Abstract
It has been nearly two decades since the discovery of Bartonella as an agent of bacillary angiomatosis in AIDS patients and persistent bacteremia and ‘nonculturable’ endocarditis in homeless people. Since that time, the number of Bartonella species identified has increased from one to 24, and 10 of these bacteria are associated with human disease. Although Bartonella is the only genus that infects human erythrocytes and triggers pathological angiogenesis in the vascular bed, the group remains understudied compared with most other bacterial pathogens. Numerous questions regarding Bartonella's molecular pathogenesis and epidemiology remain unanswered. Virtually every mammal harbors one or more Bartonella species and their transmission typically involves a hematophagous arthropod vector. However, many details regarding epidemiology and the public health threat imposed by these animal reservoirs is unclear. A handful of studies have shown that bartonellae are highly-adapted pathogens whose parasitic strategy has evolved to cause persistent infections of the host. To this end, virulence attributes of Bartonella include the subversion of host cells with effector molecules delivered via a type IV secretion system, induction of pathological angiogenesis through various means, including inhibition of apoptosis and activation of hypoxia-inducing factor 1, use of afimbrial adhesins that are orthologs of Yersinia adhesin A, incorporation of lipopolysaccharides with low endotoxic potency in the outer membrane, and several other virulence factors that help Bartonella infect and persist in erythrocytes and endothelial cells of the host circulatory system.
Keywords: angiogenesis, Bartonella, bartonellosis, hemotrophy, infection, virulence
Bartonella bacilliformis
Bartonella are widely regarded in the biomedical community as emerging bacterial agents of infectious disease. In fact, modern-day bartonelloses are preceded by a long history and an incidence that inversely correlates with the human condition. Archaeological evidence suggests that these pathogens have afflicted humans for several millennia. Anthropomorphic pottery jugs (huacos) and carvings made by pre-Columbian Indians in endemic regions of South America frequently bear the telltale angiomatous lesions of Oroya fever, termed verruga peruana, a sequela of chronic infection by Bartonella bacilliformis (Figure 1). Dissemination of knowledge regarding the dangers of exposure to the pathogen was minimal at this time, since the indigenous people had no written language, and the disease was geographically restricted to the high Andes. In fact, the disease potential of B. bacilliformis would not be fully appreciated for several centuries. In 1540, Spanish conquistadores led by Pizarro were likely the first foreigners to experience an epidemic of acute febrile illness followed by ‘warts full of blood’ in the survivors [1]. Later, during the building of the highest portion of the Central Railroad of Peru, Oroya fever resulted in 8000 fatalities in only 4 years (1869–1873). It was not until the late 19th century that a Peruvian medical student, Daniel Alcides de Carrión, demonstrated that verruga peruana lesions could be used as an inoculum to elicit the more acute febrile syndrome (Oroya fever). Tragically, the experiment was done using his own body as a model, and he died of Oroya fever at 28 years of age. To this day, the acute and chronic manifestations of B. bacilliformis infection are referred to as Carrión's disease, and Carrión is a national hero in Peru. Several lines of evidence suggest that B. bacilliformis is the sole representative of an ‘ancestral’ lineage from which other host-adapted ‘modern’ Bartonella species have evolved (see ‘Erythrocyte Parasitism’).
Figure 1. Angiomatous lesions caused by Bartonella bacilliformis.
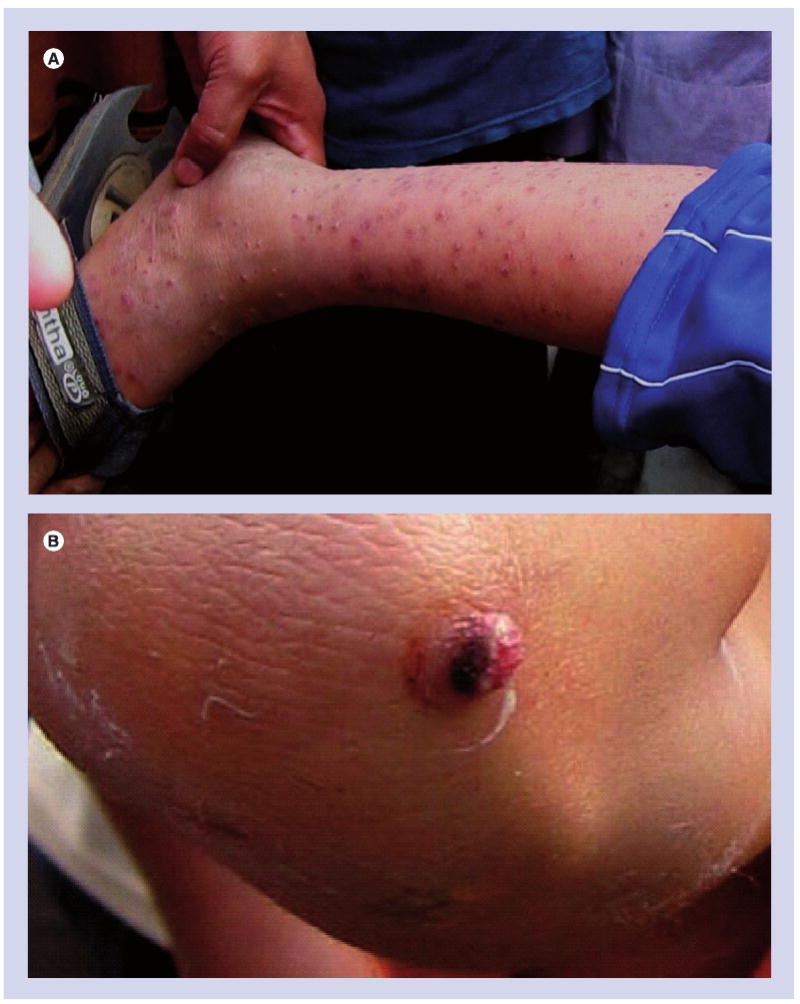
(A) Numerous verruga peruana and (B) a close-up of a lesion on a child living in a mountain village near Casma, Peru, following an outbreak of Oroya fever in 2008.
Courtesy of David Pascual.
Bartonella quintana
The second major historical event involving Bartonella coincided with World Wars I and II. Trench warfare, POW facilities and concentration camps were ideal scenarios for the transmission of louse-borne trench fever, caused by Bartonella quintana (formerly Rochliamaea or Rickettsia quintana). In fact, trench fever epidemics were commonplace during World War I and its morbidity for troops was only surpassed by influenza; a major pandemic at this time. For almost five decades following World War II, trench fever was observed only sporadically in developing nations. Then, in the mid-1990s, B. quintana was recognized as a re-emerging agent of ‘urban trench fever’, afflicting a significant portion of homeless inner-city populations throughout the world [2]. Interestingly, homeless people share many of the same risk factors for trench fever as troops or prisoners afflicted during war, including malnutrition, poor hygiene, louse infestation, alcohol abuse and an immunocompromised state.
Bartonella henselae
The third major historical event involving Bartonella, and one that helped clarify the genus' phylogenetic relatedness to other α-proteobacteria, including rhizobia and Brucella, was the increase in cases of AIDS-related complex in the late 1980s. In an interesting clinical detective story, both Bartonella henselae and B. quintana were shown by several labs to be frequent secondary pathogens of AIDS patients [3,4]. In a majority of these cases, bartonellosis manifested as one or a combination of vascular pathologies, including bacillary angiomatosis (BA) of the skin (similar to verrugas), bacillary peliosis of the liver and spleen, nonculturable endocarditis and chronic bacteremia.
Currently, there are two dozen recognized Bartonella species, 10 of which are associated with human disease [5]. Nevertheless, the majority of reported human bartonelloses are caused by three species: B. bacilliformis, B. quintana and B. henselae, and these pathogens are the focus of this article.
Transmission & colonization of the human host
Bartonella are transmitted to humans via two routes: hematophagous insects, such as phlebotamine sand flies (B. bacilliformis), human body lice (B. quintana) and cat fleas (B. henselae); and animal scratches and bites (B. henselae). In certain instances, the distinction between vector and reservoir is blurred by a lifelong infection of the vector, as seen in human body lice infected with B. quintana [6] and perhaps in sand flies infected with B. bacilliformis [7]. The role of ticks in transmission to humans is suspected and supported by indirect evidence [8]. It is also important to note that unlike B. quintana and B. bacilliformis, B. henselae also utilizes nonhuman reservoirs (e.g., cats and dogs [8]).
When Bartonella gains access to the human circulatory system, it disseminates from the point of inoculation by passive means. Motility conferred by lophotrichous flagella (B. bacilliformis) may contribute to spreading, but the importance of this appendage in host colonization has not been conclusively demonstrated. What is clear is that Bartonella can colonize secondary foci at considerable distances from the primary site of infection, and there is a preference for highly vascularized tissues like heart valves, liver and spleen (B. quintana and B. henselae), or cooler areas of the body, such as the vascular bed of the skin (B. bacilliformis). It is in the context of these particular locations that Bartonella interacts with its two major host cell types – erythrocytes and endothelial cells.
Erythrocyte parasitism
One of the most fascinating aspects of a Bartonella infection is the intracellular parasitism of erythrocytes, a practice termed hemotrophy. Although bacterial pathogens of other vertebrates, such as Anaplasma, also employ this parasitic strategy, in this regard, Bartonella is unique amongst bacterial pathogens of humans. Of the three major pathogenic Bartonella, only B. henselae and B. quintana establish chronic intraerythrocytic bacteremias. In stark contrast, B. bacilliformis' hemotrophy is relatively short-lived and potentially life-threatening, presumably due to splenic culling of infected cells (nearly every erythrocyte in circulation) and the attendant drop in hematocrit (∼80% decrease).
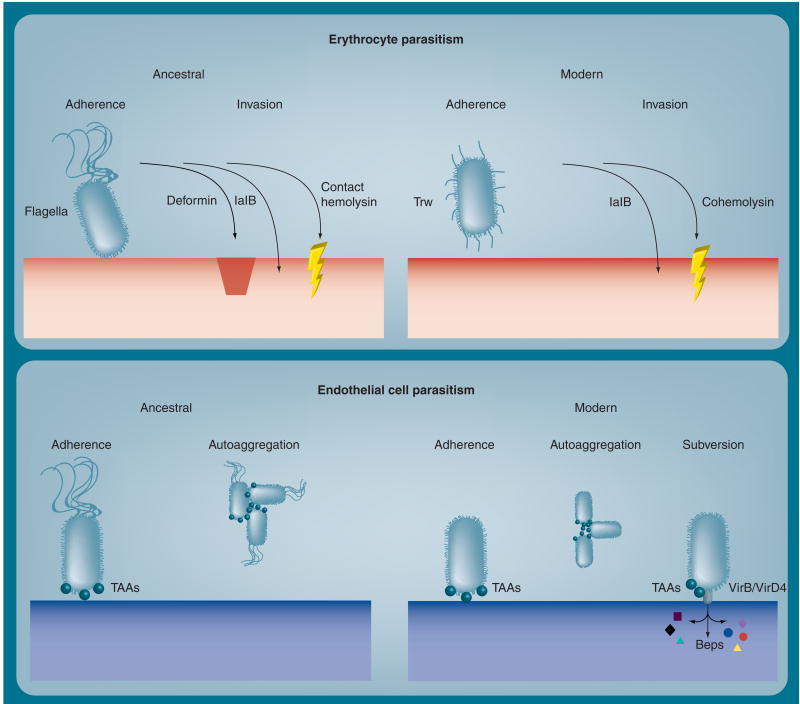
Factors that contribute to erythrocyte tropism include flagella-mediated motility, and surface proteins associated with the Trw type IV secretion system (T4SS) (Figure 2). Remarkably, genes for flagella-based motility, and Trw are mutually exclusive; the ‘ancestral’ Bartonella lineage, B. bacilliformis, is flagellated and lacks Trw, whereas more recently descended lineages, including B. quintana and B. henselae (‘modern’ Bartonella), are nonflagellated but possess Trw (Table 1) [5,9]. Early work showed that B. bacilliformis' binding to erythrocytes was absent in nonmotile or killed bacteria [10,11]. Later research showed that if B. bacilliformis' motility is impaired by antiflagellin antibodies, bacterial association and invasion of human erythrocytes is significantly decreased in vitro, suggesting that motility increases the pathogen's ability to parasitize host erythrocytes [12].
Figure 2. Virulence factors utilized by ancestral (Bartonella bacilliformis) and modern Bartonella species (Bartonella henselae and Bartonella quintana) to parasitize human erythrocytes and endothelial cells.
A summary of the depicted determinants and their occurrence in pathogenic Bartonella are provided in Table 1.
Bep: Bartonella effector protein; TAA: Trimeric autotransporter adhesin.
Table 1. Alphabetical listing of proven or likely virulence determinants of Bartonella, based on current literature, phenotypes (contact hemolysin, deformin and LPS) and GenBank listings (all others).
| Virulence determinant | Bartonella bacilliformis | Bartonella quintana | Bartonella henselae |
|---|---|---|---|
| BatR/BatS | Present | Present | Present |
| Cohemolysin | ? | Present | Present |
| Contact hemolysin | Present | Absent | Absent |
| C-terminal protease | Present | Present | Present |
| Deformin* | Present | ? | ? |
| Flagella-mediated motility | Present | Absent | Absent |
| Hbp family‡ | Present§ | Present§ | Present§ |
| Heme uptake system¶ | Present# | Present | Present |
| IalA/IalB** | Present | Present | Present |
| LPS with low endotoxicity | Present | Present | Present |
| TAAs*‡ | Present | Present | Present |
| Trw pilin system | Absent | Present | Present |
| VirB/VirD4 and Beps*§ | Absent | Present | Present |
Deformin (deformation factor) has been reported once for Bartonella henselae [13].
Heme-binding proteins. Also referred to as phage-associated protein of 31 kDa (Pap31) of B. henselae.
Three Hbps in Bartonella bacilliformis, five in both B. henselae and Bartonella quintana.
Heme receptor, periplasmic heme-binding protein, ABC transporter/permease and TonB.
A TonB ortholog is not encoded in this genome; possibly uses TolC.
Invasion-associated locus A and B for a nudix hydrolase and erythrocyte invasion protein, respectively.
Trimeric autotransporter adhesins [26]; an orthologous group of YadA-like outer membrane proteins/afimbrial adhesins. Designated as variably-expressed outer membrane proteins in B. quintana, Bartonella repetitive protein A in Bartonella vinsonii, and Bartonella adhesin A in B. henselae.
Type IV secretion system and translocated Beps.
BatR: Bartonella regulator; BatS: Bartonella sensor; Bep: Bartonella effector protein; Hbp: Heme-binding protein; LPS: Lipopolysaccharide; TAA: Trimeric autotransporter adhesin.
Another factor thought to enhance hemotrophy by B. bacilliformis and, possibly, B. henselae, is deformation factor or ‘deformin’ (Figure 2) [13]. Deformin is released into the culture medium during in vitro growth and can independently cause extensive pitting and invagination of erythrocyte cell membranes. To what extent these membrane changes contribute to Bartonella's colonization of erythrocytes in vivo is unknown. Initial analyses showed that deformin was a heat-sensitive protein of 130 kDa (native molecular mass) [14,15], whereas subsequent research showed that it was a small, hydrophobic molecule of 1400 Da with a high affinity for albumin [16]. Clarification of deformin's biochemical nature, its occurrence in other Bartonella species and role in hemotrophy in vivo warrant additional research, especially given the marked morphological changes that it can cause in erythrocytes.
More recent work has revealed that the Trw T4SS, with its multiple variant copies of pilin (TrwL) and pilus-associated component (TrwJ), is involved in erythrocyte adhesion in Bartonella tribocorum (a rodent pathogen) and is required to establish chronic intraerythrocytic bacteremia [17]. The Trw systems of B. quintana and B. henselae may play analogous roles in human infections (Figure 2). Interestingly, the absence of a TrwB (VirD4) ortholog suggests that Bartonella's Trw system has lost the ability to translocate T4SS effector molecules [5]. Instead, the multigenic nature and coexpression of Trw components is thought to provide a means of helping to establish host range by virtue of recognition and binding to cognate erythrocyte receptor(s). The nature of B. bacilliformis' erythrocyte adhesin(s) is a mystery, despite its ability to practice hemotrophy without a Trw system.
The nature of erythrocyte ligands(s) participating in Bartonella's adherence remains unclear. Early work by Walker and Winkler showed that B. bacilliformis has a binding preference for human erythrocytes over those from other mammals and that the receptor(s) is sensitive to α- or β-glucosidase treatment [11]. Later work revealed that several erythrocyte proteins, including glycophorins A and B, are recognized by B. bacilliformis and that the carbohydrate moieties are important for the pathogen's binding [18].
Erythrocyte invasion by Bartonella is an intriguing and central event in pathogenesis. Early work with B. bacilliformis identified an invasion-associated gene, termed ialB (Figure 2), which could confer an erythrocyte-invasive phenotype on laboratory-adapted strains of Escherichia coli [19]. Later work showed that ialB-knockout mutants of B. bacilliformis were severely impaired (∼50% reduction) in their ability to invade human erythrocytes in vitro, and the phenotype could be restored by trans-complementation [20]. Two studies localized the IalB protein to the inner and outer membrane fractions of B. bacilliformis and B. henselae, respectively [20,21]; a contradiction that warrants clarification. Interestingly, ialB expression is responsive to two environmental cues that would likely be encountered by B. bacilliformis in the context of the sandfly midgut. For example, ialB expression and IalB synthesis are highest under arthropod-like conditions (20°C, pH 5.0) when compared with human-like conditions (37°C, pH 7.2) [22]. These cues could conceivably ‘prime’ the bacterium during its time in the vector, enhancing its virulence for subsequent transmission to humans when the sandfly feeds again in a few days. The mechanism whereby IalB augments erythrocyte invasion by Bartonella is unknown but clearly intriguing.
Finally, Bartonella's hemolytic proteins may play a role in hemotrophy (Table 1 & Figure 2). For example, B. bacilliformis produces a contact-dependent hemolysin that is maximally expressed during exponential-phase growth [23]. The hemolysin acts independently of deformin and may be responsible for the β-hemolytic phenotype observed with B. bacilliformis after several days of growth on blood agar. It is tempting to speculate that contact-dependent hemolysin is utilized by B. bacilliformis to escape from vacuoles or host cells during intracellular parasitism. A second group of hemolytic proteins is the cohemolysins. By definition, cohemolysin activity is only apparent in the presence of a β-hemolysin, where it causes a synergistic hemolysis reaction (i.e., a CAMP test reaction). Work by Litwin and Johnson identified a cohemolysin, termed Cfa, of B. henselae that is a 180-kDa autotransporter protein with orthologs present in B. quintana. The potential virulence function of Cfa is intriguing, especially since its α domain is secreted into the culture medium during in vitro growth and the protein has some homology to repeat in toxin (RTX) hemolysins [24]. The virulence function of Cfa, characterization of Cfa orthologs from other Bartonella and analysis of other autotransporter proteins of bartonellae, such as B. henselae's acidic repeat protein [25], need further investigation.
Adherence to & invasion of endothelial cells
Bartonella's YadA-like outer membrane proteins (omps) constitute a family of afimbrial adhesins for endothelial cells (ECs). Bartonella YadA-like omps are defined as trimeric autotransporter adhesins (TAAs), in reference to their trimeric coiled–coil surface structure, use of type V secretion during export and a conserved role in adherence to host cells [26]. Bartonella TAAs were once thought to be type IV pili, based upon their superficial resemblance to these appendages under high magnification [27]. TAAs are large proteins (up to ∼330-kDa monomers) that form four major domains, including a C-terminal β-barrel anchor in the outer membrane (which also serves as a pore during secretion), a highly repetitive and variable coiled–coil stalk domain, and a short, conserved neck region that bridges the stalk to a head domain consisting of larger β-helices [26]. Bartonella TAA orthologs include: Bartonella adhesin A (BadA) of B. henselae, the variably-expressed omps (Vomps) of B. quintana, and Bartonella repetitive protein A (BrpA) of B. vinsonii. To prevent confusion by the disparate nomenclature of the various Bartonella orthologs, we will collectively refer to them as TAAs.
B. henselae's TAA (BadA) is essential for binding ECs through its recognition of host cell β1-integrins [28]. In addition, BadA mediates binding to extracellular matrix proteins, such as collagens and fibronectin, the latter of which may serve to bridge the adhesin and β1 integrins [28,29]. This TAA also significantly inhibits phagocytosis by J774 cells in vitro as compared with wild-type strains [28]. Recent work has shown that the head of BadA is crucial for binding EC and collagen, whereas the stalk is essential for fibronectin binding [30]. This same study also showed that just the BadA head domain can promote auto-aggregation of B. henselae cells, as observed in strains expressing intact BadA.
B. quintana's TAAs (Vomps) are also involved in auto-aggregation of the bacterium and collagen IV binding [31]. Interestingly, differential expression of Vomps by various strains of B. quintana does not appear to correlate with the ability of the pathogen to adhere to cocultured macrophage (THP-1) or epithelial (HeLa 229) cells [32]. A recent investigation also showed that a vomp null-mutant strain of B. quintana was unable to establish bacteremia in the macaque model of infection [33]. However, a bacteremic state could be generated in macaques by utilizing a partial-null mutant containing vompC and vompD genes. Remarkably, natural mutants for vompA and vompB arise in vivo in chronically infected macaques [31]. Characterizing Vomp receptors on host cells would be an interesting study, as they may be differentially employed to parasitize a variety of tissue types within the host.
Recent work has shown that Bartonella's heme-binding proteins (Hbps, also called Pap31), may represent another set of potential adhesins for ECs and/or extracellular matrix constituents such as fibronectin and heparin [34]. Given the relatedness of Hbps to gonococcal opacity (Opa) proteins, their extremely hydrophobic nature and surface location in bartonellae, an adhesin function is certainly possible and would effectively provide a multifunctional role for the Hbps.
Following adherence to ECs, Bartonella likely enters the cell by an actin-dependent process. For example, in B. bacilliformis, internalization was shown to involve the actin GTPases, Cdc42, Rho and Rac [35], and in B. henselae the process culminates in the formation of blebs containing several internalized bacteria [36]. A noteworthy assemblage has also been observed during EC internalization of B. henselae, termed the ‘invasome’. This basket-like structure develops over a comparatively longer time period (24 h) than typical internalization, involves extensive actin reorganization during its formation and provides for internalization of a large aggregate of bartonellae [37].
Recent evidence indicates that B. henselae avoids the normal endocytic pathway by utilizing a specialized Bartonella-containing vacuole (BCV) that does not acidify or fuse with lysosomes in ECs or is delayed in macrophages [38]. The BCV provides an ideal compartment for bacterial replication at a neutral pH and without the onslaught of innate immune effectors or degradative enzymes stored in the lysosomes. The mechanism whereby endosome maturation is inhibited is unknown, but Bartonella obviously plays an active role in the process, as dead bacteria are processed via normal phagolysosome formation. Screening of a mutant library of B. henselae for reduced evasion of the endocytic pathway identified vapA5 (a hypothetical virulence gene), hbpD (encoding HbpD) and cycA (encoding an amino acid transporter). Each of these mutations could be complemented in trans with the wild-type gene to restore the lysosome-evasion phenotype [38].
Host cell subversion
Type IV secretion systems are elegant macromolecular machines used to deliver bacterial effector molecules into a host cell. T4SS effectors, in turn, subvert the recipient cell to accommodate the pathogen's specific growth requirements and conditions [39]. T4SS are found in a growing list of bacterial pathogens, including Coxiella burnetii, Agrobacterium tumefaciens and Helicobacter pylori, where they provide efficient injection of effector molecules involved in processes, such as inhibiting apoptosis, enhancing tumorigenicity and remodeling of the host cell's actin cytoskeleton [40]. B. bacilliformis, the ancestral Bartonella lineage, lacks a T4SS, whereas descendent lineages (modern species), such as B. henselae and B. quintana possess two T4SS, including the VirB/VirD4 (T4SS effector translocation) and Trw (pilin) system (Table 1 & Figure 2) [9]. Acquisition of T4SS operons has undoubtedly allowed certain Bartonella species to expand their host range and to subjugate host cells in ways that are not possible in B. bacilliformis. In this section, we will focus on the VirB/VirD4 system of B. quintana and B. henselae, as Trw's role as an adhesin for host erythrocytes was described previously.
The VirB/VirD4 T4SS of Bartonella was inadvertently discovered during cloning and characterization of B. henselae's immunodominant 17-kDa protein. The 17-kDa antigen is actually a VirB5 homolog and is encoded in the virB operon [41,42]. Subsequent work has shown that the VirB/VirD4 T4SS is primarily involved in establishing EC parasitism [43] and is responsible for some of the EC pathology observed during bartonellosis (Figure 2), including the extensive actin-based cytoskeletal reconfiguration, activation of nuclear factor (NF)-κB leading to a proinflammatory state which fosters chronic inflammation and inhibition of EC apoptosis [40]. T4SS-translocated proteins responsible for the changes in ECs are referred to as Bartonella effector proteins (Beps). Work by Schulein et al. revealed that seven Beps (Beps A–G) are encoded downstream of the virB/virD4 locus of B. henselae [44]. Each Bep contains at least one conserved, approximately 140 amino acid sequence at its C-terminus termed a Bartonella intracellular delivery (BID) domain, followed by a positively charged tail. If additional BID domains are present (e.g., in Beps E–G), they are apparently employed in effector functions rather than translocation [5]. Beps A–F also contain an N-terminal region, referred to as filamentation induced by cAMP (FIC) domain, whose function is unclear. Potential tyrosine phosphorylation domains are also present in Beps D–F [5], and phosphorylation has been demonstrated for BepD, following its translocation into the EC cytosol [44]. More recent work by Selbach et al. has shown that effector phosphorylation events constitute the foundation of an ‘interactome’, wherein a T4SS effector is ‘activated’ by a host cell tyrosine kinase [45]. In turn, the phosphorylated effector recruits and binds a variety of cellular proteins via their SH2 domains, to ultimately subvert various host cell activities that are mediated by cell signaling pathways. With regards to Bartonella effectors, phosphorylated BepE, BepF and BepD were found to interact with seven host cell proteins (Shp-1 and -2, Csk, Grb-2 and -7, Crk and Ras-GAP1) [45].
Characterizing the function(s) of the Beps is a fascinating topic currently under investigation by several labs. BepA has been shown to sufficiently inhibit EC apoptosis through its host cell membrane association and upregulation of cAMP levels in the cytosol [46]. Interestingly, BepA's BID domain can independently cause EC membrane localization and inhibition of apoptosis. More recently, BepG was shown to be required for invasome-mediated uptake of B. henselae into ECs by promoting cytoskeletal F-actin rearrangement, and the process is induced at the expense of the more rapid, endocytic uptake and formation of BCVs [47]. The small GTPases Rac1 and Cdc42, but not RhoA, are required for subsequent invasome formation through their regulation of the changes that take place in the EC's actin cytoskeleton during internalization [47].
Pathological angiogenesis
The most notorious and unique manifestation of infection by the three major Bartonella species is the ability to induce pathological angiogenesis with concomitant production of pseudoneoplastic lesions in the human vasculature (i.e., verruga peruana, BA and bacillary peliosis). Although these lesions have been reported in immunocompetent patients [48], they are much more common in immunocompromised individuals infected with B. henselae and B. quintana. Therefore, immune status is a key risk factor [3,4]. By contrast, verruga peruana is a common manifestation of Oroya fever, regardless of immune status [49]. The mechanism whereby angiogenesis arises varies with Bartonella species and is multifactorial in nature (Figure 3).
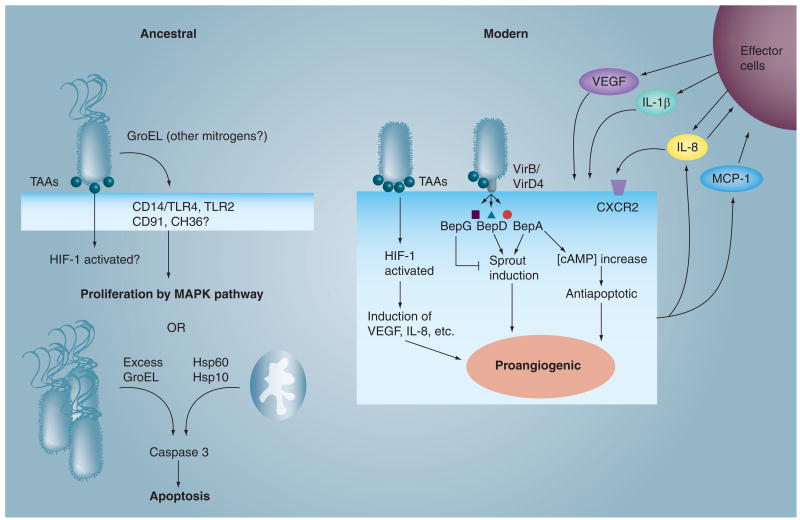
Figure 3. Potential effectors of ancestral (Bartonella bacilliformis) and modern Bartonella species (Bartonella henselae and Bartonella quintana) that trigger pathological angiogenesis in endothelial cells.
TAAs are likely required by both ancestral and modern Bartonella, although little is known about the B. bacilliformis orthologs. Secreted, extracellular GroEL may serve as a B. bacilliformis mitogen [52], whereas excess GroEL produced by intracellular B. bacilliformis in human umbilical vein endothelial cells (ECs) induces apoptosis, perhaps because it is an ortholog of Hsp60 [54]. Modern Bartonella evoke proangiogenic conditions in ECs by activating HIF-1 through TAA (e.g., BadA) interactions with the host cell membrane [28,55]. In turn, activated HIF-1 induces the expression of several angiogenic mediators, including IL-8 and VEGF [54]. The VirB/VirD4 type IV secretion system delivers BepA to the cytosol, where it triggers an increase in cytosolic cAMP, which inhibits apoptosis [46]. In addition, both BepA and BepD induce sprout formation in ECs, whereas BepG is antagonistic to this process [64]. The entire infection is accompanied by activation of NF-κB, which contributes to a proinflammatory state [56]. MCP-1 and IL-8 from ECs are thought to serve as chemotaxins for recruitment of various effector cells – an additional source of VEGF, IL1β and IL-8 (top right hand corner).
Bep: Bartonella effector protein; CD: Cluster of differentiation; CXCR: CXC motif chemokine receptor; HIF: Hypoxia-inducible factor; Hsp: Heat-shock protein; GroEL: Molecular chaperone; MCP: Monocyte/macrophage chemoattractant protein; TAA: Trimeric autotransporter adhesin; TLR: Toll-like receptor.
B. bacilliformis
Early work with B. bacilliformis implicated a soluble, bacterial protein of over 12 kDa that was specifically mitogenic for cultured human umbilical vein ECs (HUVECs). HUVECs treated with the factor showed proliferation rates that were approximately threefold greater than controls. This study also demonstrated that the factor was able to elicit neovascularization into a surgically implanted sponge in rats [50].
A subsequent study by this group showed that live B. bacilliformis was able to stimulate proliferation of HUVECs in cocultures [51]. Later research revealed that GroEL, a chaperonin, is involved in B. bacilliformis' mitogenicity for cultured HUVECs. Interestingly, B. bacilliformis GroEL is actively secreted by the bacterium, and does not affect apoptosis of the host cell through inhibition of the caspase 3 pathway [52], as observed in B. henselae [53]. Whether GroEL is mitogenic or if it protects a mitogen(s) through its chaperonin activity is unclear; however, correlations between mitogenic cell fractions and GroEL's location within the bacterium (i.e., the soluble fraction is more mitogenic than the insoluble fraction), an increase in mitogenic activity following heat shock of the bacterium, and the ability of anti-GroEL and anti-GroES antibodies to decrease mitogenicity, suggest that GroEL is mitogenic for ECs. Using the same assay system, it is also clear that B. bacilliformis' mitogenic activity is significantly greater than B. henselae's [52], underscoring disparities in the angiogenic mechanisms employed by different bartonellae. Interestingly, if GroEL is overexpressed by B. bacilliformis during intracellular infection of HUVECs it induces apoptosis, perhaps owing to the fact that it is an ortholog of mitochondrial heat-shock protein (Hsp)60; an inducer of programmed cell death [54].
B. henselae & B. quintana
Research with B. henselae and B. quintana has revealed a set of mechanisms involved in pathological angiogenesis that are distinct from those of B. bacilliformis, even though hemangiomatous lesions from all three organisms (i.e., verruga peruana and BA) are virtually indistinguishable (Figure 3).
The importance of a TAA in angiogenesis was first demonstrated in B. henselae, where activation of hypoxia-inducible factor (HIF)-1, a key proangiogenic regulator, was dependent on BadA expression [28]. Subsequent work showed that HIF-1 was activated in HUVECs and epithelial cells infected by BadA-expressing B. henselae [55]. More recent work has shown that a B. henselae strain expressing just the head domain of BadA can induce a proangiogenic state in cocultured epithelial cells (HeLa 229), as gauged by HIF-1 activation and secretion of VEGF and IL-8, relative to uninfected controls [30]. In a similar study with B. quintana, TAA-deficient strains (vomp) were unable to induce a proangiogenic state (as measured by expression of VEGF in cocultured THP-1 and HeLa229 cells) [32].
Nuclear factor-κB activation during the inflammatory response in infected ECs was demonstrated by Fuhrman et al. [56]. Live B. henselae, or their purified omps, were able to cause EC activation as evidenced by NF-κB activation, NF-κB-dependent upregulation of E-selectin and ICAM-1 and, enhanced rolling and adhesion of polymorphonuclear neutrophils to the activated ECs. Proinflammatory conditions undoubtedly provide for increased chemotaxis and infiltration of potential effector cells (monocytes, macrophages and, possibly, polymorphonuclear neutrophils) to establish chronic inflammation and proangiogenic conditions.
Early work with B. henselae showed enhanced proliferation and migration of HUVECs in coculture and identified a trypsin-sensitive mitogen in the insoluble fraction of bacterial lysates [57]. However, subsequent work showed that the proliferative factor was actually secreted into the culture medium and was specific to ECs [58]. More recent work suggests that inhibition of apoptosis via BepA activity, not mitogenicity, is the means by which B. henselae increases the number of HUVECs in coculture [46]. Data also show that an antiapoptotic factor is released into the bacterial culture medium and, as previously demonstrated, is specific for ECs [53].
Additional studies have shown that B. henselae infection induces the expression of potentially angiogenic cytokines and growth factors in vitro that may act in a paracrine and/or autocrine fashion to stimulate the growth of ECs. Kempf et al. showed that TAA (BadA)-expressing B. henselae (referred to as ‘piliated’ at this time) induce the synthesis of VEGF by EA.hy 926 or HeLa cells, but not HUVECs, in cocultures [59]. HUVEC cultures supplemented with conditioned medium from EA.hy 926–B. henselae cocultures showed proliferation rates of 30–70-fold greater than controls. Kempf et al. also showed that EA.hy 926–B. henselae cocultures induced synthesis of IL-8, another angiogenic factor and chemotaxin [60]. Finally, this study demonstrated that B. henselae growth correlated with host cell growth rates, suggesting that paracrine stimulation might provide additional host cells for bacterial colonization. Resto-Ruiz et al. demonstrated that VEGF and IL-1β could be detected within 6–12 h in medium obtained from THP-1 macrophage cells cocultured with B. henselae, and that the conditioned medium was mitogenic for human microvascular ECs (HMEC-1s) [61]. Interestingly, HMEC-1s, but not THP-1 cells, were stimulated to produce IL-8 within 6 h of infection. McCord et al. demonstrated that B. henselae or purified low Mr omps can upregulate monocyte/macrophage chemoattractant protein-1 (MCP-1) production by cultured HMEC-1s [62]. The MCP-1 could conceivably attract monocyte–macrophage effector cells into the area of Bartonella infection and elicit a proangiogenic environment through their VEGF secretion. In a subsequent study, McCord et al. demonstrated that a variety of cell types (HUVECs, HMEC-1s, THP-1 monocytes and HepG2 hepatocytes) produced IL-8 in response to a B. henselae infection and that infected ECs upregulated expression of the IL-8 receptor, CXCR2 [63]. Infected ECs were also shown to form capillary tubes in vitro [63].
Finally, new research has shown that B. henselae's T4SS effector, BepA (in addition to inhibiting apoptosis [46]) or just BepA's C-terminal portion containing a BID domain and charged carboxyl terminus, promotes capillary tube (‘sprout’) formation by HUVEC spheroids [64]. B. quintana's ortholog (BepA2) was also shown to possess similar activity. By contrast, B. henselae BepG inhibits sprout formation (and also triggers extensive cytoskeletal rearrangement in ECs [46]). Interestingly, BepD also possesses stimulatory activity but at a lower level than BepA [64]. These data suggest that modern Bartonella species, with their T4SS, may help regulate angiogenic activity through opposing activities of BepA/BepD and BepG effectors.
Taken as a whole, data suggest that pathological angiogenesis induced during a Bartonella infection:
Is triggered by TAA-mediated activation of HIF-1 in host cells;
Involves antagonistic T4SS effectors–BepA/BepD versus BepG in modern Bartonella to promote or inhibit sprout formation of ECs, respectively;
Is fostered by BepA-mediated inhibition of EC apoptosis;
Is enhanced by a proinflammatory microenvironment involving NF-κB activation in infected ECs;
Involves paracrine stimulation with effector cell-secreted VEGF, IL1β and IL-8;
Involves autocrine stimulation with IL-8 from infected ECs;
Is augmented by soluble bacterial protein effectors (mitogens) secreted by Bartonella during infection (Figure 3).
Replication & persistence in the host
Heme acquisition
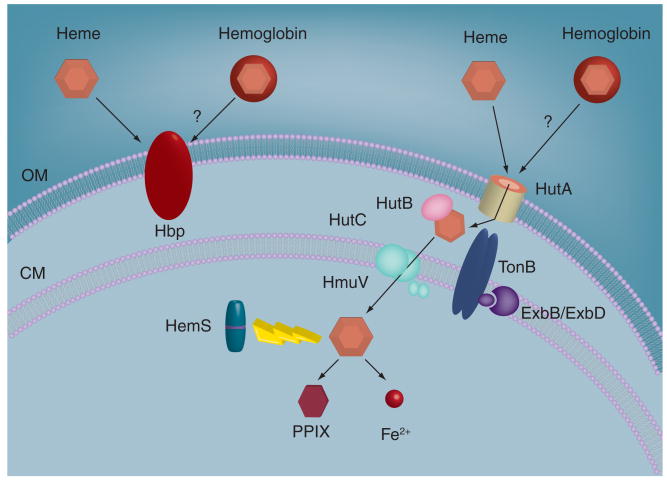
A hallmark characteristic of all Bartonella species is a requirement for heme, owing to their inability to synthesize protoporphyrin IX (PPIX) or heme (Fe2+–PPIX). Common sources of heme include hemin (Fe3+–PPIX), hemoglobin and host erythrocytes. However, unlike many pathogens, Bartonella cannot utilize heme-rich scavenger molecules of the host, such as lactoferrin or transferrin. Research has shown that acquisition of heme involves two main sets of virulence determinants including a paralagous gene family encoding Hbps (Pap31) and a heme uptake locus (Figure 4).
Figure 4. Heme acquisition system of Bartonella.
A paralagous family of Hbps serves to bind heme to the surface, where it likely serves as an antioxidant barrier and nutrient reservoir. A heme receptor (HutA) is energized by TonB to transport heme to the periplasm where it is subsequently bound and shuttled by HutB to an ABC transporter/permease (HutC/HmuV). Transported heme is utilized for biosynthesis of heme-containing proteins or is stored or degraded by cytosolic HemS into ferrous iron and PPIX.
CM: Cytosolic membrane; Exb: Participant in TonB-mediated energy transduction for outer membrane proteins; Hbp: Heme-binding protein; HemS: Heme degradation/storage protein; Hmu: Heme import protein; Hut: Heme utilization locus; OM: Outer membrane; PPIX: protoporphyrin IX; Ton: Energy transducer for outer membrane proteins.
The Hbps are encoded by three (B. bacilliformis) or five (B. quintana and B. henselae) paralagous genes that are homologs to the group 3 omp gene family of Brucella (Table 1) [65]. The hbp genes of B. quintana are differentially expressed in response to ambient heme concentration and temperature, and they are positively regulated by the α-proteobacterial iron response regulator (Irr). Interestingly, Bartonella utilizes a unique promoter regulatory element for Irr binding, termed an ‘H-box’ [66]. Two subsets of hbp genes have been identified, including those induced under conditions resembling a human (e.g., hbpA, hbpD and hbpE at 37°C and low heme), and hbpC, which is induced under conditions simulating the louse vector (25°C, high heme) [67]. Hbps are heme-binding omps [68], however, their role in heme uptake is unclear, despite a predicted β-barrel structure that resembles porins [69]. A previous report demonstrated complementation of E. coli hemA using cloned B. henselae hbpA [70]. However, this complementation study was done at a heme concentration that may have caused outer membrane leakiness and given a false-positive reaction [70]. Furthermore, heme translocation by Gram-negative bacteria is normally TonB-dependent, but the Hbps lack a consensus TonB box for TonB energization of heme transport. With these caveats, one potential role for the Hbps is to provide a surface reservoir of heme. This arrangement would resemble closely related rhizobia, which bind plant-derived leghemoglobin to their surface in order to establish an antioxidant barrier through heme's intrinsic peroxidase activity. It would also provide a reservoir for the essential growth factor.
A more likely candidate for heme acquisition and transport is the heme utilization locus of Bartonella. This multiprotein heme-acquisition machine closely resembles those described in other Gram-negative bacteria and consists of a TonB-dependent heme receptor (heme utilization locus [Hut]A), an ABC transport system (HutB, HutC and HmuV), a possible heme degradation/storage protein (HemS) and a TonB transducer for energizing HutA and other omps [71]. In B. quintana, the locus encoding this complex is repressed under high hemin concentrations and is negatively regulated by Irr. The hemS–hutB–hutC–hmuV genes are cotranscribed and located immediately downstream of hutA. Like the hbp genes, an ‘H-box’ for Irr regulation is present in the divergent promoter region that separates hutA and tonB, and in the promoter region upstream of the hemS–hmuV operon. Complementation analyses demonstrate that B. quintana HutA can serve as a heme receptor in E. coli hemA at low concentrations and that heme transport is TonB-dependent [71].
Coping with intracellular stress
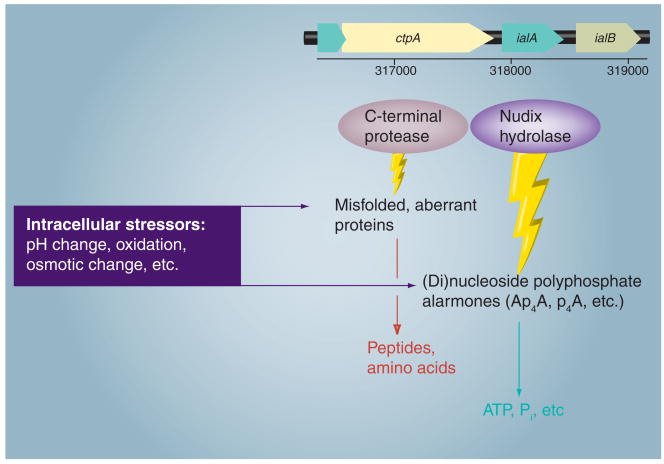
The intracellular environment requires significant adaptations to cope with a long list of stressors, including reactive oxygen species, changes in pH, fluctuations in osmolarity and misfolded proteins. Bartonella would also be subject to temperature fluctuations when cycling between mammal hosts and arthropod vectors. As mentioned earlier, hemin bound to surface Hbps may provide an antioxidant barrier and nutritive reservoir for Bartonella. In addition, early work on the invasion-associated locus of B. bacilliformis revealed two genes immediately upstream of ialB, whose corresponding proteins have been shown to serve as antistress effectors in other pathogenic bacteria, including a nudix hydrolase (ialA) and a carboxy-terminal processing protease (CtpA).
Bartonella's nudix hydrolase (ialA) is a representative from a large family of related enzymes that hydrolyze (di)nucleoside polyphosphate alarmones (e.g., Ap4A, p4A) produced during nutritional, oxidative and temperature stress in cells (Figure 5). The products of hydrolysis include ATP and inorganic phosphate, which are subsequently recycled by the bacterium [72]. ialA orthologs have been implicated as virulence determinants of B. bacilliformis and E. coli during invasion of human erythrocytes and microvascular endothelial cells, respectively [19,73]. Work with Salmonella enterica serovar Typhimurium has also shown that deletion mutants for ygdP and apaH (ialA homologs) display a significant increase in Ap4N levels and a concomitant decrease in invasiveness for cultured, human epithelial cells [74]. Although not well studied, research involving bacterial pathogens indicates that maintenance of NpnN levels by nudix hydrolases, such as ialA, is essential to an invasive phenotype [75].
Figure 5. Bartonella enzymes used to degrade misfolded or aberrant proteins and (di)nucleoside polyphosphate alarmones generated during parasitism of host cells, including a C-terminal protease (CtpA) and Nudix hydrolase (IalA).
Genes for these enzymes are located immediately upstream of ialB, a gene involved in erythrocyte parasitism (a linkage map from Bartonella bacilliformis is shown).
Early work also identified a gene immediately upstream of ialA that encodes a tail-specific protease that possesses a conserved PDZ domain, called CtpA [76]. In a role that is similar to IalA, CtpA (and related COG0793 members) degrade aberrant or misfolded proteins that arise from stress or anomalous processing (Figure 5). Orthologs of CtpA have been shown to play a role in Salmonella enterica serovar Typhimurium's survival in macrophages [77], and possibly enhancing a mucoid phenotype in cystic fibrosis-associated strains of Pseudomonas aeruginosa by degrading aberrant forms of MucA, an alginate production regulator [78]. Although tail-specific proteases are typically located in the periplasm, CtpA may actually service both the cytosol and periplasm of Bartonella by translating two forms of CtpA using alternative start sites [76].
Another coping mechanism that warrants further exploration is the two-component regulatory system of Bartonella, and its role in regulating genes involved in virulence and countering stress imposed by the host and arthropod vector. Termed Bartonella two-component regulator/sensor (BatR/BatS) by its discoverer [BIRTLES R, UNPUBLISHED DATA], the BatR/BatS is an ortholog of Brucella's BvrR/BvrS two-component regulatory system. In Brucella, BvrR/BvrS is involved in the invasion of host cells and maintenance of semi-permeability and homeostasis of the outer membrane [79]. Brucella mutants for either gene (bvrR or bvrS) are rapidly eliminated from infected mice and are unable to replicate within macrophages or epithelial cells [80]. BvrR's role in regulating the group 3 omp gene family [81] (homologs to Bartonella's hbp gene family) suggested early on that a similar regulation might exist between BatR/BatS and the hbp genes. Unsurprisingly, recent work has shown that B. henselae's BatR is an important transcriptional regulator during infection of ECs, where the regulon includes several virulence genes including the hbps, T4SS and TAAs [DEHIO M ET AL.; UNPUBLISHED DATA]. Interestingly, BatR/BatS serves as a pH sensor that upregulates genes at neutral pH and represses them at alkaline pH; possibly evolved to respond to environmental cues encountered by Bartonella in the context of the mammalian circulatory system and arthropod vector, respectively.
Pacifying the immune system
Arguably the most difficult hurdles for a pathogen to overcome in order to establish a chronic, persistent infection is the host's innate and adaptive immune responses. Two general strategies are employed by pathogenic microorganisms in this regard; they can attempt to dismantle immune system effectors or their regulation using a variety of strategies (molecular mimicry, synthesis of superantigens to induce a ‘cytokine storm’, secretion of IgA protease and so on), or they can underwhelm the immune system by utilizing subinflammatory or antagonistic molecules. Bartonella employs the latter strategy, as exemplified by its low-potency lipopolysaccharide (LPS). The absence of classical manifestations of endotoxic shock during persistent infections of bartonellosis is evidence to this effect.
The LPS of Bartonella henselae has a deep-rough structure without an O-chain polysaccharide and contains an unusual penta-acylated lipid A with a long-chain fatty acid [82]. The absence of O-side chain could conceivably decrease complement fixation and provide a degree of serum resistance on Bartonella, but this possibility has not been explored. The unusual fatty acid composition renders Bartonella's endotoxin at least 1000-fold less potent at Toll-like receptor (TLR)4 activation (as measured by IL-8 production), as compared with LPS from Salmonella [82]. Remarkably, Bartonella's LPS possesses antagonistic properties for TLR4 and does not activate TLR2 [82,83]. These LPS attributes undoubtedly contribute to the establishment and maintenance of persistent infection, since the bacterium's major surface component is subinflammatory and antagonistic to the host's innate immune response. Interestingly, long-chain fatty acids are a conserved feature in the LPS of intracellular bacteria that establish long-term symbioses with their host, including Legionella, Chlamydia and closely related rhizobia.
Future perspective
Understanding the complete story of Bartonella's molecular pathogenesis obviously requires more research. Specific areas that are ripe for investigation include: identification of virulence determinants used by the ancestral lineage, B. bacilliformis (which is comparatively much more virulent), versus those employed by more modern Bartonella species, such as B. henselae and B. quintana (which possess a T4SS), and how the T4SS has moderated virulence; continued work on characterizing differential mechanisms involved in pathological angiogenesis induced by ancestral versus modern Bartonella species; a more thorough analysis of the BatR/BatS two-component regulatory system and its virulence-associated regulon; elucidating, in detail, the functional relatedness between TAAs and other putative autotransporters from various Bartonella species; analysis of virulence gene expression and function in arthropod vectors (fleas, lice and sandflies); characterization of the remaining Bep T4SS effectors and analysis of their roles in EC subversion; and clarification of a handful of opaque issues including: deformin's biochemical nature, IalB's mechanism of enhancing erythrocyte invasion and deciphering HemS's role in cytosolic heme degradation or storage. Challenges to progress include a lack of alternative and inexpensive animal models of infection for human-specific Bartonella species (e.g., B. quintana and B. bacilliformis). Although the macaque is a useful model for these bartonellae, it is both expensive and labor-intensive to use. A second hurdle is to counter the current decline in interest to support basic research on Bartonella. The funding problem has undoubtedly been compounded by the current global economic situation, and the fact that these so-called ‘emerging’ agents have been recognized for almost two decades. Considerable work remains to be done on these fascinating pathogens.
Executive summary.
History
A total of 24 Bartonella species have been described to date. Ten are pathogenic for humans and three species (Bartonella bacilliformis, Bartonella quintana and Bartonella henselae) are the most frequent causes of bartonellosis. These pathogens can cause intraerythrocytic bacteremia, pathological angiogenesis and multisystemic involvement.
B. bacilliformis is the ancestral-type species; infections have been chronicled since pre-Columbian times by people in endemic regions of South America.
B. quintana is the agent of trench fever and is re-emerging in homeless, inner-city populations where it causes ‘urban trench fever’.
B. henselae is the agent of cat-scratch disease and is emerging as a secondary pathogen of AIDS patients.
Transmission & colonization of humans
Bartonella are transmitted by hematophagous arthropods (human body louse, cat fleas, phlebotamine sandflies and ticks) or animal trauma (scatches and bites).
Humans are the sole reservoir for B. quintana and B. baciliformis; B. henselae infects a variety of mammals (e.g., cats, dogs and humans).
Erythrocyte parasitism
All Bartonella species parasitize erythrocytes (hemotrophy).
B. bacilliformis infection can cause an acute drop in hematocrit, whereas B. quintana and B. henselae cause chronic intraerythrocytic bacteremia without anemia.
B. bacilliformis and, possibly B. henselae, secrete a hydrophobic factor (deformin), which pits and invaginates erythrocyte cell membranes.
Based on work with B. tribocorum, Trw orthologs are likely to be used as erythrocyte adhesins by B. quintana and B. henselae.
Flagella-mediated motility is used by B. bacilliformis to parasitize erythrocytes.
IalB somehow enhances erythrocyte invasion and is maximally expressed under arthropod-like conditions.
Hemolytic factors may enhance Bartonella hemotrophy, including a contact-dependent hemolysin and an autotransporter cohemolysin.
Endothelial cell parasitism
Bartonella possess YadA-like outer membrane proteins (outer membrane proteins; afimbrial adhesins), designated trimeric autotransporter adhesins (TAAs) based on their structure and function in other bacterial pathogens.
B. henselae's TAA (BadA) is an adhesin for endothelial cells (ECs) via β1-integrins and binds various extracellular matrix proteins. BadA also inhibits phagocytosis by J774 macrophages and causes autoaggregation.
B. quintana's TAAs (variably-expressed outer membrane proteins) bind to collagen IV, cause autoaggregation and are essential in establishing chronic bacteremia in macaques.
EC internalization of B. bacilliformis, and other Bartonella, involves the small GTPases, Cdc42, Rho and Rac.
B. henselae internalization involves an invasome or endocytic uptake.
Bartonella abrogate or inhibit lysosome fusion with the phagosome, creating a special vacuole, termed a Bartonella-containing vacuole.
Subverting the host cell
B. quintana and B. henselae, but not B. bacilliformis, possess a type IV secretion system (T4SS) for delivery of Bep effectors.
Delivery of Beps via the VirB/VirD4 T4SS is responsible for EC cytoskeletal changes, activation of NF-κB and inhibition of apoptosis.
BepA is involved in EC membrane localization and inhibition of apoptosis. BepG enhances invasome formation in ECs at the expense of the endocytic pathway.
Bartonella-induced angiogenesis
B. bacilliformis synthesizes a soluble, proteinaceous mitogen for human umbilical vein ECs (HUVECs) that is angiogenic in vivo.
Extracellular B. bacilliformis GroEL is involved in mitogenicity for HUVECs, whereas excess, intracellular GroEL can trigger apoptosis.
B. henselae infection results in activation of HIF-1, which in turn, induces proangiogenic mediators, such as VEGF and IL-8. B. henselae activation of HIF-1 requires its TAA, BadA.
B. henselae inhibits apoptosis of HUVECs via T4SS-translocated BepA.
B. henselae upregulates expression of VEGF in EA.hy 926, HeLa and THP-1 cells and IL-1β in cocultured THP-1 cells. VEGF and IL-1β probably act on ECs in a paracrine fashion.
B. henselae induces IL-8 in THP-1, hepatocyte, EA.hy 926 and HMEC-1 cells. IL-8 probably acts on ECs in an autocrine and paracrine loops.
B. henselae upregulates MCP-1 in HMEC-1 cells. MCP-1 probably recruits monocyte–macrophage effector cells to the site of infection.
B. henselae BepA and BepD stimulate, whereas BepG inhibits, sprout formation by HUVEC spheroids.
Bacterial replication & persistence
Bartonella have an absolute growth requirement for host-derived heme.
Hbps bind heme to the bacterial surface, where it may provide an antioxidant barrier and reservoir for the nutrient.
Bartonella utilize a heme-uptake complex that resembles those of other Gram-negative pathogens.
(Di)nucleoside polyphosphate alarmones and misfolded proteins arising in the intracellular niche are degraded by IalA and CtpA, respectively.
BatR/BatS, a two-component system, regulates several virulence genes expressed during EC parasitism.
Bartonella lipopolysaccharide is subinflammatory; it is an antagonist for Toll-like receptor 4 and does not activate Toll-like receptor 2.
Acknowledgments
This work was supported by Public Health Service grant R01 AI053111 from the NIH to Michael F Minnick.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Michael F Minnick, The University of Montana, Division of Biological Sciences, 32 Campus Drive, Missoula, MT 59812, USA, Tel.: +1 406 243 5972, Fax: +1 406 243 4184, mike.minnick@mso.umt.edu.
James M Battisti, The University of Montana, Division of Biological Sciences, 32 Campus Drive, Missoula, MT 59812, USA, Tel.: +1 406 243 5285, Fax: +1 406 243 4184, jim.battisti@mso.umt.edu.
Bibliography
Papers of special note have been highlighted as:
• of interest
- 1.deEstete M. Relación del viaje de Hernando Pizarro (1553) as cited by Hermilio Vadizán in Apuntes para la historia de la verruga peruana. Anal Fac Med Lima. 1925;10:34–44. [Google Scholar]
- 2.Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131–135. doi: 10.1086/313890. [DOI] [PubMed] [Google Scholar]
- 3.Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 4.Koehler JE, Quinn FD, Berger TG, LeBoit PE, Tappero JW. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 5.Dehio C. Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction. Cell Microbiol. 2008;10:1591–1598. doi: 10.1111/j.1462-5822.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Thorough review of Bartonella type IV secretion systems (T4SS) and their role in host-cell parasitism.
- 6.Fournier PE, Minnick MF, Lepidi H, Salvo E, Raoult D. Experimental model of human body louse infection using green fluorescent protein-expressing Bartonella quintana. Infect Immun. 2001;69:1876–1879. doi: 10.1128/IAI.69.3.1876-1879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertig M. Plebotomus and Carrión's disease. Am J Trop Med Hyg. 1942;22(Suppl):1–80. [Google Scholar]
- 8.Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. 2008;22:1–15. doi: 10.1111/j.1365-2915.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- 9.Saenz HL, Engel P, Stoeckli MC, et al. Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat Genet. 2007;39:1469–1476. doi: 10.1038/ng.2007.38. [DOI] [PubMed] [Google Scholar]; • Interesting investigation that includes phylogeny of the ancestral Bartonella lineage, Bartonella bacilliformis, and more recently radiating lineages (‘modern Bartonella’), based on possession or absence of the T4SS.
- 10.Benson LA, Kar S, McLaughlin G, Ihler GM. Entry of Bartonella bacilliformis into erythrocytes. Infect Immun. 1986;54:347–353. doi: 10.1128/iai.54.2.347-353.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker TS, Winkler HH. Bartonella bacilliformis: colonial types and erythrocyte adherence. Infect Immun. 1981;31:480–486. doi: 10.1128/iai.31.1.480-486.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherer DC, DeBuron-Connors I, Minnick MF. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect Immun. 1993;61:4962–4971. doi: 10.1128/iai.61.12.4962-4971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwaki-Egawa S, Ihler GM. Comparison of the abilities of proteins from Bartonella bacilliformis and Bartonella henselae to deform red cell membranes and to bind to red cell ghost proteins. FEMS Microbiol Lett. 1997;157:207–217. doi: 10.1111/j.1574-6968.1997.tb12775.x. [DOI] [PubMed] [Google Scholar]
- 14.Mernaugh G, Ihler GM. Deformation factor: an extracellular protein synthesized by Bartonella bacilliformis which causes deformation of erythrocyte membranes. Infect Immun. 1992;60:937–943. doi: 10.1128/iai.60.3.937-943.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu YH, Lu ZY, Ihler GM. Purification of deformin, an extracellular protein synthesized by Bartonella bacilliformis which causes deformation of erythrocyte membranes. Biochim Biophys Acta. 1995;1234:173–183. doi: 10.1016/0005-2736(94)00271-p. [DOI] [PubMed] [Google Scholar]
- 16.Derrick SC, Ihler GM. Deformin, a substance found in Bartonella bacilliformis culture supernatants, is a small, hydrophobic molecule with an affinity for albumin. Blood Cells Mol Dis. 2001;27:1013–1019. doi: 10.1006/bcmd.2001.0475. [DOI] [PubMed] [Google Scholar]
- 17.Seubert A, Hiestand R, de la Cruz F, Dehio C. A bacterial conjugation machinery recruited for pathogenesis. Mol Microbiol. 2003;49:1253–1266. doi: 10.1046/j.1365-2958.2003.03650.x. [DOI] [PubMed] [Google Scholar]
- 18.Buckles EL, McGinnis Hill E. Interaction of Bartonella bacilliformis with human erythrocyte membrane proteins. Microb Pathog. 2000;29:165–174. doi: 10.1006/mpat.2000.0381. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell SJ, Minnick MF. Characterization of a two-gene locus from Bartonella bacilliformis associated with the ability to invade human erythrocytes. Infect Immun. 1995;63:1552–1562. doi: 10.1128/iai.63.4.1552-1562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman SA, Minnick MF. Establishing a direct role for the Bartonella bacilliformis invasion-associated locus B (IalB) protein in human erythrocyte parasitism. Infect Immun. 2001;69:4373–4381. doi: 10.1128/IAI.69.7.4373-4381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chenoweth MR, Greene CE, Krause DC, Gherardini FC. Predominant outer membrane antigens of Bartonella henselae. Infect Immun. 2004;72:3097–3105. doi: 10.1128/IAI.72.6.3097-3105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman SA, Minnick MF. Differential expression of the invasion-associated locus B (ialB) gene of Bartonella bacilliformis in response to environmental cues. Microb Pathog. 2003;34:179–186. doi: 10.1016/s0882-4010(03)00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrix LR. Contact-dependent hemolytic activity distinct from deforming factor of Bartonella bacilliformis. FEMS Microbiol Lett. 2000;182:119–124. doi: 10.1111/j.1574-6968.2000.tb08884.x. [DOI] [PubMed] [Google Scholar]
- 24.Litwin CM, Johnson JM. Identification, cloning, and expression of the CAMP-like factor autotransporter gene (cfa) of Bartonella henselae. Infect Immun. 2005;73:4205–4213. doi: 10.1128/IAI.73.7.4205-4213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litwin CM, Rawlins ML, Swenson EM. Characterization of an immunogenic outer membrane autotransporter protein, Arp, of Bartonella henselae. Infect Immun. 2007;75:5255–5263. doi: 10.1128/IAI.00533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VAJ. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 2006;14:264–270. doi: 10.1016/j.tim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Batterman HJ, Peek JA, Loutit JS, Falkow S, Tompkins LS. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect Immun. 1995;63:4553–4556. doi: 10.1128/iai.63.11.4553-4556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riess T, Andersson SGE, Lupas A, et al. Bartonella adhesin A mediates a proangiogenic host cell response. J Exp Med. 2004;200:1267–1278. doi: 10.1084/jem.20040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riess T, Raddatz G, Linke D, Schäfer A, Kempf VAJ. Analysis of Bartonella adhesin A expression reveals differences between various B. henselae strains. Infect Immun. 2007;75:35–43. doi: 10.1128/IAI.00963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser PO, Riess T, Wagner CL, et al. The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell Microbiol. 2008;10:2223–2234. doi: 10.1111/j.1462-5822.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Chomel BB, Schau MK, et al. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc Natl Acad Sci USA. 2004;101:13630–13635. doi: 10.1073/pnas.0405284101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulte B, Linke D, Klumpp S, et al. Bartonella quintana variably expressed outer membrane proteins mediate vascular endothelial growth factor secretion but not host cell adherence. Infect Immun. 2006;74:5003–5013. doi: 10.1128/IAI.00663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKichan JK, Gerns HL, Chen YT, Zhang P, Koehler JE. A SacB mutagenesis strategy reveals that the Bartonella quintana variably expressed outer membrane proteins are required for bloodstream infection of the host. Infect Immun. 2008;76:788–795. doi: 10.1128/IAI.01174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Unique report on the virulence function of Bartonella quintana trimeric autotransporter adhesins (Vomps) in the context of the macaque model of infection.
- 34.Dabo SM, Confer AW, Anderson BE, Gupa S. Bartonella henselae Pap31, an extracellular matrix adhesin, binds the fibronectin repeat III13 module. Infect Immun. 2006;74:2513–2521. doi: 10.1128/IAI.74.5.2513-2521.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma A, Davis GE, Ihler GM. Infection of human endothelial cells with Bartonella bacilliformis is dependent on Rho and results in activation of Rho. Infect Immun. 2000;68:5960–5969. doi: 10.1128/iai.68.10.5960-5969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brouqui P, Raoult D. Bartonella quintana invades and multiplies within endothelial cells in vitro and in vivo and forms intracellular blebs. Res Microbiol. 1996;147:719–731. doi: 10.1016/s0923-2508(97)85119-4. [DOI] [PubMed] [Google Scholar]
- 37.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalization of the bacterial aggregate by a unique structure, the invasome. J Cell Sci. 1997;110:2141–2154. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 38.Kyme PA, Haas A, Schaller M, Peschel A, Iredell J, Kempf VAJ. Unusual trafficking pattern of Bartonella henselae-containing vacuoles in macrophages and endothelial cells. Cell Microbiol. 2005;7:1019–1034. doi: 10.1111/j.1462-5822.2005.00531.x. [DOI] [PubMed] [Google Scholar]; • Excellent study describing the ability of Bartonella to inhibit phagolysosome formation in endothelial and macrophage cells.
- 39.Juhas M, Crook DW, Hood DW. Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell Microbiol. 2008;10:2377–2386. doi: 10.1111/j.1462-5822.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid MC, Schulein R, Dehio M, Denecker G, Carena I, Dehio C. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol Microbiol. 2004;52:81–92. doi: 10.1111/j.1365-2958.2003.03964.x. [DOI] [PubMed] [Google Scholar]
- 41.Padmalayam I, Karem K, Baumstark B, Massung R. The gene encoding the 17-kDa antigen of Bartonella henselae is located within a cluster of genes homologous to the virB virulence operon. DNA Cell Biol. 2000;19:377–382. doi: 10.1089/10445490050043344. [DOI] [PubMed] [Google Scholar]
- 42.Schmiederer M, Anderson B. Cloning, sequencing, and expression of three Bartonella henselae genes homologous to the Agrobacterium tumefaciens virB region. DNA Cell Biol. 2000;19:141–147. doi: 10.1089/104454900314528. [DOI] [PubMed] [Google Scholar]
- 43.Schulein R, Dehio C. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol Microbiol. 2002;46:1053–1067. doi: 10.1046/j.1365-2958.2002.03208.x. [DOI] [PubMed] [Google Scholar]
- 44.Schulein R, Guye P, Rhomberg TA, et al. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc Natl Acad Sci USA. 2005;102:856–861. doi: 10.1073/pnas.0406796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selbach M, Paul FE, Brandt S, et al. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe. 2009;5:397–403. doi: 10.1016/j.chom.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Schmid MC, Scheidegger F, Dehio M, et al. A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog. 2006;2:e115. doi: 10.1371/journal.ppat.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhomberg TA, Truttmann MC, Guye P, Ellner Y, Dehio C. A translocated protein of Bartonella henselae interferes with endocytic uptake of individual bacteria and triggers uptake of large bacterial aggregates via the invasome. Cell Microbiol. 2009;11:927–945. doi: 10.1111/j.1462-5822.2009.01302.x. [DOI] [PubMed] [Google Scholar]; • Interesting report describing the function of the Bartonella T4SS effector, BepG.
- 48.Tappero JW, Koehler JE, Berger TG, et al. Bacillary angiomatosis and bacillary splenitis in immunocompetent adults. Ann Intern Med. 1993;118:363–365. doi: 10.7326/0003-4819-118-5-199303010-00007. [DOI] [PubMed] [Google Scholar]
- 49.Chian CA, Arrese JE, Pierard GE. Skin manifestations of Bartonella infections. Int J Dermatol. 2002;41:461–466. doi: 10.1046/j.1365-4362.2002.01489.x. [DOI] [PubMed] [Google Scholar]
- 50.Garcia FU, Wojta J, Broadley KN, Davidson JM, Hoover RL. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am J Pathol. 1990;136:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia FU, Wojta J, Hoover RL. Interactions between live Bartonella bacilliformis and endothelial cells. J Infect Dis. 1992;165:1138–1141. doi: 10.1093/infdis/165.6.1138. [DOI] [PubMed] [Google Scholar]
- 52.Minnick MF, Smitherman LS, Samuels DS. Mitogenic effect of Bartonella bacilliformis on human vascular endothelial cells and involvement of GroEL. Infect Immun. 2003;71:6933–6942. doi: 10.1128/IAI.71.12.6933-6942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirby JE, Nekorchuk DM. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc Natl Acad Sci USA. 2002;99:4656–4661. doi: 10.1073/pnas.072292699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smitherman LS, Minnick MF. Bartonella bacilliformis GroEL: effect on growth of human vascular endothelial cells in infected cocultures. Ann NY Acad Sci. 2005;1063:286–298. doi: 10.1196/annals.1355.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kempf VAJ, Lebiedziejewski M, Alitalo K, et al. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation. 2005;111:1054–1062. doi: 10.1161/01.CIR.0000155608.07691.B7. [DOI] [PubMed] [Google Scholar]; • Important study describing HIF-1 activation and its role in pathological angiogenesis, with demonstration of HIF-1 activation in bacillary angiomatosis lesions.
- 56.Fuhrmann O, Arvand M, Gohler A, et al. Bartonella henselae induces NF-κB-dependent upregulation of adhesion molecules in cultured human endothelial cells: possible role of outer membrane proteins as pathogenic factors. Infect Immun. 2001;69:5088–5097. doi: 10.1128/IAI.69.8.5088-5097.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conley T, Slater L, Hamilton K. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J Lab Clin Med. 1994;124:521–528. [PubMed] [Google Scholar]
- 58.Maeno N, Oda H, Yoshiie K, Wahid MR, Fujimura T, Matayoshi S. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb Pathog. 1999;27:419–427. doi: 10.1006/mpat.1999.0315. [DOI] [PubMed] [Google Scholar]
- 59.Kempf VAJ, Volkmann B, Schaller M, et al. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol. 2001;3:623–632. doi: 10.1046/j.1462-5822.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida S, Ono M, Shono T, et al. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor α-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Resto-Ruiz SI, Schmiederer M, Sweger D, et al. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect Immun. 2002;70:4564–4570. doi: 10.1128/IAI.70.8.4564-4570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Landmark study demonstrating a role, and providing a model, for IL-8's involvement during Bartonella henselae-induced angiogenesis.
- 62.McCord AM, Burgess AWO, Whaley MJ, Anderson BE. Interaction of Bartonella henselae with endothelial cells promotes monocyte/macrophage chemoattractant protein 1 gene expression and protein production and triggers monocyte migration. Infect Immun. 2005;73:5735–5742. doi: 10.1128/IAI.73.9.5735-5742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCord AM, Resto-Ruiz SI, Anderson BE. Autocrine role for interleukin-8 in Bartonella henselae-induced angiogenesis. Infect Immun. 2006;74:5185–5190. doi: 10.1128/IAI.00622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheidegger F, Ellner Y, Guye P, et al. Distinct activities of Bartonella henselae type IV secretion effector proteins modulate capillary-like sprout formation. Cell Microbiol. 2009;11(7):1088–1101. doi: 10.1111/j.1462-5822.2009.01313.x. [DOI] [PubMed] [Google Scholar]; • Fascinating study that relates T4SS effectors to the pathological angiogenesis triggered by modern Bartonella species.
- 65.Cloeckaert A, Vizcaíno N, Paquet JY, Bowden RA, Elzer PH. Major outer membrane proteins of Brucella spp.: past, present and future. Vet Microbiol. 2002;90:229–247. doi: 10.1016/s0378-1135(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 66.Battisti JM, Smitherman LS, Sappington KN, Parrow NL, Raghavan R, Minnick MF. Transcriptional regulation of the heme binding protein gene family of Bartonella quintana is accomplished by a novel promoter element and iron response regulator. Infect Immun. 2007;75:4373–4385. doi: 10.1128/IAI.00497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Battisti JM, Sappington KN, Smitherman LS, Parrow NL, Minnick MF. Environmental signals generate a differential and coordinated expression of the heme receptor gene family of Bartonella quintana. Infect Immun. 2006;74:3251–3261. doi: 10.1128/IAI.00245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carroll JA, Coleman SA, Smitherman LS, Minnick MF. Hemin-binding surface protein from Bartonella quintana. Infect Immun. 2000;68:6750–6757. doi: 10.1128/iai.68.12.6750-6757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minnick MF, Sappington KN, Smitherman LS, Andersson SGE, Karlberg O, Carroll JA. Five-member gene family of Bartonella quintana. Infect Immun. 2003;71:814–821. doi: 10.1128/IAI.71.2.814-821.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmermann R, Kempf VA, Schiltz E, Oberle K, Sander A. Hemin binding, functional expression, and complementation analysis of Pap 31 from Bartonella henselae. J Bacteriol. 2003;185:1739–1744. doi: 10.1128/JB.185.5.1739-1744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parrow NL, Abbott J, Lockwood AR, Battisti JM, Minnick MF. Function, regulation, and transcriptional organization of the hemin utilization locus of Bartonella quintana. Infect Immun. 2009;77:307–316. doi: 10.1128/IAI.01194-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cartwright JL, Britton P, Minnick MF, McLennan AG. The ialA invasion gene of Bartonella bacilliformis encodes a (di) nucleoside polyphosphate hydrolase of the MutT motif family and has homologs in other invasive bacteria. Biochem Biophys Res Comm. 1999;256:474–479. doi: 10.1006/bbrc.1999.0354. [DOI] [PubMed] [Google Scholar]
- 73.Badger JL, Wass CA, Kim KS. Identification of Escherichia coli K1 genes contributing to human brain microvascular endothelial cell invasion by differential fluorescence induction. Mol Microbiol. 2000;36:174–182. doi: 10.1046/j.1365-2958.2000.01840.x. [DOI] [PubMed] [Google Scholar]
- 74.Ismail TM, Hart CA, McLennan AG. Regulation of dinucleoside polyphosphate pools by the YgdP and ApaH hydrolases is essential for the ability of Salmonella enterica serovar Typhimurium to invade cultured mammalian cells. J Biol Chem. 2003;278:32602–32607. doi: 10.1074/jbc.M305994200. [DOI] [PubMed] [Google Scholar]
- 75.McLennan AG. The Nudix hydrolase superfamily. Cell Mol Life Sci. 2006;63:123–143. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell SJ, Minnick MF. A carboxy-terminal processing protease gene is located immediately upstream of the invasion-associated locus from Bartonella bacilliformis. Microbiology. 1997;143:1221–1233. doi: 10.1099/00221287-143-4-1221. [DOI] [PubMed] [Google Scholar]
- 77.Bäumler AJ, Kusters JG, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiling SA, Jansen JA, Henley BJ, et al. Prc protease promotes mucoidy in mucA mutants of Pseudomonas aeruginosa. Microbiology. 2005;151:2251–2261. doi: 10.1099/mic.0.27772-0. [DOI] [PubMed] [Google Scholar]
- 79.López-Goñi I, Guzmán-Verri C, Manterola L, Sola-Landa A, Moriyón I, Moreno E. Regulation of Brucella virulence by the two-component system BvrR/BvrS. Vet Microbiol. 2002;90:329–339. doi: 10.1016/s0378-1135(02)00218-3. [DOI] [PubMed] [Google Scholar]
- 80.Sola-Landa A, Pizarro-Cerda J, Grillo MJ, et al. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol Microbiol. 1998;29:125–138. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 81.Guzman-Verri C, Manterola L, Sola-Landa A, et al. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc Natl Acad Sci USA. 2002;99:12375–12380. doi: 10.1073/pnas.192439399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zahringer U, Lindner B, Knirel YA, et al. Structure and biological activity of the short-chain lipopolysaccharide from Bartonella henselae ATCC 49882T. J Biol Chem. 2004;279:21046–21054. doi: 10.1074/jbc.M313370200. [DOI] [PubMed] [Google Scholar]
- 83.Popa C, Abdollahi-Roodsaz S, Joosten LA, et al. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun. 2007;75:4831–4837. doi: 10.1128/IAI.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]