Abstract
Objective
To examine the association between routine childhood vaccinations and survival among infants in Guinea-Bissau.
Design
Follow up study.
Participants
15 351 women and their children born during 1990 and 1996.
Setting
Rural Guinea-Bissau.
Main outcome measures
Infant mortality over six months (between age 0-6 months and 7-13 months for BCG, diphtheria, tetanus, and pertussis, and polio vaccines and between 7-13 months and 14-20 months for measles vaccine).
Results
Mortality was lower in the group vaccinated with any vaccine compared with those not vaccinated, the mortality ratio being 0.74 (95% confidence interval 0.53 to 1.03). After cluster, age, and other vaccines were adjusted for, BCG was associated with significantly lower mortality (0.55 (0.36 to 0.85)). However, recipients of one dose of diphtheria, tetanus, and pertussis or polio vaccines had higher mortality than children who had received none of these vaccines (1.84 (1.10 to 3.10) for diphtheria, tetanus, and pertussis). Recipients of measles vaccine had a mortality ratio of 0.48 (0.27 to 0.87). When deaths from measles were excluded from the analysis the mortality ratio was 0.51 (0.28 to 0.95). Estimates were unchanged by controls for background factors.
Conclusions
These trends are unlikely to be explained exclusively by selection biases since different vaccines were associated with opposite tendencies. Measles and BCG vaccines may have beneficial effects in addition to protection against measles and tuberculosis.
Introduction
Measles vaccine is strongly associated with better childhood survival in developing countries.1,2 Since this effect cannot be explained by the specific prevention of measles,1,3,4 standard measles vaccine may be associated with a non-specific beneficial activation of the immune system.1 This effect would be observed only in areas with high mortality.5 Similar studies of BCG, polio, and diphtheria, tetanus, and pertussis vaccines have not been carried out in countries with a high mortality.
Worldwide, BCG is the most widely used vaccine and has been recommended for tuberculosis control in developing countries for more than 40 years. The protection provided by BCG is controversial as it has variable efficacy in different settings.6,7 Routine vaccinations with diphtheria, tetanus, and pertussis vaccine and polio vaccine provide good protection against the specific diseases. The recommended schedule is based on studies of seroconversion and protection and on assumed feasibility of the schedule.8 The effect of these vaccines has been assumed to be proportionate to the impact of the specific infections.
Guinea-Bissau in West Africa is one of the world's poorest countries. It has the sixth highest childhood mortality according to Unicef estimates.9 Since the early 1990s we have followed a representative cohort of 10 000 mothers and their children from the rural areas of Guinea-Bissau. Because the survival of recipients of routine vaccines has not been investigated in areas with high mortality, we examined the association between vaccination and survival in rural Guinea-Bissau.
Participants and methods
Cohort study
A longitudinal study of women of fertile age and their prospectively registered offspring was initiated in 1990 in the five most populous regions of Guinea-Bissau. The study was set up to assess mortality, including perinatal, childhood, and maternal mortality,10 and to monitor the use of health services. In each region, 20 clusters of 100 women were selected by the method recommended by the Expanded Programme on Immunisation for surveys of immunisation coverage. The children were followed to death, migration, or the age of 5 years; there was no loss to follow up because it was always possible to get information on all children from relatives living in the same compound. Data were collected by a mobile team of five to six assistants from the Bandim Health Project.
Vaccination status
The vaccination schedule recommended in Guinea-Bissau is BCG and polio at birth; diphtheria, tetanus, and pertussis and polio at 6, 10, and 14 weeks; and measles at 9 months of age. At each visit, vaccination status was determined by inspection of the immunisation card. Children who had no date on their card or who were declared to have received no vaccination were considered unvaccinated. We excluded children whose cards could not be inspected because the mother was absent or the card could not be found. Since we could not advise communities about the team's visit, many mothers were away on the day of the visit. Most mothers in rural Guinea-Bissau keep their children's vaccination cards with their personal belongings locked in a trunk. Hence, if the mother was not present, the vaccination card could not be seen.
Analysis and statistical methods
We estimated the effect of vaccinations by analysing mortality according to the vaccination status assessed at the initial visit. Information on deaths was obtained at the subsequent visit, and therefore children had to be visited twice to be included in the study. Intervals between visits were mostly 5 to 7 months but could be longer for logistic reasons, particularly because of inaccessibility during the rainy season. To minimise variability in length of follow up, mortality was assessed between the initial visit and the date of the following visit or six months later, if the following visit occurred more than six months later. As children were 0-6 months old when first seen, the impact of BCG, polio, and diphtheria, tetanus, and pertussis vaccines was assessed between the initial visit and the following visit or six months later. Survival over a longer follow up period would be confounded increasingly by the effect of measles vaccine. We calculated estimates separately for recipients of polio and diphtheria, tetanus, and pertussis vaccines, but these were virtually identical as the two vaccines are always administered together. The effect of measles vaccine was examined between the second visit at 7-13 months of age and the subsequent visit or six months later.
We examined differences in the prevalence ratio for background factors for vaccinated and unvaccinated children using a generalised linear model with binomial variability and a logarithmic link function adjusting for age.11,12 We also corrected for cluster using generalised estimation equations with an exchangeable correlation structure corresponding to a random cluster effect.13
To estimate the mortality ratio for vaccinated and unvaccinated children, we used a Cox proportional hazards model,14 taking length of follow up into consideration and adjusting by stratification for age, period, season, and cluster. By stratifying for cluster we also controlled for differences between the five regions; in fact, we compared survival for vaccinated and unvaccinated children in the same village. Vaccination coverage has changed over time as have death rates, and control for period was therefore important. Since most vaccines are given in campaigns during the dry season from November to June, and mortality is higher in the rainy season, it was also essential to control for season. Neither season nor period affected the estimates of mortality associated with vaccination, and the results are therefore not reported here (data available on request). Other background factors were controlled for in the same Cox model. Mortality differences between vaccinated and unvaccinated children were also estimated with other statistical models, but the results were essentially the same (data available on request).
Results
Study population and vaccination coverage
Between February 1990 and April 1996, we registered 15 351 women of fertile age and 11 460 pregnancies; 392 women moved before giving birth. The 11 068 pregnancies resulted in 333 abortions, and 437 stillbirths. This left 10 298 children, of whom 686 died, 90 moved before the first visit, and 770 were too young to have received two visits and could therefore not be included in the survival analysis. The remaining 8752 children were alive at the first visit and their survival ascertained at the second visit; 8104 were under 7 months old when first seen. Of the 8752 children, 429 died and 214 moved between the first and second visit, and 752 had no third visit before the end of the study, leaving 7357 children to be followed between the second and third visit. Of the 7357 children, 323 died and 183 moved before the third visit.
At the first visit, 5754 (66%) had their vaccination card inspected; 2981 (34%) were absent and 17 (0.2%) had lost their card. Eight per cent of the children received BCG within the first two days of life and 50% (3388/6771) within the first month, the median age of vaccination being 31 days. The median age for first diphtheria, tetanus, and pertussis dose was 87 days.
Of the 8104 children aged 0-6 months, 5274 had a vaccination card examined or had had no card; 63% (3301/5274) were vaccinated with BCG. Of the 2830 children whose vaccination card was not seen at the first visit, 1094 had their card examined at the following visit and 53% (576/1094) had had BCG vaccination before the first visit. Hence, children whose card was not seen at the initial visit constitute a travelling group less likely to turn up at the following visit and with a lower BCG coverage (relative risk=0.82, 95% confidence interval 0.77 to 0.88). For the assessment of measles vaccine, 7357 children were alive at the second visit and examined at the third visit; 4230 (58%) had their vaccination card inspected, 3091 (42%) were absent, and 36 (0.5%) had lost the card or had missing information. The median age for measles vaccination was 10.6 months. Table 1 shows vaccine coverage at ages 0 and 18 months.
Table 1.
Coverage for routine vaccinations at different ages, Guinea-Bissau, 1990-6
| Age (months) | Coverage (%)
|
|||||
|---|---|---|---|---|---|---|
| BCG | DTP (1)* | DTP (3)† | Polio (1)* | Polio (3)† | Measles | |
| 0-2 | 49 | 17 | 0 | 17 | 0 | 0 |
| 3-5 | 76 | 65 | 9 | 65 | 9 | 0 |
| 6-8 | 85 | 81 | 32 | 81 | 31 | 6 |
| 9-11 | 87 | 85 | 45 | 85 | 44 | 38 |
| 12-14 | 90 | 89 | 58 | 89 | 57 | 65 |
| 15-17 | 92 | 92 | 64 | 92 | 64 | 77 |
DTP=diphtheria, tetanus, and pertussis vaccine. *One dose. †Three doses.
We examined differences in background factors for vaccinated and unvaccinated children controlling for age and cluster (table 2). Vaccinated children had more contact with the health system, their mothers being more likely to have received tetanus vaccination during pregnancy and to have given birth outside the home. Vaccinated children had larger arm circumference and their mothers tended to be younger and have fewer children, to have a latrine in their compound, and not to belong to the Balanta or Pepel ethnic groups.
Table 2.
Prevalence ratio of background factors for vaccinated and unvaccinated children according to type of vaccine, Guinea-Bissau, 1990-6
| Background factor | No (%) whose vaccine card was not seen* | No (% ) in vaccine group* | Prevalence ratio (95% CI)†
|
||
|---|---|---|---|---|---|
| BCG | DTP | Measles | |||
| Sex (male/female) | 1423/2826 (50.4) | 1680/3300 (50.6) | 1.00 (0.97 to 1.04) | 0.93 (0.94 to 1.03) | 0.98 (0.91 to 1.06) |
| Mother had tetanus vaccine (yes/no) | 609/2830 (21.5) | 1756/3301 (53.2) | 1.20 (1.15 to 1.25) | 1.20 (1.13 to 1.27) | 1.18 (1.07 to 1.31) |
| Birth at home (yes/no) | 1924/2619 (73.5) | 2234/3283 (68.1) | 0.89 (0.86 to 0.93) | 0.93 (0.88 to 0.99) | 0.84 (0.75 to 0.94) |
| Mid upper arm circumference (<125/⩾125 mm) | 249/505 (49.3) | 1132/2608 (43.4) | 0.98 (0.93 to 1.03) | 0.90 (0.85 to 0.97) | 0.93 (0.83 to 1.03) |
| Maternal age (14-19/⩾20) | 627/2825 (22.1) | 702/3297 (21.3) | 1.04 (0.99 to 1.08) | 1.14 (1.04 to 1.17) | 1.07 (0.95 to 1.20) |
| Birth order (1-3/⩾4) | 1305/2824 (46.2) | 1409/3298 (42.7) | 1.01 (0.98 to 1.05) | 1.07 (1.02 to 1.12) | 1.08 (0.99 to 1.18) |
| Previous dead child (⩾1/0) | 1483/2819 (52.6) | 1704/3298 (51.8) | 0.98 (0.94 to 1.01) | 0.94 (0.90 to 0.98) | 0.98 (0.89 to 1.08) |
| Mother's schooling (some/0) | 346/2774 (12.5) | 479/3263 (14.7) | 1.00 (0.95 to 1.05) | 1.06 (0.99 to 1.13) | 1.05 (0.90 to 1.23) |
| Well (yes/no) | 879/2684 (32.8) | 1132/3129 (36.2) | 1.04 (0.99 to 1.08) | 1.07 (1.00to 1.13) | 1.11 (0.98 to 1.25) |
| Latrine (yes/no) | 957/2707 (35.4) | 1336/3146 (42.5) | 1.07 (1.02 to 1.13) | 1.11 (1.04 to 1.18) | 1.15 (1.03 to 1.28) |
| Ethnic group | |||||
| Balanta v others‡ | 616/2826 (21.6) | 474/3299 (14.4) | 0.84 (0.77 to 0.93) | 0.84 (0.73 to 0.97) | 0.72 (0.56 to 0.93) |
| Fula v others‡ | 643/2826 (22.8) | 819/3299 (24.8) | 1.01 (0.92 to 1.10) | 1.04 (0.94 to 1.15) | 0.95 (0.80 to 1.13) |
| Mandinga v others‡ | 428/2826 (15.2) | 730/3299 (22.1) | 1.00 (0.92 to 1.08) | 1.01 (0.89 to 1.15) | 0.92 (0.77 to 1.10) |
| Pepel v others‡ | 791/2826 (28.0) | 764/3299 (23.2) | 0.85 (0.76 to 0.95) | 0.89 (0.73 to 1.08) | 0.86 (0.66 to 1.12) |
DTP=diphtheria, tetanus, and pertussis vaccine.
Percentage in first category of background factor.
Prevalence ratio between vaccinated and unvaccinated children for first category of factor, adjusted for age and cluster. Analysis is based on 5274 children in the BCG analysis (table 3), 3972 in diphtheria, tetanus, and pertussis analysis, and 3414 in the measles analysis (table 5) plus the 2830 children whose card was not seen.
Ethnic groups other than Balanta, Fula, Mandinga, or Pepel.
BCG vaccine
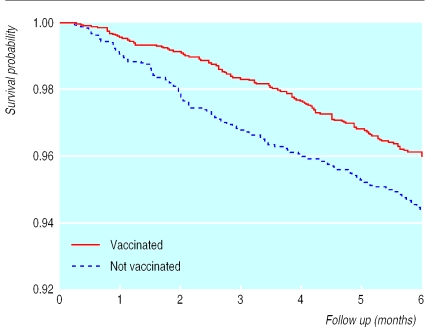
Mortality for the 5274 children aged 0-6 months was lower in the group vaccinated with any vaccine compared with those not vaccinated, the mortality ratio being 0.74 (95% confidence interval 0.53 to 1.03). For children vaccinated with BCG the mortality ratio was 0.72 (0.54 to 0.96) (table 3, fig 1). The ratio became 0.55 (0.36 to 0.85) after age, diphtheria, tetanus, and pertussis vaccine, and cluster were adjusted for; estimates varied between 0.50 and 0.58 and were significant when background factors were controlled for. If we excluded children who were considered unvaccinated because they had no vaccination card, the mortality ratio for BCG vaccine among children who were seen was 0.33 (0.17 to 0.65).
Table 3.
Mortality during six months of follow up according to BCG vaccination status and age at intial visit, Guinea-Bissau, 1990-6
| Age (months) | Mortality (%)
|
|
|---|---|---|
| Vaccinated | Not vaccinated | |
| 0-1 | 3.0 (21/705) | 5.2 (55/1058) |
| 2-3 | 3.1 (37/1187) | 4.8 (26/539) |
| 4-6 | 4.8 (67/1409) | 4.3 (16/376) |
| Total | 3.8 (125/3301) | 4.9 (97/1973) |
Figure 1.
Kaplan-Meier survival curves for children who did and did not receive BCG vaccine. Six months' follow up of 5274 children aged 0-6 months at initial visit, Guinea-Bissau, 1990-6
The effect of BCG was stronger when the analysis was restricted to infants aged 0 to 2 months, who would not receive measles vaccine during the next six months (mortality ratio 0.43 (0.25 to 0.75)). Since children weighing less than 2500 g are not supposed to receive BCG, unvaccinated children could represent a negatively selected group. After 31 days of age all children would presumably weigh more than 2500 g; we therefore analysed data on children aged 1 to 2 months who were either unvaccinated or vaccinated with BCG after 31 days of life. The protective effect of BCG was high in this subgroup (0.19 (0.04 to 0.95)).
Diphtheria, tetanus, and pertussis and polio vaccines
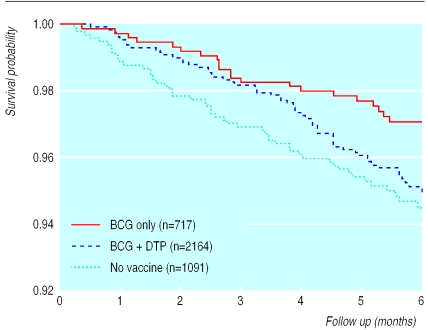
Since diphtheria, tetanus, and pertussis and polio vaccines are administered from 6 weeks of age, the analyses were limited to children aged 1.5-6 months at the initial visit. Of the 5274 children examined at 0-6 months of age, 3972 were aged 1.5-6 months; 1822 had no dose of diphtheria, tetanus, and pertussis vaccine (72 died before the second visit), 1295 had received one dose (62 died), and 855 had had two or more doses (32 died). After age, BCG vaccination, and cluster were adjusted for in a Cox analysis estimating the effect of one and two to three doses separately, one dose of diphtheria, tetanus, and pertussis vaccine was associated with a mortality ratio of 1.84 (1.10 to 3.10) and two to three doses with a ratio of 1.38 (0.73 to 2.61) compared with children who had received no dose of these vaccines (table 4). Estimates were similar for polio vaccine, one dose being associated with a mortality ratio of 1.81 (1.07 to 3.05) and two to three doses with a ratio of 1.39 (0.73 to 2.64). Mortality was also increased in the analysis combining one to three doses of diphtheria, tetanus, and pertussis (1.72 (1.03 to 2.87)) and diphtheria, tetanus, and pertussis or polio vaccine (1.67 (1.00 to 2.77) fig 2). Estimates for one dose of diphtheria, tetanus, and pertussis vaccine varied between 1.72 and 1.98 when background factors were controlled for. However, adjustment for arm circumference increased the effect of one dose of diphtheria, tetanus, and pertussis to 2.50 (1.31 to 4.78). If we excluded children considered unvaccinated because they had no card, the mortality ratio for one to three doses of diphtheria, tetanus, and pertussis vaccine among children whose card was seen was 1.78 (1.05 to 3.02)
Table 4.
Mortality during six months of follow up among children who did and did not receive one dose of diphtheria, tetanus, and pertussis vaccine* according to BCG vaccination status and age at inital visit, Guinea-Bissau, 1990-6
| Age (months) | Mortality (%)
|
|
|---|---|---|
| Vaccinated | Not vaccinated | |
| 1.5-3 | ||
| No BCG | 10.0 (1/10) | 4.9 (36/734) |
| BCG | 3.9 (28/709) | 2.5 (14/560) |
| 4-6 | ||
| No BCG | 11.1 (1/9) | 4.2 (15/357) |
| BCG | 5.6 (32/567) | 4.1 (7/171) |
| Total | 4.8 (62/1295) | 4.0 (72/1822) |
Children who had received more than one dose of diphtheria, tetanus, and pertussis vaccine are not included.
Figure 2.
Kaplan-Meier survival curves for recipients of BCG , recipients of BCG and at least one dose of diphtheria, tetanus, and pertussis (DTP) or polio vaccine, and non-recipients of all vaccines. Six months' follow up of 3972 children aged 1.5-6 months at initial visit, Guinea-Bissau, 1990-6
Measles vaccine
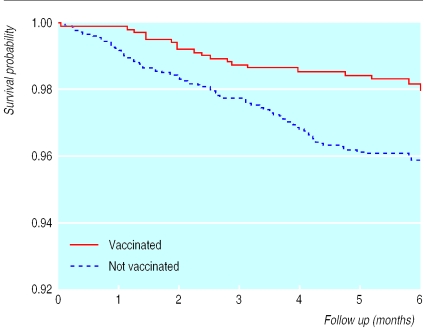
Children aged 7 to 13 months who had received measles vaccine at the second visit had a mortality ratio of 0.51 (0.30 to 0.85) compared with unvaccinated children (table 5 ; fig 3). The ratio was 0.48 (0.27 to 0.87) after age, BCG vaccination, and cluster were adjusted for and varied from 0.45 to 0.56 when background factors were controlled for, all estimates except one being significant. The estimate was unaffected by controls for other vaccinations. If we excluded children considered unvaccinated because they had no card, the mortality ratio for measles vaccine among children whose card was seen was 0.48 (0.27 to 0.87). The reduction in mortality was unrelated to measles deaths; 9 of 94 deaths in the unvaccinated and 1 of 19 deaths in the vaccinated groups were reported to be due to measles (table 5). When deaths from acute measles were excluded, the mortality ratio was 0.51 (0.28 to 0.95) for children vaccinated against measles compared with initially unvaccinated children.
Table 5.
Mortality during six months of follow up according to measles vaccination status and age at start of six months, Guinea-Bissau, 1990-6
| Age (months) | Mortality (%)
|
|
|---|---|---|
| Vaccinated | Not vaccinated | |
| 7-8 | 3.3 (3/90) | 4.4 (48/1089) |
| 9-11 | 1.6 (11/701) | 3.6 (41/1130) |
| 12-13 | 2.0 (5/245) | 3.1 (5/159) |
| Total | 1.8 (19/1036) | 4.0 (94/2378) |
Figure 3.
Kaplan-Meier survival curves for recipients and non-recipients of measles vaccine. Six months' follow up of 3414 children initially aged 7-13 months, Guinea-Bissau, 1990-6
Early and later vaccination
Many children, although classified as unvaccinated initially, received vaccination before the next visit. As information was unavailable for children who moved, died, or were travelling, these additional vaccinations could not be included in the survival analysis. To estimate the effect of BCG and diphtheria, tetanus, and pertussis or polio vaccination of initially unvaccinated children, we assessed mortality between the second and third visits for children vaccinated before or after the first visit; for measles vaccine we examined mortality between the third or fourth visits. Of 1168 children who had not received BCG vaccine at the first visit, 765 (65.5%) had received BCG at the second visit. There was no difference in mortality between those who received vaccine early or late (mortality ratio 1.36 (0.73 to 2.53) after age, diphtheria, tetanus, and pertussis vaccine, and cluster were adjusted for). Of 1082 children who had not received diphtheria, tetanus, and pertussis vaccine at the first visit, 772 (71.3% had received at least one dose at the second visit, with no difference between those who received vaccine early or late (1.10 (0.59 to 2.03)). Of 1371 children who had not received measles vaccine at the second visit, 864 (63.0%) had received measles vaccine at the third visit, with no difference in mortality between those who received the vaccine early or late (1.07 (0.40 to 2.87)).
Infants whose mothers were not at home to show a vaccination card could constitute a special group in which vaccination had a different effect. We therefore examined whether the effect of measles vaccination on children absent at the first visit but seen at the second visit was different from that in other children. There was no such difference; the mortality ratio for measles vaccination compared with no measles vaccination was 0.39 (0.13 to 1.19) for children whose card had not been seen in the first round and 0.52 (0.27 to 0.99) for children whose vaccination card had been seen during the first round.
Discussion
Research on vaccines in developing countries recommended by the World Health Organization has emphasised serological responses and protection against specific diseases.1 The aim of the research has been to optimise vaccine schedules for control, elimination, or eradication of disease. In modelling exercises, vaccination against diphtheria, pertussis, tetanus, and polio has been assumed to save 1.5-2.0% of the children in areas with high infant mortality.15 However, these assumptions are not supported by data, and few studies of the effect of routine vaccinations other than measles on mortality have been carried out in developing countries.16 Case-control studies in Benin and Brazil found that BCG vaccination is associated with better survival.17,18 We reported from Senegal that children receiving diphtheria, tetanus, and pertussis vaccination had slightly higher mortality (mortality ratio=1.59 (0.76 to 3.33)),1 and a case-control study from Benin of 74 children who died had a similar finding (odds ratio=2.20 (0.93-5.22)).17
Methodological issues
In Guinea-Bissau, we found that BCG and measles vaccines were associated with better survival and diphtheria, tetanus, and pertussis and polio vaccines with higher mortality compared with no vaccination. The estimates are unlikely to be due to registration problems. The initial recruitment for the study was based on a random selection of clusters. The survival analysis had no loss to follow up, and the statistical model compared only vaccinated and unvaccinated children from the same community. We got information on vaccination status for around two thirds of the children, a high proportion given that the communities were not informed beforehand about the day of the visit. Furthermore, there was no indication that those not presenting a vaccination card reacted differently to the vaccines. Vaccines are appreciated by mothers in rural Guinea-Bissau, as shown by the high coverage for BCG and diphtheria, tetanus, and pertussis and polio (table 1), and there would seem no reason to fake vaccination dates on cards. However, some vaccinated children may have been registered as unvaccinated because a nurse forgot to note the date on the child's card or a guardian reported the child not having a card. Such misclassification would lessen rather than exaggerate the mortality differences presented here.
For methodological reasons, our estimates may be conservative. For all vaccines, children vaccinated before the initial visit and those vaccinated later had the same mortality in the following period. Children who received vaccine after the first visit may have had their mortality changed to that of children who had been vaccinated at the first visit. This would reduce the mortality ratio for recipients versus non-recipients of vaccine.
The effects we observed could be due to behavioural differences between mothers of vaccinated and unvaccinated children. Mothers of vaccinated children apparently had more frequent contact with the health care system (table 22). Having “concerned mothers” could possibly explain a beneficial effect associated with BCG and measles vaccines; however, the negative association with diphtheria, tetanus, and pertussis and polio vaccines is difficult to explain. Alternatively, sick children could be brought more often to health centres and therefore receive more vaccines. This seems unlikely as vaccine recipients had larger arm circumferences than unvaccinated children (table 2) and it would not explain the better survival of children who received BCG and measles vaccines.
Do BCG and measles vaccines have a non-specific beneficial effect?
The reduction in mortality after measles and BCG vaccination is larger than the proportion of deaths attributed to these diseases among infants and young children. As in previous studies,1 exclusion of measles deaths did not change the mortality ratio between vaccinated and unvaccinated children. A similar analysis could not be made for BCG since infant tuberculosis is poorly defined. Tuberculosis is estimated to have a much smaller effect on childhood mortality than measles.19 However, we found that the effect of BCG vaccine was as large as that of measles vaccine. It therefore seems implausible that the positive effect is merely due to BCG protecting against primary tuberculosis. The better survival of BCG recipients is unlikely to be related to non-vaccination of low birthweight infants because when we restricted the analysis to children vaccinated after 1 month of age the protective effect of BCG was strengthened. The effect of BCG was not due to early recipients constituting a group with better survival. Future studies should assess the extent to which the impact of BCG in childhood can be explained by prevention of tuberculosis.
BCG vaccine has been used to stimulate the immune system in certain chronic diseases, including cancer.20,21,22,23 In Guinea-Bissau and the Gambia, early BCG vaccination protected against atopy,24 and BCG vaccination at birth was associated with the stimulation of a Th1 type immune response.25 In Bissau, children with a BCG scar or a positive tuberculin test have lower mortality than vaccinated children without a scar or tuberculin reaction (unpublished data). The better survival associated with BCG vaccine could therefore reflect a non-specific protection against other infections as suggested for measles vaccine.1 Children vaccinated against diphtheria, tetanus, pertussis, and polio were more atopic than non-vaccinated children in Bissau.24 The fact that the adjuvant of diphtheria, tetanus, and pertussis, aluminium hydroxide, is a strong promoter of a Th2 type immune response in mice may be important.26
Implications of results
Our results will have to be interpreted with caution. As randomised trials could not have been organised, other forms of control of internal consistency and of possible differences between vaccinated and unvaccinated children have been necessary. Although the vaccinated and unvaccinated children were not directly comparable, controlling for differences in cultural and social background factors did not affect the mortality estimates for the different vaccines. Since virtually all children do eventually get BCG, polio, and diphtheria, tetanus, and pertussis vaccines in Guinea-Bissau, we essentially measured the effect of vaccination in the short period before some children had been vaccinated. Since subsequent mortality did not differ between children vaccinated early and late, the results cannot be ascribed to children who are vaccinated having a different mortality. Our results were also consistent with the few studies reported previously from other areas.1,17,18 Unless selection bias is switching back and forth at different ages, it seems difficult to explain both good survival for BCG and measles vaccine recipients and poor survival for recipients of diphtheria, tetanus, and pertussis and polio vaccines. Some of these vaccines are therefore likely to have major non-specific effects on child survival.1
We need to determine the biological basis and magnitude of the non-targeted effects of different vaccines. Such effects will be seen only in developing countries with high mortality, where vaccine induced forms of immunostimulation could affect the response to other infections. 25 If BCG vaccine has beneficial effects, these should be considered when testing new, more specific vaccines against tuberculosis in the future. The use of a new vaccine could be associated with higher mortality, as was the case when the high titre measles vaccine was introduced.27 The combined effect of BCG and polio and diphtheria, pertussis, and tetanus vaccines was protective against childhood mortality (mortality ratio 0.74, 95% confidence interval 0.53 to 1.03). Should diphtheria, pertussis, and tetanus or polio vaccines be found out to have negative effects on mortality despite their protection against specific diseases, new vaccine formulations might improve the effect of the vaccination programme.
Our observations emphasise the importance of immunisations in developing countries; vaccinated children had much lower mortality. However, it may be possible to obtain even better results. The current vaccination schedule, emphasising three early doses of diphtheria, tetanus, and pertussis and polio vaccine, is associated with low coverage for measles vaccine.1 A stronger emphasis on BCG and measles vaccines would contribute to lower mortality. Hence, changes in the vaccination schedules28 or type of vaccines used in developing countries could improve coverage and child survival.
What is already known on this topic
Measles vaccine may be associated with a non-specific survival benefit in countries with a high mortality
No attempt has been made to assess the effect of other routine immunisations on mortality in developing countries
What this study adds
BCG and measles vaccines were associated with reductions in mortality in rural Guinea-Bissau
The effect for measles vaccine could not be explained by the prevention of measles infection
Diphtheria, tetanus, and pertussis and polio vaccines were associated with higher infant mortality
Non-specific effects of routine immunisations should be considered when planning immunisation programmes in developing countries
Footnotes
Funding: Ministry of Public Health, Guinea-Bissau; Unicef, Guinea-Bissau; Danish Council for Development Research; Danish Medical Research Council; and European Union's science and technology for development programme (TS3*CT91*0002 and ERBIC 18 CT95*0011).
Competing interests: None declared.
References
- 1.Aaby P, Samb B, Simondon F, Coll Seck AM, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ. 1995;311:481–485. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aaby P, Andersen M, Sodemann, Jakobsen M, Gomes J, Fernandes M. Reduced childhood mortality following standard measles vaccination at 4-8 months compared with 9-11 months of age. BMJ. 1993;307:1308–1311. doi: 10.1136/bmj.307.6915.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenig MA, Khan MA, Wojtyniak B, Clemens JD, Chakraborty J, Fauveau V, et al. The impact of measles vaccination upon childhood mortality in Matlab, Bangladesh. Bull WHO. 1990;68:441–447. [PMC free article] [PubMed] [Google Scholar]
- 4.Aaby P, Pedersen IR, Knudsen K, da Silva MC, Mordhorst CH, Helm-Petersen NC, et al. Child mortality related to seroconversion or lack of seroconversion after measles vaccination. Pediatr Infect Dis J. 1989;8:197–200. [PubMed] [Google Scholar]
- 5.Aaby P, Samb B, Simondon F, Knudsen K, Coll Seck AM, Bennett J, et al. A comparison of vaccine efficacy and mortality during routine use of high-titre Edmonston-Zagreb and Schwarz standard measles vaccines in rural Senegal. Trans R Soc Trop Med Hyg. 1996;90:326–330. doi: 10.1016/s0035-9203(96)90275-8. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 7.Fine PEM. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay MEB, Rao M, Begg NT, Redhead K, Atwell AM. Antibody response to accelerated immunisation with diphtheria, tetanus, and pertussis vaccine. Lancet. 1993;342:203–205. doi: 10.1016/0140-6736(93)92298-8. [DOI] [PubMed] [Google Scholar]
- 9.United Nations Children's Fund. The state of the world's children 1998. Oxford: Oxford University Press; 1998. [Google Scholar]
- 10.Høj L, Stensballe J, Aaby P. Maternal mortality in Guinea-Bissau: the use of verbal autopsy in a multi-ethnic population. Int J Epidemiol. 1999;28:70–76. doi: 10.1093/ije/28.1.70. [DOI] [PubMed] [Google Scholar]
- 11.McCullagh P, Nelder JA. Generalized linear models. 2nd ed. London: Chapman and Hall; 1989. [Google Scholar]
- 12.Zochetti C, Consonni D, Bertazzi PA. Estimation of prevalence rate ratios from cross-sectional data. Int J Epidemiol. 1995;24:1064–1065. doi: 10.1093/ije/24.5.1064. [DOI] [PubMed] [Google Scholar]
- 13.Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press; 1994. [Google Scholar]
- 14.Cox DR, Oakes D. Analysis of survival data. London: Chapman and Hall; 1984. [Google Scholar]
- 15.Jamison DT, Torres AM, Chen LC, Melnick JL. Poliomyelitis. In: Jamison DT, Mosley WH, Measham AR, Bodadilla JL, editors. Disease control priorities in developing countries. New York: Oxford University Press; 1993. pp. 117–129. [Google Scholar]
- 16.Ivanoff B, Robertson SE. Pertussis: a worldwide problem. In: Brown F, Greco D, Mastrantonio P, Salmaso S, Wassilak S, eds. Pertussis vaccine trials. Dev Biol Stand. 1997;89:3–13. [PubMed] [Google Scholar]
- 17.Velema JP, Alihonou EM, Gandaho T, Hounye FH. Childhood mortality among users and non-users of primary health care in a rural West African community. Int J Epidemiol. 1991;20:474–479. doi: 10.1093/ije/20.2.474. [DOI] [PubMed] [Google Scholar]
- 18.Niobey FML, Duchiade MP, Vasconcelos AGG, de Cavalho ML, Leal MC, Valente JG. Fatores de risco para morte por pneumonia em menores de um ano em uma região metropolitana do sudeste do Brasil. Un estudo tipo caso-controle. Rev Saúde Públ, São Paolo. 1992;26:229–238. doi: 10.1590/s0034-89101992000400004. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization, United Nations Children's Fund. State of the world's vaccines and immunization. Geneva: WHO; 1996. [Google Scholar]
- 20.Lugosi L. Theoretical and methodological aspects of BCG vaccine from discovery of Calmette e Guerin to molecular biology. A review. Tuberc Lung Dis. 1992;73:252–261. doi: 10.1016/0962-8479(92)90129-8. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal SR. Surgery, recall antigens, immunity, and bacillus Calmette-Guerin vaccination. Am J Surg. 1988;156:1–3. doi: 10.1016/s0002-9610(88)80158-2. [DOI] [PubMed] [Google Scholar]
- 22.Herr HW, Schwalb DM, Zhang ZF, Sogani PC, Fair WR, Whitmore WF, et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13:1404–1408. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 23.Ravaud A, Eghbali H, Trojani M, Hoerni-Simon G, Soubeyran P, Hoerni B. Adjuvant bacillus Calmette-Guerin therapy in non-Hodgkin's malignant lymphomas: long-term results of a randomized trial in a single institution. J Clin Oncol. 1990;8:608–614. doi: 10.1200/JCO.1990.8.4.608. [DOI] [PubMed] [Google Scholar]
- 24.Aaby P, Shaheen S, Heyes C, Goudiaby A, Shiell A, Jensen H, et al. Early BCG vaccination and reduction in atopy in Guinea-Bissau. Clin Exp Allergy. 2000;30:644–650. doi: 10.1046/j.1365-2222.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- 25.Marchant A, Goetghebuer T, Ota M, Wolfe I, Ceesay S, Corrah T, et al. Newborns develop a T helper 1 type response to BCG vaccination. J Immunol. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 26.Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aaby P, Knudsen K, Whittle H, Thårup J, Poulsen A, Sodemann M, et al. Long-term survival after Edmonston-Zagreb measles vaccination: increased female mortality. J Pediatr. 1993;122:904–908. doi: 10.1016/s0022-3476(09)90015-4. [DOI] [PubMed] [Google Scholar]
- 28.Garly ML, Martins CL, Balé C, da Costa F, Dias F, Whittle H, et al. Early two-dose measles vaccination schedule in Guinea-Bissau: good protection and coverage in infancy. Int J Epidemiol. 1999;28:347–352. doi: 10.1093/ije/28.2.347. [DOI] [PubMed] [Google Scholar]