Abstract
Blood pressure is regulated by a number of key molecules involving G-protein-coupled receptors, ion channels and monomeric small G-proteins. The relative contribution of these different signaling pathways to blood pressure regulation remains to be determined. Tamoxifen-induced, smooth muscle-specific inactivation of the L-type Cav1.2 Ca2+ channel gene in mice (SMAKO) reduced mean arterial blood pressure (MAP) in awake, freely moving animals from 120 ± 4.5 to 87 ± 8 mmHg. Phenylephrine (PE)- and angiotensin 2 (AT2)-induced MAP increases were blunted in SMAKO mice, whereas the Rho-kinase inhibitor Y-27632 reduced MAP to the same extent in control and SMAKO mice. Depolarization-induced contraction was abolished in tibialis arteries of SMAKO mice, and development of myogenic tone in response to intravascular pressure (Bayliss effect) was absent. Hind limb perfusion experiments suggested that 50% of the PE-induced resistance is due to calcium influx through the Cav1.2 channel. These results show that Cav1.2 calcium channels are key players in the hormonal regulation of blood pressure and development of myogenic tone.
Keywords: blood pressure/Ca2+ channels/RhoA/SMAKO/smooth muscle cells
Introduction
Chronically elevated arterial blood pressure leads to many fatal diseases such as myocardial infarction or stroke. This illustrates why a detailed understanding of the key factors involved in blood pressure regulation would be very valuable for modern medicine. Arterial blood pressure is determined by vascular tone, i.e. the contractile activity of vascular smooth muscle cells (SMCs) in the walls of small arteries and arterioles. Regulation of the contractile state of resistance vessels is maintained by an interplay of vasoconstrictor and vasodilator stimuli from circulating hormones, neurotransmitters and also directly by blood pressure (Bayliss effect; Bayliss, 1902). Vascular smooth muscle contraction is triggered by Ca2+/calmodulin-dependent phosphorylation of the regulatory myosin light chain. Ca2+ influx through ion channels and Ca2+ release from intracellular stores have been proposed as the major source of this activator Ca2+ (Davis and Hill, 1999). On the other hand, contractility of vascular smooth muscle is regulated not only by [Ca2+]i, but also by membrane potential- and Ca2+-independent mechanisms. The small GTPase RhoA and its upstream activators and downstream effectors have been invoked as major players in these Ca2+-independent mechanisms (Somlyo et al., 1999). To date, the relative contribution of calcium influx through L-type Ca2+ channels to blood pressure regulation and the role of Ca2+ -independent mechanisms have been discussed highly controversially.
In the microcirculation, Ca2+ influx through L-type Ca2+ channels has been supposed to play a particularly important role in myogenic reactivity and tone (Davis and Hill, 1999). Vascular voltage-gated Ca2+ channels are modulated by several signaling systems and appear to be activated by vasoconstrictors that stimulate the Gq/11/G12/13 signaling pathway, e.g. norepinephrine (Nelson et al., 1988). The autoregulatory Bayliss effect (i.e. constriction of the vessel after an increase of transmural pressure) is probably based on graded membrane depolarization in vessels in response to pressure. Membrane depolarization would open voltage-gated Ca2+ channels and increase vasoconstriction, whereas hyperpolarization would close them and induce vasodilatation (Jaggar et al., 1998).
Here, we have investigated the role of the L-type Cav1.2 Ca2+ channel for blood pressure regulation by combining a smooth muscle-specific, temporally controlled gene knockout approach with in vivo analysis of blood pressure in awake mice, video-microscopy of isolated resistance-like vessels under isobaric conditions and hind limb perfusion. Our results suggest that the Cav1.2 Ca2+ channel is required for autoregulation and maintenance of vascular tone in response to depolarization and pressure. Agonists activating the vascular Gq/11 and G12/13 pathways activate not only the L-type Cav1.2 Ca2+ channels but also the RhoA pathway in resistance vessels.
Results
Generation of SMAKO mice
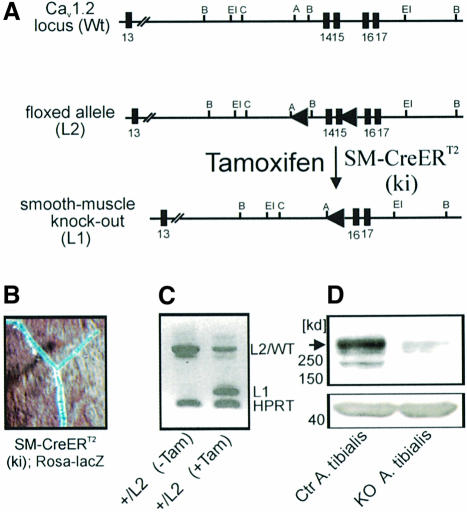
Mice globally lacking the Cav1.2 L-type Ca2+ channel die in utero before day 15 post-coitum (Seisenberger et al., 2000). To circumvent embryonic lethality, the tamoxifen-inducible Cre/loxP recombination system was used to inactivate the Cav1.2 gene specifically in SMCs (Figure 1A; see Materials and methods for details). SMC-specific Cre/loxP recombination was achieved by expressing the tamoxifen-inducible Cre recombinase under control of the SM22 promoter [SM-Cre ERT2(ki) mice; Kuhbandner et al., 2000]. Smooth muscle-specific alpha 1.2 calcium channel knockout (SMAKO) mice before tamoxifen injection are viable, have normal body weight, breed normally and are indistinguishable from control littermates. The phenotype changes dramatically after tamoxifen-induced inactivation of the Cav1.2 gene: between 21 and 28 days after the first tamoxifen injection, SMAKO mice show general signs of severe illness (lowered activity, relieving posture). Post-mortem examination revealed that the mice suffer from a complete ileus (bowel paralysis) combined with urinary retention. SMAKO mice die between 28 and 35 days after tamoxifen injection, probably due to complications of the ileus (peritonitis, shock). The control mice which were also treated with tamoxifen showed no change in phenotype.
Fig. 1. SMC-specific inactivation of the Cav1.2 gene. (A) Schematic representation of the wild-type (WT), the knockout (L1) and the conditional Cav1.2 alleles (L2). The numbers indicate the exon number. SMC-specific activation of tamoxifen-inducible Cre recombinase [SM-Cre ERT2(ki)-Cre] results in the deletion of Cav1.2 exons 14 and 15. Restriction sites are A, Acc65I; B, BamHI; C, ClaI; EI, EcoRI. (B) Detection of lacZ gene expression in tibialis arteries of a tamoxifen-treated SM-Cre ERT2(ki); Rosa-lacZ mouse. Blue staining indicates Cre-mediated recombination. (C) RT–PCR analysis of mesenteric arteries of +/L2 mice before (–Tam) and after (+Tam) tamoxifen treatment. L2/Wt and L1 bands represent L2/wild-type and L1 transcripts, respectively. The L1 band after tamoxifen treatment (+Tam) is generated by Cre-mediated recombination; the remaining L2/WT band is due to the remaining WT allele after recombination. (D) Western analysis of proteins from tibialis arteries using an anti-Cav1.2 antibody demonstrates the absence of Cav1.2 protein (arrowhead) in vessels from SMAKO mice. β-actin (∼43 kDa) was used as loading control.
By crossing SM-Cre ERT2(ki) mice with lacZ reporter mice, we confirmed that Cre-mediated recombination occurs in SMCs of small vessels (Figure 1B). Cre-mediated recombination could also be observed in other vessels and non-vascular tissues containing smooth muscle, e.g. aorta, renal artery, colon, small intestine and urinary bladder, but not in heart or cerebral cortex (not shown).
RT–PCR analysis of cDNA obtained from branches of mesenteric arteries of +/L2 mice after tamoxifen treatment again demonstrated the recombination event (conversion of L2 to L1) in small vessels. The mesenteric arteries of these mice still contain the wild-type Cav1.2 gene (+ or WT allele, respectively) after Cre-mediated recombination (Figure 1C).
Complete loss of L-type currents in smooth muscle cells from tibialis arteries
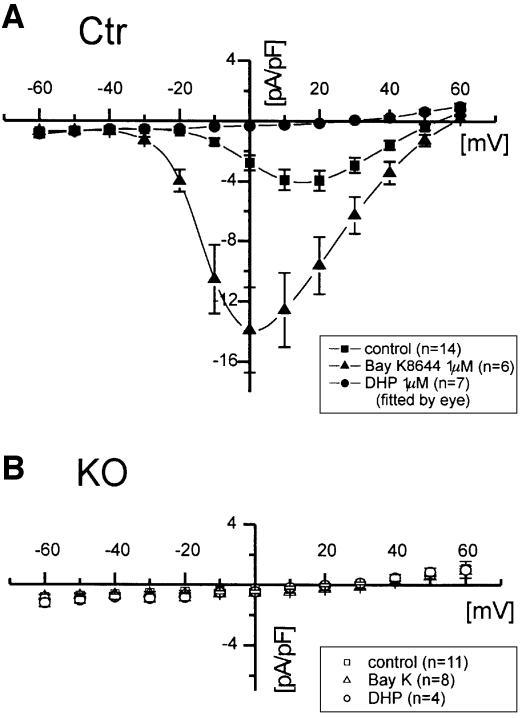
To quantify the recombination efficiency in smooth muscle of SMAKO mice, we then analyzed single SMCs by electrophysiology for the presence of L-type Ca2+ channels and small, resistance-sized (∼100–150 µM) vessels for the presence of Cav1.2 protein. Immunoblotting of whole-cell protein extracts from the tibialis artery using a Cav1.2-specific antibody revealed that the Cav1.2 protein was reduced to <10% in SMAKO mice (Figure 1D). The remaining signal probably represents Cav1.2 protein from other vascular cells such as endothelium, blood cells and fibrocytes. This assumption is confirmed by the analysis of L-type Ba2+ currents (IBa) in freshly isolated control and SMAKO SMCs from tibialis arteries. No L-type current was detected in myocytes from SMAKO mice (n = 0/18 SMCs), while 90.5% (n = 19/21 cells) of the control SMCs had typical L-type current. The maximal IBa density was –4.1 ± 0.7 and –14.0 ± 0.4 pA/pF in the absence and presence of 1 µM Bay K 8644, respectively (Figure 2). In three out of eight SMAKO cells, BayK 8644 increased IBa to –0.8 ± 0.27 pA/pF, which amounts to ∼5% of the Bay K 8644 current measured in control cells.
Fig. 2. Kinetics and pharmacology of smooth muscle L-type IBa. (A and B) Results obtained with SMCs isolated from the tibialis artery of control (Ctr) and SMAKO mice (KO), respectively. Current voltage (I–V) relationships were recorded in the absence and presence of 1 µM Bay K 8644 or nisoldipine. The HP was 80 mV. Cells were depolarized to potentials between –60 and +60 mV with 10 mV increments for 100 ms at 0.2 Hz.
The calcium channel blocker nisoldipine (1 µM) blocked IBa in control SMCs by 93% (Figure 2) but did not change the current of SMAKO myocytes (Figure 2). There was no difference in membrane capacitance between SMCs from control and SMAKO mice, indicating that gene inactivation did not affect cell size and morphology (22 ± 1.6 pF, n = 19, in control and 19.5 ± 1.6 pF, n = 18, in SMAKO mice).
The electrophysiological data and the immunoblots confirm the notion that tamoxifen-induced activation of the Cre recombinase effectively inactivated the Cav1.2 gene in arterial SMCs.
Lack of Cav1.2 attenuates blood pressure and response to vasoconstrictors
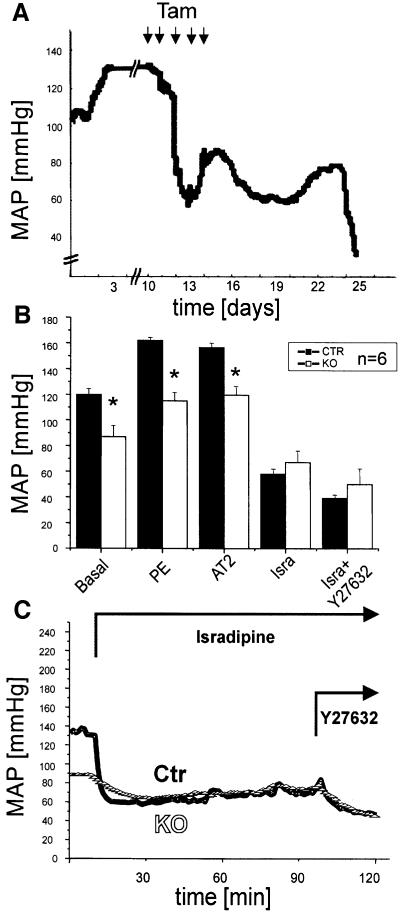
We followed the changes in mean arterial blood pressure (MAP) in individual mice during and after tamoxifen-induction of the Cre recombinase by telemetry (Figure 3A). In general, inactivation of the Cav1.2 gene resulted in a decrease of MAP from 120 ± 4.5 to 87 ± 8 mmHg (Figure 3B). Tamoxifen treatment had no short- or long-term effects on MAP in control animals (n = 3, not shown). The MAP was decreased further in control and SMAKO mice to ∼60 mmHg 1 h after i.p. injection of 0.5 mg/kgbody-weight isradipine (Figure 3B and C). This additional drop in MAP indicates that Cav1.2 channels at other, non-vascular sites could contribute to the regulation of blood pressure. Blockade of cardiac Cav1.2 channels, for example, could lead to blood pressure reduction by a negative inotropic action. Furthermore, the effect of dihydropyridines such as isradipine on blood pressure is attributed not only to their blocking action on smooth muscle L-type Ca2+ channels, but also to release of nitric oxide (NO) and endothelium-derived hyperpolarizing factor (EDHF) from the vascular endothelium, which will result in vessel relaxation and thus blood pressure reduction (Fisslthaler et al., 2000; Hirasawa and Pittman, 2003).
Fig. 3. Reduced arterial blood pressure in SMAKO mice. Blood pressure was measured in conscious, freely moving mice by telemetry. (A) Original tracing showing the effect of tamoxifen application (1 mg/day for 5 days) leading to Cre-mediated Cav1.2 gene recombination on MAP in a SMAKO mouse. (B) Basal blood pressure and effect of an i.p. bolus (200µl) injection of PE (10 µg/kg), AT2 (5 µg/kg), isradipine (Isra; 0.5 mg/kg) and Y-27632 (30 mg/kg) on MAP in awake control (Ctr) and SMAKO (KO) mice analyzed 21–28 days after tamoxifen injection. (C) Original tracing of an experiment statistically analyzed in (B). Arrows indicate time of i.p. injection of compounds.
Although treatment with the α-adrenergic agonist phenylephrine (PE) and with angiotensin 2 (AT2) increased MAP in both genotypes, the blood pressure response to these compounds was significantly lower in SMAKO mice, indicating that G-protein-coupled pathways leading to smooth muscle contraction either directly (e.g. via channel modulation by phosphorylation) or indirectly (e.g. via membrane potential changes) affect the Cav1.2 L-type calcium channel (control mice: 160 ± 2 mmHg, PE; 159 ± 2 mmHg, AT2; SMAKO mice: 117 ± 4 mmHg, PE; 119 ± 4 mmHg, AT2; P < 0.05). We inferred from these results that both receptors signaled not only through the Cav1.2 channel but also by an increase in the release of Ca2+ from intracellular stores or through activation of the RhoA pathway. Therefore, we tested whether or not the residual basal MAP obtained in the presence of isradipine could be lowered further by inhibition of the Rho pathway. One hour after i.p. injection of a high dose of the p160 Rho-kinase inhibitor Y-27632 (30 mg/kgbody-weight), MAP in control and SMAKO mice pre-treated with isradipine was reduced further from 58 ± 3 to 40 ± 1.5 mmHg and from 66 ± 6 to 50 ± 8 mmHg, respectively (Figure 3B and C).
Blunted vasoconstrictor effect in vessels from SMAKO mice
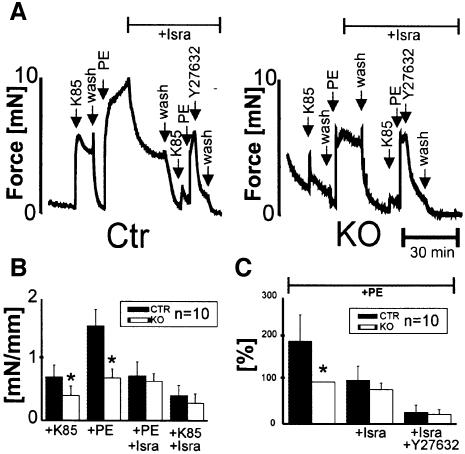
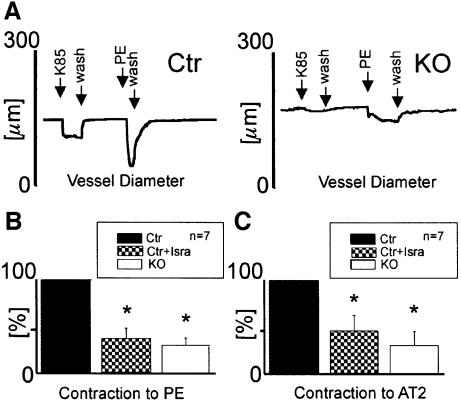
The results shown so far indicated that the L-type Ca2+ channel is functionally absent in vascular SMCs from SMAKO mice. To support this notion further, we measured isometric and isobaric force development in aortic rings and pressurized tibialis arteries, respectively. The contribution of the Cav1.2 channel to the regulation of arterial tone by G-protein-coupled receptors was evaluated by examining the effects of PE and AT2 on arterial diameter and force development. PE caused an increase of 1.6 ± 0.3 mN/mm in isometric force development normalized to vessel length in the control (Figure 4A and C). In contrast, PE increased the force only by 0.7 ± 0.15 mN/mm in aortic rings from SMAKO mice (Figure 4B and C). Similarly, reduction of vessel diameter in response to PE and AT2 in tibialis artery sections pressurized to 60 mmHg from SMAKO mice was significantly (P < 0.01) attenuated to 28 ± 7 and 30 ± 15% of the control value, respectively (Figure 5A–C). After application of isradipine (1 µM), the responses to PE and AT2 reached the value of SMAKO mice in both models of vessel contraction (Figures 4A–C and 5A–C). Again, we evaluated the effect of the Rho-kinase inhibitor Y-27632 on Gq/11/G12/13-mediated vessel contraction in the absence of Cav1.2 protein (SMAKO mice) or after pharmacological blockade of the L-type calcium channels by 1 µM isradipine. Under both conditions, Y-27632 almost completely relaxed aortic rings pre-contracted with PE (Figure 4A, B and D).
Fig. 4. Decreased isometric force generation of aortic rings from SMAKO mice. (A) Original tracings and (B) statistical analysis of contractions of isolated aortic rings from control (Ctr) and SMAKO (KO) mice. Force generation in response to 85 mM K+ (K85) and 1 µM PE measured with or without 1 µM isradipine (Isra) or 5 µM Y-27632 as indicated. Force in (B) is normalized to vessel length. (C) Relaxation of aortic rings pre-contracted with 1 µM PE by 1 µM isradipine and 5 µM Y-27632. Force levels are expressed as a percentage of the maximum contractile force in SMAKO mice. *P < 0.05 versus the same condition in control mice.
Fig. 5. Tibialis arteries from SMAKO mice show a blunted response to vasocontracting agonists. (A) Original tracings showing the effect of superfusion with 85 mM K+ (K85) and 1 µM PE on luminal diameter in vessels from control (Ctr) and SMAKO (KO) mice. (B and C) Contractile response of tibialis arteries from control mice before and after application of 1 µM isradipine (Isra) and from SMAKO mice. Vessels were superfused with (B) 1 µM PE or (C) 500 nM AT2. *P < 0.01.
Arterial myogenic tone is absent in SMAKO mice
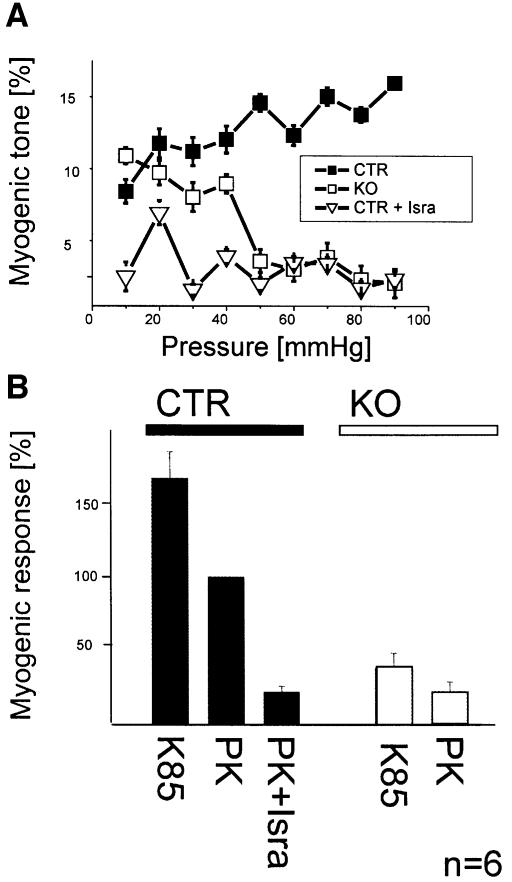
Elevation of intravascular pressure constricts small arteries (Bayliss, 1902; Knot and Nelson, 1998). This autoregulatory behavior of vessels, also termed myogenic tone, has been supposed to play a key role in regulation of tissue blood flow in vivo (Johnson et al., 1981). We investigated myogenic tone using video-microscopy of pressurized resistance-sized tibialis artery preparations. Vasoconstriction induced by elevating intravascular pressure was almost totally inhibited in SMAKO vessels compared with control arteries (control mice: 16.5 ± 0.4% myogenic tone at 90 mmHg; SMAKO mice: 2.2 ± 1% myogenic tone; P < 0.01; Figure 6A). Isradipine treatment of control vessels reduced myogenic tone to the level found in SMAKO mice (2.5 ± 0.55% myogenic tone).
Fig. 6. Pressure- and depolarization-induced myogenic tone is absent in SMAKO mice. (A) Myogenic responses of tibialis arteries of SMAKO (KO) mice and control mice (Ctr) before and after treatment with isradipine (Isra). Myogenic tone is expressed as a percentage of the difference to the passive vessel diameter in Ca2+-free solution at each given pressure. (B) Myogenic response at 60 mmHg of control (Ctr) and SMAKO (KO) tibialis arteries superfused with 85 mM K+ (85K) or 5.6 mM K+ (PK) with or without 1 µM isradipine (Isra). The myogenic response is shown as a percentage of the diameter in control vessels at 5.6 mM K+ concentration.
Elevation of intravascular pressure depolarizes the SMCs within the arterial wall (Brayden and Nelson, 1992). To link the lack of Cav1.2 channels in tibialis arteries from SMAKO mice with the compromised myogenic tone response, we examined the effect of depolarization by 60 mM K+ on the diameter of SMAKO and control arteries at constant pressure. Membrane depolarization constricted tibialis arteries from control mice by 73 ± 20%. Depolarization-induced contraction was absent in SMAKO and in isradipine-treated vessels, indicating that depolarization activates Cav1.2 Ca2+ channels leading to increased intracellular Ca2+ and vasoconstriction (Figure 6B). Similarly, depolarization by 85 mM K+ in isometric aortic ring preparations caused significantly less force development in SMAKO than in control mice (0.42 ± 0.2 versus 0.73 ± 0.2 mN/mm; P < 0.01; Figure 4A–C).
Multiple roles of Cav1.2 channels in the microcirculation
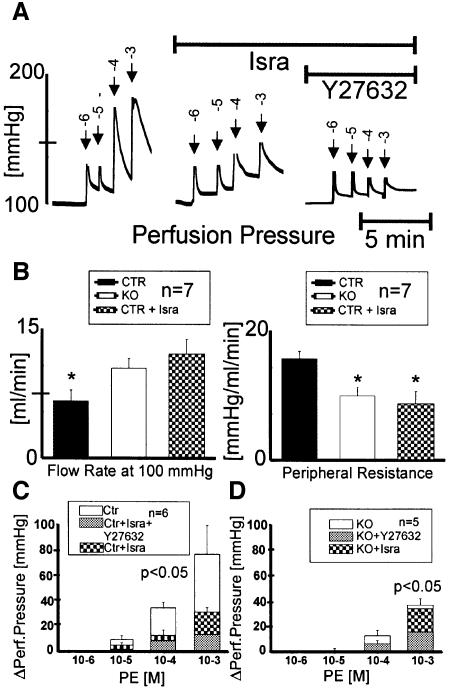
The contractile activity of vascular SMCs in the walls of small arteries and arterioles is the major determinant of blood pressure (Jackson, 2000). To test whether our results found in larger vessels could also be applied to microcirculatory hemodynamics, we used the perfused hind limb model (Figure 7). Resistance at 100 mmHg perfusion pressure was reduced to ∼60% in SMAKO compared with control hind limbs (Figure 7B), indicating the loss of autoregulatory myogenic tone (Brandes et al., 2000). Isradipine treatment of control preparations reduced peripheral vessel resistance in the hind limbs to SMAKO levels (control mice: 16.2 ± 0.9 mmHg/ml/min; control mice + isradipine: 8.8 ± 1.4 mmHg/ml/min; SMAKO mice: 10.3 ± 0.9 mmHg/ml/min; P < 0.01). Perfusion pressure changes to bolus application of 100 µl of 1 mM PE were blunted by isradipine treatment and in SMAKO mice [change in perfusion pressure (ΔPerfusion pressure) in control mice: 68 ± 19 mmHg; in control mice + isradipine: 27 ± 3 mmHg; in SMAKO mice: 35.5 ± 5 mmHg; P < 0.01; Figure 7A, C and D], indicating that ∼50% of the PE-induced resistance increase was causally linked to calcium influx through Cav1.2 channels. The Rho-kinase inhibitor Y-27632 decreased the PE-induced resistance increase further by 45% in SMAKO mice (SMAKO mice + Y-27632: 15.8 ± 2 mmHg; control mice + isradipine + Y-27632: 11.9 ± 2 mmHg; P < 0.01; Figure 7C and D), supporting a role for RhoA signaling in the microvasculature.
Fig. 7. SMAKO mice analyzed in a perfused hind limb model show reduced tone and vasoconstrictor responses. Both hind limbs were perfused via the infrarenal aorta abdominalis with modified Krebs–Henseleit solution. (A) Original tracing showing the effect of bolus (200 µl) applications of PE on perfusion pressure in the absence or presence of 1 µM isradipine (Isra) and 5 µM Y-27632 in a control mouse. Numbers above the arrows indicate the concentration of the agonist applied with each bolus (log mol). Flow rate was adjusted before application of PE to compensate perfusion pressure reduction by the vasorelaxing compounds. (B) Parameters of the perfused hind limb of control mice with and without 1 µM isradipine and of SMAKO mice. Peripheral resistance is expressed as perfusion pressure divided by flow rate. (C and D) Vasocontraction response at constant flow to bolus (200 µl) applications of PE (log mol) in the absence or presence of 1 µM isradipine (Isra) and 5 µM Y-27632 in (C) control mice (Ctr) or (D) SMAKO mice. Vasocontraction response is shown as ΔPerfusion pressure [mmHg] (change in perfusion pressure) at constant flow.
Discussion
The present study reports for the first time on a tissue-specific inactivation of the Cav1.2 gene in SMCs by using the temporally controlled Cre/loxP system. With this technique, we were able to analyze the contribution of the smooth muscle L-type Cav1.2 channel to blood pressure regulation in the absence of calcium channel blockers. The genetic approach is superior to pharmacological experiments for various reasons. Higher concentrations of calcium channel blockers will affect the cardiac Cav1.2 channel in vivo and thereby decrease cardiac contractility and blood pressure. Dihydropyridines such as isradipine act voltage dependently (for a review see Hofmann et al., 1999), which makes it difficult to block L-type Ca2+ channels in isolated cells or tissues at physiological membrane potentials. Furthermore, L-type Ca2+ channel blockers have multiple unwanted side effects. For example, L-type Ca2+ channel antagonists have been shown to induce release of EDHF (Fisslthaler et al., 2000) from vascular endothelium and to attenuate production of the vasoconstricting endothelin-1 in endothelial cells (Yakubu and Lefffler, 2002). All these mechanisms will interfere with both in vivo and in vitro experiments using L-type Ca2+ channel blockers.
A concern with any tissue-specific knockout approach utilizing the Cre/lox technique is the selectivity and efficiency of the deletion. Based on western analyses, on the electrophysiological results and the effect of high concentrations of isradipine, we estimate the efficiency of the vascular Cav1.2 deletion to be close to 100%. Although it is possible that a small subset of vascular SMCs still expressed Cav1.2 channels, the physiological analyses with SMAKO vessels and SMCs clearly show that Cav1.2 protein was functionally absent in SMAKO mice.
Intracellular Ca2+ plays a pivotal role in regulating electromechanical coupling in muscle, including vascular smooth muscle. L-type Ca2+ channels are activated in the membrane potential range found in microvascular smooth muscles. This makes them particularly suited to couple vessel membrane depolarization to contraction (Davis and Hill, 1999). Our results indeed support the concept that the Cav1.2 channel is required to couple pressure-induced membrane potential changes to the myogenic response, because the autoregulatory Bayliss effect and membrane potential-activated smooth muscle contractions were absent in SMAKO mice.
As the population of ion channels in SMCs is most probably heterogeneous, in various vascular beds the contribution of Cav1.2 channels to the regulation of myogenic tone could be different. For example, it has been shown that T-type Ca2+ channels are involved in maintenance of myogenic tone in renal (Hansen et al., 2001) and cremaster muscle arterioles (VanBavel et al., 2002). However, the functional significance of these findings remains unclear as these studies primarily rely on the use of the T-type Ca2+ channel blockers mibefradil and Ni2+, which also affect L-type calcium channels (Lacinová et al., 1995; Klugbauer et al., 1999). Further studies with SMAKO mice might help to reveal the role of T-type Ca2+ channels in smooth muscle.
In agreement with the loss of the autoregulation of myogenic tone, basal MAP was reduced by 33 mmHg in SMAKO mice. Why do SMAKO mice still have a functional, albeit attenuated blood pressure regulation? Arterial blood pressure is not only determined by autoregulatory myogenic mechanisms, but also by an interplay of vasoconstrictors (most importantly AT2 and norepinephrine) and vasodilators such as Prostacyclin, NO and EDHF. The vasoconstricting effect of PE and AT2 was reduced but not abolished. Apparently, these hormones use multiple pathways to induce smooth muscle contraction, including the calcium-dependent and -independent pathway. The mechanism leading to membrane depolarization and activation of L-type calcium channels in response to PE, AT2 or pressure increase is not clear. A number of possibilities have been suggested such as activation of a non-specific cation conductance or a Ca2+-dependent chloride conductance. Further studies with Cav1.2-deficient mice will help clarify this mechanism. The hind limb perfusion experiments suggested that 50% of the PE-induced resistance is due to calcium influx through the Cav1.2 channel. Only 20% of the resistance was caused by the calcium-independent pathway involving Rho-kinase. Rho/Rho-kinase-mediated signaling has been suggested to play a key role in the regulation of force and velocity of actomyosin cross-bridge cycling in smooth muscle by inhibition of myosin phosphatase-mediated dephosphorylation of the regulatory light chain of myosin II (Somlyo et al., 1999). Recent evidence suggests that the modulation of vascular smooth muscle Ca2+ sensitivity by RhoA is a major determinant of vascular tone in resistance arteries and hence an important factor in the control of systemic blood pressure. Different vasoconstrictors have been shown to exert their effects at least in part through activation of the RhoA signaling cascade (Sakurada et al., 2001; Sauzeau et al., 2001; Bolz et al., 2003). Furthermore, pharmacological inhibition of Rho kinase normalized blood pressure in three genetically different rat models of hypertension, while it only caused a slight fall in blood pressure in control rats (Uehata et al., 1997). Residual vasoconstriction or blood pressure changes in response to G-protein-coupled receptor agonists in SMAKO mice were clearly dependent on Rho-kinase signaling, both in vivo and in the microcirculation. These experiments confirm the notion that, in addition to Cav1.2 Ca2+ channel activation by membrane potential changes, the Ca2+ channel-independent Rho-kinase pathway contributes to blood pressure regulation by G-protein-coupled vasoconstrictors.
Clearly, the SMAKO mouse represents a unique model, wherein the complex interplay of blood pressure-regulating molecules can be analyzed in detail.
Materials and methods
All experiments were conducted in accordance with the ‘Guide for the use and care of laboratory animals’ and approved by the Regierung von Oberbayern.
Conditional inactivation of the CaV1.2 gene in smooth muscle cells
As described previously (Seisenberger et al., 2000), two different Cav1.2 alleles were generated by Cre-mediated recombination in embryonic stem cells (L1 and L2; Figure 1A). In L1, exons 14 and 15 which encode the IIS5 and IIS6 transmembrane segments and the pore loop in domain II were deleted. Additionally, this deletion causes an incorrect splicing from exon 13 to part of an intron upstream of exon 16, and thereby generates a premature stop codon in exon 16 and a loss of function allele. L2 contains the ‘floxed’ exons 14 and 15 and encodes a functional Cav1.2 gene. To generate SMAKO mice, the Cav1.2L2/L2 mouse (i.e. a mouse homozygous for the L2-allele) was crossed with a mouse expressing a tamoxifen-inducible Cre recombinase under control of the SM22 promoter [SM-Cre ERT2(ki)] (Kuhbandner et al., 2000). The resulting Cav1.2L2/L2, SM-Cre ERT2(ki)Cre/Cre mice were then mated with Cav1.2+/L1 mice (i.e. mice carrying one L1-allele and one wild-type allele) to obtain the smooth muscle-specific knockout Cav1.2L1/L2, SM-Cre ERT2(ki)+/Cre (i.e. SMAKO mice) and control animals [Cav1.2+/L2, SM-Cre ERT2(ki)+/Cre; control]. The background mouse strain was C57BL/6. Both lines were viable and showed no gross abnormalities. To induce smooth muscle-specific Cre recombination (conversion of L2 to L1 allele in vivo), adult SMAKO and control mice were treated with freshly prepared tamoxifen solution (Sigma) by i.p. injection once a day for 5 days at a dosage of 1 mg a day. Tamoxifen was dissolved in miglyol oil (Caelo) at a concentration of 10 mg/ml.
RT–PCR on mRNA of mesenteric arteries
Mesenteric sheets containing small branches of mesenteric arteries were isolated and cleaned of connective tissue. Poly(A) mRNA was isolated from the vessels using Dynabeads Oligo(dT)25 (Dynal Biotech). The following buffers were used: GTC buffer [4 M guanidine thiocyanate, 20 mM Na-acetate pH 5.4, 0.1 mM dithiothreitol (DTT), 0.5% lauroyl sarcosinate (w/v), 6.5 µl/ml mercaptoethanol], binding buffer (100 mM Tris–HCl pH 8.0, 20 mM EDTA, 400 mM LiCl) and washing buffer (10 mM Tris–HCl pH 8.0, 0.15 M LiCl, 1 mM EDTA). The mRNA was eluted with diethylpyrocarbonate (DEPC)-treated water. Random hexamer primers and Superscript Reverse Transkriptase II (Life Technologies) were used for cDNA synthesis. The following primers were used: for PCR amplifying Cav1.2 (E13 5′-aCAGCC AATAAAGCCCTCCT-3′ and Lef 1 5′-GGCTTCTCCATCACC TCCTGTT-3′) and for HPRT (QG 197 5′-GTAATGATCAGT CAACGGGGGAC-3′ and QG 198 5′-CCAGCAAGCTTGCAA CCTTAACCA-3′).
Western blot analysis and lacZ staining
The tibialis ateries were pulverized under liquid nitrogen and boiled in 2% SDS/50 mM Tris for 10 min. The resulting homogenates (80 µg of protein) were separated by 7% SDS–PAGE, blotted on a PVDF membrane (Millipore) and probed with a Cav1.2-specific antibody (Chemicon). Equal loading of slots was ascertained by the use of a monoclonal β-actin antibody (Abcam). Antibodies were visualized by the ECL system (NEN). LacZ staining was done as described (Kuhbandner et al., 2000).
Smooth muscle cell isolation
The vessel dissection and cell isolation procedures were slightly modified from the procedures described by Kleppisch et al. (1995). Briefly, control and SMAKO littermates were killed by cervical dislocation and arteries were cut into 2 mm segments and placed in Ca2+-free isolation solution of the following composition: 60 mmol/l NaCl, 85 mmol/l sodium glutamate, 5.6 mmol/l KCl, 2 mmol/l MgCl2, 10 mmol/l glucose, 10 mmol/l HEPES pH 7.4. After a 10 min equilibration (37°C), artery segments were placed in Ca2+-free isolation solution (37°C) containing 1 mg/ml albumin, 0.7 mg/ml papain and 1 mg/ml DTT. After 40 min exposure to papain, artery segments were placed for 10–15 min in a second isolation solution containing 0.1 mmol/l CaCl2 and a collagenase type F and hyaluronidase mixture (1 mg/ml each). The tissue was subsequently washed twice (10 min each) in Ca2+-free isolation solution and triturated with a polished wide-bore pipet. Cells were stored on ice and used the same day.
Electrophysiology
Whole-cell currents were recorded at room temperature using fire-polished electrodes with resistances of 2.7–3.5 MΩ. Pipettes were filled with 112 mM CsCl, 1 mM MgSO4, 3 mM Na2-aTP, 5 mM HEPES, 10 mM EGTA pH 7.4. Extracellular bath solution was 104 mM NaCl, 5.4 mM CsCl, 20 mM TEACl, 5 mM BaCl2, 1 mM MgCl2, 1 mM NaH2PO4, 10 mM glucose, 5 mM HEPES pH 7.4. The holding potential (HP) was 80 mV. To measure current–voltage (I–V) relationships for IBa of L-type Ca2+ channel, trains of test pulses were applied once every 5 s for 100 ms from –60 to 60 mV with 10 mV increments. Data were collected and stored on an EPC-9 computer under control of Pulse software (HEKA electronics). Total cell membrane capacitance was determined by compensation mechanisms of the EPC9 computer and used as a measurement of membrane area. Stock solutions were: Bay K 8644 10 mM in ethanol; nisoldipine 20 mM in ethanol. On the experimental day, stock solutions were freshly diluted to the indicated concentrations with the extracellular solution.
Telemetric blood pressure recordings
Male SMAKO and control littermate mice (8–12 weeks old) were treated with tamoxifen solution (Sigma) by i.p. injection once a day for 5 days at a dosage of 1 mg a day. Animals were kept on a 12 h light/dark cycle. Blood pressure signals from the aortic arch were measured in conscious, unrestrained animals with surgically implanted, miniaturized telemetry devices (Datascience Corp.). For long-term measurements of MAP, mice were implanted with the transmitter, allowed to recover for 2 weeks and then treated with tamoxifen. Immediately after implantation of the transmitter, mice were returned to their home cages (placed on top of telemetry receivers), where they continued to be monitored daily throughout the study for general condition, body weight, food and water intake, state of surgical wound healing, and any signs of morbidity. MAP, heart rate and locomotor activity were recorded continuously until 28 days after tamoxifen injection. For short-term measurements of MAP, mice pre-treated with tamoxifen (21–28 days before) were implanted with the transmitter, allowed to recover from anesthesia and then directly measured when fully awake. Vasoactive compounds were applied i.p. as a bolus (200 µl) in phosphate-buffered saline (PBS). Sixty second MAP recordings were obtained every 90 s for 15 min before and until 120 min after drug administration. Recordings obtained after a stable drug effect was observed were used for statistical analysis.
Measurement of arterial diameter
Tibialis arteries were quickly isolated, dissected free of connective tissue and transferred to a arteriograph (Danish Myosystems) filled with ice-cold physiological saline solution (PSS) containing: 119 mmol/l NaCl, 4.7 mmol/l KCl, 24.0 mmol/l NaHCO3, 1.2 mmol/l KH2PO4, 1.6 mmol/l CaCl2, 1.2 mmol/l MgSO4, 0.023 mmol/l EDTA and 11.0 mmol/l glucose pH 7.4. The vessels were cannulated with glass pipets and secured with nylon thread. The arteriograph was then placed on the stage of an inverted microscope, and the artery visualized with a monochrome CCD camera coupled to a calibrated video system to measure arterial diameter. The arteries were slowly pressurized to 60 mmHg under no-flow conditions and warmed to 37°C while being continuously superfused (5 ml/min) with PSS bubbled with 95% O2/5% CO2 (pH 7.35–7.40 in the bath). After an equilibration period of ∼20 min, arteries showed stable myogenic tone at 60 mmHg. Afterwards, the effect of different pharmacological agents on vessel diameter was evaluated. Obtaining the maximal dilated diameter in a calcium-free PSS concluded all experiments. Pressure–diameter curves were obtained by increasing the pressure in 10 mmHg steps from 10 to 100 mmHg. Pressure–diameter curves were repeated in the presence of calcium-free PSS.
Organ bath experiments with aortic rings
Aortae were dissected, cut into segments (2–3 mm), and mounted on wire triangles. Segments were placed in organ chambers (Danish Myosystems), connected to force transducers, and gradually stretched to 1 g. Studies were performed in modified Krebs–Henseleit (KH) buffer (118.3 mmol/l NaCl, 4.7 mmol/l KCl, 1.8 mmol/l CaCl2, 1.2 mmol/l MgSO4, 1.2 mmol/l KH2PO4, 25 mmol/l NaHCO3, 0.026 mmol/l EDTA, 11.1 mmol/l glucose pH 7.40 aerated with 95% O2/5% CO2).
Perfused hind limb
After sacrifice, the infrarenal aorta was prepared, and a catheter (1 mm, Hugo Sachs, Germany) was introduced, advanced to the iliac arteries and tied with a 6/0 prolene stitch (Ethicon). The inferior caval vein was slit open longitudinally to prevent venous congestion. A roller pump was used to perfuse the hind limb constantly with filtered KH solution. A pressure transducer and a compliance chamber were connected to a side port of the perfusion system. The flow rate was gradually increased to achieve a perfusion pressure of ∼100 mmHg. At this perfusion pressure, a considerable amount of spontaneous myogenic tone is present, which made pre-constriction unnecessary. When a substance produced a pronounced change in resistance, flow rate was adjusted. When a stable pressure plateau was reached, PE was applied in increasing concentrations as a bolus (100 µl) in KH solution, in the presence or absence of inhibitors (isradipine, Y-27632). For inhibitor studies, agonist-induced responses were recorded in the absence and then in the presence of the inhibitor to facilitate paired comparison of the results. Vasodilator responses were measured as changes in perfusion pressure.
Data analysis
Data are given as mean values ± SEM. Statistical significances were evaluated by either ANOVA followed by Dunnet’s ad hoc tests for unpaired comparisons, or by paired Students’ t-test when comparing data obtained in the same cell.
Acknowledgments
Acknowledgements
We thank Mrs Susanne Paparisto for excellent technical assistance. Supported by the Deutsche Forschungsgemeinschaft (SFB 391) and Fond der Chemischen Industrie.
References
- Bayliss W.M. (1902) On the local reactions of the arterial wall to changes of internal pressure. J. Physiol., 28, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz S.S., Vogel,L., Sollinger,D., Derwand,R., Boer,C., Pitson,S.M., Spiegel,S. and Pohl,U. (2003) Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation, 108, 342–347. [DOI] [PubMed] [Google Scholar]
- Brandes R.P., Schmitz-Winnenthal,F.H., Feletou,M., Godecke,A., Huang,P.L., Vanhoutte,P.M., Fleming,I. and Busse,R. (2000) An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc. Natl Acad. Sci. USA, 97, 9747–9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J.E. and Nelson,M.T. (1992) Regulation of arterial tone by activation of calcium-dependent potassium channels. Science, 256, 532–535. [DOI] [PubMed] [Google Scholar]
- Davis M.J. and Hill,M.A. (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev., 79, 387–423. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B., Hinsch,N., Chataigneau,T., Popp,R., Kiss,L., Busse,R. and Fleming,I. (2000) Nifedipine increases cytochrome P4502C expression and endothelium-derived hyperpolarizing factor-mediated responses in coronary arteries. Hypertension, 36, 270–275. [DOI] [PubMed] [Google Scholar]
- Hansen P.B., Jensen,B.L., Andreasen,D. and Skott,O. (2001) Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ. Res., 89, 630–638. [DOI] [PubMed] [Google Scholar]
- Hirasawa M. and Pittman,Q.J. (2003) Nifedipine facilitates neurotransmitter release independently of calcium channels. Proc. Natl Acad. Sci. USA, 100, 6139–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F., Lacinová,L. and Klugbauer,N. (1999) Voltage-dependent calcium channels: from structure to function. Rev. Physiol. Biochem. Pharmacol., 139, 35–87. [DOI] [PubMed] [Google Scholar]
- Jackson W.F. (2000) Ion channels and vascular tone. Hypertension, 35, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar J.H. et al. (1998) Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol. Scand., 164, 577–587. [DOI] [PubMed] [Google Scholar]
- Johnson P.C. (1981) The myogenic response. In Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. American Physiology Society, Bethesda, MD, pp. 409–442. [Google Scholar]
- Kleppisch T. and Nelson,M.T. (1995) Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc. Natl Acad. Sci. USA, 92, 12441–12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N., Marais,E., Lacinova,L. and Hofmann,F. (1999) A T-type calcium channel from mouse brain. Pflugers Arch., 437, 710–715. [DOI] [PubMed] [Google Scholar]
- Knot H.J. and Nelson,M.T. (1998) Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol., 508, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhbandner S., Brummer,S., Metzger,D., Chambon,P., Hofmann,F. and Feil,R. (2000) Temporally controlled somatic mutagenesis in smooth muscle. Genesis, 29, 15–22. [DOI] [PubMed] [Google Scholar]
- Lacinová L., Welling,A., Bosse,E., Ruth,P., Flockerzi,V. and Hofmann,F. (1995) The block of the expressed L-type calcium channel is modulated by the β3 subunit. FEBS Lett., 373, 103–117. [DOI] [PubMed] [Google Scholar]
- Nelson M.T., Standen,N.B., Brayden,J.E. and Worley,J.F.,3rd (1988) Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature, 336, 382–385. [DOI] [PubMed] [Google Scholar]
- Sakurada S., Okamoto,H., Takuwa,N., Sugimoto,N. and Takuwa,Y. (2001) Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am. J. Physiol., 281, C571–C578. [DOI] [PubMed] [Google Scholar]
- Sauzeau V., Le Mellionnec,E., Bertoglio,J., Scalbert,E., Pacaud,P. and Loirand,G. (2001) Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ. Res., 88, 1102–1104. [DOI] [PubMed] [Google Scholar]
- Seisenberger C. et al. (2000) Functional embryonic cardiomyocytes after disruption of the L-type α1C (Cav1.2) calcium channel gene in the mouse. J. Biol. Chem., 275, 39193–39199. [DOI] [PubMed] [Google Scholar]
- Somlyo A.P., Wu,X., Walker,L.A. and Somlyo,A.V. (1999) Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev. Physiol. Biochem. Pharmacol., 134, 201–234. [DOI] [PubMed] [Google Scholar]
- Uehata M. et al. (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature, 389, 990–994. [DOI] [PubMed] [Google Scholar]
- VanBavel E., Sorop,O., Andreasen,D., Pfaffendorf,M. and Jensen,B.L. (2002) Role of T-type calcium channels in myogenic tone of skeletal muscle resistance arteries. Am. J. Physiol., 283, H2239–H2243. [DOI] [PubMed] [Google Scholar]
- Yakubu M.A. and Leffler,C.W. (2002) L-type voltage-dependent Ca2+ channels in cerebral microvascular endothelial cells and ET-1 biosynthesis. Am. J. Physiol., 283, C1687–C1695. [DOI] [PMC free article] [PubMed] [Google Scholar]