Abstract
Sphingolipids (SLs) play critical roles in eukaryotic cells in the formation of lipid rafts, membrane trafficking, and signal transduction. Here we created a SL null mutant in the protozoan parasite Leishmania major through targeted deletion of the key de novo biosynthetic enzyme serine palmitoyltransferase subunit 2 (SPT2). Although SLs are typically essential, spt2– Leishmania were viable, yet were completely deficient in de novo sphingolipid synthesis, and lacked inositol phosphorylceramides and other SLs. Remark ably, spt2– parasites maintained ‘lipid rafts’ as defined by Triton X-100 detergent resistant membrane formation. Upon entry to stationary phase spt2– failed to differentiate to infective metacyclic parasites and died instead. Death occurred not by apoptosis or changes in metacyclic gene expression, but from catastrophic problems leading to accumulation of small vesicles characteristic of the multivesicular body/multivesicular tubule network. Stage specificity may reflect changes in membrane structure as well as elevated demands in vesicular trafficking required for parasite remodeling during differentiation. We suggest that SL-deficient Leishmania provide a useful biological setting for tests of essential SL enzymes in other organisms where SL perturbation is lethal.
Keywords: gp63-major surface protease-leishmanolysin/lipophosphoglycan/sphingoid base synthesis/trpanosomatid parasite/virulence
Introduction
Leishmania are covered with a variety of interrelated glycosylphosphatidylinositol (GPI)-anchored molecules, including lipophosphoglycan (LPG), small glycosylinositolphospholipids (GIPLs), proteophosphoglycan (PPG) and GPI-anchored proteins. Numerous studies have suggested ways in which these molecules may play a role in steps important for Leishmania survival and pathogenesis (reviewed in Ilgoutz and McConville, 2001; Sacks and Kamhawi, 2001). These include survival in the sand fly vector, where the flagellated promastigote form differentiates from a replicating procyclic to a non-replicating, infectious metacyclic form, and in the mammalian host, where parasites ingested by macrophages differentiate into the non-flagellated amastigotes that ultimately cause disease.
In contrast to GPI-anchored molecules, the roles of sphingolipids (SLs), a diverse family of ubiquitous eukaryotic membrane-anchored lipids, are largely unknown, although they comprise 5–10% of Leishmania membrane lipids (Kaneshiro et al., 1986). SLs, ceramides and sphingoid bases (SBs) play crucial roles in general membrane function, cell-to-cell recognition, regulation of cell growth and differentiation, intracellular signaling, apoptosis and modulation of the immune response (Merrill et al., 1993; Vaux and Korsmeyer, 1999; Kolter et al., 2002; Maceyka et al., 2002). Previous studies showed that Leishmania parasites synthesize inositol phosphorylceramide (IPC), which is absent in mammals but present in fungi and plants (Kaneshiro et al., 1986; Lester and Dickson, 1993), and ceramide-anchored molecules in Trypanosoma cruzi may alter host cell signaling (Lederkremer and Bertello, 2001; DosReis et al., 2002; McConville et al., 2002).
SLs are enriched at key points within both exocytic and endocytic pathways and play important roles in trafficking (Gruenberg, 2001; Ikonen, 2001; Funato et al., 2002). Leishmania and the related trypanosomes have an unusual polarized organization of secretory and degradative pathways, where endocytosis and exocytosis are restricted to a small invagination termed the ‘flagellar pocket’ (Landfear and Ignatushchenko, 2001). Macromolecular flux through this organelle is intense due to the demands associated with maintenance and remodeling of its surface during the infectious cycle, as well as from nutrient acquisition. Current knowledge about vesicular trafficking is relatively limited, and suggests that there are significant differences with other eukaryotes. One involves the multivesicular tubule (MVT), a terminal lysosome-like organelle (Ilgoutz et al., 1999; Weise et al., 2000; Ghedin et al., 2001; Mullin et al., 2001; Waller and McConville, 2002). The MVT contains vesicles of ∼50 nm in size, which appear to arise from an upstream organelle resembling multivesicular bodies (MVBs) (Mullin et al., 2001; Katzmann et al., 2002; Waller and McConville, 2002). In mammalian cells, MVB-derived vesicles are rich in SLs and cholesterol, while the limiting membranes of the late endosome and MVB are poor in these lipids (Kobayashi et al., 2002; Mobius et al., 2003; Wubbolts et al., 2003). In Leishmania exogenous ceramides accumulate in the MVT rather than in the Golgi (Ghedin et al., 2001; Mullin et al., 2001; Waller and McConville, 2002).
De novo SL biosynthesis starts with the condensation of l-serine and palmitoyl-CoA into 3-ketosphinganine (3-ketodihydrosphingosine or 3-KDS). This is catalyzed by the pyridoxal 5′-phosphate-dependent enzyme serine palmitoyltransferase (SPT, EC 2.3.1.50), which is encoded by two subunits, SPT1 and SPT2 (reviewed by Hanada, 2003). Deletion or inhibition of SPT2 in yeast and mammalian cells leads to severe growth retardation and death (Pinto et al., 1992a; Hanada et al., 2000). Here we used targeted deletion of Leishmania SPT2 to show that SLs are not required for growth in log phase, or formation of detergent resistant membranes (DRMs) associated with lipid ‘rafts’.
Results
Identification of genes encoding the subunits of Leishmania major serine palmitoyltransferase
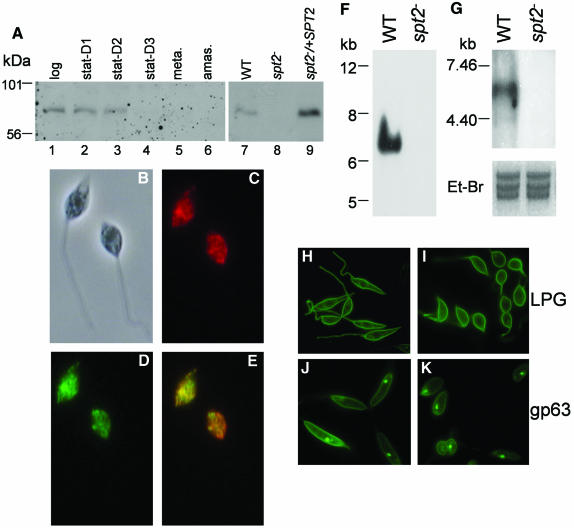
Two ORFs with homology to the SPT subunits SPT1 and SPT2 were identified from the L.major genome project data. Each was recovered by PCR from genomic DNA and sequenced (GenBank AY235575, AY235574). The SPT1 and SPT2 genes predicted proteins of 488 and 538 amino acids, which showed orthologous relationships to those of other eukaryotes (35–36% and 35–46% identity, respectively, for comparisons with human, yeast or Arabidopsis). Leishmania major SPT2 contained the catalytic site motif (368GTFTKSFG375), where K372 is thought to bind pyridoxal 5′-phosphate (reviewed by Hanada, 2003). Southern blot analysis of L.major genomic DNA showed that SPT2 was single-copy (data not shown). Western blot analysis with anti-SPT2 antibody showed that SPT2 occurred at high levels in log and early stationary promastigotes, but was down-regulated in late stationary promastigotes, metacyclic and amastigote stages (Figure 1A, lanes 1–6). Similar data were obtained by northern blot analysis (data not shown). Leishmania major SPT2 was localized to the endoplasmic reticulum, as revealed by the similar distributions of a functional GFP–SPT2 fusion protein with the ER marker BIP (Figure 1B–E).
Fig. 1. Characterization of the SPT2 gene and its expression in L.major. (A) Developmental expression of SPT2 protein. Whole cell lysates (1 × 106) from WT log promastigotes (lanes 1 and 7), WT stationary promastigotes (days 1–3, lanes 2–4), metacyclics (lane 5), WT amastigotes (lane 6), spt2– log promastigotes (lane 8) and spt2–/+SPT2 log promastigotes (lane 9) were subjected to immunoblot analysis with affinity purified anti-SPT2 antibody. (B)–(E) GFP–SPT2 is localized to the ER. WT L.major cells were transfected with pXG-GFP–SPT2 and grown in the presence of 50 µg/ml G418. (B) Phase contrast. (C) Immunofluorescent staining with rabbit anti-T.brucei BIP antibody followed by Texas Red-labeled goat anti-rabbit IgG. (D) GFP fluorescence of GFP–SPT2. (E) Merge of (C) and (D). (F) Southern blot analysis of genomic DNA from WT and spt2– digested with EcoRI. The probe was the entire SPT2 ORF. (G) Northern blot analysis of total RNA from WT and spt2– null mutant. (H)–(K) Distribution of LPG and gp63 are unaltered in log phase spt2–. Localizations of LPG and gp63 in log phase WT (H and J, respectively) and spt2– (I and K) were determined by immunofluorescence microscopy.
Targeted replacement of L.major SPT2
To test the requirement for de novo SL synthesis in Leishmania we chose to delete the catalytic SPT2 subunit. Homologous gene replacement works efficiently in Leishmania although two rounds of targeting are necessary. Remarkably, spt2– colonies bearing homozygous Δspt2::HYG/Δspt2::PAC replacements were readily obtained, whether or not SBs were provided. Hybridizations with a SPT2 ORF probe confirmed the lack of the SPT2 gene and transcript in these lines (Figure 1F and G). As a control we reintroduced the SPT2 gene on an episomal Leishmania expression vector (pXG-SPT2), and this ‘add-back’ line is termed spt2–/+SPT2. This line showed overexpression of SPT2 (Figure 1A, lanes 7–9), without any apparent adverse effects.
spt2– promastigotes grow somewhat more slowly in log phase but crash in stationary phase and fail to generate infective metacyclics
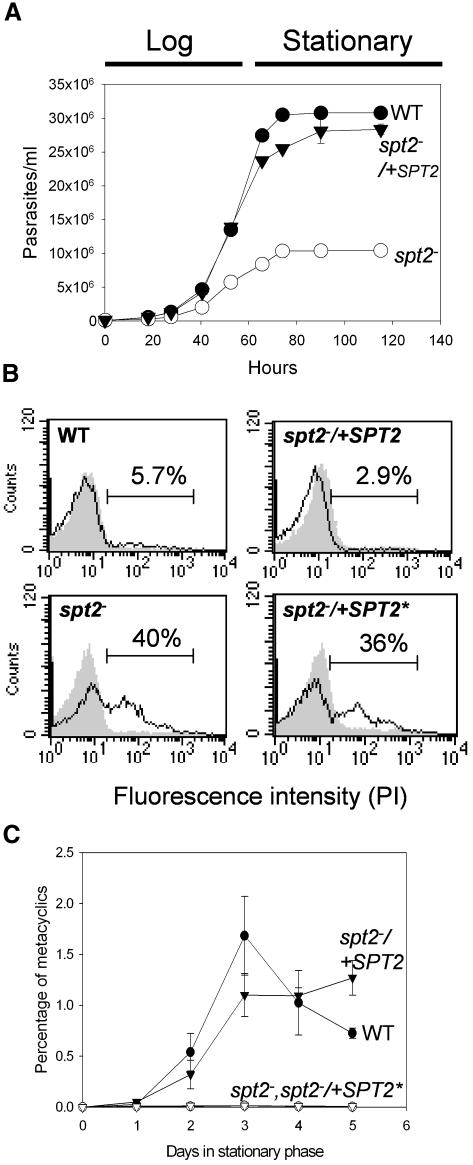
In log phase, spt2– promastigotes grew somewhat more slowly than wild type (WT), with a doubling time of ∼9.5 h versus 7.3 h, and attained lower densities in stationary phase (8–10 × 106/ml versus 2.5–3.2 × 107/ml; Figure 2A). During exponential growth both WT and spt2– showed good viability, as judged by dye exclusion (Figure 2B, shaded peaks). Immunofluorescence microscopy with antibody against LPG or the GPI-anchored protein gp63 showed normal surface expression in log-phase spt2–, although these cells appeared somewhat less elongate (Figure 1H–K).
Fig. 2. Deletion of SPT2 results in slower growth during log phase, poor viability during stationary phase and failure to differentiate. (A) WT, spt2– or spt2–/+SPT2 were inoculated into M199 medium at 2 × 105 cells/ml and cell densities were measured. Experiments were carried out in duplicate and error bars represent standard deviations. (B) Flow cytometry of WT, spt2–, spt2–/+SPT2 or spt2–/+SPT2* (spt2– transfected with a modified SPT2 that contains a K372A mutation that abolishes the binding to pyridoxal 5′-phosphate: Supplementary data) labeled with 0.5 µg/ml of propidium iodide (PI). Shaded peaks represent log phase cells, and unfilled peaks indicate day 3 stationary phase cells (3 days after reaching stationary phase). The percentage of PI-positive cells in each stationary culture is shown. (C) spt2– parasites are defective in metacyclogenesis. WT, spt2–, spt2–/+SPT2 or spt2–/+SPT2* cells were grown to stationary phase (day 3) and the percentage of metacyclics was determined by the peanut agglutinin method.
Coincident with entry into stationary phase, the spt2– parasites showed progressively higher frequencies of cell shape abnormalities and loss of viability (Figure 2B, unfilled peaks). After 3 days in stationary phase, 35–50% of the spt2– cells were permeable to propidium iodide (PI), versus 5.7% of WT (Figure 2B), consistent with microscopic evaluation (data not shown). Restoration of WT SPT2 but not a catalytic site mutant SPT2* (K372A) expression in spt2– fully reversed this defect (Figure 2B, spt2–/+SPT2 and spt2–/+SPT2*). Thus while not required in log phase, SPT2 played a vital role in stationary phase.
The stationary phase defect was particularly interesting, as cessation of growth marks the onset of differentiation to the infective metacyclic stage (Sacks and Perkins, 1984). Metacyclics are distinguished by morphology, reactivity with lectins and/or monoclonal antibodies, sedimentation, expression of stage-specific genes such as the HASP and SHERP gene family, and infectivity (Knuepfer et al., 2001; Sacks and Perkins, 1985; Späth and Beverley, 2001). By these criteria spt2– failed to produce metacyclics: e.g., WT metacyclics appeared after entry into stationary phase and peaked after 3–4 days (Figure 2C, WT), whereas few were found in the spt2– parasites at any point (<0.01%, Figure 2C, spt2–). Importantly, normal metacyclic levels returned when SPT2 but not SPT2* expression was restored (Figure 2C), or when spt2– was grown in the presence of SBs (below). Unsurprisingly, stationary phase spt2– cells showed poor infectivity in macrophage and animal infections (data not shown).
spt2– lacks de novo synthesis of IPCs and ceramide
Log phase parasites were pulse labeled with [3H]serine, and cellular lipids were extracted and separated by thin layer chromatography (Figure 3). Leishmania major SLs occur as ceramides and IPCs (Denny et al., 2001; Ralton et al., 2002), which were distinguished from other phospholipids arising through metabolism of serine such as phosphatidylserine (PS) or phosphatidylethanolamine (PE) by their mobility and resistance to mild base treatment. In WT abundant labeling of ceramides and IPCs was seen, which was absent in spt2–, (Figure 3). Ceramide and IPC synthesis was restored in the spt2– parasites after re-expression of SPT2 (Figure 3, spt2–/+SPT2). Similarly, treatment of WT with 10 µM myriocin, a specific inhibitor of SPT (Mandala and Harris, 2000) did not limit growth, but inhibited synthesis of both IPCs and ceramides (Figure 3, WT + Myr).
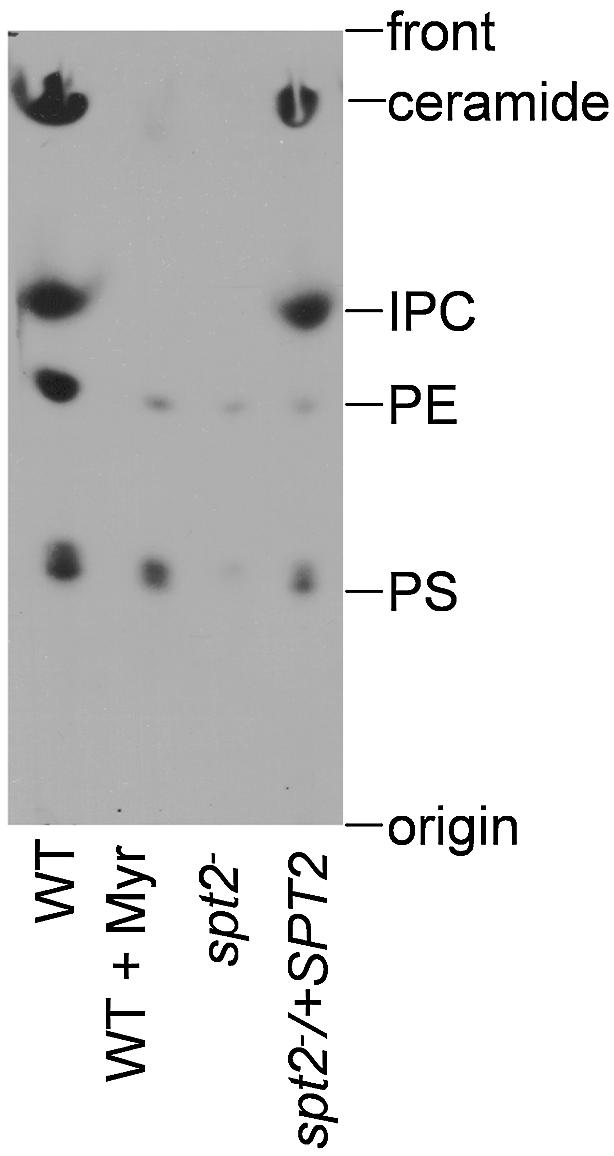
Fig. 3. Leishmania major WT (grown in the absence or presence of 10 µM myriocin), spt2– and spt2–/+SPT2 cells were cultured to mid-log phase (∼5 × 106 cells/ml) and pulse labeled with [3H]serine as described in Materials and methods. Cellular lipids were extracted and resolved by TLC. Each lane contained material from 3 × 107 cells. Abbreviations: IPC, inositolphosphorylceramide; PE, phosphatidylethanolamine; PS, phosphatidylserine.
spt2– lacks sphingolipids
We used electrospray ionization mass spectrometry (ESI/MS, negative ion mode) to examine SLs in total lipid fractions. The molecular species represented by [M–H]– or [M+Cl]– ions were determined by collisionally activated dissociation (CAD) of the parent ion and mass analyses of the resultant product ions as described in Materials and methods.
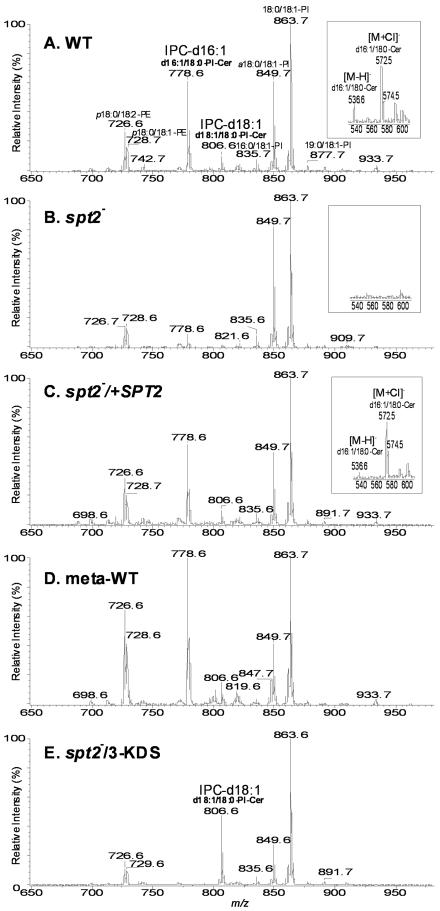
In log phase WT parasites, a variety of IPCs and alkyl/acyl and acyl/acyl glycerophospholipids were identified by their [M–H]– ions (Figure 4). Glycerophospholipids identified included 1-alkyl (18:0)-2-acyl (18:1)-PI (1-O-octadecanyl-2-octadecenoyl-sn-glycero-3-phosphoinositol, represented by [M–H]– at m/z 849.7, Figure 4A), which is probably a precursor in GPI anchor biosynthesis (McConville and Bacic, 1989; Ilgoutz and McConville, 2001), and diacyl (18:0/18:1)-PI (1-stearoyl-2-oleoyl-sn-glycero-3-phosphoinositol, represented by [M–H]– at m/z 863.7, Figure 4A). IPC species were represented by ions at m/z 778.6 and 806.7 corresponding to IPC-d16:1 (phosphoryl inositol N-stearoylhexadecesphing-4-enine, d16:1/18:0-PI-Cer) and IPC-d18:1 (phosphoryl inositol N-stearoylsphingosine, d18:1/18:0-PI-Cer), respectively, with IPC-d16:1 being more abundant (Figure 4A) (Kaneshiro et al., 1986). Correspondingly, in WT parasites the ceramide precursor of IPC-d16:1 (d16:1/18:0-PI-Cer) was represented by low abundance [M–H]– and [M+Cl]– ions at m/z 536.6 and 572.5, respectively (Figure 4A inset). Thus, Leishmania preferentially incorporate myristoyl-CoA over palmitoyl-CoA into IPCs. In contrast, enzymic studies of yeast SPT showed that while palmitoyl Co-A was the most active, C12-C18 acyl-CoAs could be incorporated efficiently (Pinto et al., 1992b) and in vivo SBs arise primarily from C16 and C18 acyl-CoA (Dickson et al., 1997).
Fig. 4. Negative-ion ESI/MS spectra of total lipids purified from log phase WT (A), spt2– (B), spt2–/+SPT2 (C), metacyclic WT (D) and spt2– cells grown with daily supplementation of 3-KDS (E). The assigned identities of peaks are indicated. Abbreviations: p18:0/18:2- PE, 1-O-octadec-1′-enyl-2-octadecadienoyl-sn-glycero-3-phosphoethanolamine (plasmalogen PE); p18:0/18:1-PE, 1-O-octadec-1′-enyl 2-octadecenoyl sn-glycero-phosphoethanolamine; d16:1/18:0-PI-Cer, phosphoryl inositol N-stearoylhexadecesphing-4-enine (IPC-d16:1); d18:1/18:0-PI-Cer, phosphoryl inositol N-stearoylsphingosine (IPC-d18:1); 18:0/18: 1-PI, 1-stearoyl-2-oleoyl-sn-glycero-3-phosphoinositol; 16:0/18:1-PI, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoinositol; a18:0/18:1-PI, 1-O-octadecanyl-2-octadecenoyl- sn-glycero-3-phosphoinositol (plasmanyl phosphoinositol). The insets in (A), (B) and (C) show the presence of N-stearoylhexadecesphing-4-enine (d16:1/18:0-Cer; [M–H]– and [M–Cl]– represent deprotonated and chloride ion addition of d16:1/18:0-Cer) in WT and spt2–/+SPT2 but not in spt2– parasites.
While spt2– lacked IPCs, as well as ceramide, it maintained WT levels of the other alkyl/acyl and acyl/acyl phospholipids (Figure 4B and inset). Restoration of SPT2 expression in the spt2–/+SPT2 line restored IPCs and ceramide expression to the levels and patterns seen in WT (Figure 4C and inset). Thus deletion of SPT2 abolished synthesis of SLs without affecting other phospholipids.
Total lipids from stationary phase or metacyclic WT parasites showed a similar profile of lipid species to that in log phase (metacyclic data is in Figure 4D; stationary phase data are not shown), although the relative abundance of IPCs and PEs increased in metacyclics (Figure 4D). Stationary phase spt2– resembled log phase in lacking IPCs and ceramides (data not shown); it was not possible to examine spt2– metacyclics due to their absence.
Rescue of spt2– parasites by exogenous SBs but not ceramides
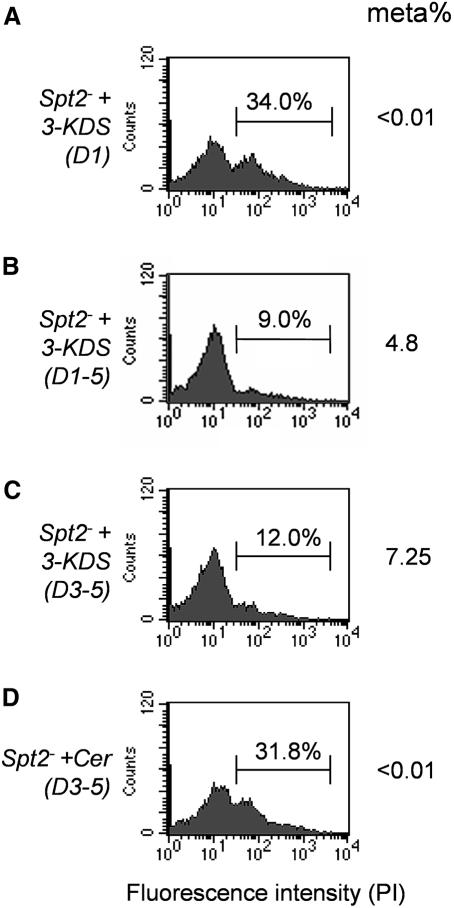
Provision of spt2– with the SPT product 3-keto-dihydrosphingosine (3-KDS) or other SBs at 2–10 µM, did not rescue spt2– (Figure 5A and data not shown; as in other organisms, SBs are toxic for L.major at higher concentrations). We found that the stability of SBs in Leishmania culture media was poor, and in other eukaryotes SLs are strictly regulated through both synthetic and degradative pathways (Hannun and Obeid, 2002; van Meer and Lisman, 2002). This prompted tests using more constant levels of SB supplementation, and daily addition of 3-KDS to 2 µM completely rescued the spt2– defects (Figure 5B). IPC synthesis was restored in these cells, although only IPC-d18:1 was made as expected (Figure 4E).
Fig. 5. spt2– parasites can be rescued by SBs but not ceramides. (A) spt2– parasites were inoculated at 2 × 105 cells/ml in M199/10% FBS medium containing 2 µM of 3-KDS. (B) spt2– parasites were inoculated at 2 × 105 cells/ml in M199/10% FBS medium and 2 µM of 3-KDS was added each day from day 1 to day 5. (C) spt2– parasites were inoculated at 2 × 105 cells/ml in M199/10% FBS medium and 2 µM of 3-KDS was added each day from day 3 to day 5. (D) Similar experiments to (C) but 2 µM of C18-ceramide was added instead of 3-KDS. At day 6 (approximately day 3 in stationary phase), the percentage of PI-positive cells and metacyclics were determined as described.
Rescue of the stationary phase defect was also obtained with dihydrosphingosine, sphingosine and phytosphingosine (PHY); as before, ESI-MS spectrometry showed that only IPC-d18:1 synthesis was restored (data not shown). Supplementation with PHY resulted in the formation of a new IPC with PHY as the long chain base (data not shown), which was not abundant in WT. Neither C16-ceramide (N-palmitoylsphingosine), C18-ceramide (N-stearoylsphingosine) nor sphingomyelin was able to rescue the stationary phase defects or restore IPC synthesis (Table I; Figure 5D; data not shown), similar to yeast and mammalian SPT-deficient mutants (Hanada et al., 1992; Pinto et al., 1992a).
Table I. Rescue of spt2– by SBs but not by ceramide.
| Expt | Addition | Log day |
Stationary day |
% PI | % metacyclic | % IPC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| 1 | SB | – | – | – | – | – | – | 40 | <0.01 | 0 |
| 2 | SB | + | + | + | + | + | + | 9 | 4.8 | 59 |
| 3 | SB | + | – | – | – | – | – | 34 | <0.01 | 11 |
| 4 | SB | + | + | – | – | – | – | 33 | <0.01 | 15 |
| 5 | SB | + | + | + | – | – | – | 23 | 0.1 | 27 |
| 6 | SB | – | – | – | + | – | – | 34 | 0.12 | 18 |
| 7 | SB | – | – | – | + | + | – | 14 | 3.2 | 45 |
| 8 | SB | – | – | – | + | + | + | 12 | 5.0 | 49 |
| 9 | Cer | + | + | + | + | + | + | 37 | <0.01 | 0 |
spt2– parasites were at inoculated at 1 × 105/ml at day 1. SB (3-KDS) or CER (C18 ceramide) was added on the days indicated by ‘+’ to a final concentration of 2 µM. Cultures entered stationary phase on day 4, and metacyclic formation (peanut agglutinin method) and IPC levels (ESI/MS) were evaluated on day 7. The relative abundance of IPCs (IPC-d18:1 + IPC-d16:1 for WT or IPC-d18:1 for SB-supplemented spt2–) was estimated by comparison with 18:0/18:1-PI whose abundance appeared constant, and normalized to the ratio seen in WT.
The SL/SB rescue studies addressed a potential concern that while spt2– completely lacked SLs, the serum required to cultivate Leishmania in vitro contains submicromolar concentrations of complex SLs such as gangliosides (Chu et al., 2000). This can now be understood since (i) mammalian SLs such as sphingomyelins, or ceramides derived from complex SLs, were unable to rescue spt2– defects; (ii) both Leishmania cells and culture media rapidly degraded SBs; and (iii) even active SBs must be added frequently at elevated levels. For these reasons, the spt2– parasite remained completely devoid of SLs.
Sphingoid bases are required in stationary phase but not in log phase
Rescue of the spt2– phenotypes with exogenous SBs permitted a test of the timing of their requirement during parasite growth. Although SPT2 mRNA and protein levels were highest in log phase, provision of SBs only during this period failed to rescue the stationary phase spt2– defects (Table I). However, if SBs were added at the first day of stationary phase and maintained for at least 2 days, rescue was strong and similar to that obtained in parasites to which SBs had been added every day (Table I; Figure 5C). Rescue of viability and metacyclogenesis correlated with IPC synthesis, with an apparent threshold of ∼30% of WT levels (Table I).
spt2– mutants continue to express the metacyclic gene marker SHERP
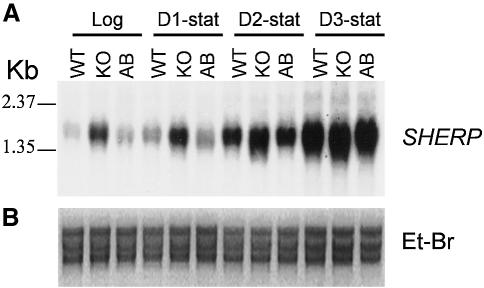
To test whether expression of metacyclic stage genes was generally altered in spt2– due to disruption of SL-dependent signaling, we examined the expression of the metacyclic stage-specific marker SHERP (Knuepfer et al., 2001). In log phase WT, spt2– and spt2–/+SPT2, SHERP mRNA levels were low, while upon entry into stationary phase SHERP transcripts increased dramatically (Figure 6). A small increase in SHERP mRNA was seen in log phase and early stationary phase spt2– (Figure 6B), the significance of which is unknown. Thus, despite the failure to form metacyclics, spt2– parasites nonetheless expressed a key metacyclic marker in stationary phase.
Fig. 6. Deletion of SPT2 does not alter the expression of metacyclic stage-specific marker SHERP. (A) Northern blotting with radio-labeled SHERP probe was performed as described. Total RNA from log phase and day 1–3 stationary cultures was analyzed. (B) Loading control (ribosomal RNA stained with ethidium bromide). KO: spt2–, AB: spt2–/+SPT2.
spt2– defects do not arise through programmed cell death
One explanation for the stationary phase spt2– defects invoked apoptosis or programmed cell death, since in other organisms SLs have both pro- and anti-apoptotic effects, and that at certain times, metazoan cells must choose between paths leading to either differentiation or death via apoptosis (reviewed by Vaux and Korsmeyer, 1999; Maceyka et al., 2002). Effectively, in stationary phase WT Leishmania chooses ‘differentiation’ while spt2– mutants choose ‘death’. We examined several key parameters described previously for cell death in Leishmania: nuclear morphology, DNA fragmentation, surface exposure of PS and altered membrane permeability (Lee et al., 2002; Zangger et al., 2002), and focused on the critical times surrounding entry into stationary phase. In no respect did stationary phase spt2– parasites show characteristics of programmed cell death (Supplementary data).
spt2– shows severe defects in membranous structures and small vesicle accumulation upon entry into stationary phase
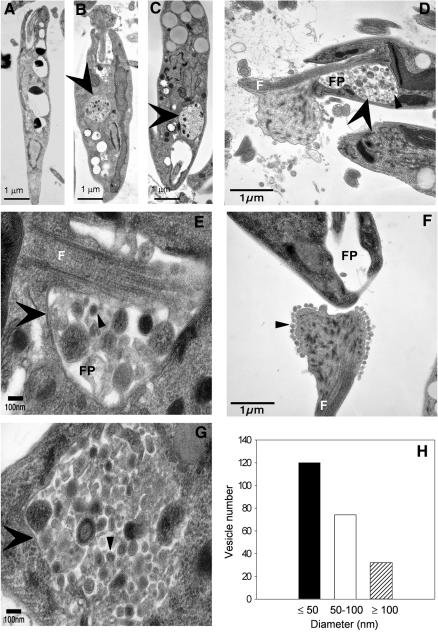
Transmission electron microscopy (EM) of stationary phase spt2– parasites revealed one or more of the following abnormalities: (i) increased vacuolization (Figure 7B and C, versus A for WT); (ii) extensive debris in the flagellar pocket (Figure 7D and E); (iii) alterations in the flagellar surface (Figure 7F), and (iv) accumulation of small vesicles with single lipid bilayer membranes in both internal vacuoles, the flagellar pocket and spilling out onto the parasite surface and flagellar membrane (Figure 7D–G). The sizes of the small vesicles in these compartments were heterogeneous, with the majority at ∼50 nm (Figure 7H). The small vesicles resembled those occurring normally within other organelles of the endocytic pathway of Leishmania such as MVBs or the lysosome-like MVT (Weise et al., 2000; Ghedin et al., 2001; Mullin et al., 2001). Virtually every stationary phase spt2– cell showed these defects, albeit to varying extents and proportions, and a variety of more severe abnormalities were found. No such defects were seen in log phase spt2–, or stationary phase WT or spt2–/+SPT2 parasites (data not shown). In other organisms SLs are enriched at various points in the endocytic pathway as well as in MVB-derived vesicles (David et al., 1998; Kasahara and Sanai, 1999; McMaster, 2001; van Meer and Lisman, 2002; Wubbolts et al., 2003), and the phenotypes seen in spt2– were consistent with a defect in these processes.
Fig. 7. spt2– parasites have severe defects in vesicular trafficking in stationary phase. (A)–(C) Transmission EM images of stationary phase WT and spt2– cells (day 3). Big arrowheads indicate vacuoles filled with small vesicles seen in spt2– (B and C) but not in WT (A) cells. (D)–(G) High magnification images of stationary phase spt2– showing MVB-like vesicles accumulated in and outside the flagellar pocket (D and E), at the surface of flagellum near the proximal end (F) and in an intracellular vacuole (G). Big arrowheads indicate flagellar pockets (D and E) or intracellular vacuoles (B, C and G). Small arrowheads indicate examples of MVB-like vesicles. F, flagellum; FP, flagellar pocket. (H) Size distribution of vesicles found in (D)–(G).
spt2– maintains the ability to generate DRMs similar to those in WT
SLs participate in the formation of membrane microdomains often termed lipid ‘rafts’ (Simons and Ikonen, 1997; Simons and Toomre, 2000; van Meer and Lisman, 2002). One common method for their recovery is the formation of detergent insoluble complexes at low but not high temperatures (DRMs) (Brown and London, 1998; London and Brown, 2000; Schuck et al., 2003), and this has been used successfully in Leishmania (Denny et al., 2001; Ralton et al., 2002; Zufferey et al., 2003). Thus, we prepared cold Triton X (TX)-100 DRMs from log and metacyclic phase WT L.major, and from log phase spt2– parasites, and probed them for the distribution of informative GPI-anchored markers.
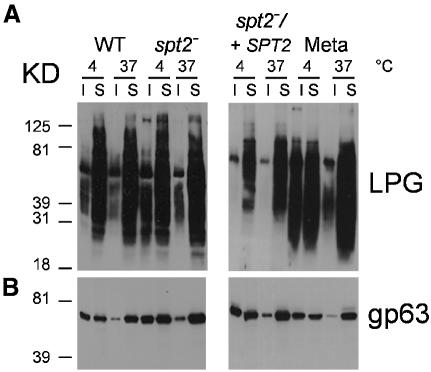
In log and metacyclic WT parasites, gp63 showed a strong association with DRM fractions, as ∼50% was insoluble to TX-100 extraction at 4°C but not at 37°C (Figure 8B). Gp63 in log phase spt2– parasites behaved identically to that in WT (Figure 8B). Similar results were obtained with other parasite DRM markers tested, and by additional criteria such as flotation in density gradients (data not shown). This suggested that the propensity of the spt2– mutant to form DRMs was unaltered, despite the absence of SLs (Figure 8).
Fig. 8. spt2– parasites maintain TX-100 DRMs. Soluble (S) and insoluble (I) fractions from log and metacyclic phase WT L.major, and from log phase spt2– and spt2–/+SPT2 parasites were subjected to western blot analysis to assess the distribution of LPG (A) and gp63 (B). Extractions were performed at 4°C or 37°C.
Previously it was proposed that in contrast to other markers, LPG was associated with buoyant DRM fractions in metacyclic, but not log phase promastigotes (Denny et al., 2002; Zufferey et al., 2003). This finding was reproduced here with log phase WT or spt2–, as LPG was soluble at both 4°C and 37°C (Figure 8A), while a significant proportion of LPG now appeared in the DRM fraction in metacyclic WT parasites (Figure 8A). This conclusion was confirmed by flotation in density gradients (data not shown). It was not possible to examine spt2– metacyclics. Since the structure of the LPG GPI anchor is invariant during development (McConville et al., 1992), relocalization of LPG to DRMs in metacyclic Leishmania may reflect a stage-specific reorganization of parasite membrane domains.
Discussion
In this study, we probed the role of de novo SL synthesis in Leishmania growth and differentiation by studying a null mutant lacking SPT2, one of the subunits of the first enzyme of the de novo SL pathway. The SPT2 protein contains the SPT catalytic site (reviewed by Hanada, 2003), and we showed that mutation of this site abolished activity in Leishmania. Unlike yeast and mammalian cells, SPT2 was not essential for L.major viability or growth during log phase in the absence of SBs. This raised the possibility that the spt2– parasites were completely lacking in SLs. We showed that the spt2– mutant lacked de novo SL synthesis (Figure 3), and that parasites grown in vitro lacked SLs including the abundant IPCs, as well as precursors such as ceramide detectable by MS analysis (Figure 4). Thus, we conclude that spt2– is completely devoid of SLs, and does not require them for near-normal growth in log phase.
In contrast, upon entry into stationary phase spt2– parasites rapidly lost viability and failed to generate the infective metacyclic stage. That these defects arose solely through loss of SL synthesis was established by first, that spt2– parasites were not rescued by expression of an active-site mutant of SPT2*, and secondly, that spt2– parasites could be rescued by exogenous SBs. Since SPT2 mRNA and protein was detected in early stationary phase but not in metacyclic promastigotes (Figure 1), presumably SLs are required in stationary phase for the completion of metacyclogenesis, but not by metacyclic parasites themselves.
To explain the phenotypes of the spt2– mutant, we considered effects related to overall membrane composition (bulk), the formation of membrane microdomains or ‘rafts’, membrane trafficking and cell signaling. In terms of ‘bulk’, apparently Leishmania can compensate for the loss of the 5–10% of the cellular lipids comprised by SLs by increasing overall lipid synthesis generally, as no specific changes in membrane composition were detected in the spt2– line in this work (other than loss of SLs; Figure 4). A similar conclusion was reached in studies of another mutant lacking the ∼10% of the cellular lipids comprised of ether phospholipids (Zufferey et al., 2003).
SLs, through interactions involving their head groups, the SB and the hydrophobic anchor, associate with sterols and other molecules to form distinct membrane microdomains (‘lipid rafts’) within eukaryotic membranes (Simons and Ikonen, 1997; van Meer and Lisman, 2002). Here we used a standard experimental criterion for ‘lipid rafts’ involving the formation of cold TX-100 DRMs. In Leishmania as in other organisms, DRMs are rich in SLs, GPI-anchored proteins and sterols (Denny et al., 2001; Ralton et al., 2002). However, the GPI-anchored protein gp63 was localized to a similar extent in DRMs in both the WT and spt2– parasites (Figure 8). Moreover, preliminary data examining other markers and/or methods for raft preparation similarly showed retention of DRMs in spt2– as well (unpublished data). Notably, retention of DRMs in the spt2– mutant does not imply that they have exactly the same properties as WT. Studies in other eukaryotes have suggested that the properties of DRMs/rafts may differ among cell types and even within different compartments within the same cell (Simons and Toomre, 2000), as discussed further below.
The remarkable ability of Leishmania to maintain DRMs without SLs may arise from two unique properties of parasite membranes. First, instead of cholesterol, Leishmania membranes contain ergosterol, which shows an increased propensity to form the underlying biophysical characteristics of rafts in studies of model membranes (Xu et al., 2001). Secondly, ether lipids comprise ∼10% of Leishmania phospholipids, in the form of alkyl/acyl PE, PI, PC and GPI-anchors of proteins and phosphoglycans (Beach et al., 1979; Wassef et al., 1985; Singh et al., 1988; Zufferey et al., 2003). Potentially, LPG and/or other abundant GPI-anchored molecules or ether lipids may compensate for the lack of SLs. While less extensively studied, ether lipids associate preferentially with lipid rafts, and studies of model membranes suggest they may also promote the formation of raft-like domains (Mattjus and Slotte, 1996; Ohvo-Rekila et al., 2002; Pike et al., 2002). Thus, the Leishmania membrane may provide a permissive environment for alterations in lipid composition, and maintenance of membrane microdomains or ‘rafts’ (Zufferey et al., 2003).
We eliminated several models for the stationary phase defect of spt2–, involving SL-dependent alterations in the metacyclic gene expression program or the induction of cell death through apoptotic mechanisms (Figure 6; Supplementary data). Instead, stationary phase spt2– parasites suffered catastrophic membranous defects that ultimately led to their demise. These defects included the formation of large vacuoles containing membranous material and smaller vesicles, general alterations in vacuolar appearance and abundance, the presence of membranous material and small vesicles in the flagellar pocket, some of which seemingly can escape and bind to the cell surface, and alterations in the flagellar surface (Figure 7). While a variety of aberrant larger vesicles were seen, the most abundant class had a size of ∼50 nm, similar to that seen within organelles of the Leishmania endocytic pathway such as the MVT or MVB, or in yeast or other organelles in the MVB pathway (Weise et al., 2000; Ghedin et al., 2001; Mullin et al., 2001; Katzmann et al., 2002; Waller and McConville, 2002; Wubbolts et al., 2003). In mammalian cells, recent data suggest that while the membranes of late endosomes and the MVB are low in SLs and cholesterol and enriched for other lipids such as lyso-bis-phosphatidic acid, the vesicles residing within the MVBs have a complementary composition enriched in SLs and cholesterol (Gruenberg, 2001; Kobayashi et al., 2002; Mobius et al., 2003; Wubbolts et al., 2003). Thus it is reasonable to propose that the accumulation of MVB-like vesicles in stationary phase spt2– arises from the lack of SLs. This further suggests that while not essential for the formation of MVB vesicles in Leishmania, SLs are required at subsequent steps in trafficking and/or degradation.
Current data suggest that stage-specific changes in the organization of the parasite membrane, as well as increased demands on membrane trafficking, contribute to the stationary phase specificity of the spt2– defects. In both Leishmania mexicana and Leishmania donovani, the activity of degradative pathways associated with the MVT/lysosomal compartments are elevated in stationary phase, a time when parasites are undergoing considerable remodeling while differentiating into the infective metacyclic form (Mullin et al., 2001; Waller and McConville, 2002). MVB vesicles are known to play key roles in degradative processes, through both ubiquitin-dependent and independent targeting routes (Gruenberg, 2001; Katzmann et al., 2002). That Leishmania membranes undergo stage-specific reorganization was shown by the association of LPG with DRMs in metacyclic but not growing cells, whereas the GPI-anchored protein gp63 was always associated with DRMs (Figure 8; McConville et al., 1992; Denny et al., 2002; Zufferey et al., 2003). Alterations in DRM properties have been associated with defects in membrane trafficking (van Meer and Lisman, 2002), and mammalian MVB-derived vesicles are rich in SLs and cholesterol and show raft-like properties in detergent extractions (Mobius et al., 2003; Wubbolts et al., 2003). Significantly, the MVT network was suggested to include lipid raft-like domains in L.donovani (Ghedin et al., 2001). In total, the coincident appearance of spt2– defects with developmental changes in membrane properties, and increased demands on membrane trafficking through the MVT/MVB pathway upon entry into stationary phase and differentiation, are consistent with the known roles and distribution of SLs.
The spt2– studies raise a number of important points that will be the subject of future studies. To definitively explore the role of SLs in Leishmania membrane formation and trafficking, it will be necessary to develop a repertoire of markers for both DRM domains, and for MVBs and MVTs. Notably, while the spt2– cells are attenuated in infections of macrophages and mice, after some delay, mice infected with high numbers of spt2– go on to develop lesions that progress rapidly (data not shown). Since amastigotes also lack SPT2 mRNA and protein (Figure 1), this may imply that L.major amastigotes, like promastigotes, may not require SBs and possibly SLs. Alternatively, amastigote acquisition of host cell SLs has been described (reviewed in McConville et al., 2002), and perhaps Leishmania satisfy amastigote SL requirements through salvage. Lastly, Leishmania offers a new perspective from which to explore sphingolipid metabolic pathways, in a setting where organisms are viable and permissive to perturbations not possible in other organisms (such as the study of DRMs lacking SLs). This feature could be adapted for use in cell-based assays exploring the function of SL metabolic genes expressed heterologously in Leishmania.
Materials and methods
Cloning and sequencing of L.major SPT2 and molecular constructs
From the L.major Genome Database, we identified a 1.6 kb DNA sequence that was homologous to the SPT2 genes from other species; this was amplified by PCR, inserted into the expression vector pXG (Ha et al., 1996), and its sequence was confirmed. The K372A mutation in the catalytic site mutant SPT2* was introduced by oligonucleotide directed mutagenesis. The SPT2 ORF was inserted into the pXG-GFP+2/ vector (B2952; Zufferey et al., 2003) to give a GFP::SPT2 fusion protein. Bacterial expression of a His-tagged SPT2 was achieved following insertion into the pET15b vector, and purified protein was used to generate antisera in rabbits. Specific details are included as Supplementary data.
Leishmania culture and transfection
Wild type L.major LV39 clone 5 (Rho/SU/59/P) cells were grown in M199 medium with supplements and 10% heat inactivated fetal bovine serum (FBS) at 26°C. Metacyclic parasites were isolated by PNA agglutination or density gradient methods (Sacks and Perkins, 1984; Späth and Beverley, 2001). Stocks of SBs and ceramides were made at 10 and 1 mM, respectively in ethanol, and stored at –20°C. Transfections by electroporation were performed as described (Kapler et al., 1990). The SPT2 coding region of the first allele was replaced by a HYG marker and that of the second by a PAC marker. Restoration of SPT2 expression to several of these spt2– mutants was achieved by transfection of pXG-SPT2. Specific details are included as Supplementary data.
Flow cytometry, fluorescence and electron microscopy
Cells were attached to slides, fixed with formaldehyade and permeabilized with ethanol. Antisera used included rabbit anti-Trypanosoma brucei BIP and monoclonal antibodies against gp63 and LPG (WIC 79.3). For electron microscopy, parasites were fixed in 2% formaldehyde/2.5% glutaraldehyde, washed, post-fixed with 1% osmium tetroxide and stained with 1% uranyl acetate; 70–80 nm sections were cut and viewed by transmission EM. For flow cytometry, 1 × 106 cells were washed once in phosphate-buffered saline (PBS) and incubated with 0.5 µg/ml of propidium iodide at room temperature for 5 min, 1 µg/ml of annexin V-FITC (Sigma) on ice for 30 min or 10 µM YOPRO-1 (Molecular Probes) at room temperature for 10 min. Flow cytometry was performed using a Becton Dickinson FACSCalibur system. Specific details are found in Supplementary data.
Metabolic labeling, lipid fractions and TLC
Log phase cells (<5 × 106/ml) were resuspended in M199/10% FCS at 1 × 108 cells/ml and labeled with [3H]serine (26 Ci/mmol) at 50 µCi/ml for 20 min at room temperature. Cells were harvested, washed with PBS, and resuspended in lysis buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 10 mM CaCl2, 0.5% NP-40, 0.5% TX-100, 1 mM PMSF, 2 µg/ml leupeptin, 5 µg/ml pepstatin A) at 5 × 107 cells/ml. After 10 min on ice, lysates were extracted with 3.75 vol of CHCl3/methanol (1:2), followed by 1.25 vol of CHCl3, and 1.25 vol of water. After centrifugation (1000g, 10 min), the upper aqueous phase was removed and the organic phase was washed once with water. The organic phase was then dried under nitrogen and dissolved in CHCl3 at the equivalent of 2 × 109 cells/ml and stored at –20°C. TLC analysis of lipids was performed using aluminum-backed silica gel plates (Sigma). Solvent contained methyl acetate/1-propanol/CHCl3/methanol/0.25% KCl (25:25:25:10:9). Autoradiography was performed after spraying plates with En3Hancer (Perkin Elmer). The presence of SBs in ceramides and IPCs was confirmed by resistance to mild base (0.1 M KOH in methanol, 37°C, 3 h).
Mass spectrometry
For MS, cells were washed in PBS and resuspended in water at 2 × 108 cells/ml, sonicated on ice (15 W, 5 s) and extracted as described for TLC. ESI/MS analyses were performed in the negative ion mode, and ESI/MS or ESI/MS/MS spectra were generated by a 1 min or 1–20 min period, respectively, of signal averaging of repeated scans. Specific details of instrumentation and protocols can be found in Supplementary data.
Preparation of DRMs and western blotting of LPG and gp63
Leishmania cell pellets (1 × 108 cells) were washed once in PBS and extracted with 1 ml of 1% TX-100 (prepared in PBS and supplemented with 1 mM PMSF, 2 µg/ml leupeptin and 5 µg/ml pepstatin A) for 10 min on ice or at 37 °C. Detergent-soluble and insoluble fractions were separated by centrifugation at 20 000g for 2 min at 4°C or 37°C. An equal volume of 2 × SDS sample buffer was added to the detergent soluble fraction (supernatant) and two volumes of 1 × SDS sample buffer were added to the detergent insoluble fraction (pellet). Samples were resolved by SDS–PAGE and electroblotted onto Hybond ECL nitrocellulose membranes (Amersham Biosciences). LPG was detected with monoclonal antibody WIC79.3 (1:1000). Gp63 was detected with monoclonal antibody 235 (1:1000), (Connell et al., 1993). An enhanced chemiluminescence (ECL) detection system (Amersham Biosciences) was used to detect signals.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank E.Handman and J.Bangs for the rabbit anti-T.brucei BIP polyclonal antibody, W.Beatty for EM analysis, L.Pike and D.K.Toomre for discussions about sphingolipids and lipid rafts, P.Denny for DRM protocols and preliminary results, everyone in the Beverley lab for insightful discussions, and A.Capul, D.Dobson, K.Robinson, L.D.Sibley and P.Stahl for comments on this manuscript. This work was funded by NIH grant AI21903 to S.M.B. J.R. was a recipient of the Lucille P.Markey Fellowship in Human Pathobiology. The Mass Spectrometry Facility is supported by NIH grants P41-RR-00954, P60-DK-20579 and P30-DK-56341.
References
- Beach D.H., Holz,G.G.,Jr and Anekwe,G.E. (1979) Lipids of Leishmania promastigotes. J. Parasitol., 65, 201–216. [PubMed] [Google Scholar]
- Brown D.A. and London,E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol., 14, 111–136. [DOI] [PubMed] [Google Scholar]
- Chu K.U., Ravindranath,M.H., Gonzales,A., Nishimoto,K., Tam,W.Y., Soh,D., Bilchik,A., Katopodis,N. and Morton,D.L. (2000) Gangliosides as targets for immunotherapy for pancreatic adenocarcinoma. Cancer, 88, 1828–1836. [PubMed] [Google Scholar]
- Connell N.D., Medina-Acosta,E., McMaster,W.R., Bloom,B.R. and Russell,D.G. (1993) Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette–Guerin expressing the Leishmania surface proteinase gp63. Proc. Natl Acad. Sci. USA, 90, 11473–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D., Sundarababu,S. and Gerst,J.E. (1998) Involvement of long chain fatty acid elongation in the trafficking of secretory vesicles in yeast. J. Cell Biol., 143, 1167–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny P.W., Field,M.C. and Smith,D.F. (2001) GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett., 491, 148–153. [DOI] [PubMed] [Google Scholar]
- Denny P.W., Nugent,P.G. and Smith,D.F. (2002) Lipid rafts in Leishmania: A role in infectivity? Molecular Parasitology Meeting, Woods Hole, MA, USA. http://e2kroos.cis.upenn.edu/mpm-2003/ [Google Scholar]
- Dickson R.C., Nagiec,E.E., Skrzypek,M., Tillman,P., Wells,G.B. and Lester,R.L. (1997) Sphingolipids are potential heat stress signals in Saccharomyces. J. Biol. Chem., 272, 30196–30200. [DOI] [PubMed] [Google Scholar]
- DosReis G.A., Pecanha,L.M., Bellio,M., Previato,J.O. and Mendonca-Previato,L. (2002) Glycoinositol phospholipids from Trypanosoma cruzi transmit signals to the cells of the host immune system through both ceramide and glycan chains. Microbes Infect., 4, 1007–1013. [DOI] [PubMed] [Google Scholar]
- Funato K., Vallee,B. and Riezman,H. (2002) Biosynthesis and trafficking of sphingolipids in the yeast Saccharomyces cerevisiae. Biochemistry, 41, 15105–15114. [DOI] [PubMed] [Google Scholar]
- Ghedin E., Debrabant,A., Engel,J.C. and Dwyer,D.M. (2001) Secretory and endocytic pathways converge in a dynamic endosomal system in a primitive protozoan. Traffic, 2, 175–188. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. (2001) The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol., 2, 721–730. [DOI] [PubMed] [Google Scholar]
- Ha D.S., Schwarz,J.K., Turco,S.J. and Beverley,S.M. (1996) Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol., 77, 57–64. [DOI] [PubMed] [Google Scholar]
- Hanada K. (2003) Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta, 1632, 16–30. [DOI] [PubMed] [Google Scholar]
- Hanada K., Nishijima,M., Kiso,M., Hasegawa,A., Fujita,S., Ogawa,T. and Akamatsu,Y. (1992) Sphingolipids are essential for the growth of Chinese hamster ovary cells. Restoration of the growth of a mutant defective in sphingoid base biosynthesis by exogenous sphingolipids. J. Biol. Chem., 267, 23527–23533. [PubMed] [Google Scholar]
- Hanada K., Nishijima,M., Fujita,T. and Kobayashi,S. (2000) Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem. Pharmacol., 59, 1211–1216. [DOI] [PubMed] [Google Scholar]
- Hannun Y.A. and Obeid,L.M. (2002) The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem., 277, 25847–25850. [DOI] [PubMed] [Google Scholar]
- Ikonen E. (2001) Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol., 13, 470–477. [DOI] [PubMed] [Google Scholar]
- Ilgoutz S.C. and McConville,M.J. (2001) Function and assembly of the Leishmania surface coat. Int. J. Parasitol., 31, 899–908. [DOI] [PubMed] [Google Scholar]
- Ilgoutz S.C., Mullin,K.A., Southwell,B.R. and McConville,M.J. (1999) Glycosylphosphatidylinositol biosynthetic enzymes are localized to a stable tubular subcompartment of the endoplasmic reticulum in Leishmania mexicana. EMBO J., 18, 3643–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro E.S., Jayasimhulu,K. and Lester,R.L. (1986) Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J. Lipid Res., 27, 1294–1303. [PubMed] [Google Scholar]
- Kapler G.M., Coburn,C.M. and Beverley,S.M. (1990) Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell. Biol., 10, 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K. and Sanai,Y. (1999) Possible roles of glycosphingolipids in lipid rafts. Biophys. Chem., 82, 121–127. [DOI] [PubMed] [Google Scholar]
- Katzmann D.J., Odorizzi,G. and Emr,S.D. (2002) Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol., 3, 893–905. [DOI] [PubMed] [Google Scholar]
- Knuepfer E., Stierhof,Y.D., McKean,P.G. and Smith,D.F. (2001) Characterization of a differentially expressed protein that shows an unusual localization to intracellular membranes in Leishmania major. Biochem. J., 356, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Beuchat,M.H., Chevallier,J., Makino,A., Mayran,N., Escola,J.M., Lebrand,C., Cosson,P. and Gruenberg,J. (2002) Separation and characterization of late endosomal membrane domains. J. Biol. Chem., 277, 32157–32164. [DOI] [PubMed] [Google Scholar]
- Kolter T., Proia,R.L. and Sandhoff,K. (2002) Combinatorial ganglioside biosynthesis. J. Biol. Chem., 277, 25859–25862. [DOI] [PubMed] [Google Scholar]
- Landfear S.M. and Ignatushchenko,M. (2001) The flagellum and flagellar pocket of trypanosomatids. Mol. Biochem. Parasitol., 115, 1–17. [DOI] [PubMed] [Google Scholar]
- Lederkremer R.M. and Bertello,L.E. (2001) Glycoinositolphospholipids, free and as anchors of proteins, in Trypanosoma cruzi. Curr. Pharm. Des., 7, 1165–1179. [DOI] [PubMed] [Google Scholar]
- Lee N., Bertholet,S., Debrabant,A., Muller,J., Duncan,R. and Nakhasi,H.L. (2002) Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ., 9, 53–64. [DOI] [PubMed] [Google Scholar]
- Lester R.L. and Dickson,R.C. (1993) Sphingolipids with inositolphosphate-containing head groups. Adv. Lipid Res., 26, 253–274. [PubMed] [Google Scholar]
- London E. and Brown,D.A. (2000) Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta, 1508, 182–195. [DOI] [PubMed] [Google Scholar]
- Maceyka M., Payne,S.G., Milstien,S. and Spiegel,S. (2002) Sphingosine kinase, sphingosine-1-phosphate and apoptosis. Biochim. Biophys. Acta, 1585, 193–201. [DOI] [PubMed] [Google Scholar]
- Mandala S.M. and Harris,G.H. (2000) Isolation and characterization of novel inhibitors of sphingolipid synthesis: australifungin, viridiofungins, rustmicin and khafrefungin. Methods Enzymol., 311, 335–348. [DOI] [PubMed] [Google Scholar]
- Mattjus P. and Slotte,J.P. (1996) Does cholesterol discriminate between sphingomyelin and phosphatidylcholine in mixed monolayers containing both phospholipids? Chem. Phys. Lipids, 81, 69–80. [DOI] [PubMed] [Google Scholar]
- McConville M.J. and Bacic,A. (1989) A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization and antigenicity. J. Biol. Chem., 264, 757–766. [PubMed] [Google Scholar]
- McConville M.J., Turco,S.J., Ferguson,M.A. and Sacks,D.L. (1992) Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J., 11, 3593–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M.J., Mullin,K.A., Ilgoutz,S.C. and Teasdale,R.D. (2002) Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev., 66, 122–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster C.R. (2001) Lipid metabolism and vesicle trafficking: more than just greasing the transport machinery. Biochem. Cell Biol., 79, 681–692. [DOI] [PubMed] [Google Scholar]
- Merrill A.H. Jr, Hannun,Y.A. and Bell,R.M. (1993) Introduction: sphingolipids and their metabolites in cell regulation. Adv. Lipid Res., 25, 1–24. [PubMed] [Google Scholar]
- Mobius W., van Donselaar,E., Ohno-Iwashita,Y., Shimada,Y., Heijnen,H.F., Slot,J.W. and Geuze,H.J. (2003) Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic, 4, 222–231. [DOI] [PubMed] [Google Scholar]
- Mullin K.A., Foth,B.J., Ilgoutz,S.C., Callaghan,J.M., Zawadzki,J.L., McFadden,G.I. and McConville,M.J. (2001) Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol. Biol. Cell, 12, 2364–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohvo-Rekila H., Ramstedt,B., Leppimaki,P. and Slotte,J.P. (2002) Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res., 41, 66–97. [DOI] [PubMed] [Google Scholar]
- Pike L.J., Han,X., Chung,K.N. and Gross,R.W. (2002) Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry, 41, 2075–2088. [DOI] [PubMed] [Google Scholar]
- Pinto W.J., Srinivasan,B., Shepherd,S., Schmidt,A., Dickson,R.C. and Lester,R.L. (1992a) Sphingolipid long-chain-base auxotrophs of Saccharomyces cerevisiae: genetics, physiology and a method for their selection. J. Bacteriol., 174, 2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto W.J., Wells,G.W. and Lester,R.L. (1992b) Characterization of enzymatic synthesis of sphingolipid long-chain bases in Saccharomyces cerevisiae: mutant strains exhibiting long-chain-base auxotrophy are deficient in serine palmitoyltransferase activity. J. Bacteriol., 174, 2575–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralton J.E., Mullin,K.A. and McConville,M.J. (2002) Intracellular trafficking of glycosylphosphatidylinositol (GPI)-anchored proteins and free GPIs in Leishmania mexicana. Biochem. J., 363, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. and Kamhawi,S. (2001) Molecular aspects of parasite–vector and vector–host interactions in leishmaniasis. Annu. Rev. Microbiol., 55, 453–483. [DOI] [PubMed] [Google Scholar]
- Sacks D.L. and Perkins,P.V. (1984) Identification of an infective stage of Leishmania promastigotes. Science, 223, 1417–1419. [DOI] [PubMed] [Google Scholar]
- Sacks D.L. and Perkins,P.V. (1985) Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am. J. Trop. Med. Hyg., 34, 456–459. [DOI] [PubMed] [Google Scholar]
- Schuck S., Honsho,M., Ekroos,K., Shevchenko,A. and Simons,K. (2003) Resistance of cell membranes to different detergents. Proc. Natl Acad. Sci. USA, 100, 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K. and Ikonen,E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons K. and Toomre,D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol., 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Singh B.N., Costello,C.E., Beach,D.H. and Holz,G.G.,Jr (1988) Di-O-alkylglycerol, mono-O-alkylglycerol and ceramide inositol phosphates of Leishmania mexicana mexicana promastigotes. Biochem. Biophys. Res. Commun., 157, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Späth G.F. and Beverley,S.M. (2001) A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol., 99, 97–103. [DOI] [PubMed] [Google Scholar]
- van Meer G. and Lisman,Q. (2002) Sphingolipid transport: rafts and translocators. J. Biol. Chem., 277, 25855–25858. [DOI] [PubMed] [Google Scholar]
- Vaux D.L. and Korsmeyer,S.J. (1999) Cell death in development. Cell, 96, 245–254. [DOI] [PubMed] [Google Scholar]
- Waller R.F. and McConville,M.J. (2002) Developmental changes in lysosome morphology and function Leishmania parasites. Int. J. Parasitol., 32, 1435–1445. [DOI] [PubMed] [Google Scholar]
- Wassef M.K., Fioretti,T.B. and Dwyer,D.M. (1985) Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids, 20, 108–115. [DOI] [PubMed] [Google Scholar]
- Weise F., Stierhof,Y.D., Kuhn,C., Wiese,M. and Overath,P. (2000) Distribution of GPI-anchored proteins in the protozoan parasite Leishmania, based on an improved ultrastructural description using high-pressure frozen cells. J. Cell Sci., 113, 4587–4603. [DOI] [PubMed] [Google Scholar]
- Wubbolts R., Leckie,R.S., Veenhuizen,P.T., Schwarzmann,G., Mobius,W., Hoernschemeyer,J., Slot,J.W., Geuze,H.J. and Stoorvogel,W. (2003) Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem., 278, 10963–10972. [DOI] [PubMed] [Google Scholar]
- Xu X., Bittman,R., Duportail,G., Heissler,D., Vilcheze,C. and London,E. (2001) Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal and disease-associated sterols and comparison of sphingomyelin, cerebrosides and ceramide. J. Biol. Chem., 276, 33540–33546. [DOI] [PubMed] [Google Scholar]
- Zangger H., Mottram,J.C. and Fasel,N. (2002) Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ., 9, 1126–1139. [DOI] [PubMed] [Google Scholar]
- Zufferey R., Allen,S., Barron,T., Sullivan,D.R., Denny,P.W., Almeida,I.C., Smith,D.F., Turco,S.J., Ferguson,M.A.J. and Beverley,S.M. (2003) Ether phospholipids and glycosylinositolphospholipids (GIPLs) are not required for amastigote virulence nor for inhibition of macrophage activation by Leishmania major. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]