Summary
TGF-β blockade significantly slows tumor growth through many mechanisms, including activation of CD8+ T-cells and macrophages. Here, we show that TGF-β blockade also increases neutrophil-attracting chemokines resulting in an influx of CD11b+/Ly6G+ tumor-associated neutrophils (TAN) that are hypersegmented, more cytotoxic to tumor cells, and express higher levels of pro-inflammatory cytokines. Accordingly, following TGF-β blockade, depletion of these neutrophils significantly blunts anti-tumor effects of treatment and reduces CD8+ T-cell activation. In contrast, in control tumors, neutrophil depletion decreases tumor growth and results in more activated CD8+ T-cells intra-tumorally. Together, these data suggest that TGF-β within the tumor microenvironment induces a population of TAN with a pro-tumor phenotype. TGF-β blockade results in the recruitment and activation of TAN with an anti-tumor phenotype.
Keywords: tumor immunology, immunosuppression, TGFβ, tumor associated macrophages, Tumor associated neutrophils, lung cancer, mesothelioma
Introduction
Mounting evidence suggests that the immunosuppressive cytokine TGF-β is over-expressed by tumors and plays a significant role in blocking immune responses and affecting tumor progression. The pivotal role of TGF-β in suppressing anti-tumor immune responses has made it a logical target for the development of antagonists (Bierie and Moses, 2006). TGF–β blockers (soluble receptors/antibodies) and TGF–β receptor inhibitors have anti-tumor effects that, in several models, are due primarily to CD8+ T-cell dependent immunologic mechanisms (Ge et al., 2006; Nam et al., 2008; Suzuki et al., 2007).
In addition to suppressing T-cell functions, it has been shown that TGF-β also has an impact on myeloid cell functions. The tumor microenvironment polarizes tumor-associated macrophages toward a pro-tumor (M2) versus an anti-tumor (M1) phenotype (Allavena et al., 2008). Since TGF-β can alter macrophage cell function and phenotype in vitro (Lee et al., 2007; Tsunawaki et al., 1988), it may play an important role in regulating macrophage phenotype in vivo as well. Although less well studied, TGF-β has also been noted to inhibit neutrophil activity (i.e degranulation) (Shen et al., 2007). Early studies suggested that TGF-β had chemoattractant activity for neutrophils at very low concentrations (Reibman et al., 1991), more recent studies have suggested that blocking the TGF-β pathway increases the recruitment of neutrophils in some types of chronic disease states (Allen et al., 2008).
In recently published studies, we used a small, orally available type I TGF–β receptor (Alk-5/Alk-4) kinase inhibitor (SM16) and showed that TGF-β receptor blockade increased the percentage and activation of intra-tumoral CD8+ T-cells and was able to augment immunotherapy (Kim et al., 2008; Suzuki et al., 2007). In addition, blockade of TGF-β function led to an influx of myeloid cells (marked by CD11b positivity on FACS) into tumors. The goals of this study were to evaluate the effect of SM16 on the myeloid cell phenotype of tumors and to explore how these changes might affect CD8+ T cell function.
Results
Inhibition of TGF-β signaling increases intra-tumoral CD11b+ cells that express neutrophil (Ly6G+) rather than macrophage (Ly6G−) markers
To evaluate the role of myeloid (CD11b+) cells, mice bearing established flank tumors from three syngeneic models were fed with chow containing SM16 or control chow. Tumors were harvested and subjected to FACS to detect CD11b+ cells and different myeloid cell markers.
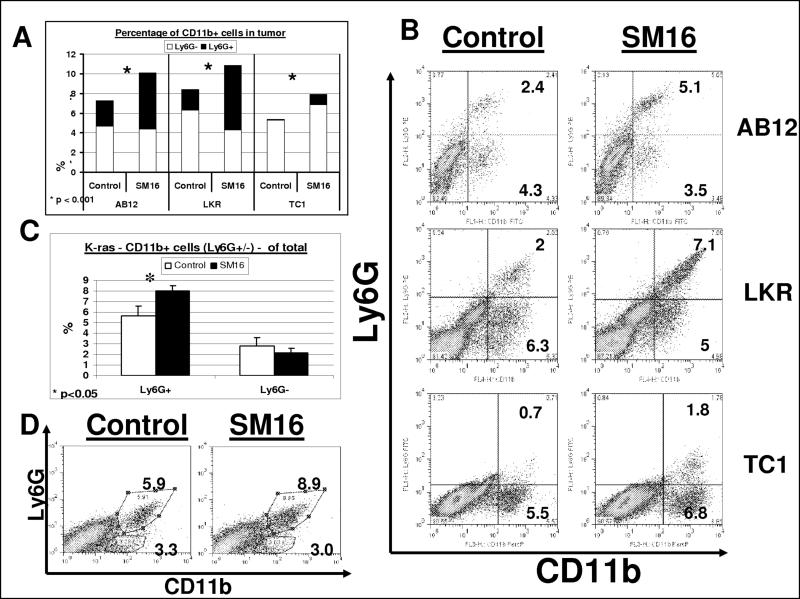
As shown in Figure 1A and 1B, administration of SM16 increased the percentage of CD11b+ cells in the tumors by 30−45% (p <0.02). To differentiate macrophages from neutrophils, we used the 1A8 anti-Ly6G antibody which is found only on neutrophils (Daley et al., 2008). SM16 treatment led to significant increases in the percentage of intra-tumoral Ly6G+ cells and only minor changes in the Ly6G− cells (mostly macrophages). As seen in Figure 1B, virtually all the Ly6G+ cells were also CD11b+.
Figure 1. SM16 causes an influx of CD11b+ Ly6G+ granulocytic cells into tumors.
Panels A-B. Flow cytometry was performed on digested tumors from animals treated for one week with control chow (left columns) or SM16 chow (right columns), in each of the three flank tumors – AB12 (n=26), LKR (n=5) and TC-1 (n=9−10). Panel A summarizes the percentage of CD11b+ cells out of all tumor cells in the three cell lines, in both groups (total). This is divided to Ly6G− cells (bottom section of each bar- white), and Ly6G+ (granulocytic) cells (top section of each bar- black). *=p<0.001. Panel B shows representative FACS tracings of CD11b versus Ly6G expression in each of the lines. The number in each quadrant is the percentage of the total tumor cells.
Panels C-D. Flow cytometry was performed on digested lungs with orthotopic tumors from mice with the conditionally expressed, K-rasG12D allele, treated for one week with control (white) or SM16 (black) chow (n=5 per group). Panel C summarizes the percentage of the different CD11b+ cells out of all lung cells ± SEM – Ly6G+ (granulocytic) cells (left) and Ly6G− cells (right). *=p<0.05. Panel D shows representative FACS tracings of CD11b versus Ly6G expression in the two groups. The number in each quadrant is the percentage of total lung cells.
To ask if neutrophils travel to areas of tumor necrosis, we performed immunohistochemistry of tumors using the Ly6G antibody. We found an increased number of Ly6G+ cells in tumors from SM16-treated mice and that the cells were primarily in the non-necrotic areas of the tumors (Supplemental Fig. 1). We also blocked TGF-β activity using a neutralizing anti-TGF-β monoclonal antibody (1D11) in the AB12 cell line and confirmed significantly increased levels of intra-tumoral neutrophils (CD11b+/Ly6G+) (data not shown).
Evaluation of myeloid cell populations in the spleens of mice treated with SM16 versus control showed no significant changes in the percentage of CD11b+ cells (12.1 ± 4.7 in control-treated vs. 13 ± 0.7 in SM16-treated mice), CD11b+/GR1+ myeloid derived suppressor cells (10.7 ± 4.3 vs. 11.7 ± 0.7) or CD11b+/Ly6G+ cells (9.2 ± 3.8 vs. 9.6 ± 0.6). There was no change in the percentage of CD11b+/Ly6G+ neutrophils in the blood in control tumor-bearing mice (41.3% of leukocytes) versus SM16 treated mice (38.3% of leukocytes). The percentage of CD11b+/Ly6G− in the blood was negligible in both groups of mice. These data suggest that the changes in TAN were not systemic, but rather due to a change in recruitment and/or persistence within the tumors.
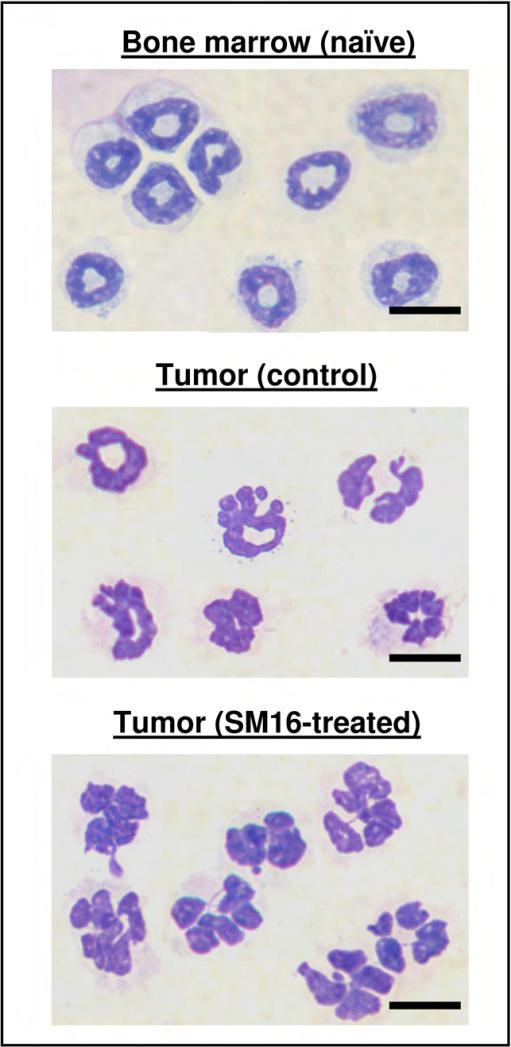
To evaluate the morphology of the TAN, intra-tumoral CD11b+/Ly6G+ cells were isolated. As seen in Fig. 2, the Ly6G+ cells isolated from flank tumors from both control untreated mice and SM16-treated mice had a clear neutrophil-like morphology. Interestingly however, most of the neutrophils in the SM16-treated tumors were more lobulated and hyper-segmented (bottom panel), losing some of the characteristic circular nuclei appearance typical of blood or bone marrow murine neutrophils (top panel), and relatively maintained in control TAN (middle panel),.
Figure 2. The morphology of TAN in control and SM16-treated mice compared to bone-marrow neutrophils.
Photomicrograph slides from bone marrow neutrophils (top panel) and from previously sorted CD11b+/Ly6G+ cells from control (middle panel) or SM16-treated mice (bottom panel). Scale bars 10 μm.
We further evaluated the pulmonary influx of CD11b+ cells in the orthotopic transgenic activated K-ras model of bronchogenic adenocarcinoma of the lung. Eight to nine weeks after activation of the K-ras mutation, we treated the mice with SM16 or control chow followed by flow cytometry of the whole lung. As seen in Figure 1C and 1D, we found a 43% increase in the percentage of neutrophils in the lungs of the SM16 mice (8 ± 0.5) compared to the control mice (5.6 ± 0.9) (p=0.03). Similar to the results in the flank models, the percentage of CD11b+/Ly6G− cells in control (2.8 ± 0.7) vs. SM16-treated (2.1 ± 0.5) mice did not increase.
TGF-β-blockade increases the mRNA for neutrophil chemoattractants
We next used real-time RT-PCR to measure the level of cytokines, chemokines, and cell adhesion molecules in flank tumors derived from AB12, LKR and TC1 cells. As we have shown previously (Kim et al., 2008; Suzuki et al., 2007), SM16 treatment resulted in changes in the tumor microenvironment manifested by increased levels of iNOS, cytokines and cell adhesion molecules, along with decreased levels of arginase (Table 1A).
Table 1. Real-Time RT-PCR analysis of whole tumors and neutrophil subsets with and without treatment with SM16.
Flank Tumors or whole lungs containing Kras-derived tumors (n=5 for each treatment group) from animals treated for 5−7 days with control or SM16 chow were harvested, digested, and had RNA extracted from either whole tumor (Panel 1A) or from isolated TAM/TAN. Equal amounts of RNA from each tumor in each group were pooled; cDNA generated, and subjected to real time RT-PCR analysis. RNA was normalized using β-actin levels. Each assay was run in at least quadruplicate.
Panel A. Fold change (SM16 to control) from whole tumors in several important inflammatory cytokines (top) and neutrophil chemoattractants (bottom). Part of the data in this panel (related to the AB12 line) was adopted from our previous publication (Kim et al., 2008).
Panel B. Comparison of mRNA expression from purified neutrophils in the two tumor groups (control and SM16), naïve bone marrow-derived neutrophils (BM) and macrophages (CD11b+ Ly6G−) from control tumors in AB12 and LKR tumors. The fold change of each molecule, using the expression level in untreated control neutrophils as the denominator, was calculated. Left panel shows data from cells purified from AB12 flank tumors. Right panel shows data from cells purified from LKR flank tumors.
Panel C. Comparison of mRNA expression from purified neutrophils isolated from the lungs of Kras lung cancer mice treated with control or SM16 chow for one week. Fold change (SM16 to control) is listed.
| A | AB12 | LKR | TC1 | |||
|---|---|---|---|---|---|---|
| Fold change | P-value | Fold change | P-value | Fold change | P-value | |
| TNFα | 5.4 | < 0.001 | 2.1 | 0.008 | 3.7 | < 0.001 |
| IFNγ | 6.2 | < 0.001 | 1.4 | 0.03 | 7.8 | 0.02 |
| IL-12 | 2.7 | 0.006 | 21.2 | < 0.001 | 1.5 | NS |
| Arginase | 0.9 | NS | 0.5 | < 0.001 | 0.2 | 0.01 |
| INOS | 5.4 | < 0.001 | 2.5 | < 0.001 | 3.1 | 0.04 |
| ICAM-1 | 2.6 | 0.04 | 1.5 | 0.02 | 1.5 | 0.002 |
| CXCL1 / KC | 3.5 | < 0.001 | 1.3 | NS | 2.1 | 0.043 |
| CXCL2 / MIP2α | 2.1 | 0.013 | 5 | < 0.001 | 3.4 | 0.02 |
| CXCL5 / LIX | 3.2 | < 0.001 | 4.1 | 0.002 | 1.8 | 0.023 |
| CCL3 / MIP1α | 2.2 | 0.02 | 4.1 | 0.04 | 2.6 | 0.001 |
| CCL5 / RANTES | 6.3 | < 0.001 | 2.6 | < 0.001 | 0.9 | NS |
| GM-CSF | 1.03 | NS | 1.1 | NS | 8.8 | < 0.001 |
| IL-17 | ND | -- | ND | -- | ND | -- |
| B | AB12 | LKR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophils | Macs. | Neutrophils | Macs. | |||||||
| BM | Control | SM16 | P-value (ctl-SM16) | TAM (Control) | BM | Control | SM16 | P-value (ctl-SM16) | TAM (Control) | |
| Arginase | ND | 1 | 0.19 | < 0.001 | 0.3 | ND | 1 | 0.55 | 0.05 | 1.05 |
| TNFα | 0.9 | 1 | 7.5 | < 0.001 | 1.3 | 0.5 | 1 | 2.4 | 0.008 | 0.7 |
| ICAM1 | 9.1 | 1 | 36.4 | < 0.001 | 66.7 | 0.9 | 1 | 6.7 | 0.002 | 0.8 |
| INOS | ND | ND | ND | NA | 1 | ND | 1 | 1.4 | 0.14 | 1.7 |
| CCL3 / MIP1α | ND | 1 | 2 | 0.002 | ND | ND | 1 | 2.7 | 0.02 | 0.9 |
| VEGF | 0.5 | 1 | 0.8 | 0.17 | 0.25 | 0.1 | 1 | 0.5 | 0.04 | 0.6 |
| CCL5 / RANTES | ND | 1 | 0.38 | 0.01 | 0.5 | |||||
| CCL2 / MCP-1 | ND | 1 | 0.18 | 0.006 | 0.4 | |||||
| C (K-Ras) | Neutrophils (SM16 / Control) | |
|---|---|---|
| Fold change | P-value | |
| Arginase | 0.04 | < 0.001 |
| TNFα | 1.4 | 0.04 |
| ICAM1 | 2.4 | < 0.001 |
| INOS | ND | NS |
| CCL3 (MIP1α) | 0.64 | < 0.001 |
| VEGF | 1.1 | NS |
| CCL5 (Rantes) | 0.37 | 0.025 |
| CCL2 (MCP-1) | 0.27 | < 0.001 |
We also assessed the message levels of selected chemokines that have an established role in the recruitment and chemoattraction of neutrophils (Kobayashi, 2008). As shown in Table 1A, we found a significant increase in the mRNA levels of 3 potent neutrophil chemoattractants: MIP-2α/CXCL2, LIX/CXCL5 and MIP1α/CCL3. We also found a 2 to 3-fold increase in two other neutrophil chemoattractants in 2 of the lines – Rantes/CCL5 (in AB12 and LKR) and KC/CXCL1 (in AB12 and TC1), as well as a 2-fold increase in the CXCL1 protein in AB12 tumors (data not shown). In the TC1 tumor, but not in the AB12 tumor, we found a significant 7-fold increase in GM-CSF – another known neutrophil chemoattractant (Wang et al., 1988). To evaluate a potential source of these chemokines, we isolated mRNA from TAMs of AB12 tumors. We found a 2.5 to 3-fold increase in the mRNA levels of CXCL2 and CXCL5 in isolated macrophages (CD11b+ Ly6G−) of tumors from SM16-treated mice, with no change in CXCL1 levels. These findings suggest that the blockade of TGF-β by SM16 induces secretion of neutrophil chemoattractants, at least partially from tumor macrophages, and expression of adhesion molecules, both of which could augment the recruitment of neutrophils into the tumor.
Intra-tumoral neutrophils are important effector cells in the anti-tumor effect of anti-TGF-β receptor treatment
We next studied the functional significance of TAN in the AB12 model by depleting the Ly6G+ cells in untreated and SM16-treated tumor-bearing animals.
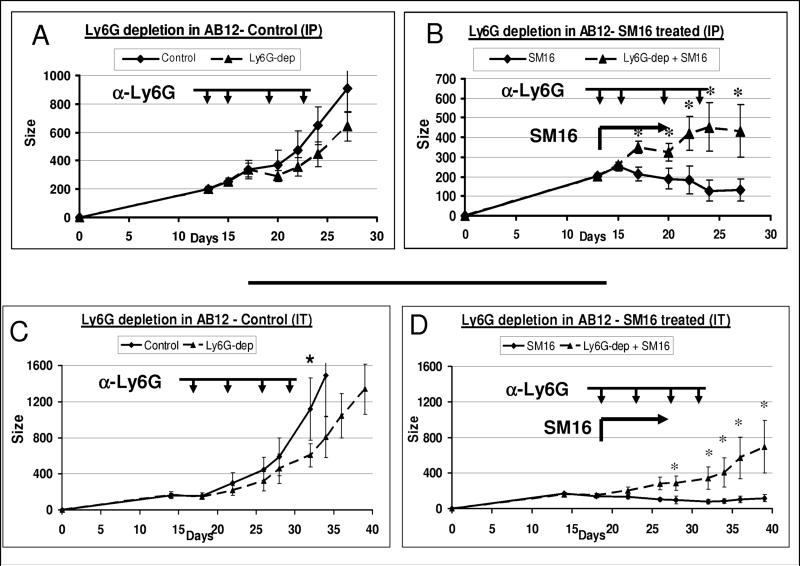
We first injected the 1A8 antibody or isotype-matched control IgG intraperitoneally. A significant reduction of 80−90% in systemic and intra-tumoral neutrophils was noted by FACS in these experiments (data not shown). Depletion of neutrophils in non-SM16 treated mice resulted in a small reduction in tumor growth (Fig. 3A). In contrast, depletion of neutrophils in SM16-treated mice resulted in a significantly reduced effect of SM16 (Fig. 3B); that is, the tumors grew more rapidly when neutrophils were depleted in the SM16-treated mice. Interestingly, when the systemic neutrophils returned in 3−4 days after the last injection, the growth inhibition with SM16 treatment appeared to also return (Fig. 3B, last time point).
Figure 3. Effect of neutrophil depletion on tumor growth and on tumor response to SM16.
Panels A-B (systemic depletion). Mice (n = 6−8 for each subgroup) bearing large AB12 tumors, were treated with either control (Panel A) or SM16 chow (panel B), starting at day 13. One group on each diet was injected with either 100 μg of the anti-Ly6G monoclonal antibody 1A8 intra-peritoneally (IP) (arrowheads) every 3−5 days during the experiment (triangles, with dashed lines) or a control IgG antibody at the same schedule and dose (diamonds with solid lines). Panel A compares mean tumor size ± SEM with or without Ly6G depletion (Ly6G-dep) in mice treated with control chow. Panel B compares mean tumor size ± SEM with or without Ly6G depletion in mice treated with SM16 chow. Groups were compared using ANOVA. *=p<0.05.
Panels C-D (intra-tumoral depletion). The experiment was repeated again with injection of 30 μg of the anti-Ly6G monoclonal antibody 1A8 or control IgG given intratumorally (IT, arrowheads). Groups were compared using ANOVA. *=p<0.05.
To determine if local depletion of neutrophils had a similar effect, we injected the antibodies at lower dose, directly into the tumors using a previously described approach (Chen et al., 2007; Yu et al., 2005) (Fig. 3 C-D). Using flow cytometry, we confirmed a 70−80% reduction in the percentage of intra-tumoral Ly6G+ cells. Local depletion of neutrophils in non-SM16-treated animals resulted in a small but significant slowing of tumor growth (p < 0.05) (Fig. 3C). Again, in contrast, when animals were treated with SM16 and also had neutrophils depleted, the SM16-induced treatment effect was significantly blunted. Treatment with SM16 reduced tumor burden by 80−90% compared to controls in the non-depleted mice, but tumor size was reduced by only 40% compared to controls in the mice that received SM16 plus repeated intra-tumoral depletion of Ly6G+ cells (Fig. 3D). It should be noted that after intra-tumoral injection, we also found a 30−50% reduction in blood neutrophils (data not shown), suggesting that the antibody may also have a systemic effect. Together, these data indicate that depletion of neutrophils causes a significant reduction in the anti-tumor effect of TGF-β blockade, suggesting that neutrophils contribute to the antitumor activity of TGFβ blockade.
Inhibition of TGF-ß signaling in vivo increases CD11b+ cell cytotoxicity via an oxygen radical-dependent mechanism
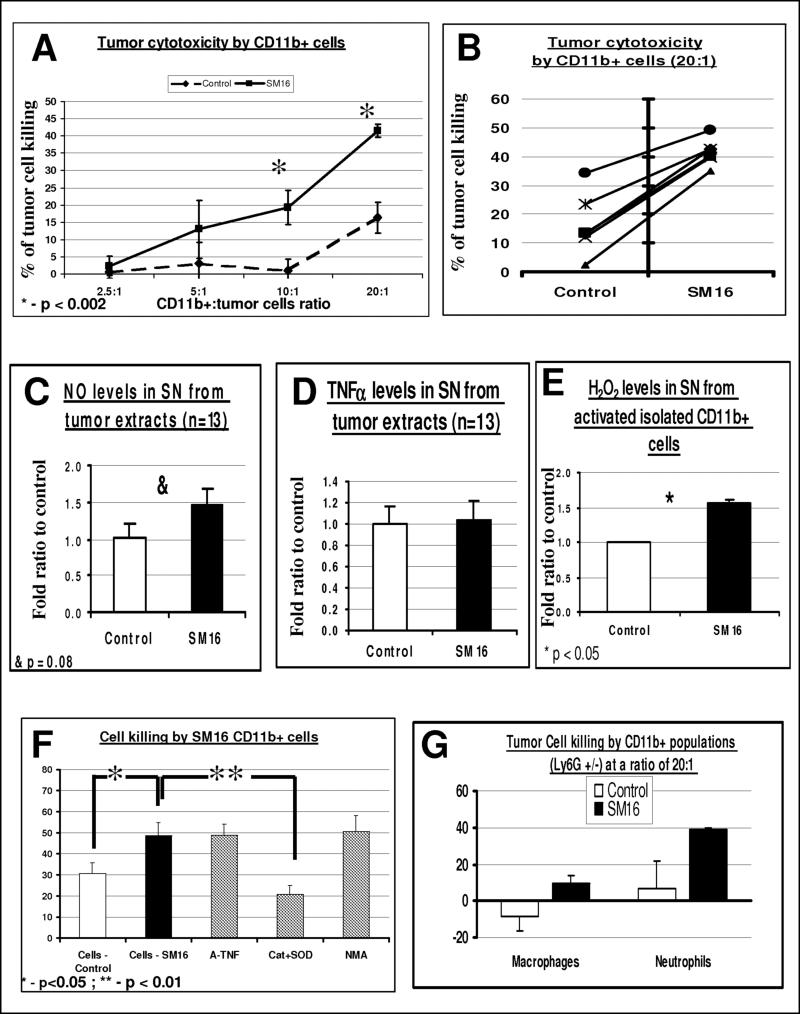
To evaluate the cytotoxic potential of the intra-tumoral myeloid cells, microbeads were used to purify CD11b+ cells from AB12 tumors resulting in more than 90% purity (by FACS) in all experiments (data not shown). The isolated CD11b+ cells were co-cultured at varying ratios with luciferase-labeled AB12 tumor cells and the number of viable tumor cells was determined after 24 hours. CD11b+ cells from untreated control mice were found to be non-cytotoxic up to a ratio of 20:1 (macrophage to tumor cell), while CD11b+ cells isolated from the tumors of SM16-treated mice showed dose-dependent cytotoxicity, with significantly more killing than control myeloid cells at ratios of 10:1 and 20:1 (Fig. 4A). Figure 4B shows data from six independent experiments with a CD11b+/tumor ratio of 20:1. The average killing induced by co-culture of CD11b+ cells from SM16-treated mice with AB12 cells was 41.6% vs. 16.3% in co-culture with CD11b+ cells from control mice (p=0.00013). Similar results were found with evaluation of LKR tumor-cells cytotoxicity, assessed by LDH levels (data not shown).
Figure 4. SM16 CD11b+ cell cytotoxicity is primarily due to Activated Neutrophils, via an oxygen radical-dependent mechanism.
Panels A-B. AB12 tumors (n=5−7 for each group) from control and SM16-treated animals were treated for 7 days and then digested and pooled. Isolated CD11b+ cells were co-cultured with AB12-luciferase cells at different ratios of effector cells (CD11b+) to tumor cells. At 24 hours, the percentage of tumor cells killed was calculated. Panel A summarizes the percentage of tumor killing ± SEM at each ratio of co-culture (n=4−6) *=p<0.002. In panel B, the individual data from 6 separate experiments with co-culture at a ratio of 20 effector cells to 1 tumor cell is shown.
Panels C-D. Pieces of harvested tumors from control and SM16-treated mice were cultured in medium for 24 hours, and the secretion of NO (panel C) and TNFα (Panel D) per mg of tissue was evaluated. The bars represent mean ± SEM, &-p=0.08.
Panel E. Isolated CD11b+ cells were cultured in wells and activated with PMA, followed by evaluation of the release of H2O2. The bars represent mean ± SEM, *=p<0.05.
Panel F. CD11b+ cells were co-cultured with AB12-Luc cells as above (panels A-B) and different inhibitors were added. The bars represent mean ± SEM. *=p<0.05, **=p<0.01.
Panel G. CD11b+ cells were sorted using anti-Ly6G antibody to neutrophils (Ly6G+) and macrophages (Ly6G−). Each of the cell subtypes were co-cultured with tumor cells at a ratio of 20 effector cells to 1 tumor cell, and cytotoxicity was evaluated as above. The bars represent mean ± SEM.
To evaluate possible mechanisms of killing by the intra-tumoral CD11b+ cells, tumor explants were isolated and put in medium for 24 hours. Tumors from SM16-treated mice secreted 40% higher levels of NO (p=0.08, Fig. 4C). In contrast, we found no change in the secretion of TNF-α (Fig. 4D). Furthermore, the level of hydrogen peroxide (H2O2) secretion in PMA-activated CD11b+ cells from SM16 treated-tumors was 45% higher than in CD11b+ cells isolated from control mice (p<0.05) (Fig 4E). Moreover, there was no reduction in tumor cell killing in the presence of either anti-mouse TNFα antibodies or N-methyl-arginine (NMA), an inhibitor of NO synthase (iNOS). In contrast, the blockade of superoxide and H2O2 by the addition of superoxide dismutase (SOD) and catalase (Cat) respectively, significantly reduced the cytotoxicity of the CD11b+ cells to tumor cells, reducing the killing to 20.8% (p=0.0039), a level lower than the killing induced by control CD11b+ cells. These data show an important role for oxygen radicals in this in vitro assay of myeloid cell-induced tumor cytotoxicity.
CD11b+ cell cytotoxicity is primarily due to activated neutrophils
CD11b+ cells were next sorted using flow cytometry to separate the tumor-associated macrophages and tumor-associated neutrophils. We confirmed that the percentage of Ly6G+ cells (by flow cytometry) and neutrophilic-like cells (by morphologic appearance) in the isolated neutrophils was >95% following isolation. Control TAM, Control TAN, SM16 TAM, and SM16 TAN were co-cultured for 24 hrs with AB12 cells and cytotoxicity to tumor cells assessed. At ratios equal or lower than 10 viable neutrophils/macrophages to each tumor cell, there was no significant killing by either TAM or TAN (data not shown). Even at a ratio of 20 macrophages per tumor cell, TAM from control mice were mildly supportive to tumor growth. TAM from SM16-treated mice, and TAN from control mice had small amounts of killing capacities (up to 10%). The only cells able to induce significant tumor cytotoxicity were the TAN from the SM16-treated mice, which at a ratio of 20 to 1, showed a killing rate similar to whole CD11b+ cells (about 40% killing) (Fig. 4G). These data show that the tumor-associated neutrophils make a significant contribution to the direct tumor cytotoxicity of myeloid cells following TGF-β blockade.
We further evaluated the expression of surface expressed “killing” molecules on TAN and found low expression of FAS-ligand and TRAIL by flow cytometry on TAN from both the control and SM16-treated mice. However, the percentage of FAS+ neutrophils increased in the tumors from SM16-treated mice by 25 % (14.3% ± 1.3 of neutrophils in control mice vs. 18.3% ± 1.4 in SM16-treated mice, p<0.05), with a similar increase in the mean fluorescence intensity (MFI) of FAS in the neutrophils (31.4 ± 2.5 vs.37.2 ± 1.4, p=0.05).
The TAN present after TGF-β-blockade have a more immunostimulatory mRNA profile than TAN from untreated mice
To study other phenotypic change in the neutrophils following TGF-β blockade, we performed real time RT-PCR of selected receptors, chemokines and cytokines in TAN. For comparison, we also examined mRNA levels in neutrophils isolated from the bone marrow of naïve mice and tumor-associated macrophages (CD11b+/Ly6G− cells) from control, tumor-bearing mice. We normalized all values to the levels found in TAN from control animals (Table 1B).
Bone marrow neutrophils had undetectable levels of arginase, iNOS, CCL3, CCL5, and CCL2, whereas (with the exception of iNOS) these genes were highly expressed in control TAN from AB12 tumors (left panel, Table 1B). Unlike many of the other cytokines examined, however, TNFα and VEGF message levels were detected in bone marrow neutrophils from naïve mice at similar levels as in control TAN. A similar pattern was seen in LKR tumors (right panel, Table 1B), except that basal VEGF levels in bone marrow neutrophils were only 10% of those found in TAN.
Of even more interest was the effect of TGF-β blockade on TAN gene expression. In both types of tumors, control TAN were found to have high levels of arginase, an important immunosuppressor of the adaptive immune system (Rodriguez and Ochoa, 2008). In fact, the levels of arginase message in control TAN were equal to or even higher than in control tumor associated macrophages. However, arginase levels were 2 to 5-fold lower in TAN from mice treated with SM16. In contrast, the level of TNF-α mRNA, an important immunostimulator, was significantly increased with TGF-β blockade by more than 7-fold in AB12 TAN and 2.4-fold in TAN from LKR tumors. The levels of CCL2 and CCL5 were significantly lower in SM16-treated TAN (AB12 tumors), whereas CCL3 was 2 to 2.7-fold higher in AB12 or LKR-bearing SM16-treated mice. ICAM-1 mRNA levels were markedly increased in SM16-treated neutrophils (6.7 to 36.4-fold). The level of ICAM-1 surface expression was increased by about 2-fold in the neutrophils of SM16-treated mice, as shown by flow cytometry (7.4% ± 0.1 of neutrophils in control mice vs. 12.6% ± 0.8 in SM16-treated mice, p<0.01). mRNA for VEGF, was reduced by 2-fold in the LKR SM16-treated tumors, but had only a non-significant trend to reduction in the AB12 tumor.
We also analyzed neutrophils isolated from lungs of tumor-bearing mice with advanced tumors from the orthotopic K-ras model and found very similar differences between the neutrophils in the SM16-treated mice and those from the control, untreated mice (Table 1C).
Anti-tumor activity, as well as neutrophil recruitment to the tumors of SM16-treated mice, is dependent on the presence of CD8+ T cells
Given that systemic CD8+ T cell depletion essentially eliminates the effect of TGF-β blockade on tumor growth (Suzuki et al., 2007) and our data showing that the anti-tumor effect was also partially neutrophil dependent (Fig. 3), we wanted to explore the intra-tumoral connections between TAN and CD8+ T cells.
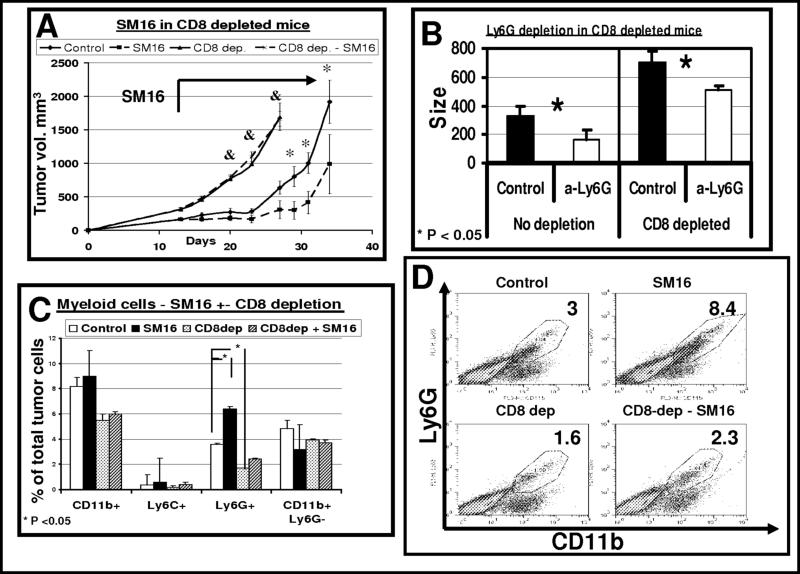
We first evaluated the effect of CD8+ T cell depletion on the phenotype of the tumor-associated myeloid cells. Animals bearing AB12 tumors were treated with SM16 with or without CD8+ T cell depletion. Systemic administration of anti-CD8 antibody resulted in depletion of more than 90% of CD8+ cells from the spleen (data not shown). As shown in Figure 5A (and similar to our previous data), all of the anti-tumor effect of SM16 was lost with CD8+ T cell depletion. Given complete loss of efficacy, we did not try to combine depletion of CD8 and depletion of neutrophils in the SM16 treated animals. However, we did study combined depletion of CD8 cells and neutrophils in control, untreated mice. As shown in Fig. 5B (left columns), and similar to our findings in Figure 3, intra-tumoral depletion of neutrophils led to a slowing of tumor growth. However, even though the tumors grew faster (as expected after CD8 depletion), we noted that the mild, but significant, anti-tumor effect of neutrophil depletion was maintained (Fig. 5B, right columns). These data suggest that the neutrophils do have some pro-tumor effect that is independent of the presence of CD8 CTLs.
Figure 5. CD8+ cell depletion blocks all of the SM16 clinical effect and reduces influx of neutrophils to the tumors.
Panel A. - Mice (n = 5−6 for each group) bearing large AB12 tumors were treated in one of four ways: 1) control chow (diamonds- control); 2) SM16 chow starting at day 13 and throughout the experiment (squares - SM16); 3) control chow, and injected with 300 μg of an anti-CD8 monoclonal antibody IP twice per week starting two days prior to tumor injection (triangles – CD8 dep.); and 4) SM16 chow and depletion of CD8+ cells (crosses – CD8-dep - SM16). Control and SM16 groups were treated with an IP control IgG antibody. The bars represent mean ± SEM. *=p<0.05, **=p<0.01 - control vs SM16; &=p<0.05 control vs. SM16 (both with CD8 depletion).
Panel B. - Mice were injected with either 300 μg of an anti-CD8 monoclonal antibody IP twice per week starting two days prior to tumor injection (right – CD8 depleted) or control IgG (left – no depletion). When tumors reach a size of approximately 100mm3, Each of these two groups (n = 10−12 for each group) were divided to two subgroups, treated for 2 weeks with either a control IgG antibody (Control, black) or 100 μg of an anti-Ly6G monoclonal antibody i.t. twice per week (α-Ly6G, white), followed by tumor measurements. The bars represent mean size ± SEM of each group. Although CD8 depletion accelerated tumor growth, the reduction in tumor growth following Ly6G depletion was maintained in this group (right panel). Differences in both groups were significant (*=p<0.05).
Panels C-D. - Mice (n = 4−5 for each group) bearing large AB12 tumors were treated in one of four ways as above. Seven days after starting treatment with SM16, flow cytometry of the tumors was performed. Panel C summarizes the percentage of CD11b+, Ly6G+, Ly6C+ and CD11b+ Ly6G− cells in the four groups out of the total number of tumor cells ± SEM, *=p<0.05. Panel D shows representative FACS tracings of CD11b versus Ly6G expression in each of the four treatment groups. The number in each panel is the percentage of the total tumor cells.
Consistent with the data shown in Figure 1, the percentage of CD11b+/Ly6G+ cells in the non-CD8 depleted tumors increased with SM16 treatment from 3.6% to 6.4% (p<0.05) (Fig. 5C). CD8 depletion, by itself, reduced the percentage of neutrophils in the tumor to 1.7% of the cells (p<0.05). Recruitment of neutrophils after treatment with SM16 in the CD8+ T cell-depleted mice was somewhat blunted, increasing to 2.4% of the cells, however, this was less than control untreated, non-depleted tumors (Fig. 5C and individual panels in Fig. 5D). This small increase in the percentage of neutrophils was not sufficient to induce an anti-tumor growth effect, as noted in Figure 5A.
Depletion of neutrophils affects the activation of CD8+ CTLs
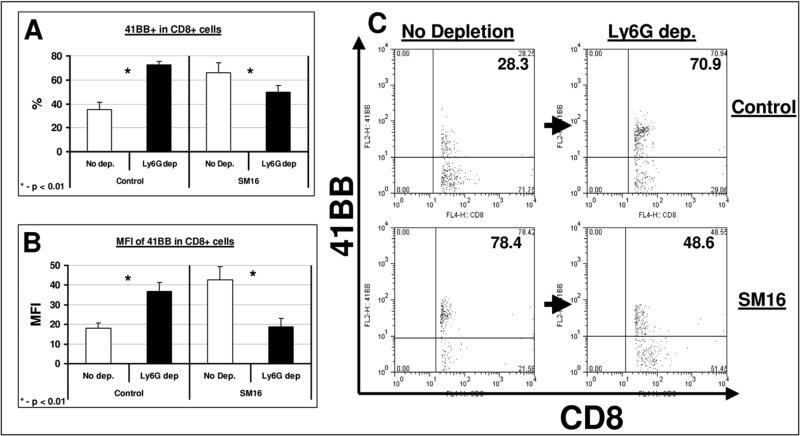
To understand the effect of TAN on CD8+ T cell activation, we evaluated the expression of two established surface activation markers, 4−1BB (CD137) and CD25. Tumor-bearing animals were left untreated or given SM16 chow with or without neutrophil depletion. Tumors were harvested one week after SM16 treatment and subjected to FACS. As we have previously shown (Kim et al., 2008; Suzuki et al., 2007; Wallace et al., 2008), SM16 treatment led to activation of CD8+ T cells compared to controls, with the percentage of cells positive for 4−1BB rising from 35.1% to 65.8% of the CD8+ T cells (p<0.05) and the MFI of 4−1BB increasing from 18.1 to 42.6 (p<0.01) (Fig. 6).
Figure 6. Neutrophil depletion increases CD8+ T-cell activity in untreated mice, but reduces CD8+ T-cell activity in SM16-treated mice.
Mice (n = 5 for each group) bearing large AB12 tumors were treated in one of four ways: control chow (control); SM16 chow (SM16); control chow and injected with 100 μg of the anti-Ly6G monoclonal antibody 1A8 IP twice per week (Ly6G-dep); SM16 chow and depletion of neutrophils (Ly6G-dep + SM16). The groups not treated with Ly6G depletion (Control and SM16) were treated with a control IgG antibody at the same schedule and dose. Seven days after starting SM16 or control chow, multi-color flow cytometry of tumors was performed. Activation of the CD8+ T-cells was measured using the activity marker 4−1BB (CD137). T cell activation was compared with and without neutrophil-depletion in the control-chow-treated tumor bearing mice (left columns) and SM16-treated tumor-bearing mice (right columns) tumors. Panel A summarizes the percentage of 4−1BB+ out of total intra-tumoral CD8+ T-cells ± SEM. Panel B summarizes the Mean Fluorescent Intensity (MFI) of 4−1BB in the intra-tumoral CD8+ T-cells ± SEM, *=P<0.01. Panel C shows representative FACS tracings of CD8 versus 4−1BB expression in each of the four treatment groups. The number in the upper right quadrant is the percentage of 4−1BB+ cells out of total CD8+ cells.
Ly6G+ depletion slightly increased the percentage of intra-tumoral CD8+ cells in control mice (NS), and did not change this percentage in SM16-treated mice (data not shown). However, depletion of neutrophils in control mice (having a “pro-tumor” phenotype) led to significantly increased CD8+ T cell activation: 72.6% of the cells were found to be 4−1BB+ in tumors from neutrophil-depleted, untreated mice vs 35.1% in control mice (p<0.01, Fig 6A and 6C). The MFI of 4−1BB in these CD8+ T cells increased from 18.1 to 36.9 in control versus depleted mice (p<0.01, Fig. 6B). This increased activation of CD8+ cells in control tumor-bearing mice following neutrophil depletion is consistent with the decreased tumor growth in neutrophil-depleted mice (Fig. 3A and 3C).
We then studied the effect of neutrophil depletion on CD8+ T cell activation in SM16-treated animals. Depletion of these neutrophils (having an “anti-tumor” phenotype) led to significant decreases in CD8+ T cell activation. The percentage of 4−1BB+ activated cells was reduced from 65.8% of the CD8+ T-cells to only 49.7% (p<0.01, Fig 6A and 6C), and the MFI was reduced from 42.6 to 18.7 (p<0.01, Fig. 6B). These results suggest that neutrophil depletion impairs both SM16-induced activation of CD8+ T cells and SM16-mediated tumor growth inhibition (see Fig. 3). Similar results of CD8 activity were found when the percentage of CD8+/CD25+ was evaluated (data not shown).
Discussion
Polarization of Tumor-Associated Neutrophil (TAN) Phenotype: “N1” versus “N2” TAN
Various types of myeloid cells have been shown to promote tumor progression by direct immune suppression (Mantovani et al., 2002), as well as by producing angiogenic factors, matrix-degrading enzymes, or growth factors (Balkwill and Coussens, 2004; Yunping et al., 2006). The best characterized have been tumor-associated macrophages (TAM), which have properties of alternatively activated macrophages, also known as M2 macrophages (Mantovani et al., 2002).
Our results indicate that, like TAM, tumor-associated neutrophils (TAN) also have differential states of activation/differentiation, suggesting a classification scheme for TAN similar to that of TAM: TAN can thus take an anti-tumorigenic (what we are calling an “N1-phenotype”) versus a pro-tumorigenic (“N2”) phenotype. The anti-tumor activities of N1 TANs include expression of more immuno-activating cytokines and chemokines, lower levels of arginase, and more capability of killing tumor cells in vitro. Since blockade of TGF-β favors the accumulation of N1 TAN that have anti-tumor activity, our data suggest that TGF–β is a major proximal cytokine within tumors that defines the TAN phenotype and skews differentiation toward the N2 pro-tumorigenic phenotype. This hypothesis is consistent with previous in vitro data showing that TGF-β can inhibit neutrophil activity and cytotoxicity (Shen et al., 2007). Our findings were similar in two different tumor types (NSCLC and mesothelioma), in 3 different mice strains, and in both flank and orthotopic (K-ras) models, suggesting the polarization of neutrophils may be a general feature of tumor microenvironment.
The presence of different TAN phenotypes explains our Ly6G− depletion data and may help to explain some of the apparent contradictions in the literature. In untreated tumors, neutrophils have been reported to support tumor growth by producing angiogenic factors and matrix-degrading enzymes (Pekarek et al., 1995; Shojaei et al., 2008), support the acquisition of a metastatic phenotype (Tazawa et al., 2003) and suppress the anti-tumor immune response (Schmielau and Finn, 2001). These observations are consistent with the hypothesis that most TAN appear to have an N2 phenotype and thus contribute to tumor growth and immunosuppression. Depleting these “pro-tumorigenic” N2 neutrophils, would thus be expected to inhibit tumor growth. This explanation is consistent with at least two other earlier studies (Nozawa et al., 2006; Pekarek et al., 1995) and with our data showing that depleting neutrophils slowed tumor growth, even in the absence of CD8+ cells (Figs. 3 and 6).
In contrast, when neutrophils assume a more tumor-cytotoxic N1 phenotype, for example, during TGF-β inhibition (our study) or after immunologic or cytokine activation, they have the potential to kill tumor cells and inhibit growth (Colombo et al., 1992a; Di Carlo et al., 2001; Hicks et al., 2006), as well as coordinate adaptive immune responses through interactions with dendritic cells (Van Gisbergen et al., 2005). Depletion of these N1 TAN would thus either augment tumor growth and/or blunt the anti-tumor effects of immunologic treatments (Kousis et al., 2007; Stoppacciaro et al., 1993; Suttmann et al., 2006). This is exactly what we observed when we depleted neutrophils in the SM16-treated animals (Fig. 3).
Our definition of TAN subsets in tumors has some similarities to phenotypic and functional distinct neutrophil populations reported in inflammation and infection models, both in vitro (Buckley et al., 2006) and in vivo (Itou et al., 2006; Tsuda et al., 2004). However, the neutrophil subsets in the Tsuda scheme were defined by morphology, not surface markers. In fact, some of their proposed neutrophil subsets did not express CD11b+, nor were there consistent differences in the secretion of TNF-α. It is likely that distinct differentiation programs occur in different disease states depending on the cytokine milieu.
One important feature of our study was the use of a highly neutrophil-specific monoclonal antibody against Ly6G (1A8) to differentiate macrophages (CD11b+/Ly6G−) from neutrophils (CD11b+/Ly6G+), as well as to specifically deplete neutrophils. This contrasts with most previous studies that used the monoclonal antibody RB6−8C5 directed against the “Granulocyte Receptor 1” (GR-1) surface antigen (Daley et al., 2008). It is now recognized that the RB6 antibody identifies two epitopes: one on the neutrophil-specific receptor, Ly6G, and a second epitope on Ly6C, an antigen expressed by many other cell types (Daley et al., 2008).
Role of TGF-β in TAN recruitment
Neutrophils, like all other leukocytes, move into tissues from the blood under the influence of specific chemokines, cytokines (like TNF-α and IFN-γ), and cell adhesion molecules located on their own surface (i.e. CD11b) and on the surface of endothelial cells (i.e. selectins, ICAM-1 and PECAM-1) (Kobayashi, 2008). TGF-β appears to inhibit endothelial adhesiveness for neutrophils and neutrophil transmigration through endothelium in vitro (Smith et al., 1996) and in different inflammatory disease states (Allen et al., 2008).
Our data show that TGF-β receptor blockade increases the number of neutrophils in tumors and suggests that this effect occurs through all three parts of the recruitment pathway including increased expression of mRNA for CXC chemokines, CC chemokines and activating cytokines within the tumor (Table 1), as well as up-regulating ICAM-1 message and protein expression on endothelial cells (Table 1 and see also (Kim et al., 2008)). Our preliminary data shows that macrophages, as well as endothelial cells are the most important in neutrophil recruitment, as previously shown in lung inflammation (Maus et al., 2003).
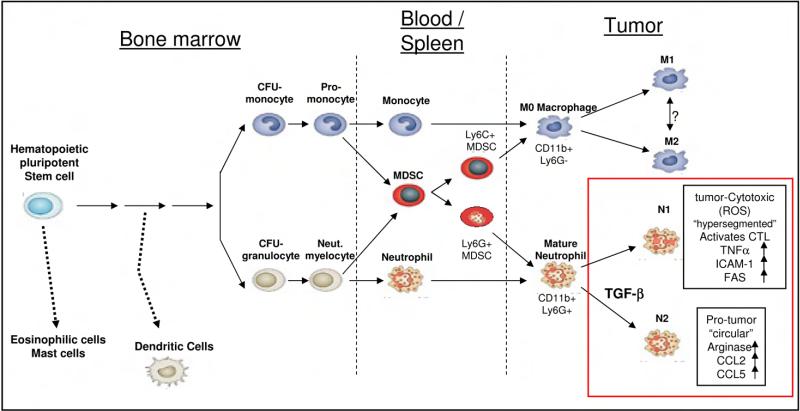
It is worth considering the relationship between TAN and myeloid derived suppressor cells (MDSC, Fig. 7). Splenic and blood MDSC are a heterogeneous population of immune suppressive cells, excessively produced in cancer, that can be either monocytic (Ly6C+) or granulocytic (Ly6G+) and function as systemic immune suppressors, and promoters of tumor angiogenesis (Gabrilovich and Nagaraj, 2009; Movahedi et al., 2008; Youn et al., 2008). It has been previously shown that MDSC can enter tumors and differentiate to mature macrophages (TAM) or neutrophils (TAN) (Kusmartsev et al., 2005). Since we have no definitive markers yet, we do not know if the N2 neutrophils within the tumors are actually granulocytic MDSC of splenic origin that were attracted to the tumor or if they are blood-derived neutrophils that were then converted to an N2 phenotype by the tumor microenvironment, specifically by the high local concentrations of TGF-β. Given the fact that we saw no effects of TGF-β blockade on the percentage of total blood neutrophils, splenic myeloid cells (CD11b+), or splenic MDSC, it appears that TGF-β blockade changes only the local chemoattraction and/or the intra-tumoral education of neutrophils, rather than changing the general phenotype of myeloid cells.
Figure 7. The origin and differentiation of myeloid-derived tumor associated cells (based on (Murdoch et al., 2008) and (Gabrilovich and Nagaraj, 2009)).
Myeloid-derived tumor associated cells originate from a common pluripotent stem cell, but separate early to monocytic and granulocytic lineages, eventually infiltrating tumors. As we show in the current study, TAN can polarize to either anti-tumor N1 TAN or pro-tumor N2 TAN, with TGF-β being an important effector in that polarization. The characteristics of the polarized TAN, as presented in the current study, are framed in the bottom right part of the figure.
Interaction of TAN with CD8+ T-cells
The role of TGF-β on the function of T-cells in vitro and in vivo has been well established through inhibitor studies (Ge et al., 2006; Kim et al., 2008; Nam et al., 2008; Suzuki et al., 2007) and through studies in transgenic mice where a dominant negative TGF-β receptor has been expressed specifically in T-cells (Gorelik and Flavell, 2000). Given the importance of CD8+ cells on the anti-tumor effect of TGF-β blockade (see also Fig. 5A), we also studied the interplay between TAN and cytotoxic CD8+ cells and found that TAN depletion affected the phenotype of intra-tumoral CD8+ T cells, however, the results were dependent on the phenotype of the TANs. Neutrophil depletion of untreated tumor-bearing animals (i.e. removal of N2 TAN) increased the activation status of CD8+ T cells. In contrast, neutrophil depletion of SM16-treated tumor-bearing animals (i.e. removal of N1 TAN) decreased the activation status of the intra-tumoral CD8+ T cells compared to T-cells in the animals treated only with SM16 (Fig. 6). These data support the idea that N2 TAN function in an immunosuppressive fashion, while N1 TAN are immunostimulatory.
The ability of neutrophils to influence CD8+ T cells has been suggested in infections (Tvinnereim et al., 2004) and in cancer (Colombo et al., 1992b; Di Carlo et al., 2001; Kousis et al., 2007), however, interpretation of these studies in the light of differential neutrophil activation status within tumors is instructive. “Proinflammatory” or N1 neutrophils promote CD8+ recruitment and activation by producing T-cell attracting chemokines (like CCL3, CXCL9, and CXCL10) and pro-inflammatory cytokines (i.e. IL-12, TNF-α, GM-CSF, and VEGF) (Scapini et al., 2000). There is also evidence that they can activate dendritic cells via cell-cell contact and through secretion of TNF-α (Van Gisbergen et al., 2005). N2 neutrophils do not produce high levels of such pro-inflammatory agents, but do produce large amounts of arginase (Table 1) which would serve to inactivate T-cell effector functions in the same way that has been proposed for M2 TAMs (Movahedi et al., 2008; Rodriguez et al., 2004).
Although there is an extensive literature on the ability of neutrophils to recruit CD8+ T cells as discussed above, there are surprisingly few studies examining the effect of CD8+ T cells on the recruitment of neutrophils. It has been shown that CD8+ T cell depletion decreases the tissue influx of neutrophils in infectious diseases (Appelberg, 1992). In the only tumor study we were able to identify, a marked decrease in TAN following CD8+ T cell depletion was shown in a model in which CT26 colon carcinoma cells transduced to express G-CSF were placed into mice (Stoppacciaro et al., 1993).
The mechanisms by which T-cells might attract and/or activate neutrophils are not known for certain, but include the ability of tumor-stimulated activated T-cells to produce GM-CSF (Aruga et al., 1997), MIP2 and KC (Sherwood et al., 2004), or cytokines such as TNF–α and IFN-γ. These cytokines may act to recruit neutrophils by stimulating tumor macrophages or endothelial cells to produce appropriate chemokines and cell adhesion molecules (Iking-Konert et al., 2008; Maus et al., 2003). Interestingly, without a basal level of tumor microenvironment “activation” provided by CD8+ T cells, as we see here in our CD8-depletion studies, blockade of TFG-β is apparently not sufficient to induce neutrophil migration.
Our data suggests at least 2 different polarized populations of tumor-associated neutrophils, pro-tumorigenic and anti-tumorigenic, similar to what is seen in macrophages. This paradigm could explain some of the apparent contradictions in the evaluation of the role of neutrophils in tumor biology.
Experimental Procedures
Animals
Mice were purchased from Taconic Labs (Germantown, NY), and Jackson Labs (Bar Harbor, ME). The Animal Use Committee at PENN approved all protocols in compliance with the Guide for the Care and Use of Laboratory Animals.
Cell lines
The murine malignant mesothelioma cell line, AB12 was derived from an asbestos-induced tumor in a Balb/C mouse. TC1 cells were derived from mouse lung epithelial cells from a C57B6 mouse, immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene (Kim et al., 2008). The murine lung cancer line LKR was derived from an explant of a pulmonary tumor from an activated Kras G12D mutant mouse that had been induced in an F1 hybrid of 129Sv.J and C57BL/6 (Wilderman et al., 2005).
Animal flank tumor models
Mice were injected on the right flank with 1 × 106 AB12, LKR, or TC1 tumor cells in the appropriate syngeneic host. The flank tumors were allowed to reach an average size of 200−250 mm3 (approximately 12−15 days). Following treatments as outlined below, tumor growth was followed with measurement twice weekly. All experiments had at least 5 mice per group and were repeated at least 2 times. When needed (i.e. for FACS, RNA, cell subsets isolation, etc.) flank tumors were harvested from the mice, minced, and digested with 2 mg/mL DNase I (Sigma, St. Louis, MO) and 4 mg/mL collagenase type IV (Sigma) at 37°C for 1 hour.
K-ras mutated orthotopic lung cancer model
The orthotopic lung cancer model using intra-tracheal Ad.Cre in transgenic K-ras mice has been previously described in detail (Wilderman et al., 2005). Briefly, to activate the conditional oncogene and induce tumors, 100 μL of saline with 3 × 1010 particles of adenovirus containing Cre recombinase (Ad.Cre) were administered to LSL KrasG12D mice intra-nasally. Eight to nine weeks after instillation of Ad.Cre,mice were treated with SM16 or control chow for 1 week and sacrificed. Lungs were excised, minced, and either subjected to flow cytometry or cell purification.
SM16, a TGF-β Receptor Kinase Inhibitor
The chemical structure and biochemical characteristics of SM16, a 430MW ALK4/ALK5 kinase inhibitor produced by BiogenIdec has been previously published (Fu et al., 2008). SM16 formulated into chow at a dose of 0.45 g/kg of chow results in therapeutic drug levels and has a measurable clinical effect on tumors (Kim et al., 2008; Suzuki et al., 2007). We further confirmed that oral administration of SM16 using formulated chow for 5 days, significantly decreases the phosphorylated Smad2 level in AB12 (Supplemental Fig. 2a) and LKR (Supplemental Fig.2b) tumors. Tumor-bearing mice were treated with SM16 or control chow ad libitum. In some experiments, we confirmed our results using the anti-TGFβ monoclonal antibody 1D11 and an isotype-matched IgG1 monoclonal antibody, 13C4, provided by Genzyme Corp (Nam et al., 2008). One hundread μg of the antibody was injected intra-peritoneally twice per week.
Flow Cytometric Analysis of Tumors and spleen after SM16 treatment
Splenocytes, blood leukocytes, lungs, and tumor cells were studied by FACS analysis as previously described (Kim et al., 2008; Suzuki et al., 2007). The following fluorescently labeled antibodies were purchased from BD Bioscience: CD8-FITC, CD8-PE, CD8-APC, CD11b-FITC, CD11b-PercP, CD11b-APC, Ly6G-FITC, Ly6G-PE, CD25-FITC, Isotype controls (FITC, PE, PercP, APC). CD206-PE was obtained from Serotec (Oxford, UK). 4−1BB (CD137)-PE was obtained from Abcam (Cambridge, UK). All flow cytometry was done using a Becton Dickinson FACS Calibur flow cytometer (San Jose, CA). Data analysis was done using FlowJo software (Ashland, OR).
In vivo depletion of CD8 T-cells and Ly6g+ neutrophils
In order to evaluate the specific role of CD8 T-cells and neutrophils in the SM!6 model and the interactions between these cells, we depleted them using monoclonal antibodies (mAb) to CD8 and/or the neutrophils marker Ly6G. Details on the mAb and the protocol used can be found in the supplemental data.
Isolation of CD11b+ cells and separation of neutrophils and macrophages
Tumors were harvested, digested as previously described, and CD11b+ cells were isolated using magnetic beads (Miltenyi Biotec, Germany) per manufacturer's instructions to purity of greater than 90% CD11b+ cells. In some experiments, the positive CD11b+ cells were further sorted using a Beckman-Coulter EPICS Elite ESP FACS Sorter (Fullerton, CA) to CD11b+/Ly6G+ (neutrophils) and Cd11b+/Ly6G− (mostly macrophages).
For isolation of naïve neutrophils, mice were euthanized, and bone marrow was harvested by flushing the femurs and tibias with HBSS media. Cells were separated by centrifugation over a 3 layer discontinuous Percoll gradient as previously described (Nick et al., 2000).
Evaluation of the morphology of tumor neutrophils
For the evaluation of the morphology of TAN in control and SM16-treated mice, slides from previously sorted CD11b+/Ly6G+ cells were prepared by centrifugation at 1,500 rpm for 10 min in a Shandon Cytospin 3 (Shandon Lipshaw, Inc., Pittsburgh, PA). The neutrophils on the slides were stained using a Hemacolor kit (EM Science, Gibbstown, NJ). Cells were evaluated under light microscopy (X100).
RNA isolation and real-time, reverse transcription-PCR
Pooled RNA from tumors of control and SM16-treated mice was isolated, and the quantification of tumor mRNA levels was performed as previously described (Suzuki et al., 2005). Details on this method and primer sequences can be found in the supplemental data.
Immunohistochemical staining of tumors
Animals bearing flank tumors were euthanized and the tumors were immediately placed in Tissue-Tek OCT compound (Sakura Finetek USA, Inc., Torrance, CA) to be stored at −80°C, followed by Sectioning and staining. Monoclonal antibodies against leukocytes (anti-CD45), macrophages (anti-CD11b), and Ly6G+ cells (anti-Ly6G) were obtained from BD Biosciences.
Evaluation of tumor cytotoxicity by immune cell subsets
We evaluated tumor cytotoxicity using the AB12 mesothelioma-cell line transfected with a luciferase reporter (AB12-Luc), co-cultured with immunocytes. The mechanism of killing was done using specific inhibitors. Details on this method and the inhibitors can be found in the supplemental data.
Evaluation of secretion of cell products (NO, TNF-α, Hydrogen-Peroxidase)
We evaluated the secretion of NO and TNFα in whole tumor explants. Details on this method can be found in the supplemental data.
Statistical Analyses
For the RT-PCR, FACS studies, and flank tumor studies comparing differences between two groups, we used unpaired Student t-tests. For FACS and flank tumor studies comparing more than two groups, we used one sided ANOVA with appropriate post hoc testing. Differences were considered significant when P<0.05. Data are presented as mean+/− SEM.
Supplementary Material
Acknowledgments
This work was funded by NCI PO1 CA 66726, NHLBI T32 HL07586, NHLBI RO1 HL068876 and NIEHS P30 ES013508-02.. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Significance
The role of tumor-associated neutrophils (TAN) in tumor biology is unclear. It is well established that the tumor microenvironment polarizes tumor-associated macrophages (TAM) toward a pro-tumor (M2) versus an anti-tumor (M1) phenotype. Our data supports a paradigm in which resident TAN acquire a pro-tumor phenotype (similar to M2), largely driven by TGF-β to become “N2” neutrophils. After TGF-β blockade, neutrophils acquire an anti-tumor phenotype to become “N1”-TAN (similar to M1). This paradigm suggests that TAN are a double-edged sword, capable of being pro- or anti-tumorigenic, depending on the tumor microenvironment. Our study also shows another mechanism by which TGF-β can enhance tumor growth and supports the potential utility of TGF-β blockade to inhibit tumor growth.
References
- Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological Reviews. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- Allen SS, Mackie JT, Russell K, Jeevan A, Skwor TA, McMurray DN. Altered inflammatory responses following transforming growth factor-[beta] neutralization in experimental guinea pig tuberculous pleurisy. Tuberculosis. 2008;88:430–436. doi: 10.1016/j.tube.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Appelberg R. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J Leukoc Biol. 1992;51:472–477. doi: 10.1002/jlb.51.5.472. [DOI] [PubMed] [Google Scholar]
- Aruga A, Aruga E, Cameron MJ, Chang AE. Different cytokine profiles released by CD4+ and CD8+ tumor-draining lymph node cells involved in mediating tumor regression. J Leukoc Biol. 1997;61:507–516. doi: 10.1002/jlb.61.4.507. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Coussens LM. Cancer: An inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Tumour microenvironment: TGF-beta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PCW, Salmon M, Matharu NM, Vohra RK, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- Chen A, Liu S, Park D, Kang Y, Zheng G. Depleting Intratumoral CD4+CD25+ Regulatory T Cells via FasL Protein Transfer Enhances the Therapeutic Efficacy of Adoptive T Cell Transfer. Cancer Res. 2007;67:1291–1298. doi: 10.1158/0008-5472.CAN-06-2622. [DOI] [PubMed] [Google Scholar]
- Colombo MP, Lombardi L, Stoppacciaro A, Melani C, Parenza M, Bottazzi B, Parmiani G. Granulocyte colony-stimulating factor (G-CSF) gene transduction in murine adenocarcinoma drives neutrophil-mediated tumor inhibition in vivo. Neutrophils discriminate between G-CSF-producing and G-CSF- nonproducing tumor cells. J Immunol. 1992a;149:113–119. [PubMed] [Google Scholar]
- Colombo MP, Modesti A, Parmiani G, Forni G. Local Cytokine Availability Elicits Tumor Rejection and Systemic Immunity through Granulocyte-T-Lymphocyte Cross-Talk. Cancer Res. 1992b;52:4853–4857. [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- Fu K, Corbley MJ, Sun L, Friedman JE, Shan F, Papadatos JL, Costa D, Lutterodt F, Sweigard H, Bowes S, et al. SM16, an Orally Active TGF-{beta} Type I Receptor Inhibitor Prevents Myofibroblast Induction and Vascular Fibrosis in the Rat Carotid Injury Model. Arterioscler Thromb Vasc Biol. 2008;28:665–671. doi: 10.1161/ATVBAHA.107.158030. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, et al. Inhibition of Growth and Metastasis of Mouse Mammary Carcinoma by Selective Inhibitor of Transforming Growth Factor-beta Type I Receptor Kinase In vivo. Clin Cancer Res. 2006;12:4315–4330. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Abrogation of TGF[beta] Signaling in T Cells Leads to Spontaneous T Cell Differentiation and Autoimmune Disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Hicks AM, Riedlinger G, Willingham MC, Alexander-Miller MA, Von Kap-Herr C, Pettenati MJ, Sanders AM, Weir HM, Du W, Kim J, et al. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. 2006;103:7753–7758. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iking-Konert C, Vogl T, Prior B, Wagner C, Sander O, Bleck E, Ostendorf B, Schneider M, Andrassy K, Hansch GM. T lymphocytes in patients with primary vasculitis: expansion of CD8+ T cells with the propensity to activate polymorphonuclear neutrophils. Rheumatology. 2008;47:609–616. doi: 10.1093/rheumatology/ken028. [DOI] [PubMed] [Google Scholar]
- Itou T, Collins LV, Thoren FB, Dahlgren C, Karlsson A. Changes in Activation States of Murine Polymorphonuclear Leukocytes (PMN) during Inflammation: a Comparison of Bone Marrow and Peritoneal Exudate PMN. Clin Vaccine Immunol. 2006;13:575–583. doi: 10.1128/CVI.13.5.575-583.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Cheng G, Haas A, Cheung HK, Zhang X, Corbley M, et al. Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer Research. 2008;68:10247–10256. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic Therapy Enhancement of Antitumor Immunity Is Regulated by Neutrophils. Cancer Res. 2007;67:10501–10510. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-Associated CD8+ T Cell Tolerance Induced by Bone Marrow-Derived Immature Myeloid Cells. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GT, Hong JH, Kwak C, Woo J, Liu V, Lee C, Kim IY. Effect of Dominant Negative Transforming Growth Factor-{beta} Receptor Type II on Cytotoxic Activity of RAW 264.7, a Murine Macrophage Cell Line. Cancer Res. 2007;67:6717–6724. doi: 10.1158/0008-5472.CAN-06-4263. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Monocytes Are Potent Facilitators of Alveolar Neutrophil Emigration During Lung Inflammation: Role of the CCL2-CCR2 Axis. J Immunol. 2003;170:3273–3278. doi: 10.4049/jimmunol.170.6.3273. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Nam J-S, Terabe M, Mamura M, Kang M-J, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, et al. An anti-transforming growth factor-beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick JA, Young SK, Brown KK, Avdi NJ, Arndt PG, Suratt BT, Janes MS, Henson PM, Worthen GS. Role of p38 Mitogen-Activated Protein Kinase in a Murine Model of Pulmonary Inflammation. J Immunol. 2000;164:2151–2159. doi: 10.4049/jimmunol.164.4.2151. [DOI] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibman J, Meixler S, Lee TC, Gold LI, Cronstein BN, Haines KA, Kolasinski SL, Weissmann G. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:6805–6809. doi: 10.1073/pnas.88.15.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunological Reviews. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-Cell receptor expression and antigen-specific T-Cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunological Reviews. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived Hydrogen Peroxide are the underlying mechanism of suppression of T-Cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- Shen L, Smith JM, Shen Z, Eriksson M, C. S, Wira CR. Inhibition of human neutrophil degranulation by transforming growth factor-beta. Clinical and Experimental Immunology. 2007;149:155–161. doi: 10.1111/j.1365-2249.2007.03376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab Invest. 2004;84:1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proceedings of the National Academy of Sciences. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR. Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol. 1996;157:360–368. [PubMed] [Google Scholar]
- Stoppacciaro A, Melani C, Parenza M, Mastracchio A, Bassi C, Baroni C, Parmiani G, Colombo MP. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J Exp Med. 1993;178:151–161. doi: 10.1084/jem.178.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stockle M, Jocham D, Bohle A, Brandau S. Neutrophil granulocytes are required for effective Bacillus Calmette-Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006;66:8250–8257. doi: 10.1158/0008-5472.CAN-06-1416. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kim S, Cheung HK, Corbley MJ, Zhang X, Sun L, Shan F, Singh J, Lee W-C, Albelda SM, Ling LE. A novel small-molecule inhibitor of Transforming Growth Factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 2007;67:2351–2359. doi: 10.1158/0008-5472.CAN-06-2389. [DOI] [PubMed] [Google Scholar]
- Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by Methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-[beta]. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Tvinnereim AR, Hamilton SE, Harty JT. Neutrophil involvement in cross-priming CD8+ T Cell responses to bacterial antigens. J Immunol. 2004;173:1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen KP, Geijtenbeek TB, Van Kooyk Y. Close encounters of neutrophils and DCs. Trends in Immunology. 2005;26:626–631. doi: 10.1016/j.it.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Wallace A, Kapoor V, Sun J, Mrass P, Weninger W, Heitjan DF, June C, Kaiser LR, Ling LE, Albelda SM. Transforming Growth Factor-beta receptor blockade augments the effectiveness of adoptive T-Cell therapy of established solid cancers. Clin Cancer Res. 2008;14:3966–3974. doi: 10.1158/1078-0432.CCR-08-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Chen ZG, Colella S, Bonilla MA, Welte K, Bordignon C, Mantovani A. Chemotactic activity of recombinant human granulocyte colony-stimulating factor. Blood. 1988;72:1456–1460. [PubMed] [Google Scholar]
- Wilderman MJ, Sun J, Jassar AS, Kapoor V, Khan M, Vachani A, Suzuki E, Kinniry PA, Sterman DH, Kaiser LR, Albelda SM. Intrapulmonary IFN-beta gene therapy using an adenoviral vector is highly effective in a murine orthotopic model of bronchogenic adenocarcinoma of the lung. Cancer Res. 2005;65:8379–8387. doi: 10.1158/0008-5472.CAN-05-0920. [DOI] [PubMed] [Google Scholar]
- Youn J-I, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu Y-X. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunping L, He Z, Krueger J, Kaplan C, Sung-Hyung L, Dolman C, Markowitz D, Wenyuan W, Cheng L, Reisfeld RA, Rong X. Targeting tumor-associated macrophages as a novel strategy against breast cancer. Journal of Clinical Investigation. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.