Abstract
Stress plays an important role in drug addiction. It can trigger relapse in abstinent addicts, and both in the everyday world and in the laboratory, a stressor can induce drug craving. Drug cues, such as the sight of drug, can also trigger subjective craving and relapse, and this effect may be amplified by stress. Underpinning this interaction may be the fact that stress and reward-predicting drug cues act on overlapping brain regions. We exposed 15 smokers undergoing functional magnetic resonance imaging to a psychosocial stressor, the Montreal Imaging Stress Task, followed by drug cues consisting of video clips of smokers. In a separate session similar video clips were shown after a non-stress control task. We observed significantly decreased neural activity during stress in the hippocampus, amygdala, bed nucleus of the stria terminalis, hypothalamus and nucleus accumbens. Following stress there was an increased neural response to drug cues in the medial prefrontal cortex, posterior cingulate cortex, dorsomedial thalamus, medial temporal lobe, caudate nucleus, and primary and association visual areas. These regions are thought to be involved in visual attention and in assigning incentive value to cues. Stress-induced limbic deactivation predicted subsequent neural cue-reactivity. We suggest that stress increases the incentive salience of drug cues.
Keywords: fMRI, stress, nicotine, tobacco, hippocampus, amygdala, nucleus accumbens
Introduction
In cigarette smokers, drug cues (stimuli previously paired with smoking) can promote craving and relapse (Abrams et al., 1988). Stress can also lead to cigarette craving in abstinent humans (Doherty et al., 1995; Swan et al., 1988), and to reinstatement of self-administration in animals (Buczek et al., 1999; Zislis et al., 2007). Furthermore, stress and drug cues may act in synergy (Liu and Weiss, 2002; Pecina et al., 2006). This is especially problematic as drug withdrawal is itself stressful, and can make individuals more vulnerable to life’s other stresses. Nicotine withdrawal, for example, is associated with dysregulation of the hypothalamic pituitary adrenal axis and increased stress reactivity (Picciotto et al., 2002).
Nicotine withdrawal is also associated with dysfunction of the dopamine system (Epping-Jordan et al., 1998), providing another avenue for stress to affect drug intake. In animal models, conditioned stimuli predictive of drug availability, i.e. drug cues, trigger dopamine release, which increases the motivation to consume the drug (Phillips et al., 2003). Acute stress also promotes dopamine release (Abercrombie et al., 1989; Pruessner et al., 2004). The areas of the brain involved in regulation of appetitive behaviors, such as feeding and drug taking, include the amygdala and hippocampus, medial prefrontal cortex (PFC), orbitofrontal cortex (OFC), insula, and striatum, all of which receive dopaminergic projections. Neuronal activity within this network of regions is also modulated by acute stress, and experimental evidence in humans and animals points to a role of dopamine in stress-induced reinstatement of drug taking (Erblich et al., 2004; Shaham and Stewart, 1995; Shaham et al., 2003).
Stress may also become a conditioned stimulus for cigarette smoking. Nicotine is anxiolytic, and some individuals smoke to relieve anxiety (Picciotto et al., 2002). According to negative reinforcement models, stress creates a negative emotional state, which then leads to the pursuit of the drug rewards to relieve these unpleasant effects. However, stress might also act as a positive reinforcer, by increasing the appetitive and incentive effects of drug cues (Koob, 2008; Pecina et al., 2006). As such, stress could act in part like drug cues, via the mesolimbic reward system, including the ventral striatum and amygdala.
We have previously used an acute psychosocial stressor, the Montreal Imaging Stress Task (MIST) (Dedovic et al., 2005), in the neuroimaging environment. When combined with functional magnetic resonance imaging (fMRI), its main effect is widespread deactivation in limbic and associated regions, including the hippocampus, amygdala, and nucleus accumbens (Pruessner et al., 2008). These regions are all modulators of the hypothalamic pituitary adrenal axis (Herman et al., 2005), and the deactivations are thought to be specifically stress-related as their intensity correlates with the salivary cortisol response (Pruessner et al., 2008). The MIST also has the ability to trigger dopamine release in the ventral striatum (nucleus accumbens), as shown by positron emission tomography (Pruessner et al., 2004).
We have also used smoking cues to map out the neural correlates of cue-reactivity (McBride et al., 2006). In the current study we combined our stress and cue reactivity paradigms in a group of young cigarette smokers (Fig. 1). All subjects underwent fMRI on two occasions. For each session they viewed two-minute blocks of smoking and control videos, then performed either the stress or non-stress control version of the MIST, followed by three blocks of different smoking and neutral videos. Our hypothesis was that stress would increase the neural response to smoking cues in brain areas involved in attention and motivation, when compared to the non-stress session. In other words, following acute stress, cues associated with nicotine use would become increasingly important and take on greater incentive salience (Berridge and Robinson, 1998). We also hypothesized that the individual brain response to stress would predict the subsequent response to smoking cues. This interaction between stress and appetitive responses could predispose the vulnerable abstinent smoker to relapse.
Figure 1. Experimental paradigm.
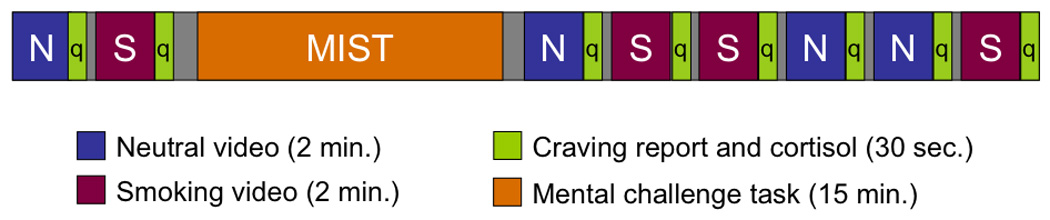
Results
Behavior and Biochemistry
Craving scores significantly increased over time (Fig. 2). A within subjects 2-factor repeated measures ANOVA (time × condition) showed an effect of time irrespective of condition (stress or non-stress), (F7, 84=8.342, p< 0.001). Craving scores were higher in the stress condition overall, but this did not reach significance (F(1, 12= 2.088, p=0.174). There was no significant time by condition interaction.
Figure 2. Craving.

Subjective craving level over time for the stress and non-stress sessions. There was a main effect of time, but no session difference or interaction effect. Error bars indicate the standard error.
A within subjects repeated measures ANOVA (time × condition) was performed to ascertain changes in saliva cortisol over the course of each fMRI session. While cortisol did show a modest increase in response to the first run of the stressful version of the MIST, there were no significant main effects of condition or time, although time neared significance (p=0.060).
In an attempt to quantify the overall secretion of cortisol during the scanning period, we computed the Area Under the Curve with respect to the ground (AUCg) (Pruessner et al., 2003). There was no significant difference in AUCg between the two sessions (stress: mean=0.191, SE=0.03; control: mean= 0.175, SE=0.02 ; p=0.635); however, the cortisol AUCg correlated with the mean craving response during the stress session (r2 = 0.527, p = 0.064), but not the non-stress session (r2 = −0.138, p= 0.620).
Functional MRI
The stressor caused widespread deactivations of the limbic and paralimbic systems (Table 1). When comparing stressful mental challenge minus baseline (stress session) to non-stressful mental challenge minus baseline (non-stress session), we found significantly greater deactivation during stress in the medial PFC including the subgenual anterior cingulate cortex (ACC) and ventromedial PFC, ventrolateral PFC, medial temporal lobe including hippocampus and amygdala, posterior insula and posterior putamen, posterior cingulate cortex (PCC), and a large bilateral peak involving the nucleus accumbens, ventral pallidum and putamen, adjacent extended amygdala, including the bed nucleus of the stria terminalis (BNST), and hypothalamus (Fig. 3). The data from each session are shown in Supplementary Table 1. The region of interest data, derived from anatomically defined masks, confirmed the t-map results, showing reductions in effect size for the hippocampus (non-stress: −0.009, stress: −0.087, p = 0.02), amygdala (non-stress: −0.05, stress: −0.20, p = 0.009), and nucleus accumbens (non-stress: 0.002, stress: −0.106, p = 0.001).
Table 1. Deactivations during stress.
Comparison of the math task minus control for the stress compared to the non stress session. Peaks listed here are areas that showed greater deactivation during the stress session. All significant peaks (p < 0.05 corrected for multiple comparisons) are listed. Brodmann areas in parentheses. Abbreviations: vmPFC: ventromedial prefrontal cortex, ACC: anterior cingulate cortex, OFC: orbitofrontal cortex, vlPFC: ventrolateral prefrontal cortex, BNST: bed nucleus of the stria terminalis, NAC: neucleus accumbens, PCC: posterior cingulate cortex, STG: superior temporal gyrus, LPs: superior parietal lobule.
| Region | T | x | y | z |
|---|---|---|---|---|
| VmPFC | 4.57 | 0 | 60 | 0 |
| VmPFC | 4.67 | 2 | 40 | 2 |
| L vlPFC (47) | 5.26 | −44 | 38 | −8 |
| L OFC | 4.86 | −24 | 42 | −20 |
| L vlPFC (45) | 4.73 | −46 | 32 | 8 |
| Subgenual ACC | 5.39 | −6 | 18 | −6 |
| R BNST/ NAC | 6.71 | 12 | 14 | −14 |
| L BNST/ NAC | 5.81 | −14 | 14 | −12 |
| R temporal pole (22) | 5.96 | 36 | 8 | −26 |
| L amygdala | 5.40 | −24 | −8 | −16 |
| L posterior insula/ putamen | 5.07 | −32 | −16 | 4 |
| R posterior insula/ putamen | 7.84 | 40 | −20 | 4 |
| L hippocampus | 5.13 | −28 | −24 | −20 |
| R hippocampus | 4.73 | 32 | −24 | −16 |
| PCC (23) | 4.71 | 0 | −32 | 32 |
| L STG (22) | 5.81 | −54 | −36 | 4 |
| L PCC (23) | 6.27 | −8 | −58 | 16 |
| R Precuneus (31) | 5.19 | 2 | −66 | 28 |
| L LPs (7) | 4.68 | −46 | −74 | 30 |
| Cuneus (18) | 5.82 | 4 | −84 | 28 |
Figure 3. Neural response to stress (deactivations).
T-map depicting reduced BOLD response when comparing the stress math task to the non-stress math task. T-values in color are overlayed on the average MRI of all subjects in MNI space. Stress responsive regions shown here include the hippocampus (a), amygdala (b), lateral hypothalamus (c), bed nucleus of the stria terminalis (d), ventral pallidum (e), and nucleus accumbens (f). Not shown: subgenual ACC/ medial OFC, as well as regions belonging to the default mode network (seeTable 1). Coronal sections from left to right taken at y = −23mm, −9mm, −4mm, −1mm, and +12mm.
Cue reactivity was assessed by comparing the response to the smoking videos minus the neutral videos, for the three blocks that occurred after the mental challenge. Data for the stress and non-stress sessions are shown in Table 2. There were more activated regions following the stress task. In the stress session only there was activation of the dorsomedial PFC, dorsal caudate, dorsomedial thalamus, and hippocampus. In both sessions, there was activation of the middle and superior temporal gyrus, precuneus and primary visual cortex. We extracted effect sizes from each scan using the peak voxels from the stress and non-stress sessions (Fig. 4). There were significantly greater smoking minus neutral video effects in the stress compared to the non-stress session in the caudate, dorsomedial thalamus, mPFC, precuneus and hippocampus (all p < 0.0001 one-tailed).
Table 2. Cue reactivity activations.
Brain regions activated in the smoking video minus control video comparison, for the to sessions. All significant peaks listed (p < 0.05 corrected for multiple comparisons). Abbreviations: dmPFC: dorsomedial prefrontal cortex, DM: dorsomedial, MTG: middle temporal gyrus, STG: superior temporal gyrus, V1: visual cortex (peak extends bilaterally to include areas 17 and 18).
| Stress | Non-Stress | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | T | x | y | z | T | x | y | z |
| R dmPFC | 4.7 | 10 | 53 | 20 | ||||
| Dorsal caudate | 5.6 | 16 | 0 | 18 | ||||
| DM Thalamus | 4.5 | 2 | −6 | 2 | ||||
| R hippocampus | 6.4 | 18 | −34 | −12 | ||||
| R MTG/ STG | 5.3 | 58 | −40 | 6 | 4.7 | 54 | −40 | 6 |
| Fusiform gyrus | 4.5 | −18 | −48 | −6 | ||||
| Precuneus | 5.6 | 2 | −58 | 44 | 5.5 | −24 | −54 | 2 |
| V1 | 6.1 | 0 | −82 | 0 | 7.2 | 16 | −80 | 8 |
Figure 4. Effect of stress on cue reactivity.
Peak voxels from the stress and non-stress sessions were used to extract effect sizes of the smoking minus control video analyses. All significant at p < 0.001.
Finally, we assessed the relationship between limbic stress-induced deactivation and subsequent smoking cue-related activation. We applied an anatomically defined mask to extract the effect size in the nucleus accumbens (from the math task) for each scanning session. When we used this as a covariate, we found, for the stress session, a significant correlation between accumbens deactivation during stress and cue-related activation (smoking videos minus neutral videos) in medial PFC (dorsomedial PFC and subgenual and dorsal ACC), caudate, amygdala, hippocampus, dorsomedial thalamus, PCC, and primary and extra-striate visual areas (Table 3, Fig. 5). The same correlation for the non-stress session yielded no significant peaks. When the hippocampus and amygdala were used to extract stress-induced deactivations the results were similar: deactivation during stress predicted smoking cue related activations in the caudate, nucleus accumbens and BNST, medial OFC, hippocampus, PCC and precuneus, only in the stress session (Supplementary Table 2).
Table 3. Stress response predicts craving response.
Correlation between the deactivation in the nucleus accumbens during math challenge and the activation to smoking cues (minus neutral). All peaks significant at p<0.001 uncorrected with 20 contiguous voxels. Abbreviations: dmPFC: dorsomedial prefrontal cortex, STG: superior temporal gyrus, V1: primary visual cortex. L: left. R: right.
| Region | T-stat | X | Y | Z |
|---|---|---|---|---|
| Stress Session | ||||
| R dmPFC | 4.67 | 10 | 50 | 22 |
| L Subgenual cingulate | 4.57 | −20 | 36 | −2 |
| R dmPFC | 4.99 | 16 | 34 | 38 |
| R Subgenual cingulate | 4.71 | 14 | 32 | 4 |
| L Anterior cingulate cortex | 4.46 | −16 | 24 | 40 |
| R Anterior cingulate cortex | 4.38 | 20 | 20 | 44 |
| R Caudate | 3.94 | 18 | 8 | 18 |
| R Amygdala | 4.12 | 14 | −6 | −26 |
| Dorsomedial Thalamus | 3.73 | −2 | −8 | 4 |
| Posterior cingulate cortex (23) | 3.83 | −4 | −26 | 32 |
| Paracentral lobule | 4.43 | −4 | −28 | 60 |
| R Hippocampus | 5.91 | 18 | −34 | −12 |
| L Hippocampus | 4.62 | −22 | −34 | −10 |
| Posterior cingulate cortex | 4.11 | −12 | −38 | 48 |
| R STG (22) | 4.34 | 58 | −44 | 10 |
| R Precuneus | 4.86 | 16 | −58 | 14 |
| L Posterior cingulate cortex (30) | 4.70 | −28 | −60 | 10 |
| V1 | 3.94 | 0 | −86 | 0 |
| Non-stress session (No significant peaks) | ||||
Figure 5. Correlation map.
T-map of brain regions where smoking-control video effect correlates with deactivation in the nucleus accumbens during stress. This image depicts the subgenual anterior cingulate cortex, dorsomedial prefrontal cortex, and posterior cingulate (top left), amygdala and hippocampus (bottom left), and caudate nucleus (top right). ((seeTable 3) for coordinates.)
Discussion
An acute psychosocial stressor increased the neural response to smoking cues in brain areas that control attention and motivation. Moreover, the brain response to stress in the hippocampus, amygdala, and nucleus accumbens predicted the subsequent neural cue-reactivity. This provides a mechanism, at the neural systems level, for the role of stress in addiction maintenance and relapse.
The fMRI results were in very close agreement with our previous published work for both the stress (Pruessner et al., 2008) and cue-reactivity (McBride et al., 2006) paradigms. The psychosocial stressor, when compared to the non-stress control, caused widespread deactivation in the limbic system (hippocampus, amygdala, nucleus accumbens, BNST), medial prefrontal and medial and lateral parietal areas (Table 1). Some of these areas belong to the default mode network of brain function (Gusnard and Raichle, 2001), which shows consistent deactivation during the performance of attention-demanding tasks, a phenomenon not thought to be specifically stress-related. In our study these default mode regions include the medial PFC and ACC, PCC, and medial and lateral parietal areas. However, other stress sensitive regions identified in the current and previous (Pruessner et al., 2008) studies are not typically considered part of the default mode network, namely the medial temporal lobe (amygdala and hippocampus), nucleus accumbens, subgenual ACC, BNST, and hypothalamus. We propose that deactivations in these limbic structures index stress per se, as opposed to focused attention on a task. In previous work, limbic deactivations were greater in stress responders than non-responders and proportional in size to cortisol release during the task (Pruessner et al., 2008). This is in distinction to deactivations in the default mode network, which usually depend on cognitive load (McKiernan et al., 2003). When we extracted effect sizes from the anatomically defined hippocampus, amygdala, and nucleus accumbens regions there was a large and statistically significant difference between the stress and non-stress versions of the task. We therefore used these deactivations as an indicator of the brain response to stress and showed that they predicted the magnitude of the subsequent neural response to the smoking cues.
Reductions in BOLD signal almost certainly imply concomitant reductions in regional neuronal activity (Devor et al., 2007; Shmuel et al., 2006). Reduced BOLD is caused by a decrease in the fraction of oxygenated hemoglobin, which is in turn typically related to reduced regional cerebral blood flow. Although there are other potential mechanisms of BOLD reduction, such as increased oxygen extraction and vascular steal, they are unlikely here (Shmuel et al., 2006). Moreover, direct measurements of cerebral blood flow with positron emission tomography have demonstrated similar reductions in limbic regions during the MIST (Pruessner et al., 2008). Reductions in BOLD are thought to index inhibitory postsynaptic potentials and neuronal inhibition, rather than changes in neuronal spiking (Devor et al., 2007; Shmuel et al., 2006).
However, it is not clear what neurochemical events are responsible for the deactivations seen in this stress paradigm. Dopamine may be involved: we have shown on two occasions that the MIST is associated with dopamine release in the ventral striatum (Pruessner et al., 2004; Soliman et al., 2008), as measured by [11C]raclopride positron emission tomography (a method that only allows measurements dopamine release in the striatum). Reductions in regional cerebral blood flow have been observed following dopamine D2 agonism in the striatum (Choi et al., 2006). More recently, painful sensory stimulation in anesthesized rodents was shown to cause bilateral reductions in striatal blood flow via a dopamine D2 receptor dependent mechanism (Shih et al., 2009). On the other hand, CRF, another neurotransmitter implicated in the stress response, causes increased inhibitory postsynaptic potentials (Koob, 2008), which are known to lead to reductions in BOLD signal (Devor et al., 2007).
Pruessner et al. (2008) have argued that hippocampal deactivation in the MIST represents a specific component of the stress response, leading to ACTH secretion via the disinhibition of the paraventricular nucleus of the hypothalamus. The extended amygdala, which includes the BNST and nucleus accumbens, was also deactivated during stress. It is thought to be a node connecting stress-regulating areas (hippocampus, amygdala, and prelimbic medial PFC) to stress effector sites such as hypothalamus and mesolimbic dopamine neurons (Herman et al., 2005).
As in our previous study (McBride et al., 2006), smoking videos, when compared to neutral videos, activated medial PFC, dorsomedial thalamus, precuneus and numerous visual areas including primary visual cortex. This activation was greater following stress and proportional to the neural response to the preceding stressor. We previously speculated that this pattern of activation reflected top-down visual attention, and was indicative of the saliency of the smoking cues. In our previous study a cognitive manipulation had a similar effect on the attentional response to smoking cues as stress did here (McBride et al., 2006): fore-knowledge of an immediately available cigarette increased neural response to the cues in medial PFC and ACC, OFC, dorsomedial thalamus, precuneus, and association and primary visual areas. The main difference is that mental stress here additionally affected cue-reactivity in medial temporal and striatal areas. It may be that the cognitive manipulation in our previous study only enhanced the neural response in areas involved in attentional control, while psychosocial stress additionally affected regions involved in motivation. A direct comparison of the two data sets is not possible however as different subjects and MRI equipment were used.
Most imaging cue reactivity studies use simple cues such as those used here, and trigger modest increases in craving. It is of interest then that a recent fMRI study using very appetitive cues that combined the sight, feel and smell of a cigarette with suggestive audio scripts demonstrated cue-responses in the regions modulated by stress in our experiment: the amygdala, hippocampus, nucleus accumbens, dorsomedial thalamus, and OFC (Franklin et al., 2007).
Numerous animal studies have demonstrated a causal link between stress response, especially in the extended amygdala, and subsequent cue reactivity and drug self-administration (Koob, 2008; Pecina et al., 2006; Shaham et al., 2003; Zislis et al., 2007). Mesolimbic dopamine stimulation could account for the increased cue reactivity following stress. In animal models, stress-induced dopamine release correlates with subsequent drug seeking (Shaham and Stewart, 1995), and in humans, dopamine gene polymorphisms that likely influence dopamine release predict stress-induced drug craving (Erblich et al., 2004). Drug-associated cues also release dopamine, a phenomenon that seems to directly promote drug seeking (Phillips et al., 2003). This would suggest that drug cues and stress act similarly on overlapping brain systems to promote appetitive behaviors, a notion that is somewhat at odds with negative reinforcement models of stress and addiction. It must be noted, however, that other neurotransmitters have also been implicated in the stress effects on drug seeking, most notably CRF and noradrenaline. CRF antagonists more reliably block stress-induced reinstatement than dopamine antagonists, while the latter are better at blocking drug cue induced reinstatement (Shaham et al., 2003). Indeed, with respect to nicotine, stress (e.g. footshock) appears to mediate reinstatement of self-administration via the CRF system (Zislis et al., 2007). It is possible then that stress and conditioned cues act on different neural systems to promote drug seeking, but that their effects are additive.
Nicotine cue-induced activation of the amygdala, hippocampus, and ventromedial PFC was enhanced by stress (Table 3). The hippocampus and ventromedial PFC were implicated in a recent meta-analysis, which showed that pre-scan negative affect predicted cue reactivity in both regions (McClernon et al., 2008). As stated above, one view of addiction is that stress acts as a negative reinforcer, whose unpleasant effects are alleviated by drug intake (Koob, 2008). However, the ACC, OFC and amygdala, along with the extended amygdala and nucleus accumbens, also play a central role in the positive aspects of appetitive behaviors such as feeding and drug taking (Cardinal et al., 2002). For example, fMRI activation of the amygdala in response to food cues is a consistent marker of desire to eat (Malik et al., 2008). Several cue reactivity imaging studies have linked activation of striatum, amygdala, and subgebual anterior cingulate cortex to drug craving (Childress et al., 1999), and behavioral therapy leading to smoking cessation was found to reduce the fMRI cue response in the amygdala (McClernon et al., 2007). It may be that stress has directly incentive properties. Indeed, in a recent study, CRF injection into the nucleus accumbens was shown to increase the incentive salience of sucrose cues in a Pavlovian to instrumental transfer task (Pecina et al., 2006). In this study, the CRF effects could not be explained by a negative reinforcement model, as the injections were never paired with sucrose reward.
Subjective desire to smoke increased throughout our procedure, confirming that the stimuli used here promote craving. There was however no difference between the stress and non-stress sessions. This may be because of ceiling effects in subjective questionnaires, or because drug cues and stress both promote subjective craving, but not synergistically. The lack of difference in cortisol between the stress and non-stress sessions may be due to the small number of participants, and to the fact that exposure to drug cues also promotes cortisol secretion (Sinha et al., 2003), which might have obscured the stress effect on cortisol.
Experimental Procedure
Subjects
Fifteen healthy regular smokers (7 females), all right handed, 18 to 40 years of age, were recruited through an advertisement posted in the local classified ads. Interested participants were pre-screened with a detailed telephone interview. In order to be included, participants had to smoke an average of 12 cigarettes per day and score at least 5 out of 10 on the Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991). Subjects gave written consent and all procedures were approved by the research ethics boards of the Institut Universitaire de Gériatrie de Montréal and the Montreal Neurological Institute. Exclusion criteria included a history of neurological or psychological illness, previous head trauma, metal in the body, prescription drug use (with the exception of oral contraceptives), and history of drug abuse (other than tobacco).
All subjects were scanned on two occasions, at least one week apart, in the middle of the afternoon (between 1 and 4 pm), and were told to smoke at their usual rate the day of the scan. Females were scanned at the same point of their menstrual cycle. On arrival to the imaging unit they had one cigarette (approximately one hour before fMRI) under the observation of one of the investigators. We had previously found that a non-abstinent state was associated with greater neural cue-reactivity using this paradigm (McBride et al., 2006). Each subject underwent two sessions, stress and non-stress, in counter-balanced order, without foreknowledge. Subjects were not told that the sessions were stress or non-stress, but that they would perform mental arithmetic tasks on both days. The paradigm is illustrated in Fig. 1.
Psychosocial Stress Paradigm
We used the MIST to induce psychosocial stress while subjects were in the fMRI scanner. This task, which is based on the Trier Mental Challenge Task (Kirschbaum et al., 1993), has been extensively described elsewhere (Dedovic et al., 2005). Stress is induced by having subjects solve difficult mental arithmetic problems presented via a computer. The key aspects of psychosocial stress, uncontrollability and social-evaluative threat, are induced by consistently adjusting the time limit on the problems to ensure a 55% failure rate, by having a continuous performance progress bar on the computer monitor, and by direct negative feedback from one of the investigators in between task blocks. The non-stress control task consists of similar mental arithmetic problems given without time constraint, progress bar, or investigator feedback. Importantly, the same number of problems is given in each block of stress and non-stress control.
For their two sessions, subjects performed either the stress or the non-stress arithmetic task. They performed three blocks of 6 minutes each. Each block consisted of 60 seconds of rest (while viewing the task interface), 120 seconds of mental arithmetic, 60 seconds of rest, and another 120 seconds of arithmetic. In between the blocks, subjects received the negative feedback (stress session only). Thus subjects performed mental arithmetic for a total of 12 minutes, and the MIST lasted 18 minutes. Throughout the fMRI session saliva samples were collected through the use of small sponges (salivettes) placed briefly in the subjects’ oral cavities. These were analyzed for cortisol concentration (µg/dl) as previously described (Pruessner et al., 2004; Pruessner et al., 2008). A total of 8 samples were collected from start to finish of the scanning session, 4 before the MIST and 4 after.
Cigarette Cue Paradigm
We used the same smoking and control cues as in our previous study (McBride et al., 2006). The smoking videos consisted of young men and women smoking in typical social settings such as bars. Control videos depicted young people getting their hair cut. Different but very similar video clips were shown during the two sessions and all subjects saw the same video clips. Videos were similar in degree of facial exposure, movement and physical characteristics of the actors. Stimulus presentation and response input were coordinated using Media Control Function software (Digivox, Montreal). Visual stimuli in video format were projected onto a screen at the foot of the scanner bed, and viewed by means of a mirror mounted on the MRI head coil. Eight two-minute video clips, alternating between smoking (S) and control (C) content, were presented in the following order: C-S prior to the MIST (stress or non-stress versions) and C-S-S-C-C-S after the MIST. Cigarette craving was assessed at the start of the imaging session and after each video with a three item questionnaire taken from a larger battery (Tiffany and Drobes, 1991). Subjects answered questions, such as “I am craving a cigarette right now”, using a computer mouse to slide a cursor along a visual analog scale.
Functional Magnetic Resonance Imaging and Analysis
Subjects underwent fMRI scanning using a 3T Siemens (Erlangen, Germany) Magnetom Trio MRI scanner at the Institut Universitaire de Gériatrie de Montréal. Each scanning session began with a high-resolution, T1-weighted, three-dimensional volume acquisition for anatomical localization (voxel size of 1mm3). This was followed by acquisition of functional data: one 5-min run for the initial C-S cue paradigm, one 18-min run for the MIST and three 5-min runs for the C-S S-C C-S cue paradigm. The fMRI images were acquired with an echo-planar T2*-weighted scans for the contrast of blood oxygenation level-dependent (BOLD) signals (TR=2.5sec; TE=30 ms; flip angle, 90°; matrix size=64×64; voxel size=3.4×3.4×3.4mm3, number of slices=40).
Anatomical data were transformed into Montreal Neurological Institute (MNI) space (Collins et al., 1994) using the ICBM152 template. The BOLD data were analyzed using fmristat (Worsley et al., 2002). First a general linear model (GLM) was applied to each run of the MIST and smoking cue acquisitions. For the MIST we defined two events: math task and rest. For the cue reactivity study we defined smoking videos, control videos (haircut), and blank screen. The contrasts of interest were task minus rest for the MIST and smoking minus haircut for cue reactivity. The blocks were analyzed separately. This first step used a fixed-effects GLM to generate effect and standard deviation (SD) images for each run for each subject and session. The effect and SD images were then transformed into MNI space using the previously calculated linear transformation. In a second step, subject data were combined using a mixed-effects GLM, which allowed the generation of effect and T-statistic maps. Data were corrected for multiple comparisons taking into account the height and extent of statistical peaks from the t-maps (Poline et al., 1997). For these analyses, data are presented at a p value of less than 0.05 corrected. The anatomical location of peaks was ascertained with the atlases of Talairach and Tournoux (Talairach and Tournoux, 1988) and Mai et al. (Mai et al., 2003).
We took deactivation in the hippocampus, amygdala, and nucleus accumbens during the mental challenge task as an index of stress effects, based on previous work (Pruessner et al., 2008). We therefore extracted the effect sizes from the GLM using regions of interest for these three structures. These regions were defined on the anatomical MRI, independently of the functional data, using the brain segmentation program ANIMAL (Collins et al., 1995). Each individual’s anatomical MRI was non-linearly registered to the ICBM152 non-linear 6th generation target (Grabner et al., 2006). Then, digital anatomical atlases were used for identifying the amygdala and hippocampus (Grabner et al., 2006) and nucleus accumbens (Chakravarty et al., 2006). These anatomical regions were defined individually for each subject and then applied to the effect size maps generated by fmristat for each session (stress and non-stress). Thus for each session we obtained an index of deactivation in each of the three regions of interest. We then entered this data as a covariate in a GLM to identify brain regions where activation to smoking cues minus neutral cues correlated with deactivation during stress. These results are presented for each session at a threshold of p < 0.001 uncorrected (minimum of 20 contiguous activated voxels).
Supplementary Material
Acknowledgements
We acknowledge funding from the Canadian Tobacco Control Research Initiative, the Canadian Institutes for Health Research, the National Institute on Drug Abuse, and the Fonds de la Recherche en Santédu Québec.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: two studies of discriminant validity. Behav Res Ther. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage. 2006;30:359–376. doi: 10.1016/j.neuroimage.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 2005;30:319–325. [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenomics J. 2004;4:102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2006;9:58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Assheuer JK. Atlas of the Human Brain, Vol. San Diego: Academic Press; 2003. [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C. A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci. 2009;29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Soliman A, O'Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology. 2008;33:2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, Rosenman RH. Risk factors for late relapse in male and female ex-smokers. Addict Behav. 1988;13:253–266. doi: 10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain, Vol., Thieme, Stuttgart. 1988 [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





