1911–21. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M, Jr., Lifson JD, Sodora DL, Villinger F, Axthelm MK, Schmitz JE, Picker LJ. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med. 2009 doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MR, Weiler AM, Weisgrau KL, Piaskowski SM, Furlott JR, Weinfurter JT, Kaizu M, Soma T, Leon EJ, MacNair C, Leaman DP, Zwick MB, Gostick E, Musani SK, Price DA, Friedrich TC, Rakasz EG, Wilson NA, McDermott AB, Boyle R, Allison DB, Burton DR, Koff WC, Watkins DI. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J Exp Med. 2008;205(11):2537–2350. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Silvera P, Flanagan B, Kitchin P, Almond N. The development of PCR based assays for the detection and differentiation of simian immunodeficiency virus in vivo. J Virol Methods. 1995;51(2−3):229–239. doi: 10.1016/0166-0934(94)00109-t. [DOI] [PubMed] [Google Scholar]
- Ruprecht RM. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol Rev. 1999;170:135–149. doi: 10.1111/j.1600-065x.1999.tb01335.x. [DOI] [PubMed] [Google Scholar]
- Shibata R, Siemon C, Czajak SC, Desrosiers RC, Martin MA. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Mackay GA, Mukherjee S, Li Z, Piatak M, Jr., Lifson JD, Narayan O, Kumar A. Pathogenic simian/human immunodeficiency virus SHIV(KU) inoculated into immunized macaques caused infection, but virus burdens progressively declined with time. J Virol. 2000a;74(22):10489–10497. doi: 10.1128/jvi.74.22.10489-10497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Mackay GA, Mukherjee S, Li Z, Piatak M, Jr., Lifson JD, Narayan O, Kumar A. Pathogenic Simian/Human Immunodeficiency Virus SHIVKU Inoculated into Immunized Macaques Caused Infection, but Virus Burdens Progressively Declined with Time. J. Virol. 2000b;74(22):10489–10497. doi: 10.1128/jvi.74.22.10489-10497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings R, Stott J, Almond N, Hull R, Lines J, Silvera P, Sangster R, Corcoran T, Rose J, Cobbold S, Gotch F, McMichael A, Walker B. Mechanisms of protection induced by attenuated simian immunodeficiency virus. II. Lymphocyte depletion does not abrogate protection. AIDS Res Hum Retrovir. 1998;14(13):1187–1198. doi: 10.1089/aid.1998.14.1187. [DOI] [PubMed] [Google Scholar]
- Ui M, Kuwata T, Igarashi T, Ibuki K, Miyazaki Y, Kozyrev IL, Enose Y, Shimada T, Uesaka H, Yamamoto H, Miura T, Hayami M. Protection of macaques against a SHIV with a homologous HIV-1 Env and a pathogenic SHIV-89.6P with a heterologous Env by vaccination with multiple gene-deleted SHIVs. Virology. 1999;265:252–263. doi: 10.1006/viro.1999.0049. [DOI] [PubMed] [Google Scholar]
- Wakrim L, Le Grand R, Vaslin B, Ch...Ret A, Matheux F, Theodoro F, Roques P, Nicol-Jourdain I, Dormont D. Superinfection of HIV-2-Preinfected Macaques after Rectal Exposure to a Primary Isolate of SIVmac251. Virology. 1996;221(2):260–270. doi: 10.1006/viro.1996.0375. [DOI] [PubMed] [Google Scholar]