Abstract
Upper airway obstruction during sleep can trigger compensatory neuromuscular responses and/or prolong inspiration in order to maintain adequate minute ventilation. The aim of this study was to investigate the strength of these compensatory responses during upper airway obstruction during propofol anesthesia. We assessed respiratory timing and upper airway responses to decreases in nasal pressure in nine propofol anesthetized normal subjects under condition of decreased (passive) and increased (active) neuromuscular activity. Critical closing pressure (PCRIT) and upstream resistance (RUS) were derived from pressure-flow relationships generated from each condition. The inspiratory duty cycle (IDC), maximum inspiratory flow(V̇I max) and respiratory rate (f)were determined at two levels of mean inspiratory airflow (V̄I; mild airflow limitation with V̄I ≥ 150ml s−1; severe airflow limitation with V̄I < 150ml s−1). Compared to the passive condition, PCRIT decreased significantly (5.3 ± 3.8 cm H2O, p < 0.05) and RUS increased (7.4 cm H2O ml−1 s, p < 0.05) in the active condition. The IDC increased progressively and comparably as V̄I decreased in both the passive and active conditions (p < 0.05). These findings imply that distinct compensatory mechanisms govern the modulation of respiratory pattern and pharyngeal patency during periods of airway obstruction under propofol anesthesia.
Keywords: Propofol anesthesia, Critical closing pressure, Inspiratory duty cycle, Upper airway, Obstructive sleep apnea
1. Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive obstruction of the upper airway that results in recurrent hypoxemia and arousal from sleep and increases in cardiovascular morbidity and mortality (Remmers et al., 1978; Malhotra and White, 2002). Upper airway obstruction during sleep can result from alterations in either the passive structural pharyngeal properties or from disturbances in neuromuscular control. Methods for quantifying the contribution of anatomic alterations have been established in both sleeping and anesthetized subjects (Isono et al., 1997; Eastwood et al., 2002a; Patil et al., 2007), while those for assessing active neuromuscular responses to upper airway obstruction have been limited to sleeping subjects (Schwartz et al., 1988; Smith et al., 1988; Patil et al., 2007).
When the upper airway first obstructs, the upper airway musculature remains in a relatively hypotonic or passive state (Schwartz et al., 1998; Patil et al., 2004, 2007). Initially, mechanoreceptor activity in airway pressure receptors and pulmonary stretch receptors can produce immediate alterations in respiratory timing in experimental animals and sleeping humans. Recent evidence suggests that a prolongation of the inspiratory duty cycle can help stabilize ventilation during periods of upper airway obstruction (Tagaito et al., 2002; Schneider et al., in press). Thereafter, upper airway obstruction can elicit compensatory neural responses that can mitigate the obstruction during spontaneous breathing in sleeping and anesthetized subjects. As upper airway obstruction persists, disturbances in gas exchange ensue, leading to increases in upper airway neuromuscular activity, improvements in airway patency and greater ventilatory stability (active state) (Schwartz et al., 1993; Seelagy et al., 1994; Rowley et al., 1997; Patil et al., 2007; McGinley et al., 2008). When compensatory mechanisms are inadequate to stabilize ventilation, upper airway obstruction often terminates in an arousal from sleep and the prompt restoration of upper airway patency (Younes, 2004). Arousals can therefore interfere with the assessment of compensatory upper airway and respiratory timing responses.
We have previously reported that passive measurements of upper airway collapsibility in sedated subjects were similar to non-REM sleep (Ayuse et al., 2004; Inazawa et al., 2005; Ikeda et al., 2006). Active compensatory neuromuscular responses, however, have not been characterized in anesthetized subjects. We hypothesized that compensatory neuromuscular responses can be readily characterized under anesthesia and that these responses increase progressively with the severity and duration of upper airway obstruction. To address this hypothesis, we examined passive and active upper airway properties and timing responses to acute and sustained periods airflow obstruction under propofol anesthesia, respectively.
2. Methods
2.1. Subjects
Upper airway pressure-flow relationships were assessed under propofol anesthesia in nine subjects (male 4, female 5), who were of 22.2 ± 3.0 years of age and had a mean body weight of 56.0 ± 9.6 kg, height of 1.60 ± 0.1m and body mass index (BMI) of 20.9 ± 2.6 kg/m2. Subjects who reported a history or snoring or anatomical deformation in upper airway (e.g., deviated septum, retrognathia) were excluded from this study. The protocol was approved by the Human Investigation Committee of the Nagasaki University School of Dentistry and written informed consent was obtained from all subjects.
2.2. Experimental procedures and monitoring
2.2.1. Polysomnographic and anesthetic monitoring
All subjects underwent routine hemodynamic monitoring (systolic and diastolic blood pressure as well as pulse rate) and electrocardiogram (ECG), polysomnographic monitoring of sleep, with bilateral electro-oculograms (EOG; left and right), electroencephalograms (EEG; C3-A2 and O2-A1) and submental electromyogram (EMG) to confirm anesthetic level. EEG signals were processed by the BIS monitor (Aspect Medical Systems, Inc., Natick, MA) in order to determine the depth of anesthesia. Oxygen saturation was measured by pulse oximetry (SpO2) and transcutaneous PCO2 (TcCO2) was measured by transcutaneous CO2 analyser (Tina, TCM4, Radiometer, Tokyo, Japan).
2.2.2. Respiratory measurements
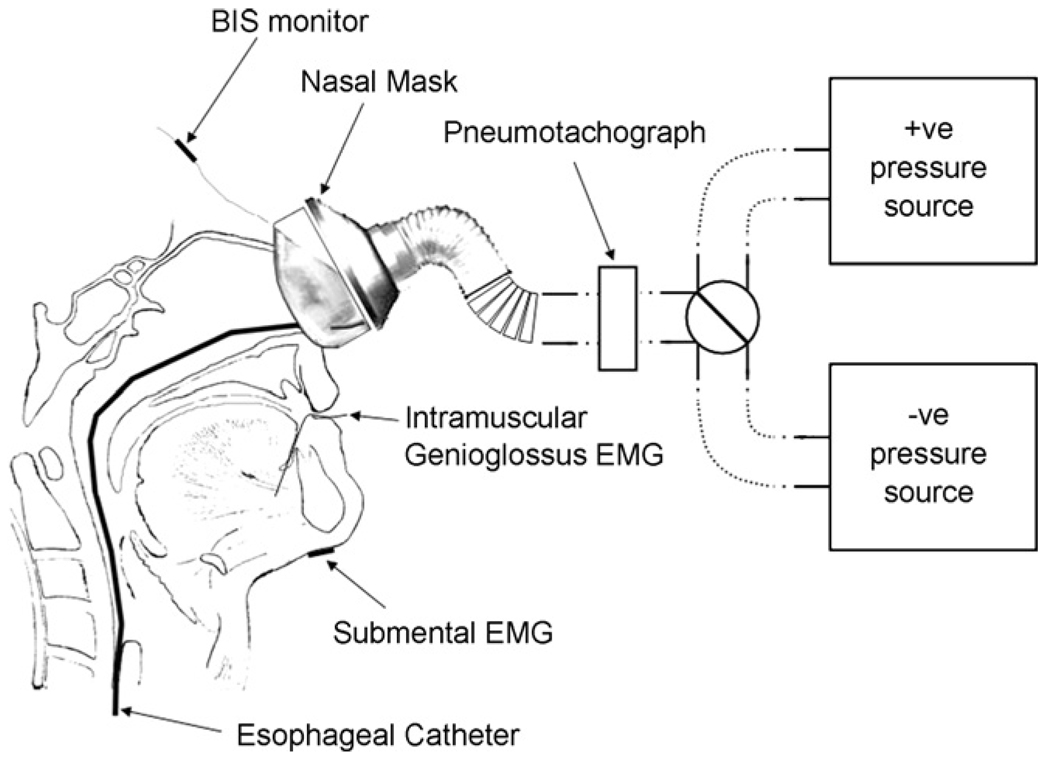
Thoracic and abdominal movements were also measured using inductance plethysmography. Esophageal pressure (PESO) was determined from a Hyatt-type esophageal balloon (Ackrad laboratories, Cranford, NJ, USA) where the tip of catheter was positioned ~45–50 cm from nares. Airflow (V̄) was measured using a pneumotachometer (model 3830, Hans Rudolph, Inc., Kansas City, MO, USA) and nasal pressure (PN) measured using a differential pressure transducer (model 1100, Hans Rudolph, Inc., Kansas City, MO, USA). The outflow from this valve was then connected in series to the pneumotachometer and nasal mask (Fig. 1). Air leaks from the mouth were prevented by applying surgical tape across the lips. The changes in PN were able to be made utilizing a pressure generator (MAP/ResMed, Martinsreid, Germany) operating over a −15 to +15 cm H2O range connected to the nasal mask. All the measurements were displayed and recorded simultaneously on a computer using data acquisition device (either Embla S7000 or A-10,Medcare, NY, USA).
Fig. 1.
Diagram of the experimental setup. A nasal mask attached to a pneumotachograph is connected via tubing to either a positive (+ve) or negative (−ve) pressure source. Genioglossus electromyography (EMGGG) was recorded from fine wire intramuscular electrodes positioned percutaneously. Respiratory effort was determined by an esophageal pressure transducer tipped catheter was inserted via the nares. Bispectral Index (BIS) monitor, electroencephalographic leads and submental surface EMG were recorded to monitor state of sedation.
2.2.3. Genioglossus EMG (EMGGG) measurements
Genioglossus EMG activity was recorded and analyzed utilizing previously described methods (Mezzanotte et al., 1992). Briefly, EMGGG was measured by using a pair of unipolar percutaneous intramuscular electrodes referenced to a single ground, thus producing a bipolar recording. Tonic EMGGG (lowest integrated genioglossus EMG activity value during expiration) was defined as the difference between electrical zero and minimum level near end-expiration. Phasic EMGGG was defined as the difference between the end-expiratory value and peak-inspiratory EMGGG (at V̇I max). The EMGGG measurements made for the “activation phase” were made at the Pn where sustained flow limitation was induced for 2 min. Measurements were expressed as a percent change from the maximal value obtained during voluntary tongue protrusion and swallows prior to anesthesia.
2.3. Experimental protocols
2.3.1. Propofol anesthesia
No premedication was given. Propofol anesthesia was induced with intravenous propofol (Dipriva; Astra Zeneca, Alderley Park, Cheshire, United Kingdom), administered via a Diprifusor (Astra Zeneca) target-controlled infusion system (Terumo TCI pump TE371, Tokyo, Japan), which calculated effect site concentration on the basis of a three-compartment pharmacokinetic algorithm (Marsh et al., 1991; Coetzee et al., 1995; Eastwood et al., 2005). The propofol target blood concentration was increased and kept constant between 1.5 and 2.0 µg/ml to obtain adequate level of anesthesia. We required the subject’s BIS value be between 40 and 60 in order to obtain an adequate level of anesthesia. At the conclusion of the measurements, all the subjects were kept in the supine position until they showed a spontaneous emergence from anesthesia.
2.3.2. Assessing passive and active upper airway and timing responses
Specific experimental paradigms for manipulating the PN were used to assess passive and active upper airway and timing characteristics during anesthesia (Patil et al., 2007; McGinley et al., 2008). During these protocols, the subject’s head was positioned in the Frankfurt plane and the mandible in the occluded neutral position. At least two series of passive and active PCRIT measurements were conducted in random order over an approximately 10min interval.
2.3.2.1. Passive PCRIT
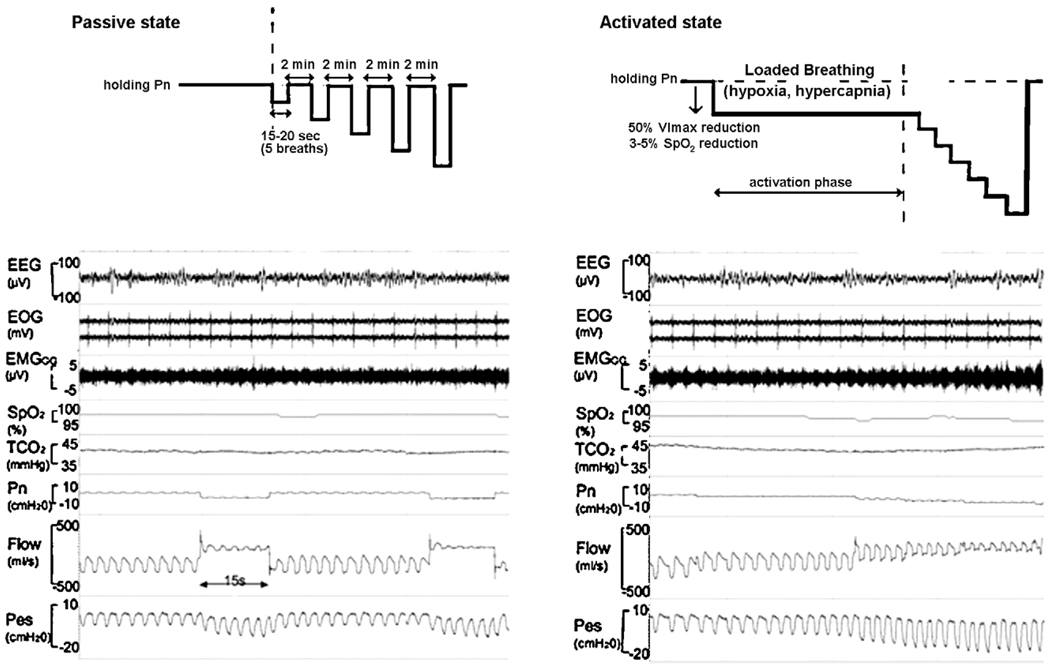
Following the establishment of an adequate level of stable anesthesia, the subjects were initially allowed to breathe under atmospheric pressure. PN was then gradually increased to a holding pressure where inspiratory airflow limitation was abolished (“passive state”), as previously described (Schwartz et al., 1998; Boudewyns et al., 2000). To establish the passive PCRIT, PN was rapidly lowered from the holding pressure to specific levels for five successive breaths before returning to the holding pressure (Fig. 2). Nasal pressure levels traversed a range of pressures encompassing zero flow (airway occlusion). Passive pressure-flow relationships were generated from at least two series of pressure ‘drops’ over this range.
Fig. 2.
A schematic of the experimental protocol for producing upper airflow obstruction in the passive state (shown at the left) and in the active state (shown at the right). The polysomnographic recording in a one subject in passive and active condition is shown electroencepalogram (EEG), electroocculograms (EOG), intramuscular genioglossus electromyogram (EMGGG), oxyhaemoglobin saturation (SpO2), transcutaneous carbon dioxide (TcCO2), nasal mask pressure (PN), pnuemotach airflow (Flow), and eosophageal pressure (PES).
In the passive condition, PN is abruptly reduced from an elevated holding pressure to a level that induced airflow limitation (plateau in airflow with increasing esophageal pressure). Note that the drop in PN from holding pressure to lower pressure levels decreases the flow.
In the active condition, a stable unobstructed breathing pattern was initially maintained at a positive holding pressure. Thereafter, PN was lowered by 2 cm H2O steps until a quasi-steady state flow limited breathing pattern associated with a 40–50% reductions in V̄I max (“activation phase”) was achieved. This level of airflow obstruction was maintained for 2 min during which genioglossal EMG activity rose, and SpO2 fell by 3–5% and TcCO2 increased by 5–10 mmHg above baseline (accounting for a 5–10s delay in TcCO2). Subsequently, PN was lowered in a stepwise fashion by 2 cm H2O every 5 breaths until zero flow was obtained or SpO2 reached a lower limit of 88–90%.
2.3.2.2. Active PCRIT
Nasal pressure was lowered step-wise to load the ventilatory control system, utilizing a modification of a previously described protocol (Patil et al., 2007; McGinley et al., 2008). In brief, a stable unobstructed breathing pattern was initially maintained at a positive holding pressure. Thereafter, PN was lowered by 2 cm H2O steps until a quasi-steady state flow limited breathing pattern was achieved in association with a 40–50% reductions in V̄I max (“activation phase”). This level of airflow obstruction was maintained for 2 min during which genioglossal EMG activity rose, the SpO2 fell by 3–5% and the TcCO2 increased by 5–10 mmHg above baseline (accounting for a 5–10 s delay in TcCO2). Subsequently, PN was lowered in a stepwise fashion by 2 cm H2O every 5 breaths until zero flow was obtained or SpO2 reached a lower limit of 88–90% (Fig. 2).
2.3.3. Severity of upper airway obstruction
All of the respiratory parameters were obtained from non-flow limited breaths at baseline and two levels of V̄I obtained from breaths during the passive and active conditions. The severity of upper airway obstruction was established based on the specific ranges of V̄I above and below a clinically and physiologically relevant cut-off of 150 ml s−1. When V̄I is below this cut-off, upper airway obstruction is known to lead to periodic obstructive hypopneas, whereas breathing patterns stabilize when V̄I exceeds this cut-off (Gleadhill et al., 1991). Mild flow limitation was therefore defined by a V̇I max of greater than 150 ml s−1 and severe flow limitation was defined as a V̇I max between 25 and 150 ml s−1.
2.4. Data analysis
2.4.1. Upper airway pressure-flow relationship
At each level of PN, breaths were evaluated for the presence of inspiratory airflow limitation, as previously described (Schwartz et al., 1988, 1989; Boudewyns et al., 2000; Ayuse et al., 2004). Inspiratory flow limitation was defined as the presence of a plateau in inspiratory airflow in association with a continued fall in esophageal pressure by at least 1 cm H2O beyond the onset of the plateau. Breaths that were associated with arousal were excluded from analysis. Non-flow limited breaths from the baseline condition at holding pressure were analyzed to determine the peak inspiratory airflow (V̄I peak) at baseline. Maximal inspiratory flow(V̇I max) and the corresponding PN were obtained for each flow limited breath in the obstructed conditions. Least-squares linear regression was used to generate the pressure-flow relationship (Gold and Schwartz, 1996), and fit by the following equation:
| (1) |
where PCRIT is the critical closing pressure (PN at zero flow) and RUS is the resistance of the portion of the tube upstream to the site of collapse. The difference between the active PCRIT and passive PCRIT (ΔPCRITA-P) was considered a measure of the strength of dynamic neuromuscular responses to upper airway obstruction.
2.4.2. Respiratory parameters (f, V̄E, IDC, V̄Ipeak, V̇I max, V̄I,VT, V̄ALV)
The respiratory parameters were obtained in baseline and two different level of flow limitation. The respiratory parameters evaluated were as follows: respiratory rate (f), minute ventilation (V̄E), inspiratory duty cycle (IDC; IDC = TI/TTOT) where TI is the duration of inspiration and TTOTAL is the duration of the inspiration and expiration; peak inspiratory airflow (V̄Ipeak) during non-flow limited breathing; maximum inspiratory airflow (V̇I max) during airflow limited breathing; mean inspiratory airflow (V̄I = VT/TI) where VT is the tidal volume.
Dead space volume (VDS) was calculated with the formula, 24 × height2 (in.)/703 ml. Alveolar ventilation (V̄ALV)was calculated by subtracting dead space ventilation, which is the product of dead space volume (VDS) and respiratory frequency (f), from minute ventilation, as shown in the following equation:
| (2) |
2.5. Statistical analysis
All statistical analyses were performed using SPSS version 16.0 (SPSS, Inc., USA). The differences between the active and passive PCRIT (ΔPCRITA-P), the passive PCRIT, the active PCRIT, RUS, and tonic and peak phasic EMGGG were statistically analyzed. The effects of the time course of the responses in IDC, V̇I max, I, V̄E, f, VT, V̄ALV were analyzed using General Linear Model for repeated measures with a post hoc protected Bonferroni adjustment test. Differences between the passive and active RUS, tonic and peak phasic EMGGG were assessed with by Mann–Whitney U- and paired T-tests. Statistical significance was assumed for p < 0.05. Results are presented as mean ± standard deviation (SD) unless otherwise noted.
3. Results
3.1. Effect of propofol anesthesia on consciousness and breathing parameters
During stable awake conditions before administration of propofol, f was 17.2 ± 1.0 breaths min−1, VT was 310.6 ± 73.5 ml, V̄E was 5503 ± 1141 ml min−1, and IDC was 0.39 ± 0.05. The mean target blood concentration of propofol was 1.54 ± 0.23 µg/ml, which produced adequate anesthesia, and a BIS score 52.5 ± 4.5. For both the passive and active states, there was no significant difference in BIS score (51.4 ± 6.5 and 53.7±5.3, respectively; p = 0.33). The EEG power spectrum was predominantly comprised of frequencies between 0.5 and 4 Hz, similar to that of natural non-REM sleep stages 3–4. The average holding pressure required to abolish flow limitation in all subjects was 3.4 ± 2.6 cm H2O. During stable anesthesia at the baseline holding pressure, the f increased slightly to 19.1 ± 2.1 breaths min−1 (p < 0.05), VT decreased to 281.6 ± 61.1ml (p < 0.05) and V̄E decreased to 5362 ± 1071 ml min−1 (p < 0.05), whereas the IDC remained constant (0.40 ± 0.04; p = 0.17)compared to quiet wakefulness.
3.2. The effect of passive and active condition
3.2.1. Passive and active EMGGG responses
TcCO2 did not differ significantly between the baseline and passive state (39.7 ± 3.7 and 41.7 ± 4.2 mmHg, respectively). In contrast, TcCO2 increased from baseline (39.8 ± 3.6mmHg) to the active condition (44.7 ± 4.8mmHg; p < 0.05). Similarly, there was no change in SpO2 for the passive state, whereas the lowest value of SpO2 fell from a baseline of 97.2 ± 0.9% to 91.3 ± 1.5% in the active condition (p < 0.05). Tonic EMGGG activity did not differ between the baseline and the passive state (5.7 ± 4.2%), but increased in the active state (12.5 ± 11.3%; p < 0.05). Similarly, peak phasic EMGGG activity increased in the active state, relative to both the baseline and passive state (baseline, 4.9 ± 3.5%; passive state, 4.9 ± 3.5%; active state, 9.5 ± 8.4%; p < 0.05).
3.2.2. Passive and active upper airway responses
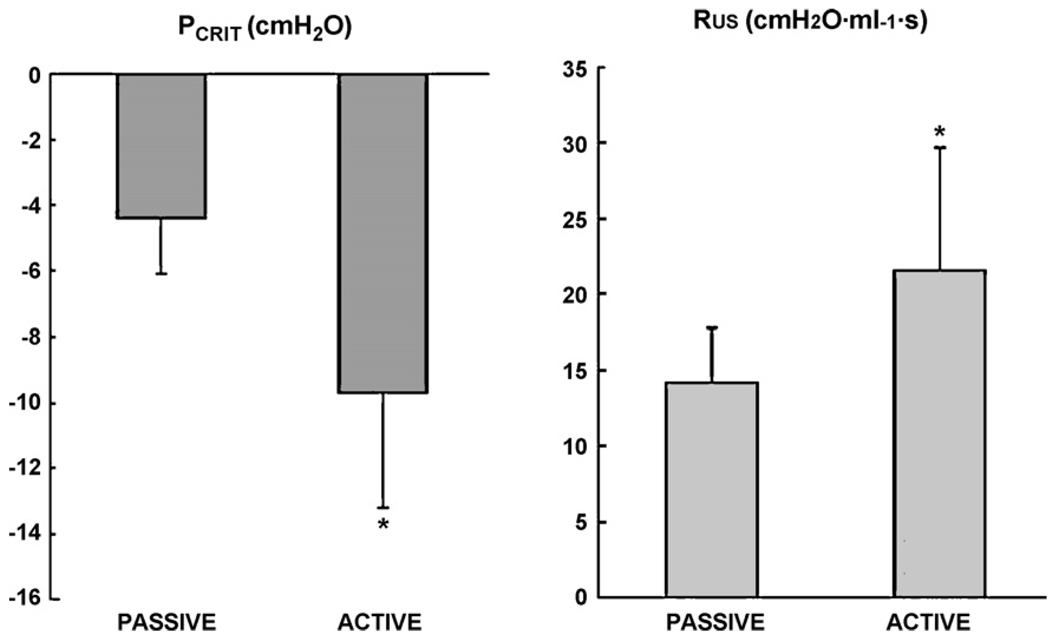
Passive PCRIT and active PCRIT values are shown up in Table 2 and Fig. 3. The mean passive PCRIT of −4.4 ± 1.7 cm H2O was significantly higher than the mean active PCRIT of −9.7 ± 3.5 cm H2O (p < 0.05). There was a significant difference between the passive RUS 14.2 ± 3.6 cm H2O ml−1 s and active RUS 21.6 ± 8.1 cm H2O ml−1 s (p = 0.02). ΔPCRITA-P was 5.3 ± 3.8 cm H2O (p = 0.003), suggesting a substantial neuromuscular responses to upper airway obstruction (Patil et al., 2007).
Table 2.
Individual upper airway characteristics of participants during propofol anesthesia.
| Passive |
Dynamic responses |
Active |
||
|---|---|---|---|---|
| PCRIT (cm H2O) | RUS (cm H2O ml−1 s) | ΔPCRITA-P (cm H2O) | PCRIT (cm H2O) | RUS (cm H2O ml−1 s) |
| −1.1 | 22.0 | 9.3 | −10.3 | 18.0 |
| −5.0 | 17.2 | 10.8 | −15.7 | 41.4 |
| −4.7 | 15.1 | 8.0 | −12.7 | 24.3 |
| −3.6 | 11.9 | 0.6 | −4.2 | 20.6 |
| −4.9 | 12.6 | 4.1 | −9.0 | 11.8 |
| −7.5 | 15.0 | 1.6 | −9.0 | 19.2 |
| −5.5 | 11.3 | 1.1 | −6.6 | 18.7 |
| −3.4 | 12.5 | 4.0 | −7.5 | 20.2 |
| −4.3 | 10.4 | 8.1 | −12.4 | 20.0 |
| −4.4 (1.7) | 14.2 (3.6) | 5.3 (3.8) | −9.7 (3.5)* | 21.6 (8.1)* |
Individual data for all subjects is presented in the table. Abbreviations for columns are as follows: critical closing pressure (PCRIT), upstream resistance (RUS), differences between passive and active PCRIT(ΔPCRITA-P).
p < 0.05 active vs. passive.
Fig. 3.
PCRIT and RUA for passive and active states. PCRIT decreased (*p < 0.05) whereas RUS increased (*p < 0.05) in the active state compared to passive state. These changes reflect an improvement in upper airway patency (see Section 4 for details).
3.2.3. Comparison of respiratory patterns between passive and active conditions
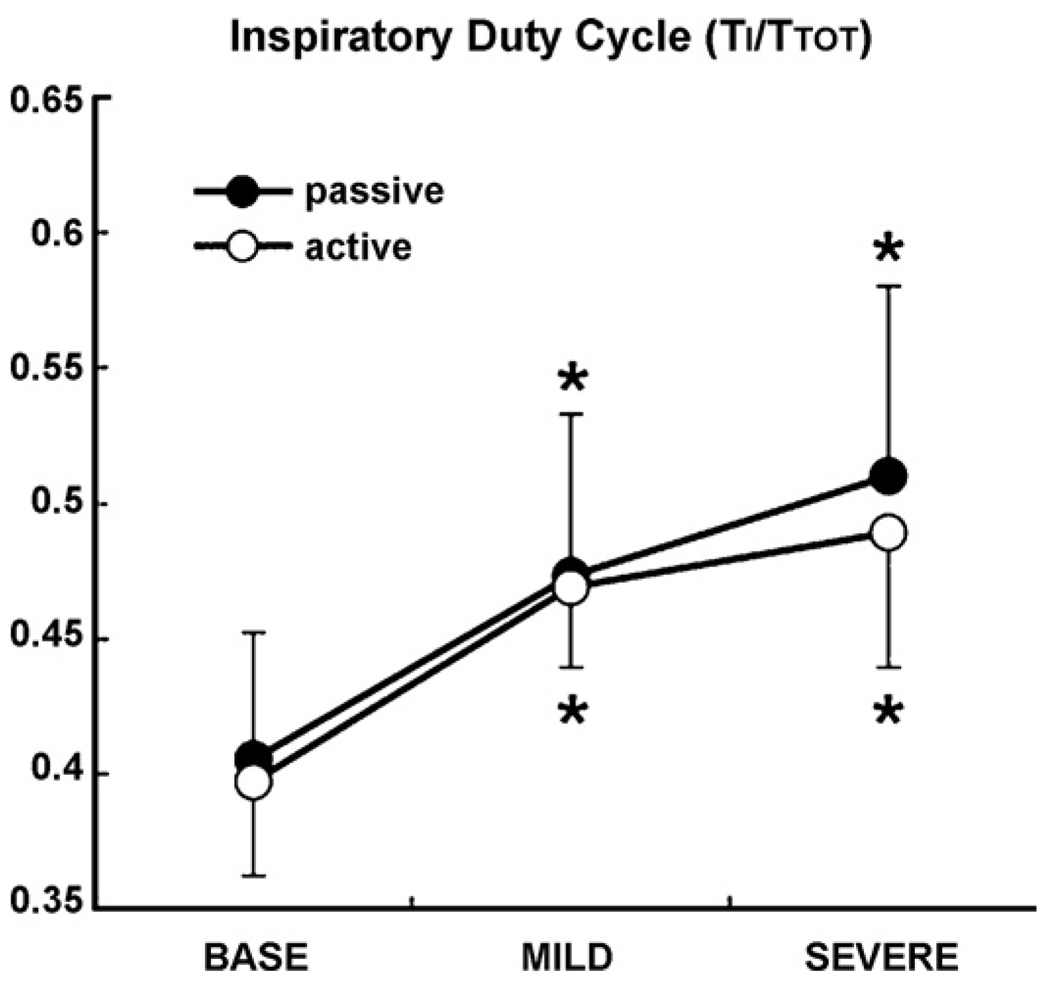
V̇I max, f, VT, V̄E, and V̄ALV are represented for each level of airflow obstruction (V̄I, baseline non-flow limited, mild obstruction and severe obstruction) in both passive and active conditions in Table 1. We observed that V̇I max decreased significantly in severe obstruction compared to V̇Ipeak in the baseline condition (p < 0.05), and compared flow-limited breaths during mild obstruction (p < 0.05) in both the passive and active conditions. VT and V̄E also decreased significantly (p < 0.05) in severe obstruction compared to in baseline condition in both passive and active conditions (p < 0.05). f did not change at different levels of airflow obstruction in the passive and active conditions. Alveolar ventilation V̄ALV significantly increased in mild obstruction in the passive and active compared to the baseline condition. Fig. 4 shows the change in IDC in different levels of V̄I in both the passive and active conditions. The IDC increased significantly as V̄I decreased in passive and active conditions, but did not differ significantly between passive and active conditions.
Table 1.
Respiratory pattern in different level of obstruction.
| Baseline | Passive |
Active |
|||
|---|---|---|---|---|---|
| Mild | Severe | Mild | Severe | ||
| PN (cm H2O) | 3.4 (2.6) | −1.4 (2.2)* | −3.7 (1.3)*,# | −4.3 (2.0)* | −8.2 (2.0)*,# |
| Upper airway | |||||
| V̇I max (ml s−1),V̇Ipeak (ml s−1) | 276.8 (50.7)a | 229.8 (13.5) | 87.1 (25.5)*# | 304.9 (63.5) | 91.0 (34.2)*,# |
| V̄I (ml s−1) | 222.6 (26.5) | 185.5 (12.7) | 60.1 (12.7)*,# | 247 (51) | 65.6 (28.8)*,# |
| Respiratory pattern | |||||
| f (breaths min−1) | 19 (2) | 20 (3) | 20 (3) | 19 (2) | 19 (3) |
| VT (ml) | 281.6 (61.1) | 265.8 (20.8) | 94.1 (34.7)* | 378 (77.9)* | 103.5 (47.7)* |
| Ventilation | |||||
| V̄E (ml min−1) | 4989 (1070) | 5076 (344) | 2206 (576)*,# | 7000 (2696) | 1901 (883)*,# |
| V̄ALV (ml min−1) | 2204 (997) | 1813 (513) | 0 | 3920 (1424)* | 0 |
Maximum inspiratory flow (V̇I max).
Peak inspiratory airflow(V̇Ipeak) refers to baseline non-flow limited condition only, mean inspiratory air flow(V̄I), respiratory rate (f), tidal volume (VT), minutes ventilation (V̄E), estimate of alveolar ventilation (V̄ALV).
p < 0.05 mild and severe vs. baseline.
p < 0.05 severe vs. mild.
Fig. 4.
IDC at three different level of airflow obstruction (baseline, mild and severe obstruction) in passive and active states. IDC significantly increased progressively in mild and severe obstruction compared to baseline (*p < 0.05 vs. baseline) in both passive and active conditions. There was no significant difference between passive and active condition.
4. Discussion
There were two major findings in our study. First, upper airway obstruction induced immediate progressive increases in the inspiratory duty cycle as the severity of airflow obstruction (V̄I) increased in the passive condition, an effect that persisted in the active condition. Second, upper airway obstruction induced significant increases in V̇I max in the active state compared to passive state. These increases in V̇I max were related to significant decreases in PCRIT from the passive to active condition, reflecting decreasing upper airway collapsibility over time. These decreases in PCRIT were also associated with significant increases in EMGGG activity, suggesting that neuromuscular activation mediated improvements in airway patency. As in our previous study in non-REM sleep (Patil et al., 2007), our findings suggest that compensatory responses in upper airway and timing parameters are well preserved under propofol anesthesia. They also suggest that immediate responses in the inspiratory duty cycle and time-dependent responses in V̄I determine the degree of ventilatory compensation to upper airway obstruction when arousal is suppressed pharmacologically.
4.1. Mechanical and neuromuscular control of upper airway patency
Our study was based on prior work demonstrating substantial increases in pharyngeal collapsibility (Patil et al., 2007) in spontaneously breathing patients during sedation and anesthesia (Drummond, 1996; Oshima et al., 1999). Although anesthesia and/or neuromuscular blockade can produce elevations in upper airway collapsibility (Isono et al., 1997; Eastwood et al., 2002a, 2005), we found that the passive PCRIT (−4.4 cm H2O) under propofol anesthesia was comparable to that found during natural non-REM stage 2 sleep (passive PCRIT =−4.5 cm H2O) in normal subjects (Patil et al., 2007; Kirkness et al., 2008). In contrast, our passive critical pressures were much more negative than that reported under isoflurane anesthesia (PCRIT = 1.2 cm H2O) (Eastwood et al., 2002b), which produced marked decreases in pharyngeal neuromuscular tone and patency. Therefore, it is likely that the collapsibility of the hypotonic pharynx is similar during propofol anesthesia to that observed during natural non-REM sleep.
As previously demonstrated in normal sleeping subjects, we also found a significant reduction in the active compared to passive PCRIT (−4.4 ± 1.7 cm H2O vs. −9.7 ± 3.5 cm H2O) under propofol anesthesia. The active PCRIT fell by 5.3 ± 3.8 cm H2O in the present study (ΔPCRITA-P) and was marginally less than that observed in non-REM sleep in normal individuals (ΔPCRITA-P, 6.9 ± 5.7 cm H2O) (Patil et al., 2007), indicating that dynamic neuromuscular responses during propofol anesthesia remain largely intact in normal anesthetized subjects as compared to natural non-REM sleep. Activation of upper airway dilator muscles (e.g., genioglossus) can mitigate the obstruction (Mezzanotte et al., 1992; Malhotra et al., 2000; Fogel et al., 2001). Despite depression of dilator activity under propofol anesthesia, dilator responses to sustained periods of upper airway obstruction were evident, and can account for increases in upper airway patency (increased in V̇I max) and for decreases in PCRIT. These responses could have also helped to maintain V̇I max in the mildly flow-limited active condition at levels that were comparable to V̇Ipeak levels observed at baseline (non-flow limited). Despite comparable levels of airflow, airflow became limited during sustained reductions in nasal pressure because negative tracheal pressure swings increased as ventilatory drive increased. RUS also increased in the active compared to passive condition, reflecting progressive decreases in intraluminal pressures (Schwartz et al., 1993; Rowley et al., 1997) and/or decreases in PCRIT caused by reductions in the level of nasal pressure (Schwartz et al., 1988; Smith et al., 1988). These findings imply that neuromuscular responses served to stabilize upper airway patency during sustained periods of airflow obstruction. The time course of these active responses is most consistent with chemo- rather than mechanoreceptor stimulation, which results from prolonged periods of upper airway obstruction and hypoventilation (Schwartz et al., 1993; Seelagy et al., 1994).
4.2. Inspiratory duty cycle responses to airflow limitation
During periods of inspiratory airflow limitation, inspiratory duty cycle (IDC) increased immediately and progressively as the severity of airflow obstruction increased. This prolongation of duty cycle occurred instantaneously, consistent with the time course to mechano- rather than chemo-reflex responses (Chow et al., 1994; Iber et al., 1995; Kimoff et al., 2001; Leevers et al., 1993; Dejours, 1962; Manchanda et al., 1996). This increase in inspiratory duty cycle (TI/TTOT) will act to restore ventilation at any given level of upper airway obstruction, as described by the relationship: V̄E = VT/TI × TI/TTOT, where VT/TI represents the mean inspiratory flow rate, which was varied experimentally with the severity of upper airway obstruction. In fact, IDC increased initially with the development of upper airway obstruction and decreased over time as obstruction abated (V̇I max rose) with the activation of pharyngeal neuromuscular responses (Tagaito et al., 2002; Schneider et al., 2003). Moreover, the IDC responses were superimposable in both passive and active conditions, suggesting that these responses are chiefly determined by the severity of upper airway obstruction (V̄), and independent of concomitant alterations in gas exchange over time (Tagaito et al., 2002; Schneider et al., 2003). These findings suggest that distinct physiologic mechanisms govern inspiratory duty cycle and upper airway responses to airway obstruction. The IDC response offers immediate relief from nocturnal hypoventilation during periods of upper airway obstruction during sleep (Schneider et al., 2003), and is well preserved during propofol anesthesia.
4.3. Methodological critique: study limitations
There were several limitations to consider in evaluating compensatory timing and pharyngeal responses to upper airway obstruction during propofol anesthesia. First, we could not produce maximum activation of dilator muscles including genioglossus muscle, because subjects’ exposure to hypoxemia was limited on ethical grounds. In OSA patients and anesthetized patients, obstruction may cause severe hypoxia and/or hypercapnia which were avoided by imposing a lower limit on the SpO2 at 90% for reasons of safety. This limitation may have attenuated the degree of activation during step-wise decreases in nasal pressure, leading to an underestimation of the full strength of the active response. Second, although propofol presumably exerted a direct suppressant effect on EMGGG activity, some degree of activation was undoubtedly present in the passive condition compared to that produced by neuromuscular blockade (Isono et al., 1997). Nevertheless, the main purpose for utilizing propofol anesthesia was to suppress arousal responses to upper airway obstruction in order to examine compensatory responses to acute and sustained upper airway obstruction. Thus, propofol anesthesia induced some degree of neuromuscular suppression in the passive state while enhancing our ability to examine dynamic upper airway and timing responses to obstruction.
4.4. Clinical implications
Our findings have two major clinical implications. First, they serve to characterize distinct upper airway and timing responses to airway obstruction that can stabilize ventilation during sleep. The preservation of compensatory neuromuscular responses in respiratory control (i.e., IDC to sustained upper airway obstruction during propofol anesthesia) suggests that upper airway patency can be restored during sleep even if arousal is pharmacologically suppressed (Younes, 2004; Jordan et al., 2007). In fact, Younes (2004) has suggested that if arousal can be prevented with sedative agents, these compensatory mechanisms should help to stabilize the breathing patterns during sleep in sleep apnea subjects. Our study further suggests that immediate responses in IDC can prevent a precipitous fall in ventilation during obstructive hypopneas (but not apneas), whereas more sustained periods of upper airway obstruction in the absence of arousal are required for the restoration of upper airway patency. Although the threshold to arousal responses and EMGGG activation is significantly depressed during propofol anesthesia, compensatory mechanisms may be still function to varying degrees. Our study implies that OSA can be mitigated by immediate timing responses and/or delayed upper airway responses, if they can restore ventilation prior to arousal or oxyhemoglobin desaturation.
Second, our findings establish that methods for assessing upper airway collapsibility during propofol anesthesia are comparable to those during non-REM sleep. In this study, our results demonstrate that the mechanical upper airway properties (passive PCRIT) and the strength of the compensatory neuromuscular responses (active PCRIT) to upper airway obstruction is similar to natural non-REM sleep and upper airway neuromuscular reflexes remain intact during propofol anesthesia. In addition, the use of propofol anesthesia holds the advantage of a short half-life and accurate control depths of anesthesia based on a target-controlled infusion system, which calculates effect site concentrations on the basis of a three-compartment pharmacokinetic algorithm (Marsh et al., 1991; Coetzee et al., 1995). Recently, Eastwood et al. (2005) reported that increasing depths of propofol anesthesia are associated with increases in upper airway collapsibility. These investigators suggested that suppression of neuromuscular drive by propofol anesthesia would facilitate studies examining the influence of mechanical factors on human upper airway collapsibility. Our findings also provide new evidence that patients can be evaluated during propofol anesthesia to model the effects of both mechanical factors and neuromuscular activity on upper airway function during sleep. Observed increases in inspiratory airflow with neuromuscular activation suggest that compensatory responses can maintain airway patency, independent of central arousal mechanisms. Pharmacologic suppression of these arousal responses can also help to standardize the assessment of passive and active upper airway properties.
In conclusion, our findings demonstrate that compensatory neuromuscular responses to upper airway obstruction remain intact during propofol anesthesia. They also imply that distinct mechanisms serve to maintain ventilation in the face of upper airway obstruction with specific latencies to responses in inspiratory duty cycle (immediate) and inspiratory airflow (delayed). Partitioning the upper airway properties between its structural and neuromuscular components could also serve to established physiologic phenotypes that aid in uncovering specific genetic susceptibilities to obstructive sleep apnea and to perioperative respiratory complications of anesthesia.
Acknowledgements
The authors would like to acknowledge the technical assistance of Mr. Joseph Maly.
Footnotes
Grants: This study was supported by grants-in-aid for scientific research no. 18592189 from Japanese Ministry of education, Science, Sports and Culture to Terumi Ayuse, and by HL50381; HL 72126 and NHMRC 353705 and NHLBI HL077137.
References
- Ayuse T, Inazawa T, Kurata S, Okayasu I, Sakamoto E, Oi K, Schneider H, Schwartz AR. Mouth-opening increases upper-airway collapsibility without changing resistance during midazolam sedation. J. Dent. Res. 2004;83:718–722. doi: 10.1177/154405910408300912. [DOI] [PubMed] [Google Scholar]
- Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O’Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–1041. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- Chow CM, Xi L, Smith CA, Saupe KW, Dempsey JA. A volume dependent apneic threshold during NREM sleep in the dog. J. Appl. Physiol. 1994;76:2315–2325. doi: 10.1152/jappl.1994.76.6.2315. [DOI] [PubMed] [Google Scholar]
- Coetzee JF, Glen JB, Wium CA, Boshoff L. Pharmacokinetic model selection for target controlled infusions of propofol. Assessment of three parameter sets. Anesthesiology. 1995;82:1328–1345. doi: 10.1097/00000542-199506000-00003. [DOI] [PubMed] [Google Scholar]
- Dejours P. Chemoreflexes in breathing. Physiol. Rev. 1962;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Comparison of sedation with midazolam and ketamine: effects on airway muscle activity. Br. J. Anaesth. 1996;76:663–667. doi: 10.1093/bja/76.5.663. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–477. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Szollosi I, Platt PR, Hillman DR. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology. 2002a;97:786–793. doi: 10.1097/00000542-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002b;359:1207–1209. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am. J. Respir. Crit. Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am. Rev. Respir. Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110:1077–1088. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- Iber C, Simon P, Skatrud JB, Mahowald MW, Dempsey JA. The Breuer–Hering reflex in humans. Effects of pulmonary denervation and hypocapnia. Am. J. Respir. Crit. Care Med. 1995;152:217–224. doi: 10.1164/ajrccm.152.1.7599827. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Ayuse T, Oi K. The effects of head and body positioning on upper airway collapsibility in normal subjects who received midazolam sedation. J. Clin. Anesth. 2006;18:185–193. doi: 10.1016/j.jclinane.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Inazawa T, Ayuse T, Kurata S, Okayasu I, Sakamoto E, Oi K, Schneider H, Schwartz AR. Effect of mandibular position on upper airway collapsibility and resistance. J. Dent. Res. 2005;84:554–558. doi: 10.1177/154405910508400613. [DOI] [PubMed] [Google Scholar]
- Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J. Appl. Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoff RJ, Sforza E, Champagne V, Ofiara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2001;164:250–255. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J. Appl. Physiol. 2008;104:1618–1624. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers AM, Simon PM, Xi L, Dempsey JA. Apnoea following normocapnic mechanical ventilation in awake mammals: a demonstration of control system inertia. J. Physiol. 1993;472:749–768. doi: 10.1113/jphysiol.1993.sp019971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- Manchanda S, Leevers AM, Wilson CR, Simon PM, Skatrud JB, Dempsey JA. Frequency and volume thresholds for inhibition of inspiratory motor output during mechanical ventilation. Respir. Physiol. 1996;105:1–16. doi: 10.1016/0034-5687(96)00037-0. [DOI] [PubMed] [Google Scholar]
- Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br. J. Anaesth. 1991;67:41–48. doi: 10.1093/bja/67.1.41. [DOI] [PubMed] [Google Scholar]
- McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J. Appl. Physiol. 2008;105:197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J. Clin. Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Masaki Y, Toyooka H. Flumazenil antagonizes midazolam-induced airway narrowing during nasal breathing in humans. Br. J. Anaesth. 1999;82:698–702. doi: 10.1093/bja/82.5.698. [DOI] [PubMed] [Google Scholar]
- Patil SP, Punjabi NM, Schneider H, O’Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am. J. Respir. Crit. Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J. Appl. Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Williams BC, Smith PL, Schwartz AR. Neuromuscular activity and upper airway collapsibility. Mechanisms of action in the decerebrate cat. Am. J. Respir. Crit. Care Med. 1997;156:515–521. doi: 10.1164/ajrccm.156.2.9607115. [DOI] [PubMed] [Google Scholar]
- Schneider H, Patil SP, Canisius S, Gladmon EA, Schwartz AR, O’Donnell CP, Smith PL, Tankersley CG. Hypercapnic duty cycle is an intermediate physiological phenotype linked to mouse chromosome 5. J. Appl. Physiol. 2003;95:11–19. doi: 10.1152/japplphysiol.01144.2002. [DOI] [PubMed] [Google Scholar]
- Schneider H, Schwartz AR, Smith PL, Patil SP, Krishnan V, Pichard L. Duty cycle responses to flow limitation predict nocturnal hypoventilation. Eur. Respir. J. doi: 10.1183/09031936.00063008. in press. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, O’Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am. J. Respir. Crit. Care Med. 1998;157:1051–1057. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J. Appl. Physiol. 1989;66:1626–1634. doi: 10.1152/jappl.1989.66.4.1626. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J. Appl. Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Thut DC, Brower RG, Gauda EB, Roach D, Permutt S, Smith PL. Modulation of maximal inspiratory airflow by neuromuscular activity: effect of CO2. J. Appl. Physiol. 1993;74:1597–1605. doi: 10.1152/jappl.1993.74.4.1597. [DOI] [PubMed] [Google Scholar]
- Seelagy MM, Schwartz AR, Russ DB, King ED, Wise RA, Smith PL. Reflex modulation of airflow dynamics through the upper airway. J. Appl. Physiol. 1994;76:2692–2700. doi: 10.1152/jappl.1994.76.6.2692. [DOI] [PubMed] [Google Scholar]
- Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J. Appl. Physiol. 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- Tagaito Y, Schneider H, O’Donnell CP, Smith PL, Schwartz AR. Ventilating with tracheal gas insufflation and periodic tracheal occlusion during sleep and wakefulness. Chest. 2002;122:1742–1750. doi: 10.1378/chest.122.5.1742. [DOI] [PubMed] [Google Scholar]
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]