Summary
Human neutrophil α-defensins (HNPs) are synthesized in vivo as inactive precursor proteins, i.e., preproHNPs. A series of sequential proteolytic events excise the N-terminal inhibitory pro peptide, leading to defensin maturation and storage in azurophilic granules. The anionic pro peptide, required for correct sub-cellular trafficking and sorting of proHNPs, inhibits the antimicrobial activity of cationic defensins, either inter- or intra-molecularly, presumably through charge neutralization. To better understand the role of the pro peptide in the folding and functioning of α-defensins and/or pro α-defensins, we chemically attached the proHNP1 pro peptide or wtpro peptide and the following artificial pro segments to the N-terminus of HNP1: polyethylene glycol (PEG), Arg10 (polyR), Ser10 (polyS), and crpro peptide – a charge-reversing mutant of the pro peptide where Arg/Lys residues were changed to Asp, and Asp/Glu residues to Lys. Comparative in vitro folding suggested that while all artificial pro segments chaperoned defensin folding, with PEG being the most efficient, the pro peptide catalyzed the folding of proHNPs likely through two independent mechanisms: solubilization of and interaction with the C-terminal defensin domain. Further, the N-terminal artificial pro segments dramatically altered the bactericidal activity of HNP1 against both E. coli and S. aureus. Surprisingly, crpro peptide and wtpro peptide showed similar properties with respect to intra-molecular and inter-molecular catalysis of defensin folding as well as α-defensin binding, although their binding modes appeared different. Our findings identify a dual chaperone activity of the pro peptide and may shed light on the molecular mechanisms by which pro α-defensins fold in vivo.
Keywords: defensin, HNP, pro peptide, native chemical ligation, chaperone
Introduction
Human defensins, expressed primarily in leukocytes and epithelial cells, are a class of cationic, Cys-rich antimicrobial mini-proteins that directly inactivate a broad range of microbes such as bacteria, fungi and enveloped viruses, playing important roles in the innate immune defense against microbial infection 1; 2; 3; 4. Defensins also modulate adaptive immunity by chemoattracting a subset of T lymphocytes and immature dendritic cells, and, by activating receptor-mediated signaling for dendritic cells maturation 5; 6; 7; 8; 9. Based on sequence homology and disulfide topology, human defensins are classified into α and β families. Human neutrophil α-defensins, also known as human neutrophil peptides (HNPs), are among the most extensively studied cationic antimicrobial proteins from mammals.
Four human neutrophil α-defensins have been identified so far 10; 11; 12; 13. The three most abundant forms, HNP1-3, which account for 5–7% of the total cellular protein in neutrophils, consist of 29–30 amino acid residues, differing by a single residue at the N-terminus 11. By contrast, a slightly larger but much less abundant HNP4 shares only ~30% sequence identity to HNP1-3. Despite the differences, both HNP1-3 and HNP4 are initially synthesized in vivo as inactive precursors (proHNPs) containing a highly homologous 44 to 45-residue anionic pro region at the N-terminus. Ganz and colleagues first showed that the pro peptide is required for correct sub-cellular trafficking and sorting of proHNP1 14; 15, and its subsequent removal by still-unidentified protease(s) yields mature α-defensins stored in azurophilic granules. Further, folded proHNP1, unlike mature α-defensins, is ineffective against bacteria, suggesting an intra-molecular inhibitory role by the pro peptide. Consistent with this premise is the finding by Ganz and colleagues that the bactericidal activity of HNP1 can be inhibited in a dose-dependent fashion by the pro peptide exogenously added 16. Recently, we demonstrated via Trp fluorescence spectroscopy that the 45-residue pro peptide from proHNP1-3 specifically binds to HNP1 inter-molecularly 17. The molecular basis of such interaction is thought to be of electrostatic nature due to the opposite net charges of HNPs and their pro peptide.
The intra-molecular functional inhibition seen in proHNPs is reminiscent of some bacterial serine proteases such as α-lytic protease and subtilisins, which are synthesized in vivo as inactive pro enzymes. The N-terminal inhibitory pro domain has been shown to be required for the folding of precursor enzymes (covalently linked) and for the refolding of denatured catalytic domains (unlinked) 18; 19, raising an intriguing question: does the pro peptide play an analogous role in proHNP folding? We have previously shown that a covalently linked pro peptide dramatically enhances the solubility of the aggregation-prone defensin domain HNP1, facilitating the folding of proHNP1 in aqueous solution 17. However, productive folding of HNPs without the pro peptide can also be achieved under highly solubilizing and/or partially denaturing conditions such as the use of organic co-solvents and/or moderately high concentrations of chemical denaturants 20. The sum of our previous findings appear to suggest that the primary mechanism by which the pro peptide assists the folding of proHNPs is inhibition of the formation of misfolded aggregates of the C-terminal defensin domain. Our hypothesis contrasts with the sum of knowledge of the chaperone roles of serine protease pro segments, which thermodynamically favor the stability of the native fold relative to intermediates or unfolded states 21.
To better understand the catalytic role of the pro peptide in the folding of proHNPs, we covalently attached highly water-soluble moieties, i.e., polyethylene glycol (PEG), (Arg)10 (polyR), and (Ser)10 (polyS), via a di-peptide linker, in place of the proHNP1 pro peptide, to the N-terminus of HNP1 using solid phase peptide synthesis. The di-peptide linker ε-aminohexanoic acid (Ahx)-Met, cleavable by cyanogen bromide, was inserted to facilitate the release of folded HNP1 for verification. Here we report comparative in vitro folding studies of PEG-HNP1, polyR-HNP1, polyS-HNP1, HNP1, proHNP1, and crproHNP1 – a charge-reversing mutant of proHNP1 where Arg/Lys residues in the pro region were changed to Asp, and Asp/Glu residues to Lys. In addition, we observed mature HNP2 interacting with isolated PEG, polyR, polyS, and crpro peptide, collectively termed artificial pro segments, as well as the proHNP1 pro peptide or wtpro peptide, by surface plasmon resonance (SPR) and Trp fluorescence spectroscopy. Finally, functional assays were performed to evaluate the effects of the artificial pro segments and wtpro peptide, either linked covalently or added exogenously, on the ability of HNP1 to kill bacteria and to induce leakage from large unilamellar vesicles (LUVs).
Results
All five pro segments solubilize HNP1 and intra-molecularly chaperone its oxidative folding
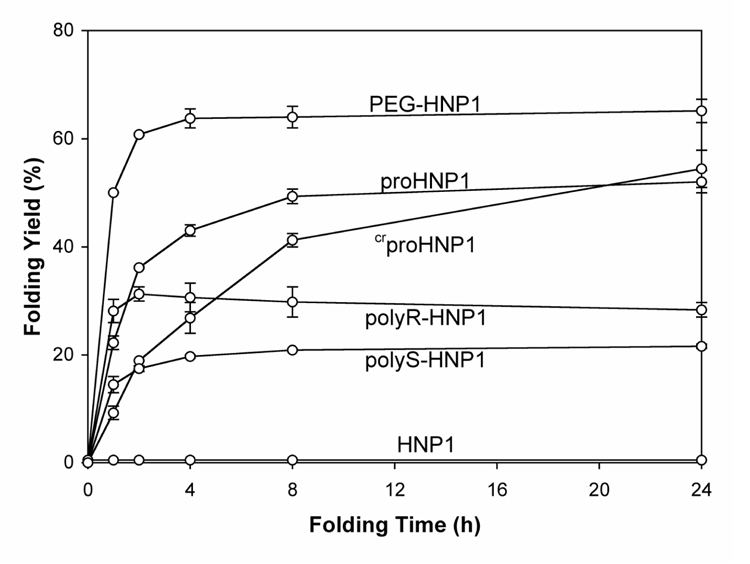
The most common protocol for productive folding of disulfide-containing polypeptides in vitro utilizes reduced and oxidized thiol pairs in the presence of low concentrations of denaturants. The conditions described in this work have been optimized to fold proHNP1 17, and are applicable to the folding of HNP1 with artificial pro segments. Comparative folding kinetics of PEG-HNP1, polyR-HNP1, polyS-HNP1, proHNP1, crproHNP1 and HNP1, 50 µM each, were performed in 2 M urea, 3 mM reduced and 0.3 mM oxidized glutathione, pH 8.1. While HNP1 massively aggregated, yielding no detectable amounts of folded defensin, as previously reported 20, no precipitation was observed with proHNP1 and any defensins with artificial pro segments. As shown in Figure 1, the ability of the four covalently attached artificial pro segments to chaperone the folding of HNP1 ranked in the following order: PEG>crpro peptide>polyR>polyS. PEG facilitated the folding of HNP1 more efficiently than did wtpro peptide. It took 8 h for proHNP1 to yield a 50% conversion to the folded product, whereas the PEG-conjugated defensin reached the same level within 1 h and over 65% within approximately 4 h. Notably, the folding yields for most defensins plateaued at around 8 h except for the folding of crproHNP1, which, despite a slow initial rate compared with proHNP1, continued to progress beyond the time point at 24 h. These results, while showing significantly different chaperone efficiencies exhibited by wtpro peptide and the artificial pro segments, suggested that a soluble defensin might be required for its productive folding.
Figure 1.
Time-dependent folding yields of PEG-HNP1, proHNP1, crproHNP1, polyR-HNP1, polyS-HNP1 and HNP1. Oxidative folding of each peptide was performed at a concentration of 50 µM in 2 M urea, 3 mM reduced and 0.3 mM oxidized glutathione, pH 8.1. Samples (2 nmol) were withdrawn at different time intervals for HPLC analysis. Yields, proportional to peak area, were calculated by integration. The data were obtained from two independent experiments.
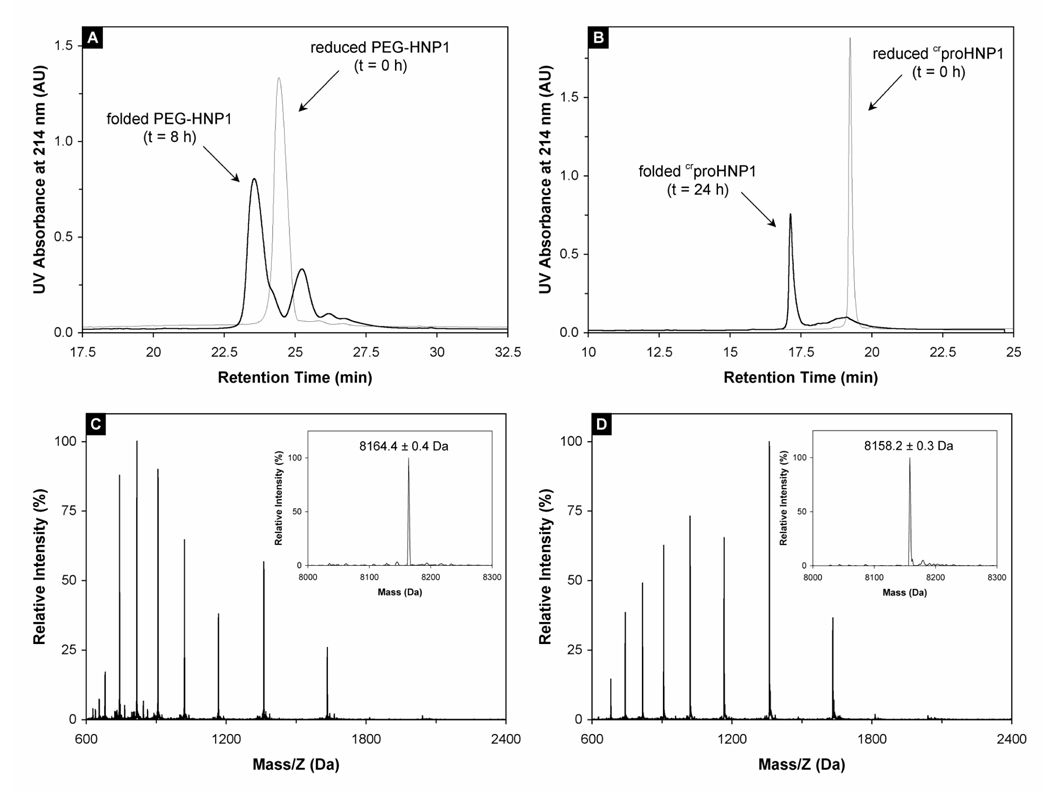
Shown in Figure 2A and 2B are representative folding reactions of PEG-HNP1 and crproHNP1 monitored by RP-HPLC at 8 h and 24 h, respectively. The chromatographic peaks of both reduced and oxidized PEG-HNP1 were noticeably broader than those of crproHNP1 because of an inherent heterogeneity of PEG chain length. The retention time of each folded species decreased versus the corresponding reduced form, presumably due to folding-associated burial of hydrophobic residues. The molecular mass of folded crproHNP1 was determined to be 8158.2 ± 0.3 Da, in agreement with the expected value of 8158.6 Da calculated based on the average isotopic compositions of oxidized crproHNP1 (Figure 2D). The mass value of folded crproHNP1 is 6 Da less than that of its reduced counterpart (Figure 2C), indicative of the formation of three disulfide bonds in the folded structure. ESI-MS analyses were also conducted on the folding products of polyR-HNP1 and polyS-HNP1, and similar results within experimental error were obtained (data not shown).
Figure 2.
(A) PEG-HNP1 before (thin line) and after (thick line) 8-h folding. RP-HPLC analysis was performed at 40 °C on a Waters Symmetry™ 300 C18 column (4.6 × 150 mm) using a linear gradient of 5–65% ACN containing 0.1% TFA at a flow rate of 1 ml/min over 30 min. 10 nmol peptide was injected. The 25.5 min peak in the 8 h trace was a major folding intermediate of PEG-HNP1. (B) crproHNP1 before (thin line) and after (thick line) 24-h folding. RP-HPLC analysis was carried out under identical chromatographic conditions. (C) Mass spectrometric analysis of unfolded crproHNP1. The measured molecular mass 8164.4 ± 0.4 Da is in agreement with the expected value of 8164.7 Da calculated based on the average isotopic compositions of reduced crproHNP1. (D) Folded crproHNP1 analyzed by ESI-MS. As expected, the folded defensin lost 6 mass units as a result of the formation of three disulfide bridges. Note that the characteristic shift in HPLC retention time and ESI charge envelope between reduced and oxidized crproHNP1 is consistent with the burial of hydrophobic residues associated with the folding of the molecule.
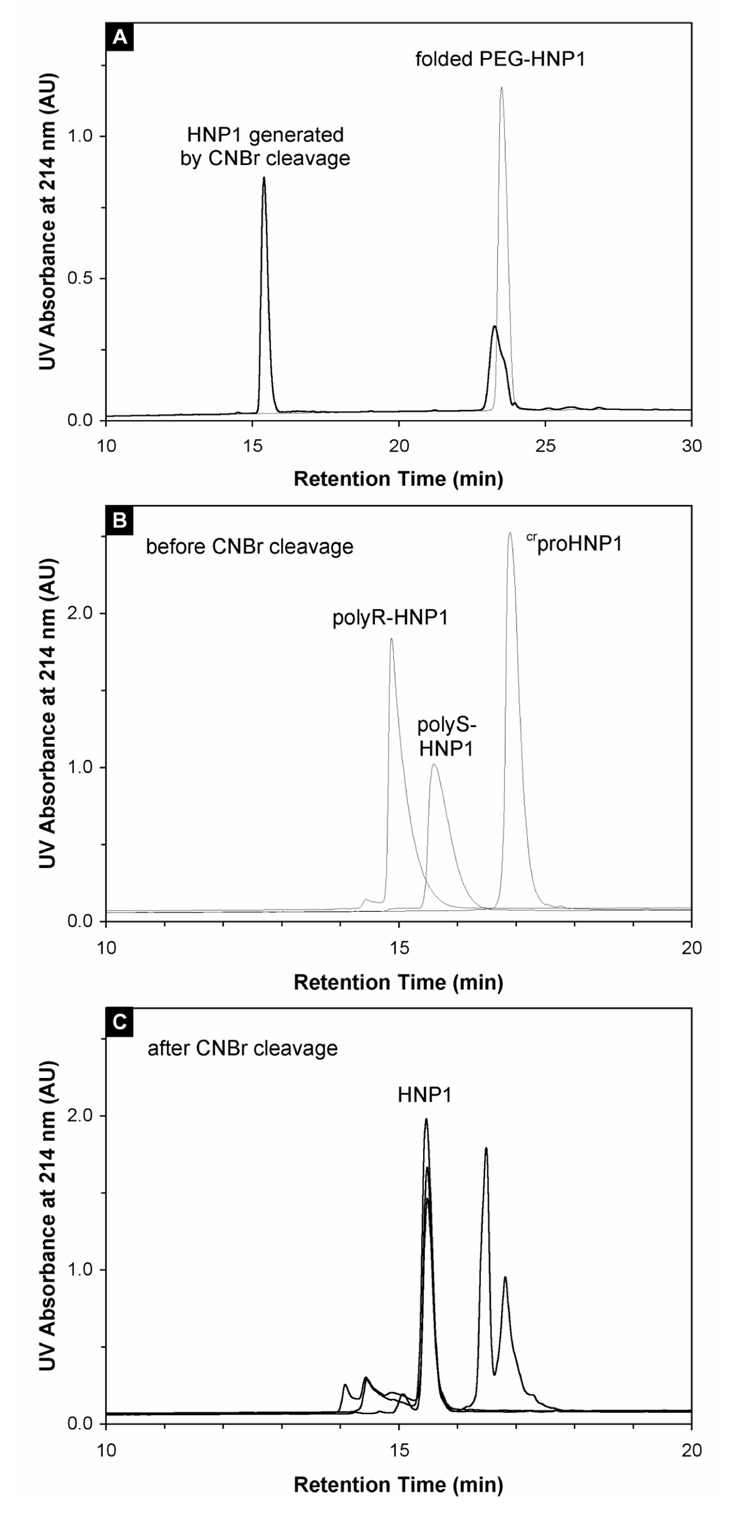
To verify that the defensin domain correctly folded in the presence of an N-terminal artificial pro segment, folded PEG-HNP1, polyR-HNP1, polyS-HNP1, and crproHNP1 were subjected to CNBr cleavage (Figure 3), and released HNP1 was thoroughly characterized. Several lines of evidence support correct folding of the defensin domain. First, all CNBr-released HNP1 molecules were chromatographically indistinguishable from each other and from a previously characterized synthetic HNP1 on analytical RP-HPLC (Fig 3A and 3C). Second, their molecular masses were determined to be 3442.1 ± 0.1 Da, in agreement with the expected value of 3442.1 Da calculated based on the average isotopic compositions of oxidized HNP1. Third, cleaved defensins were assayed along with a previously characterized HNP1 in the killing of E. coli and S. aureus, and showed identical activities. Finally, disulfide mapping of the chemically released HNP1 from PEG-HNP1 and crproHNP1 was performed using the previously published procedures 17; 22, confirming that the defensin domain adopted native disulfide pairings in the structure, i.e., Cys1-Cys6, Cys2-Cys4 and Cys3-Cys5.
Figure 3.
(A) Cleavage of 100 µM folded PEG-HNP1 by 25 mg/ml CNBr in 2.5% TFA. For comparison, 5 nmol of folded PEG-HNP1 dissolved in water was injected as control (thin line). An identical amount of protein was analyzed after one-hour cleavage by CNBr (thick line), showing a more than 75% conversion to HNP1. (B) Folded polyR-HNP1, polyS-HNP1 and crproHNP1 analyzed by RP-HPLC, prior to CNBr cleavage. (C) CNBr cleavage of polyR-HNP1, polyS-HNP1 and crproHNP1 monitored by RP-HPLC. The common cleavage product-HNP1 eluted at 15.5 min. Peaks eluted after 16 min were cleaved products derived form crpro peptide, whereas peaks eluted before HNP1 were cleaved products derived from polyR and polyS. The chromatographic data were collected under the same conditions as described above. The chromatographic data presented in this figure were collected under the same conditions as described in the legend of Figure 2.
wtPro peptide and crpro peptide catalyze the folding of polyS-HNP1 inter-molecularly but not of proHNP1 or crproHNP1
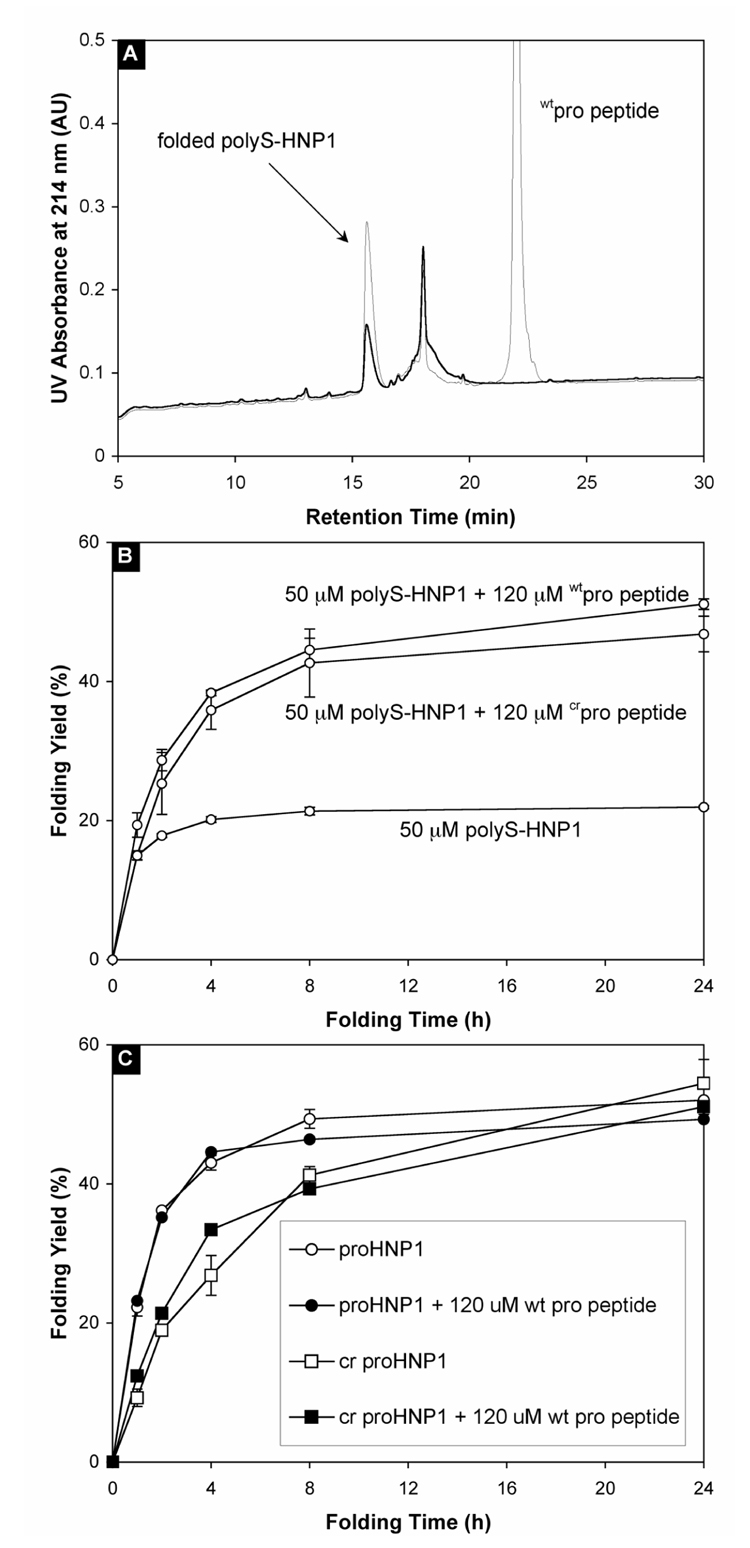
We have previously demonstrated that exogenously added wtpro peptide neither alleviates the aggregation of HNP1 nor improves its folding efficiency 20. To investigate whether similar results can be obtained in a soluble defensin system, we compared the folding reactions of soluble polyS-HNP1 (50 µM) in the presence and absence of 120 µM wtpro peptide (Figure 4A). A dramatic improvement in the folding efficiency of polyS-HNP1 was observed with wtpro peptide. As shown in Figure 4B, in the presence of wtpro peptide, the folding yield of polyS-HNP1 almost doubled within 4 h, and continued to increase over time to more than 50% at 24 h. By contrast, the amount of folded polyS-HNP1 leveled off at ~20% after 8 h without wtpro peptide. A similar chaperone effect by crpro peptide was also observed with polyS-HNP1, although crpro peptide was slightly less efficient than wtpro peptide (Figure 4B). Importantly, isolated wtpro peptide at the same concentration (120 µM) did not catalyze the folding of proHNP1 or crproHNP1 (Figure 4C). The catalytic effect of an intra-molecular pro peptide on proHNP1 folding was apparently equivalent to that of wtpro peptide in excess on the folding of polyS-HNP1. These findings suggest that the pro peptide chaperones the folding of pro defensins intra-molecularly, which cannot be further improved through exogenously adding wtpro peptide to the system. We presume that the intra-molecular interaction between the pro peptide and the C-terminal defensin domain is so entropically favorable compared to the intermolecular interaction that the kinetic effect of the pro peptide added exogenously is not detectable.
Figure 4.
(A) HPLC-monitored folding at 24 h of 50 µM polyS-HNP1 in the presence (thin line) and absence (thick line) of 120 µM wtpro peptide. (B) Time-dependent folding yields for polyS-HNP1 alone, and polyS-HNP1 in the presence of either wtpro peptide or crpro peptide. The data were obtained from three separate experiments. (C) Time-dependent folding yields for both proHNP1 and crproHNP1 in the presence and absence of wtpro peptide.
Both wtpro peptide and crpro peptide specifically bind to HNP2 inter-molecularly, but in different modes
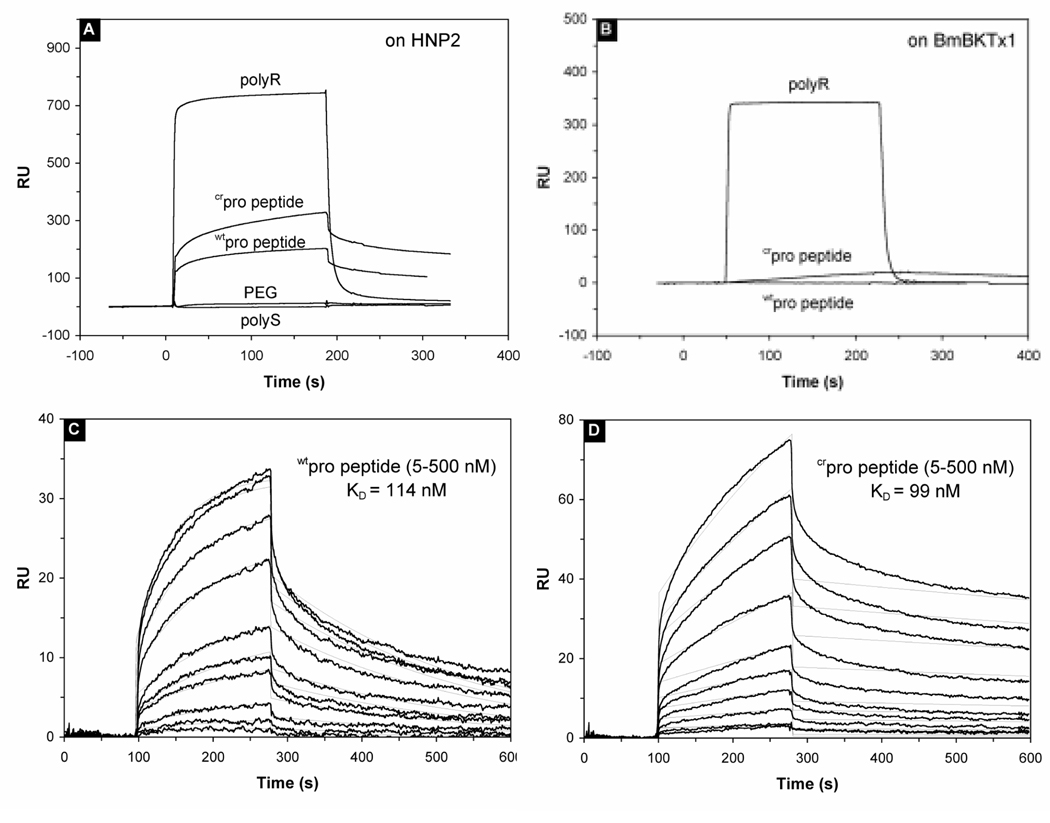
The SPR experiments showed that both wtpro peptide and crpro peptide bind specifically to immobilized HNP2 with dissociation equilibrium constants (Kd) of 114 and 99 nM, respectively (Figure 5A, 5C and 5D). In contrast, PEG and polyS exhibited, as expected, no binding to the immobilized defensin at the concentration tested. It is surprising that crpro peptide, despite 12 charge-reversing mutations in the sequence (27%), bound to HNP2 with a similar affinity compared with wtpro peptide. Nevertheless, the binding result was supported by folding kinetic measurements, where crpro peptide and wtpro peptide displayed similar chaperone activity. Interestingly, compared with wtpro peptide and crpro peptide, polyR bound to HNP2 at much faster on and off rates (Figure 5A). We speculated that rather than specific interactions with the defensin, the binding of polyR to HNP2 was due to a non-specific association with protein molecules in general. To verify this, we immobilized BmBKTx1, a cationic and Cys-rich peptide toxin of 31 amino acid residues from scorpion, and analyzed binding kinetics of wtpro peptide, crpro peptide and polyR under the same condition. As shown in Figure 5B, polyR was the only substance bound in meaningful quantities to the sensor chip with a kinetics similar to that on HNP2, supporting that wtpro peptide or crpro peptide specifically bound to the defensin, whereas polyR did not.
Figure 5.
Binding characteristics of pro peptides to HNP2 determined by surface plasmon resonance. Binding of 1 µM of the indicated peptides, measured in resonance units (RU’s), to 700 RU’s of HNP2 (A) or 850 RU’s of the scorpion toxin BmK37 (B) was examined as a function of time. The association and dissociation phases of wtpro peptide (C) and crpro peptide (D) binding to HNP2 were monitored by following the changes in SPR signal, given in RU. For both peptides, a concentration range of 500, 400, 300, 200, 100, 75, 50, 25, 10, and 5 nM was used.
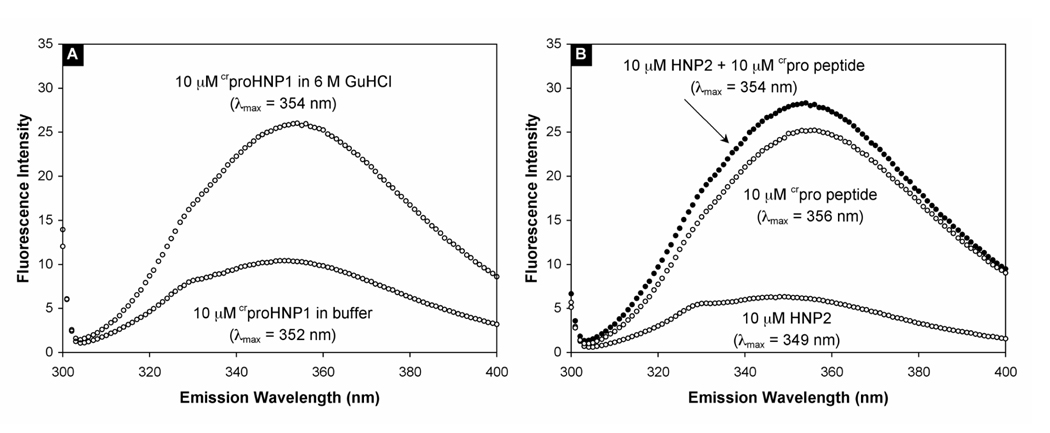
wtPro peptide alone is structurally disordered in aqueous solution. Its only fluorescent residue, Trp, is fully exposed to solvents 17. We have previously shown that the hallmark of both inter-and intra-molecular complex formation is the concomitant blue-shift and quenching of the Trp fluorescence 17. In aqueous buffer, the Trp fluorescence of crproHNP1, emitting maximally at 352 nm, is also quenched (Figure 6A). When dissolved in 6 M GuHCl, where intra-molecular interactions in crproHNP1 are fully disrupted, the Trp fluorescence intensified by 2.5 times, with the maximum emission wavelength slightly shifted to 354 nm. This phenomenon, also seen with proHNP1, is consistent with an intra-molecular interaction in crproHNP1 under non-denaturing conditions. However, crpro peptide displayed a distinct mode of binding to HNP2 compared with wtpro peptide. When crpro peptide was mixed with an equal molar concentration of HNP2 in aqueous buffer, no Trp quenching was observed (Figure 6B), suggesting that the structural orientation of crpro peptide is different from that of wtpro peptide in the binary complex with HNP2. This is probably not surprising in light of the extensive re-arrangement of charged residues in the pro peptide sequence.
Figure 6.
(A) The fluorescence spectra of 10 µM crproHNP1 in 10 mM phosphate buffer, pH 7.4, and of 10 µM crproHNP1 in 10 mM phosphate, 6 M GuHCl, pH 7.4. (B) The fluorescence spectra of 10 µM HNP2, 10 µM crpro peptide, and a mixture of both at 10 µM each in 10 mM phosphate buffer, pH 7.4. The spectroscopic data were collected at room temperature with an excitation wavelength of 295 nm, and averaged from multiple scans with a deviation of λmax of ± 1 nm.
Pro segments dramatically impact bactericidal and membrane activities of defensin in vitro
To evaluate the functional ramification of covalently attaching pro segments to HNP1, PEG-HNP1, polyR-HNP1, polyS-HNP1, crproHNP1, proHNP1 and HNP1 were assayed for their ability to kill E. coli and S. aureus. As shown in Figure 7A and 7B, attachment of polyR significantly enhanced the bactericidal activity of HNP1 against both strains. For S. aureus, complete killing was achieved by polyR-HNP1 at 1.6 µM and by HNP1 at 25 µM. Similarly, while HNP1 at the highest concentrations reduced E. coli survival by 2–3 orders, polyR-HNP1 achieved complete killing at 6.4 µM. By contrast, polyS-HNP1 showed markedly reduced activity against both strains, underscoring the influence of the N-terminal pro region in defensin function. It should be pointed out that the augmented bactericidal activity seen with polyR-HNP1 might be attributed to enhanced electrostatic interactions between the significantly more positively charged defensin and the bacterial cell wall and membrane. Since polyR alone was a potent cytotoxic agent against both E. coli and S. aureus (data not shown), an additive or synergistic effect in the bacterial killing by polyR-HNP1 was highly probable.
Figure 7.
Survival curves for HNP1, PEG-HNP1, proHNP1, crproHNP1, polyR-HNP1 and polyS-HNP1 against E. coli ATCC 25922 (A) and S. aureus ATCC 29213 (B). Each curve is the mean of triplicate experiments. Points scored as zero survival could not be plotted. Bactericidal activity of 10 µM HNP2 (circle) against E. coli (C) and S. aureus (D), titrated by an increasing amount of wtpro peptide ranging from 0.35 µM to 90 µM. The survival curve for wtpro peptide alone is depicted by the diamond marker (◊). Error bars indicate the standard error in triplicate experiments. Bactericidal activity of 10 µM HNP2 (square) against E. coli (E) and S. aureus (F), titrated by crpro peptide ranging from 0.35 µM to 90 µM. The survival curve for crpro peptide alone is depicted by filled circles. Bars indicate the standard error of the mean of triplicate experiments.
As was first reported by Ganz and colleagues and subsequently confirmed by us 16; 17, proHNP1 was totally inactive against E. coli and S. aureus across the entire concentration range used, presumably due to intra-molecular inhibition of cationic HNP1 by the anionic pro peptide. Consistent with this result, titration of 10 µM HNP2 with an increasing amount of wtpro peptide showed a dose-dependent inhibition of the bactericidal activity against both strains (Figure 7C and 7D). PEG-HNP1 exhibited no bactericidal activity against E. coli and was extremely weak against S. aureus, a result similar to the finding for polyS-HNP1. Surprisingly, crproHNP1, while virtually inactive against S. aureus, was more potent than HNP1 against E. coli. The underlying molecular basis of the anomaly remains obscure and deserves further investigation.
The results from the titration of 10 µM HNP2 by crpro peptide implied heterogeneity of mechanism (Figure 7E and 7F). Charge reversing in the pro sequence resulted in a cationic crpro peptide, which was, by itself, an effective bactericidal agent against both strains. This was in stark contrast to the inactive anionic wtpro peptide (Figure 7C and 7D). As the concentration of crpro peptide increased from 0.35 to 90 µM in the presence of 10 µM HNP2, the bactericidal activity showed a steep transition phase at intermediate concentrations. The fact that the presumed binary complex of crpro peptide and HNP2 was active against both strains compared with crproHNP1, which was active only against E. coli, may be consistent with our assertion that crpro peptide adopts a different orientation relative to the defensin domain in crproHNP1 from that in the crpro peptide-HNP2 complex.
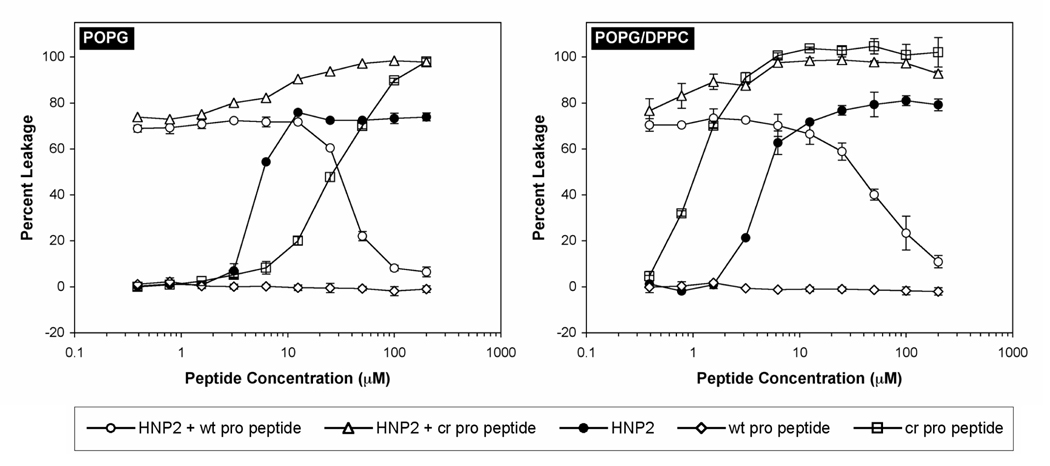
LUVs encapsulating fluorescent indicators have been widely used for studying membrane-active peptides including defensins 23; 24; 25; 26. We have previously demonstrated that the bactericidal activity of analogs of HNPs correlates to their ability to induce leakage of the low molecular weight fluorophore/quencher pair (ANTS/DPX) from liposomes composed of POPG/DPPC phospholipids 27; 28. In this report, we used two types of liposomes, POPG/DPPC (1:1) and POPG, and the results are illustrated in Figure 8. Two important conclusions can be readily drawn from the membrane leakage experiments. First, wtpro peptide was totally inactive across the entire concentration range used (0.4 to 200 µM), while crpro peptide was capable of inducing membrane leakage in a dose-dependent manner. In fact, crpro peptide was more effective than HNP2 with respect to POPG/DPPC. Second, wtpro peptide, when added to 20 µM HNP2, inhibited defensin-induced leakage from LUVs in a concentration-dependent fashion. By contrast, titration of 20 µM HNP2 by varying concentrations of crpro peptide showed an additive effect. These results are in good agreement with the findings from the bactericidal activity assays, thus validating the model membrane system as a useful tool for functional as well as mechanistic evaluation of various defensin molecules.
Figure 8.
Percent leakage from POPG and DPPC/POPG (1:1) LUVs induced by HNP2, wtpro peptide, and crpro peptide. Leakage inhibition experiments were performed at a fixed concentration of HNP2 (20 µM) with varying amounts of either wtproHNP1 (circle) or crpro peptide (triangle). All peptides were incubated with 600 µM LUVs for one hour before readings were taken. Bars indicate the standard error of the mean of triplicate experiments.
Discussion
One of the central tenets in biology states that the three-dimensional structure of a protein is encoded entirely in its amino acid sequence 29. However, how a linear polypeptide transforms into a uniquely folded, functional protein remains poorly understood. It has become apparent that various cellular accessory molecules or molecular chaperones aid protein folding in vivo by catalyzing specific disulfide or peptide bond isomerization reactions, and more generally, by interacting with incorrectly folded or denatured proteins to prevent their aggregation 30. Given that human α-defensins 1–4 are the most abundant cellular protein in neutrophils and extremely prone to aggregation in reduced form, molecular chaperoning could play a major role in averting α-defensin aggregation and facilitating correct α-defensin folding inside cells.
Molecular chaperoning in protein folding is commonly described in terms of inter-molecular interactions. Intra-molecular chaperoning by pro-regions also exists, notably to assist the folding of some extracellular bacterial proteases such as α-lytic protease and subtilisins 18; 19, where the N-terminal pro-domain transiently acts as a template inhibitor upon which the downstream protease domain folds 31; 32. A subsequent intra-molecular, autocatalytic processing event initiates pro domain degradation, leading to enzyme maturation. Significantly, the pro domain also catalyzes the refolding of denatured α-lytic protease and subtilisins inter-molecularly. In fact, when an active enzyme is denatured, efficient renaturation can be achieved only in the presence of an exogenously added pro domain. For α-lytic protease, the pro region catalyzes correct folding of the protease domain by stabilizing the folding transition state and lowering an extremely large folding barrier that separates the metastable native state from a kinetically trapped, stable, molten globule-like intermediate state 33. Mutational studies of subtilisins indicated that the ability of the pro peptide to chaperone the refolding of a denatured enzyme correlated with its ability as a competitive inhibitor for the active enzyme 34.
The mode of chaperoning of the pro peptide in α-defensins appears to be less restricted than that of the pro region in α-lytic protease and subtilisins. The first indication of such a difference came from the studies by Ganz and colleagues of the in vivo processing of preproHNP1 14; 15, who demonstrated that the N-terminal 19 amino acid residues of the pro peptide could be truncated without negatively impacting correct subcellular trafficking and sorting of HNP1, although further deletion of the succeeding 13 residues was detrimental. This finding indicates that more than 40% of the pro peptide is dispensable for defensin biosynthesis, casting doubt on the stringency of sequence-specific domain-domain interactions generally required for molecular chaperoning.
Our results reported here suggest dual roles of the pro peptide in chaperoning the folding of α-defensins. First, the highly soluble pro peptide non-specifically promotes the formation of the native defensin fold by disfavoring aggregation of the C-terminal defensin domain in reduced form. Solubilizing artificial pro segments such as polyS, polyR, and PEG in particular, while fully incapable of specific binding to HNP1, all catalyzed the correct folding of α-defensins. Consistent with this finding is that HNP1 without the pro peptide, as has been shown previously 20, could be productively folded under solubilizing solvent conditions. This mode of chaperoning is reminiscent of the highly soluble maltose-binding protein in a fusion protein expression system, where fusion increases the solubility and promotes correct folding of the attached protein 35. Second, the pro peptide chaperones the folding of pro defensins via specific intra-molecular interactions. An unambiguous support for this mechanism comes from the catalysis of the folding of polyS-HNP1 by exogenously added wtpro peptide and crpro peptide, which, as demonstrated in this work, were capable of specifically binding to the defensin domain. As is the case with α-lytic protease and subtilisins, the enhanced folding efficiency of polyS-HNP1 (inter-molecularly) or proHNPs (intra-molecularly) can be attributed to the pro peptide interacting with native-like defensin folding intermediates, thus lowering the free energy of the transition state in the folding pathway.
An intriguing finding is that PEG-HNP1 folded more efficiently than both proHNP1 and crproHNP1, and much more so than either polyR-HNP1 or polyS-HNP1. A plausible explanation may lie in the effect of “macromolecular crowding” – a phenomenon existent in living cells. Dobson and colleagues suggested that macromolecular crowding enhances the chaperone activity of the protein folding catalyst protein disulfide isomerase, which under crowded conditions is particularly effective in preventing the aggregation of reduced proteins inside cells 36. PEG, due to its size when fully hydrated, is arguably a more effective crowding agent than polyR and polyS, and possibly a better stabilizing agent for the transition and/or folded state of defensin. Although our folding experiments were carried out at very low protein concentrations, covalently linked PEG likely exhibited more pronounced “crowding” effects due entropically to a much higher “effective concentration.” Alternatively, polyR and polyS were less effective than PEG in promoting defensin folding due to the formation of “soluble aggregates” of polyR-HNP1 and polyS-HNP1. In fact, it is known that protein folding yields directly correlate to the suppression of protein aggregation by added polyalcohols such as glycerol, and in turn, to the number of hydroxyl groups in them 37.
While the precise reason remains unknown and deserves further investigation, the paradigm of a highly soluble molecule promoting the folding of a covalently linked, sparingly soluble polypeptide may apply to both naturally occurring pro peptides and synthetic nonpeptide chaperones. It should be noted that PEG has been widely used in conjugation with therapeutic proteins for performance enhancement 38; 39. Although recent advances have expanded the utility of post-translational PEGylation chemistries, several types of modifications are still beyond the current progress of chemistries for the modification of peptides produced in vivo. Solid phase peptide synthesis makes possible specific non-peptide chemical modifications beyond the scope of any in vivo expression system 40. Site-specific PEGylation of unfolded polypeptides should have an added benefit of facilitating folding reactions in various pharmaceutical applications.
The results obtained from the functional characterizations of various defensins are rather interesting and mechanistically revealing. It is commonly believed that defensins directly kill bacteria by permeabilizing the cytoplasmic membrane and inducing leakage of intracellular contents 41; 42; 43; 44; 45. Electrostatic forces mediate the self-promoted uptake of cationic defensins across the cell wall and their subsequent association with anionic bacterial cytoplasmic membranes 46. Since hydrophobicity-mediated burial of amphiphilic defensin molecules into the lipid phase of microbial membranes is an obligate step leading to eventual membrane disruption and cell lysis, the interplay between charge and hydrophobicity largely dictates the antimicrobial activity and specificity of defensins 24. This mode of action was vividly manifested by the two opposing examples, polyR-HNP1 and polyS-HNP1, where the cationic pro segment significantly augmented the bactericidal activity of HNP1, while the neutral, hydrophilic polyS abolished it. For polyR-HNP1, the enhanced activity may come either directly from the cytotoxic effect of the poly-Arg sequence as polyR itself effectively killed both E. coli and S. aureus, or from a polyR-promoted interaction between the defensin and the anionic microbial membrane. Nevertheless, the question of how the N-terminal hydrophilic polyS eradicated the defensin activity remains difficult to answer. Similarly, PEG-HNP1 is also inactive against bacteria. PEG neither associates specifically with nor changes the conformation of its polypeptide partner 47. However, PEGylation can enhance the apparent size of the small defensin molecules to such an extent that it interferes with the sequence of events that results in membrane disruption. In the same vein, PEG may interfere with the structural amphiphilicity of HNP1, blocking electrostatic binding to the bacterial cell wall and membrane or hydrophobic interactions with the lipid phase of the membrane. These explanations share in common the general interpretation that N-terminal modifications sterically hinder HNP1, rendering it inactive.
Finally, it is important to note that N-terminal extension of active HNP1 by a neutral, ten-residue poly-serine sequence was sufficient to diminish its bactericidal activity, suggesting that charge neutralization of cationic HNP1 by its anionic pro peptide may not be the only molecular mechanism in play for the functional inhibition of α-defensins. Further, conclusive evidence is lacking to support that such inhibition is mediated primarily by interactions between oppositely charged residues in proHNP1. In fact, our observation that crpro peptide bound to HNP2 hinted otherwise. A series of single and multiple mutations in wtpro peptide have been constructed in our laboratory, and the results suggest that hydrophobic forces are also important for α-defensin and pro peptide interactions (unpublished results). Structural studies are needed to definitively establish the molecular basis for the inhibition of α-defensins by their pro peptide. Interestingly, Ouellette and colleagues recently showed that replacement of all acidic residues (Asp/Glu) by Gly in the pro region of mouse proCryptdin-4 converted the functionally inactive pro α-defensin into an active molecule ([DE/G]-proCrp4) with respect to bacterial killing and membrane permeabilization 48. They attributed the lack of activity of proCrp4 to charge neutralization of cryptdin-4 by the covalently linked anionic pro peptide. Their findings on [DE/G]-proCrp4 are analogous to our results on crproHNP1. However, as was demonstrated in this report, a positively charged crpro peptide alone is sufficient to induce membrane leakage and to kill bacteria. Thus, it is plausible that the reported activity shown by [DE/G]-proCrp4 was, at least in part, due to its cationic pro peptide.
Materials and Methods
Solid phase peptide synthesis
Chemical synthesis of HNP1, HNP2 and proHNP1 was reported elsewhere 17; 20. The synthesis of crproHNP1 via native chemical ligation 49; 50 was essentially as described for proHNP1. The amino acid sequences of HNP1, HNP2 and their pro peptide are shown below.
ACYCRIPACI AGERRYGTCI YQGRLWAFCC (HNP1)
CYCRIPACI AGERRYGTCI YQGRLWAFCC (HNP2)
EPLQARADEVAAAPEQIAADIPEVVVSLAWDESLAPKHPG SRKNM (pro)
The synthesis of PEG-HNP1 is briefly described as follows. The CNBr-cleavable dipeptide linker εAhxMet was first coupled sequentially on resin to extend the N-terminus of HNP1, using a machine-assisted Boc chemistry tailored from the HBTU activation/DIEA in situ neutralization protocol developed by Kent and colleagues 51; 52. After deprotection and cleavage in HF, the crude peptide εAhxMet-HNP1 was purified by preparative reversed phase (RP) HPLC, followed by oxidative folding using the same procedure previously described for HNPs. The molecular masses of reduced and oxidized/folded εAhxMet-HNP1 were verified by electrospray ionization mass spectrometry (ESI-MS).
Attachment of PEG to εAhxMet-HNP1 was carried out by reacting excess amounts of succinamide-activated PEG (average MW 5000 Da, Nektar) with folded and purified εAhxMet-HNP1 according to manufacturer-suggested protocols. Since the N-terminal ε-amino group is the only possible anchoring site in the entire molecule, the PEGylation reaction was site-specific and proceeded to completion overnight at room temperature. εAhx was chosen over α-amino acids due to its stronger nucleophilicity and less steric hindrance. After preparative RP-HPLC purification and lyophilization, the ligated PEG-εAhxMet-HNP1, or PEG-HNP1, was dissolved in 6 M GuHCl, pH 8.1, to which DTT in large excess was added to reduce the three disulfide bonds in HNP1. The reduction was monitored on analytical RP-HPLC based on different retention times of oxidized and reduced species. After HPLC desalting, fully reduced PEG-HNP1 was lyophilized and stored at −20 °C prior to folding experiments.
PolyR-HNP1 and polyS-HNP1 were prepared as follows. Two short peptide thioesters, (Arg)10-εAhxMet-Ala-αCOSCH2CO-Leu and (Ser)10-εAhxMet-Ala-αCOSCH2CO-Leu, were synthesized on custom-made Boc-Ala-αCOSCH2CO-Leu-PAM resin, HF-cleaved/deprotected, and purified by RP-HPLC. Native chemical ligation of each peptide thioester to fully reduced and purified HNP2 was performed according to the standard procedures published 17, resulting in (Arg)10-εAhxMet-Ala-HNP2 and (Ser)10-εAhxMet-Ala-HNP2, i.e., polyR-HNP1 and ployS-HNP1, respectively.1 The ligation products were ascertained by ESI-MS.
For binding studies with HNP2, isolated pro segments were individually prepared by base hydrolysis of corresponding peptides to remove the C-terminal thioester moiety. Succinamide-derived PEG was deactivated by treatment with excess hydroxylamine, and subsequently dialyzed against water (MWCO 2000). All peptides used in this work were chromatographically homogeneous and quantified by UV absorbance measurements using molar extinction coefficients calculated based on a published algorithm 53. Note that HNP2 instead of HNP1 was used in bi-molecular interaction studies because base hydrolysis-generated wtpro peptide and crpro peptide already contained an added Ala residue at the C-terminus.
Folding kinetics and product characterization
Comparative folding of PEG-HNP1, polyR-HNP1, polyS-HNP1, HNP1, proHNP1, and crproHNP1 was carried out by dissolving reduced peptide in 8 M urea containing 12 mM reduced glutathione and 1.2 mM oxidized glutathione to a concentration of 200 µM, followed by an immediate four-fold dilution with 0.25 M NaHCO3, pH 8.1. 80 µl was withdrawn at different time intervals, to which 20 µl of glacial acetic acid was added to quench the reaction. 50 µl (2 nmol) was injected for HPLC analysis, which was performed at 40 °C on a Waters Symmetry™ 300 C18 column (5 µm, 4.6 × 150 mm) using a linear gradient of 5–65% acetonitrile containing 0.1% TFA at a flow rate of 1 ml/min over 30 min. The folding yields were calculated based on the ratio of the integrated areas of the two peaks given by folded protein and fully reduced starting material.
Correct defensin folding is often accompanied by accumulation of a chromatographically homogeneous species with a shortened retention time on RP-HPLC and by a loss of six mass units due to formation of three disulfide bridges. For structural verification, chemical release of HNP1 from folded PEG-HNP1, polyR-HNP1, polyS-HNP1, proHNP1, and crproHNP1 was achieved quantitatively by treatment of 100 µM folded protein with 25 mg/ml CNBr in 2.5% TFA. The molecular mass of released HNP1 was ascertained by ESI-MS, and, its disulfide connectivity (Cys1-Cys6, Cys2-Cys4, Cys3-Cys5) was independently verified by mass mapping of peptide fragments generated by proteolytic digestion as well as Edman degradation according to the published procedures 17; 22.
Trp fluorescence spectroscopy
Fluorescence spectroscopic studies of crproHNP1, HNP2, and crpro peptide were performed in 10 mM phosphate, pH 7.4, in the presence and absence of 6 M GuHCl, on a Varian (Cary) Eclipse florescence spectrophotometer with a 1-ml quartz cuvette. The excitation wavelength was 295 nm, and the width of both slits set to 5 nm. 295 nm instead of the maximal excitation wavelength of ~280 nm was used to minimize spectral interference from Tyr fluorescence.
Surface plasmon resonance
HNP2 interacting with isolated pro segments, i.e., wtpro peptide, crpro peptide, polyR, polyS and PEG, was evaluated by surface plasmon resonance on a BIAcore 3000 instrument. Synthetic HNP2 in 10 mM acetate buffer, pH 5.5, was covalently coupled to CM5 carboxylated dextran chips using N-hydroxysuccinimide (NHS) and N-ethyl-N’-(3-diethylamino-propyl)carbodiimide chemistry. Uncoupled NHS-ester groups were blocked with 1 M ethanolamine, and about 700 resonance units (RU’s) of HNP2 were coupled to the sensor chip by this procedure. Kinetic analysis was carried out at 25 °C in 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20, pH 7.4. Different concentrations of pro segments were injected at a flow rate of 20 µl/min over each flow cell, and binding was experimentally determined by comparing the number of RU’s observed as a function of time to an activated and blocked flow cell containing no covalently bound HNP2. Non-specific binding of pro segments to the control flow cell was insignificant. BmBKTx1, a cationic peptide toxin from scorpion 54, was also immobilized on the chip (850 RU’s) to evaluate non-specific binding of pro segments to non-defensin molecules.
LUVs leakage assay
LUVs encapsulating the low molecular weight fluorophore/quencher pair (8-aminonaphthalene-1,3,6-trisulfonic acid sodium salt and p-xylenebis(pyridinium) bromide, or ANTS/DPX) were prepared using the standard extrusion method. Specifically, phospholipids (palmitoyl-oleoyl-phosphatidylglycerol or POPG and dipalmitoyl-phosphatidylcholine or DPPC) were dissolved in chloroform at a desired molar ratio, dried as a film by solvent evaporation. After removal of residual solvent, the lipid film was hydrated in the fluorescent solution containing 5 mM HEPES, 12.5 mM ANTS, 45 mM DPX, and 20 mM NaCl, pH 7.0, freeze-thawed for 10 cycles and extruded 10 times through 0.4-µm polycarbonate membranes. LUVs were separated from unencapsulated materials by gel filtration chromatography using a Sepharose CL-4B column eluted with 5 mM HEPES, 100 mM NaCl, pH 7.4.
Leakage of ANTS from LUVs, monitored on a LS-55 Perkin Elmer luminescence spectrometer, was characterized by an increase in fluorescence, which was quenched by DPX when encapsulated together inside liposomes 55. 270 µl ANTS/DPX-encapsulated LUVs were added to each well of a 96-well plate to a final lipid concentration of 600 µM. 30 µl H2O was added to the first well of each row as a blank, and 30 µl 2.5% (v/v) Triton X-100 to the last (twelfth) well as the control for 100% leakage. Upon addition of 30 µl of a twofold dilution series of peptide and incubation for 1 h, the fluorescence signal was recorded at 515 nm with an excitation wavelength of 353 nm, 10 nm bandwidths and a 390 nm cut-off filter in the emission path. Percent leakage is expressed as:
where Ft is the fluorescence determined at different time points after addition of peptide, F0 is the background fluorescence of the “blank” cells, and F100 is the fluorescence of the control cells containing 0.25% Triton X-100. For titration experiments, 20 µM HNP2 was pre-incubated for 5 min with varying concentrations of wtpro peptide or crpro peptide (0.4 to 200 µM) before being added to LUVs.
Bactericidal activity assay
Antimicrobial assays against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 were conducted using a previously described 96-well turbidimetric method dubbed virtual colony counting 56. A two-fold dilution series of peptide ranging from 0.2 to 50 µM was used in the dose-dependent survival assay. For titration experiments, a fixed concentration of HNP2 at 10 µM was pre-incubated for 5 min with varying concentrations of wtpro peptide or crpro peptide (from 0.35 to 90 µM) before being mixed with bacteria, and wtpro peptide or crpro peptide alone was used as a control.
Acknowledgement
This work was supported by the National Institutes of Health Grants AI056264 and AI061482 (to W.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
HNP1 differs from HNP2 by an extra Ala residue at the N-terminus. Since native chemical ligation requires an N-terminal Cys in the second (downstream) peptide, HNP2, which begins with an N-terminal Cys residue, was used in the reaction. To generate HNP1-based ligation products, however, an Ala residue was inserted after the dipeptide linker in the first (upstream) peptide.
References
- 1.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 3.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 4.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 5.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 7.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 8.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 9.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffox MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 10.Gabay JE, Scott RW, Campanelli D, Griffith J, Wilde C, Marra MN, Seeger M, Nathan CF. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989;86:5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. Primary structures of three human neutrophil defensins. J Clin Invest. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilde CG, Griffith JE, Marra MN, Snable JL, Scott RW. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J Biol Chem. 1989;264:11200–11203. [PubMed] [Google Scholar]
- 13.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valore EV, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79:1538–1544. [PubMed] [Google Scholar]
- 15.Liu L, Ganz T. The pro region of human neutrophil defensin contains a motif that is essential for normal subcellular sorting. Blood. 1995;85:1095–1103. [PubMed] [Google Scholar]
- 16.Valore EV, Martin E, Harwig SS, Ganz T. Intramolecular inhibition of human defensin HNP-1 by its propiece. Journal of Clinical Investigation. 1996;97:1624–1629. doi: 10.1172/JCI118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Prahl A, Powell R, Ericksen B, Lubkowski J, Lu W. From pro defensins to defensins: synthesis and characterization of human neutrophil pro α-defensin-1 and its mature domain. J Pept Res. 2003;62:53–62. doi: 10.1034/j.1399-3011.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XL, Ohta Y, Jordan F, Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989;339:483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]
- 19.Silen JL, Agard DA. The alpha-lytic protease pro-region does not require a physical linkage to activate the protease domain in vivo. Nature. 1989;341:462–464. doi: 10.1038/341462a0. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Powell R, Lu W. Productive folding of human neutrophil α-defensins without the pro-peptide. Journal of the American Chemical Society. 2003;125:2402–2403. doi: 10.1021/ja0294257. [DOI] [PubMed] [Google Scholar]
- 21.Truhlar SM, Cunningham EL, Agard DA. The folding landscape of Streptomyces griseus protease B reveals the energetic costs and benefits associated with evolving kinetic stability. Protein Sci. 2004;13:381–390. doi: 10.1110/ps.03336804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim J, Lubkowski J, Lu W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human β-defensin 3. Proc Natl Acad Sci U S A. 2003;100:8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hristova K, Selsted ME, White SH. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J Biol Chem. 1997;272:24224–24233. doi: 10.1074/jbc.272.39.24224. [DOI] [PubMed] [Google Scholar]
- 24.Fujii G, Selsted ME, Eisenberg D. Defensins promote fusion and lysis of negatively charged membranes. Protein Sci. 1993;2:1301–1312. doi: 10.1002/pro.5560020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanabe H, Qu X, Weeks CS, Cummings JE, Kolusheva S, Walsh KB, Jelinek R, Vanderlick TK, Selsted ME, Ouellette AJ. Structure-activity determinants in paneth cell alpha-defensins: loss-of-function in mouse cryptdin-4 by charge-reversal at arginine residue positions. J Biol Chem. 2004;279:11976–11983. doi: 10.1074/jbc.M310251200. [DOI] [PubMed] [Google Scholar]
- 26.Wimley WC, Selsted ME, White SH. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 1994;3:1362–1373. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie C, Prahl A, Ericksen B, Wu Z, Zeng P, Li X, Lu WY, Lubkowski J, Lu W. Reconstruction of the Conserved {beta}-Bulge in Mammalian Defensins Using D-Amino Acids. J Biol Chem. 2005;280:32921–32929. doi: 10.1074/jbc.M503084200. [DOI] [PubMed] [Google Scholar]
- 28.Xie C, Zeng P, Ericksen B, Wu Z, Lu WY, Lu W. Effects of the terminal charges in human neutrophil alpha-defensin 2 on its bactericidal and membrane activity. Peptides. 2005 doi: 10.1016/j.peptides.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- 30.Fersht A. Structure and Mechanism in Protein Science. New York: W. H. Freeman and Company; 1999. [Google Scholar]
- 31.Sauter NK, Mau T, Rader SD, Agard DA. Structure of alpha-lytic protease complexed with its pro region. Nat Struct Biol. 1998;5:945–950. doi: 10.1038/2919. [DOI] [PubMed] [Google Scholar]
- 32.Jain SC, Shinde U, Li Y, Inouye M, Berman HM. The crystal structure of an autoprocessed Ser221Cys-subtilisin E-propeptide complex at 2.0 A resolution. J Mol Biol. 1998;284:137–144. doi: 10.1006/jmbi.1998.2161. [DOI] [PubMed] [Google Scholar]
- 33.Sohl JL, Jaswal SS, Agard DA. Unfolded conformations of alpha-lytic protease are more stable than its native state. Nature. 1998;395:817–819. doi: 10.1038/27470. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Hu Z, Jordan F, Inouye M. Functional analysis of the propeptide of subtilisin E as an intramolecular chaperone for protein folding. Refolding and inhibitory abilities of propeptide mutants. J Biol Chem. 1995;270:25127–25132. doi: 10.1074/jbc.270.42.25127. [DOI] [PubMed] [Google Scholar]
- 35.Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999;8:1668–1674. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Berg B, Ellis RJ, Dobson CM. Effects of macromolecular crowding on protein folding and aggregation. Embo J. 1999;18:6927–6933. doi: 10.1093/emboj/18.24.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra R, Seckler R, Bhat R. Efficient refolding of aggregation-prone citrate synthase by polyol osmolytes: how well are protein folding and stability aspects coupled? J Biol Chem. 2005;280:15553–15560. doi: 10.1074/jbc.M410947200. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto Y, Tsutsumi Y, Yoshioka Y, Nishibata T, Kobayashi K, Okamoto T, Mukai Y, Shimizu T, Nakagawa S, Nagata S, Mayumi T. Site-specific PEGylation of a lysine-deficient TNF-alpha with full bioactivity. Nat Biotechnol. 2003;21:546–552. doi: 10.1038/nbt812. [DOI] [PubMed] [Google Scholar]
- 39.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 40.Kochendoerfer GG, Chen SY, Mao F, Cressman S, Traviglia S, Shao H, Hunter CL, Low DW, Cagle EN, Carnevali M, Gueriguian V, Keogh PJ, Porter H, Stratton SM, Wiedeke MC, Wilken J, Tang J, Levy JJ, Miranda LP, Crnogorac MM, Kalbag S, Botti P, Schindler-Horvat J, Savatski L, Adamson JW, Kung A, Kent SB, Bradburne JA. Design and chemical synthesis of a homogeneous polymer-modified erythropoiesis protein. Science. 2003;299:884–887. doi: 10.1126/science.1079085. [DOI] [PubMed] [Google Scholar]
- 41.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 43.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipids bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 44.Sitaram N, Nagaraj R. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim Biophys Acta. 1999;1462:29–54. doi: 10.1016/s0005-2736(99)00199-6. [DOI] [PubMed] [Google Scholar]
- 45.Blondelle SE, Lohner K, Aguilar M. Lipid-induced conformation and lipid-binding properties of cytolytic and antimicrobial peptides: determination and biological specificity. Biochim Biophys Acta. 1999;1462:89–108. doi: 10.1016/s0005-2736(99)00202-3. [DOI] [PubMed] [Google Scholar]
- 46.Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 47.Digilio G, Barbero L, Bracco C, Corpillo D, Esposito P, Piquet G, Traversa S, Aime S. NMR structure of two novel polyethylene glycol conjugates of the human growth hormone-releasing factor, hGRF(1–29)-NH2. J Am Chem Soc. 2003;125:3458–3470. doi: 10.1021/ja021264j. [DOI] [PubMed] [Google Scholar]
- 48.Weeks CS, Tanabe H, Cummings JE, Crampton SP, Sheynis T, Jelinek R, Vanderlick TK, Cocco MJ, Ouellette AJ. Matrix metalloproteinase-7 activation of mouse paneth cell pro-alpha-defensins: SER43 down arrow ILE44 proteolysis enables membrane-disruptive activity. J Biol Chem. 2006;281:28932–28942. doi: 10.1074/jbc.M602041200. [DOI] [PubMed] [Google Scholar]
- 49.Dawson PE, Kent SB. Synthesis of native proteins by chemical ligation. Annu Rev Biochem. 2000;69:923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]
- 50.Dawson PE, Muir TW, Clark_Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 51.Schnolzer M, Alewood P, Jones A, Alewood D, Kent SB. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int J Pept Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 52.Kent SB. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1988;57:957–989. doi: 10.1146/annurev.bi.57.070188.004521. [DOI] [PubMed] [Google Scholar]
- 53.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu CQ, Brone B, Wicher D, Bozkurt O, Lu WY, Huys I, Han YH, Tytgat J, Van Kerkhove E, Chi CW. BmBKTx1, a novel Ca2+-activated K+ channel blocker purified from the Asian scorpion Buthus martensi Karsch. J Biol Chem. 2004;279:34562–34569. doi: 10.1074/jbc.M312798200. [DOI] [PubMed] [Google Scholar]
- 55.Ellens H, Bentz J, Szoka FC. H+- and Ca2+-induced fusion and destabilization of liposomes. Biochemistry. 1985;24:3099–3106. doi: 10.1021/bi00334a005. [DOI] [PubMed] [Google Scholar]
- 56.Ericksen B, Wu Z, Lu W, Lehrer RI. Antibacterial activity and specificity of the six human {alpha}-defensins. Antimicrob Agents Chemother. 2005;49:269–275. doi: 10.1128/AAC.49.1.269-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]