Abstract
In colon cancer, enteric bacteria and dietary factors are major determinants of the microenvironment but their effect on cellular invasion is not known. We therefore incubated human HCT-8/E11 colon cancer cells with bacteria or bacterial conditioned medium on top of collagen type I gels. Listeria monocytogenes stimulate cellular invasion through the formation of a soluble motility-promoting factor, identified as a 13mer β-casein-derived peptide (HKEMPFPKYPVEP). The peptide is formed through the combined action of Mpl, a Listeria thermolysin-like metalloprotease, and a collagen-associated trypsin-like serine protease. The 13mer peptide was also formed by tumour biopsies isolated from colon cancer patients and incubated with a β-casein source. The pro- invasive 13mer peptide-signalling pathway implicates activation of Cdc42 and inactivation of RhoA, linked to each other through the serine/threonine p21- activated kinase 1. Since both changes are necessary but not sufficient, another pathway might branch upstream of Cdc42 at phosphatidylinositol 3-kinase. Delta opioid receptor (δOR) is a candidate receptor for the 13mer peptide since naloxone, an δOR antagonist, blocks both δOR serine phosphorylation and 13mer peptide-mediated invasion.
Keywords: bacteria/β-casein-derived peptides/cell motility/collagen invasion/Rho GTPases signalling
Introduction
Bacteria participate in colon cancer development. The stages of development in which these bacteria are implicated and their mechanisms of action are, however, not clear. Bacteria may participate in earlier stages of intestinal carcinogenesis (Hill et al., 1978; Parsonnet, 1995), in line with results from transgenic and chemically induced animal models (Ellmerich et al., 2000; Kado et al., 2001; Newman et al., 2001). The example of Helicobacter pylori has demonstrated that bacteria may act also epigenetically at later stages of cancer development, influencing cytokine profiles and cell motility (Higashi et al., 2002; Churin et al., 2003). The relationship between H.pylori infection and increased risk of gastric carcinoma is well documented. For colon cancer the situation is less clear, because of the greater complexity of the intestinal flora (Swidsinski et al., 1998).
Invasion is a crucial step in the development of cancer since it is responsible for malignancy either through locoregional spread or through metastasis to distant organs. The cross-talk between cancer cells and host elements modulates invasion-associated activities such as cell–cell adhesion, cell–matrix interaction, proteolysis, ectopic survival, growth and motility (Liotta and Kohn, 2001; Trusolino et al., 2001). In the ecosystem of primary colon cancer, invasion is modulated by environmental factors (Emami et al., 2001). Commensal and pathogenic bacteria are also abundant in this ecosystem but, so far, their participation in invasion has not been investigated. Here, we addressed the question of whether stimulation of invasion is one of the mechanisms by which bacteria promote colon cancer development. We originally worked with Listeria monocytogenes since one of its virulence factors, Internalin A (InlA), is a ligand of E-cadherin, a transmembrane cell–cell adhesion molecule that, in combination with the cytoplasmic catenins, forms an invasion-suppressor complex (Vleminckx et al., 1991; Mengaud et al., 1996). We found that Listeria stimulated colon cancer cell invasion and that this activity, not related to the InlA/E-cadherin interaction, was also found with other bacteria isolated from laboratory stock cultures or from tumour biopsies of colon cancer patients. These bacteria assist in the production of a pro-invasive and motility-promoting 13mer peptide that is derived from β-casein. Interpretation of our findings in view of known positive and negative invasion pathways (Nguyen et al., 2002) points to a crucial role for activation and inactivation of small GTPases of the Rho family.
Results
Listeria monocytogenes stimulates cancer cell invasion through production of a soluble pro-invasive factor
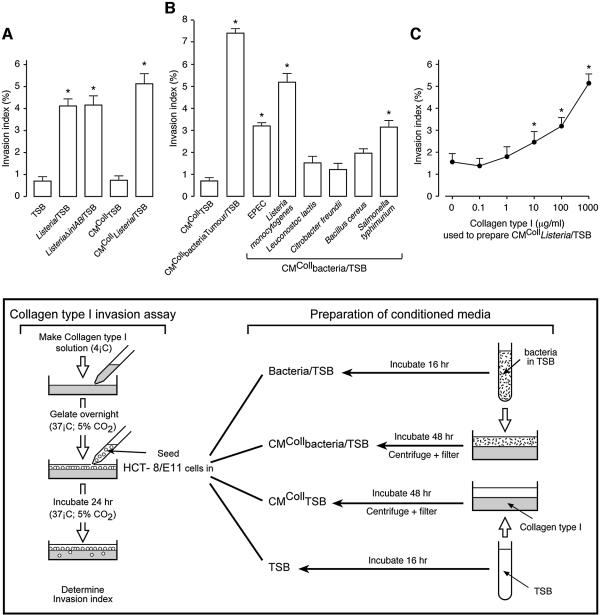
Considering the hypothesis that Listeria InlA might neutralize the invasion-suppressor function of E-cadherin, we incubated HCT-8/E11 human colon cancer cells, on top of collagen type I gels (Vleminckx et al., 1991; Barbier et al., 2001; De Corte et al., 2002), with wild-type L.monocytogenes or with its InlA- and InlB-deficient mutant (ΔinlAB) (Gaillard et al., 1991) (Figure 1, box). Listeria monocytogenes growing in tryptic soy broth (TSB) (Listeria/TSB), but not TSB alone, significantly stimulated cancer cell invasion (Figure 1A). Interestingly, the ΔinlAB mutant (ListeriaΔinlAB/TSB) also stimulated invasion, indicating that InlA/E-cadherin binding and bacterial entry into the cancer cells are not required in this process. To find out whether stimulation of invasion was due to the direct contact between bacteria and cancer cells or to the release of a soluble pro-invasive factor, moderately washed L.monocytogenes (see Materials and methods), growing in TSB, on top of collagen gels and without cancer cells, were incubated for 48 h. Supernatants of such cultures were collected, filtered and tested in the collagen invasion assay (conditioned medium [CM]CollListeria/TSB). CMColl Listeria/TSB stimulated cancer cell invasion (Figure 1A), in contrast with TSB incubated on collagen without bacteria or cancer cells (CMCollTSB). These observations point to a pro-invasive factor, generated in the absence of cancer cells, and released into the medium when bacteria and collagen are cultured together.
Fig. 1. Bacteria stimulate cancer cell invasion into collagen type I gels. (A) HCT-8/E11 cells were incubated, on top of collagen type I gels, in the presence of: TSB, L.monocytogenes wild-type or ΔinlAB mutant grown in TSB (Listeria/TSB or ListeriaΔinlAB/TSB, respectively), CM of TSB or of L.monocytogenes wild-type grown in TSB and produced for 48 h on top of collagen type I gels (CMCollTSB or CMCollListeria/TSB, respectively). The experimental design is presented schematically in the box. Invasion indices (%) were determined as in Materials and methods. Bars represent mean values of at least three independent experiments and flags indicate standard deviations. *Significantly different from TSB at p < 0.005. (B) HCT-8/E11 cells were incubated, on top of collagen type I gels, in the presence of CMColl of different bacterial strains grown in TSB and isolated from laboratory stock cultures or from the tumours of colon cancer patients (CMCollbacteria/TSB or CMCollbacteriaTumour/TSB, respectively). Invasion indices (%) were determined as in (A). *Significantly different from CMCollTSB at p < 0.005. (C) HCT-8/E11 cells were incubated, on top of collagen type I gels, in the presence of CMCollListeria/TSB, produced on gels with different collagen concentrations. Invasion indices (%) were determined as in (A). Dots represent mean values of at least three independent experiments and flags indicate standard deviations. *Significantly different from CMCollListeria/TSB prepared with 0 µg/ml of collagen type I at p < 0.005.
Pro-invasive activity was also generated with CMColl of enteropathogenic Escherichia coli (EPEC), of Salmonella typhimurium or of bacteria isolated from tumour biopsies of colon cancer patients and grown in TSB (CMCollbacteria Tumour/TSB) (Figure 1B), demonstrating that stimulation of cancer cell invasion is not restricted to Listeria.
Collagen type I participates in the production of the pro-invasive factor
To investigate the role of collagen type I in the production of the pro-invasive factor, we prepared CM Listeria on top of gels of different collagen type I concentrations (CMCollListeria/TSB). The pro-invasive activity of CMCollListeria/TSB increased with the concentration of collagen used for its preparation (Figure 1C), indicating a dose-dependent contribution of collagen to the production of the pro-invasive factor.
The pro-invasive factor is a β-casein-derived peptide
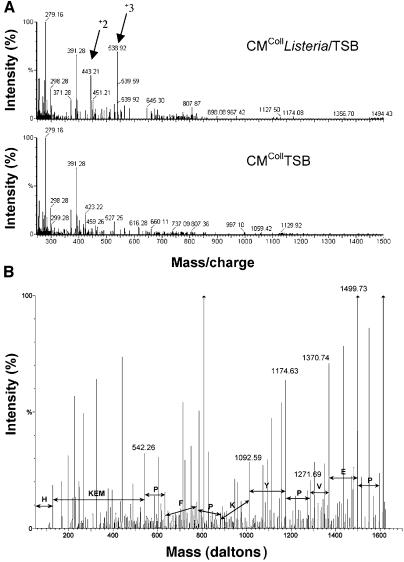
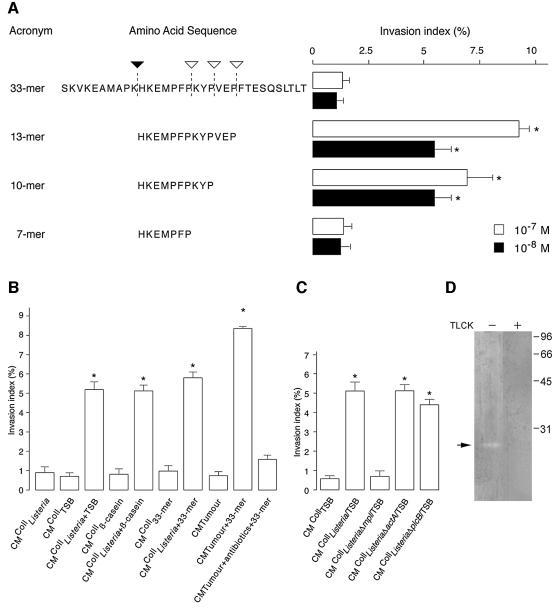
To identify the pro-invasive factor, lyophilized CMColl Listeria/TSB was subjected to reverse phase-high performance liquid chromatography (RP-HPLC). Fractions, stimulating invasion, were reproducibly detected. The pro-invasive activity of such fractions was destroyed by Pronase®, a broad-spectrum protease of Streptomyces griseus, but not affected by incubation at 95°C (data not shown), suggesting the involvement of a small peptide. Pro-invasive fractions were further purified by three additional RP-HPLC separations, using different eluting buffers. Comparison of the mass spectra of the pro-invasive CMCollListeria/TSB with the inactive CMCollTSB allowed unique assignment of doubly charged (443.21 and 807.87 mass/charge) and triply charged (538.92 mass/charge) peptides as major differential ions (Figure 2A). Fragmentation analysis led to a 7 amino acid peptide (7mer peptide) (HKEMPFP, mass of 884.42 Da) and to a 13 amino acid peptide (13mer peptide) (HKEMPF PKYPVEP with M as methionine sulphoxide, mass of 1613.74 Da) (Figure 2B). The 13mer peptide corresponds to the sequence spanning residues histidine 106 to proline 118 of bovine (Bos taurus) β-casein (Mascot, BLAST, SWISS-PROT databases). A homologous sequence is also present in sheep (Oves aries) and goat (Capra hircus) β-casein and 75% of homology is shared with porcine (Sus scrofa) β-casein. No homology was found with human β-casein sequences. The 7mer peptide is derived from the N-terminus of the 13mer. From both peptides, only the synthetic 13mer was pro-invasive at concentrations above 10–8 M (Figure 3A) while the 7mer was inactive even at concentrations up to 10–4 M.
Fig. 2. The pro-invasive factor is a β-casein-derived peptide. (A) Differential MS-spectra of CM prepared on collagen with (CMCollListeria/TSB) or without (CMCollTSB) bacteria. RP-HPLC-MS analysis of CMCollListeria/TSB revealed doubly (+2) and triply (+3) charged ions of 443.21, 807.87 and 538.92 mass/charge, absent in CMCollTSB. Vertical lines represent relative intensity (%) of ions. Numerical values indicate mass/charge of reference ions. (B) Identification of the doubly charged ion 807.87 by fragmentation analysis. The identified sequence is represented by the b-ion series HKEMPFPKYPVEP. Annotations of y-ions, internal fragment ions and ions with loss of methane sulfenic acid are omitted. Vertical lines represent ions relative intensity (%). Numerical values indicate mass/charge of reference ions.
Fig. 3. Mpl, a Listeria thermolysin-like metalloprotease, and a collagen-associated trypsin-like serine protease produce pro-invasive peptides. (A) To investigate the generation of pro-invasive peptides, a synthetic β-casein 33mer peptide (33mer) was incubated for 48 h in the presence of exhaustively washed bacteria and/or collagen type I. Peptides generated by proteolysis were sequenced by RP-HPLC-MS/MS and their pro-invasive activity measured in the collagen assay. Closed and open arrowheads indicate cleavage sites for a collagen-associated protease and a L.monocytogenes protease, respectively (left panel). The effect of synthetic β-casein peptides (33, 13, 10 and 7mer) on HCT-8/E11 cell invasion was also evaluated (right panel). Invasion indices (%) were determined as in Figure 1A. *Significantly different from 33mer at p < 0.005. (B) HCT-8/E11 cells were incubated, on top of collagen type I gels, with: CM of exhaustively washed bacteria (CMCollListeria) prepared with TSB (CMCollListeria+TSB), β-casein (CMCollListeria+β-casein) or the synthetic 33mer peptide (CMCollListeria+33mer), CM of such β-casein sources prepared in absence of bacteria (CMCollTSB, CMCollβ-casein, CMColl33mer), CM from tumour biopsies of colon cancer patients, prepared in the absence (CMTumour) or in the presence of the synthetic 33mer peptide with or without antibiotics (CMTumour+antibiotics+33mer or CMTumour+33mer, respectively). Invasion indices (%) were determined as in Figure 1A. *Significantly different from CMCollTSB at p < 0.005. (C) HCT-8/E11 cells were incubated, on top of collagen type I gels, with CMCollTSB, CMCollListeria wild-type (CMCollListeria/TSB), Δmpl (CMCollListeriaΔmpl/TSB), ΔactA (CMCollListeriaΔactA/TSB) or ΔplcB (CMCollListeriaΔplcB/TSB) mutants, grown in TSB. Invasion indices (%) were determined as in Figure 1A. *Significantly different from CMCollTSB at p < 0.005. (D) β-casein zymography reveals a trypsin-like serine protease with caseinolytic activity in collagen type I solution. Collagen solution was loaded on a SDS gel containing β-casein as substrate. After electrophoresis, gel was incubated in a serine-protease buffer with (+) or without (–) 1 µM TLCK. Arrow indicates a ∼21 kDa protein, revealed by Coomassie® staining.
The identification of a pro-invasive β-casein-derived peptide pointed to the bacterial culture medium (TSB) as the peptide source. Therefore we prepared, on collagen, CM with exhaustively washed bacteria (CMCollListeria) and confirmed, by RP-HPLC-tandem mass spectrometry (RP-HPLC-MS/MS), the absence of TSB remnants. Indeed, CMCollListeria lost its pro-invasive activity (Figure 3B), in contrast with CM of moderately washed bacteria (CMCollListeria/TSB) (Figure 1A), suggesting adsorption of TSB remnants to the bacterial surface. CM prepared on collagen in the presence of TSB or β-casein, without bacteria (CMCollTSB or CMCollβ-casein), were inactive. The addition of TSB or β-casein, during the preparation of CMCollListeria (CMCollListeria+TSB or CMCollListeria+β-casein), restored its pro-invasive activity (Figure 3B), suggesting proteolysis of inactive into pro-invasive β-casein-derived peptides.
The combined action of Mpl, a Listeria thermolysin-like metalloprotease, and a collagen-associated trypsin-like serine protease generates the pro-invasive β-casein-derived peptide
To understand how the pro-invasive β-casein-derived peptide was produced, we synthesized an inactive β-casein 33mer (Figure 3A), by extending 10 amino acids to the N- and the C-terminus of the 13mer peptide (SKVKEAMAPKHKEMPFPKYPVEPFTESQSLTLT). The 33mer peptide was then incubated on collagen for 48 h, with or without exhaustively washed Listeria (CMCollListeria+33mer or CMColl33mer, respectively). For each of these media, we measured the pro-invasive activity and analysed the cleavage products formed. CMColl33mer did not stimulate cancer cell invasion (Figure 3B) but generated two cleavage products (SKVKEAMAPK and HKEMPFPKYPVEPFTESQSLTLT) at the lysine–histidine (K–H) bound, as confirmed by RP-HPLC-MS/MS (Figure 3A, solid arrowhead). Furthermore, CMListeria+33mer, prepared in the absence of collagen, generated four major cleavage products (SKVKEAMAPKHKEMPFP, KYPVEPFTESQSLTLT, VEPFTESQSLTLT and FTESQSLTLT) (Figure 3A, open arrowheads). Complementary peptides (such as SKVKEAMAPKHKEMPFPKYP and SKVKEAMAPKHKEMPFPKYPVEP) were not found, probably due to additional cleavage. However, CMColl Listeria+33mer, prepared in the presence of collagen, generated an inactive cleavage product (7mer peptide HKEMPFP) and two others with pro-invasive activity (10mer HKEMPFPKYP and 13mer peptide HKEMPFPKYPVEP) (Figure 3A). RP-HPLC-MS/MS analysis demonstrated that Listeria cleaves at the C-terminus of proline residues in front of a hydrophobic amino acid residue (Figure 3A). Such cleavage pattern, typical for thermolysin-like proteases (PeptideCutter, ExPASy Database), suggested the participation of Mpl, a Listeria 35 kDa metalloprotease involved in the maturation of phosphatidylcholine-specific phospholipase C (PC-PLC or PlcB) (Marquis et al., 1997) and in the cleavage of actin and β-casein into smaller peptides (Coffey et al., 2000). CMColl prepared with L.monocytogenes Δmpl (CMColl ListeriaΔmpl/TSB), a transposon-induced mutant (Gaillard et al., 1986), failed to generate pro-invasive activity (Figure 3C) and to cleave the 33mer peptide into pro-invasive peptides. However, CMColl of two other strains, bearing mutations on actA (CMCollListeriaΔactA/TSB) or plcB (CMCollListeriaΔplcB/TSB), genes located on the same mpl operon (Kocks et al., 1992; Schlüter et al., 1998) still generated pro-invasive activity. These results strongly support Listeria Mpl as the enzyme responsible for cleavage of β-casein, delineating the C-terminus of the pro-invasive 13mer peptide.
To characterize the collagen-associated protease that cleaves the 33mer at the N-terminus, we performed zymography of CMColl33mer, using bovine β-casein as substrate. However, no enzymatic activity was detected (data not shown). Zymography with the collagen type I solution itself showed a single caseinolytic band of 21 kDa, sensitive to Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), an irreversible trypsin-like serine protease inhibitor (Figure 3D). This result suggests that a collagen-associated trypsin-like serine protease cleaves β-casein, delineating the N-terminus of the pro-invasive 13mer peptide.
The pro-invasive peptide is also generated by colon cancer biopsies
To investigate whether the enzymes converting inactive into pro-invasive peptides are also present in man, tumour biopsies from colon cancer patients were incubated with the 33mer peptide (CMTumour+33mer). RP-HPLC-MS/MS of this pro-invasive CM revealed, 1 h after 33mer peptide addition, similar cleavage products (HKEMPFPKYPVEP) as the ones found in vitro. Antibiotic treatment of colon cancer biopsies, 12 h prior to 33mer addition, abolished the formation of the 13mer peptide (RP-HPLC-MS/MS). Neither traces of the 13mer peptide nor pro-invasive activity were found in supernatants of biopsies cultured without the 33mer peptide (CMTumour) (Figure 3B). These results suggest that the enzymes needed to generate a pro-invasive peptide from a β-casein source are also present in the human colon.
The pro-invasive synthetic peptides stimulate cancer cell motility and invasion through activation of Cdc42 and inactivation of RhoA
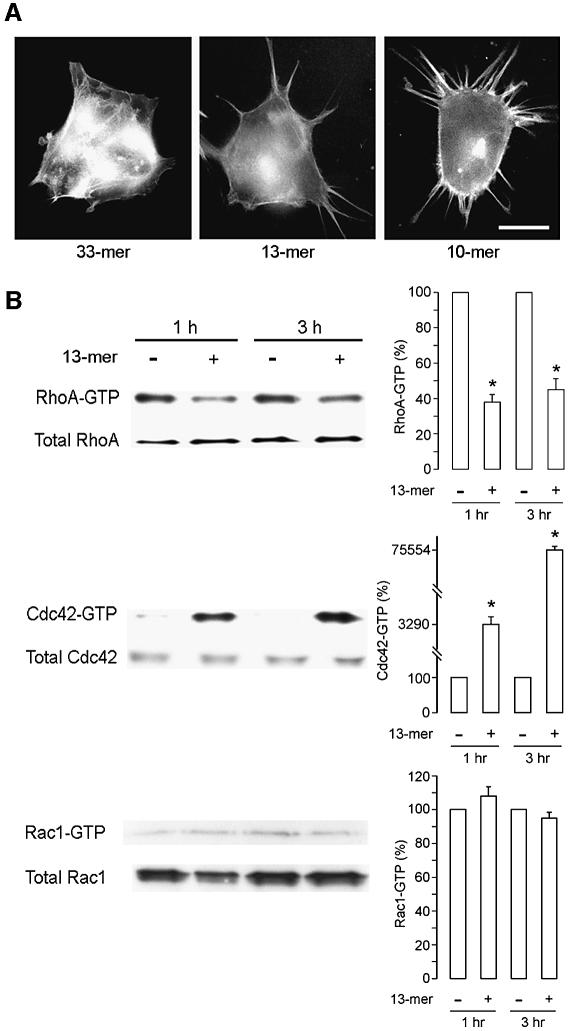
The effect of pro-invasive synthetic peptides on HCT-8/E11 cell motility and on actin cytoskeleton organization was investigated by F-actin staining complemented by time-lapse video microscopy to distinguish between retraction fibers and cellular extensions (Figure 4A). Interestingly, long filopodia were formed within 30 min of addition of the pro-invasive 10mer and 13mer but not of the inactive 33mer peptide. Stimulation of motility by the pro-invasive peptides was also demonstrated in wound healing (see Supplementary figure 1 available at The EMBO Journal Online).
Fig. 4. Pro-invasive peptides induce filopodia and modulate small GTPases. (A) HCT-8/E11 cells, seeded on a glass surface, were treated with synthetic β-casein peptides (33, 13 and 10mer at 10–7 M). Actin filaments were visualized with FITC-phalloidin. Scale bar represents 20 µm. (B) Effect of the 13mer peptide on RhoA, Cdc42 and Rac1 activity levels. HCT-8/E11 cells were treated for 1 or 3 h with (+) or without (–) the synthetic 13mer peptide (10–7 M), followed by solubilization in lysis buffer. Equal volumes of cell lysates were pulled-down with GST-C21 Rhotekin for RhoA or GST-PAK167–150 for Cdc42 and Rac1. Activated GTPases, bound to the GST-fusion proteins, were resolved by SDS–PAGE. Immunoblots for total GTPases confirmed that equal amounts of proteins were engaged in the pull-downs (left panel). Immunoblots were quantified with the Quantity One Software, with the untreated level of activated GTPases as 100% (right panel). Bars represent mean values of three independent experiments and flags indicate standard deviations. *Significantly different from untreated at p < 0.005.
The formation of filopodia suggests the implication of small GTPases of the Rho family (Nobes and Hall, 1995). We therefore investigated the effect of the pro-invasive 13mer peptide on the activation state of Rho GTPases by pull-down assays (Sander et al., 1998). One hour after addition of the 13mer peptide, we observed significant activation of Cdc42 but no change of Rac1. Moreover, activation of Cdc42 was accompanied by inactivation of RhoA (Figure 4B).
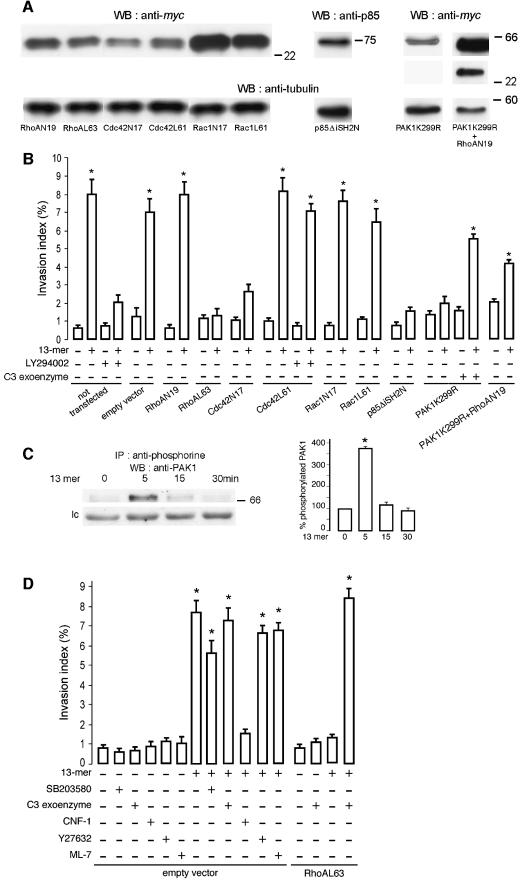
We wondered whether the 13mer peptide-mediated invasion also required Cdc42 activation and RhoA inactivation. Therefore, HCT-8/E11 cells were transiently transfected with dominant-negative (RhoAN19, Cdc42N17 or Rac1N17) or constitutively active (RhoAL63, Cdc42L61 or Rac1L61) GTPase constructs (Figure 5A). Twenty-four hours after transfection, cells were incubated with or without the 13mer peptide on top of collagen gels. None of the transfections were sufficient to stimulate HCT-8/E11 cell invasion in absence of the 13mer peptide (Figure 5B). Dominant-negative (Rac1N17) and constitutively active (Rac1L61) Rac1 transfected cells were sensitive to the pro-invasive 13mer peptide. In contrast, cells transfected with dominant-negative Cdc42 (Cdc42N17) or constitutively active RhoA (RhoAL63) were resistant. Invasion of cells expressing constitutively active RhoA was achieved by treatment with the 13mer peptide only when the RhoA inhibitor C3 exoenzyme (Clostridium botulinum exoenzyme C3 transferase) was added (Figure 5D). Consistent with the pull-down assays, these results demonstrate that 13mer peptide-mediated invasion and motility require Cdc42 activation and RhoA inactivation.
Fig. 5. Cdc42 and PI3K but not Rac1 are necessary for the 13mer peptide-mediated invasion. (A) Dominant-negative or constitutively active RhoA, Cdc42 or Rac1, dominant-negative PI3K or kinase-dead PAK1 or double kinase-dead PAK1 and dominant-negative RhoA expression in transiently transfected cells. Total lysates were immunoblotted with anti-myc or anti-p85 antibodies. Loading of equal amounts of protein was controlled by anti-α-tubulin immunoblotting. (B) HCT-8/E11 cells were transiently transfected with an empty vector or with dominant-negative RhoA (RhoAN19), Cdc42 (Cdc42N17) or Rac1 (Rac1N17), constitutively active RhoA (RhoAL63), Cdc42 (Cdc42L61) or Rac1 (Rac1L61), dominant-negative PI3K (p85ΔiSH2N), kinase-dead PAK1 (PAK1K299R) or kinase-dead PAK1+dominant-negative RhoA (PAK1K299R+RhoAN19) constructs. Twenty-four hours after transfection, cells were tested in the collagen invasion assay with (+) or without (–) the 13mer peptide (10–7 M). The PI3K inhibitor LY294002 (10–5 M) or the C3 exoenzyme (6.6 µg/ml) were added in selected assays. Invasion indices (%) were determined as in Figure 1A. *Significantly different from untreated (–) cells, transfected with an empty vector, at p < 0.005. (C) The 13mer peptide stimulates PAK1 serine- phosphorylation. Lysates of HCT-8/E11 cells, treated (for 0, 5, 15 or 30 min) with the 13mer peptide (10–7 M), were immunoprecipitated using an anti-phosphoserine antibody and immunoblotted with an anti-PAK1 antibody. A non-identified serine phosphorylated protein, crossreacting with the anti-PAK1 antibody, is presented as loading control (lc). Immunoblots were quantified as in Figure 4B (right panel), considering as 100% the phosphorylation level of untreated cells. (D) Cells were transiently transfected with an empty vector or with the constitutively active RhoA (RhoAL63) construct. Twenty-four hours after transfection, cells were incubated, on top of collagen type I gels, with (+) or without (–) the 13mer peptide (10–7 M) and/or SB203580 (1mg/ml), C3 exoenzyme (6.6 µg/ml), CNF1 (10–9 M), Y27632 (10–6 M) or ML-7 (5 × 10–6 M). Invasion indices (%) were determined as in Figure 1A. *Significantly different from untreated (–) cells, at p < 0.005.
PAK1 and PI3K are elements of the positive 13mer peptide-mediated invasion pathway
The serine/threonine p21-activated kinase 1 (PAK1) is a downstream target of activated Cdc42 (Manser et al., 1994). The implication of PAK1 in 13mer peptide-mediated invasion was evidenced by the increase on PAK1 serine phosphorylation 5 min after 13mer peptide addition (Figure 5C) and by the resistance of HCT-8/E11 cells, transiently transfected with a kinase dead PAK1 mutant (PAK1K299R) (Figure 5A and B). In conformity with Adam et al. (2000), these transfectants bear prominent stress fibers (data not shown), suggesting activation of the RhoA/ROCK/MLC pathway. Indeed, pro-invasive activity was observed when 13mer peptide-treated kinase dead PAK1 cells were incubated with C3 exoenzyme or cotransfected with dominant-negative RhoA (PAK1 K299R+RhoAN19) (Figure 5A and B).
Phosphatidylinositol 3-kinase (PI3K) serves the positive invasion pathway upstream of Cdc42. HCT-8/E11 cells treated with LY294002, a specific PI3K inhibitor, or transfected with a dominant-negative p85α (p85ΔiSH2N), the regulatory subunit of class IA PI3Ks, were resistant towards induction of invasion by the 13mer peptide (Figure 5A and B). In contrast, the pro-invasive effect of the peptide could not be neutralized by LY294002 in cells that expressed constitutively active Cdc42 (Cdc42L61) (Figure 5B).
To investigate the participation of other molecules in this invasion pathway, HCT-8/E11 cells were treated with the p38 specific mitogen-activated protein kinase (MAPK) inhibitor SB203580, the C3 exoenzyme, the Rho GTPases activator CNF1 (E.coli cytotoxic necrotizing factor 1), the Rho-associated coil-coiled kinase (ROCK) inhibitor Y27632, or the myosin light chain kinase (MLCK) inhibitor ML-7, in the presence or absence of the 13mer peptide. None of these modulators was able to stimulate invasion in the absence of the 13mer peptide and only CNF1 counteracted the 13mer peptide-mediated invasion (Figure 5D).
The pro-invasive peptide induces dephosphorylation of FAK downstream of RhoA
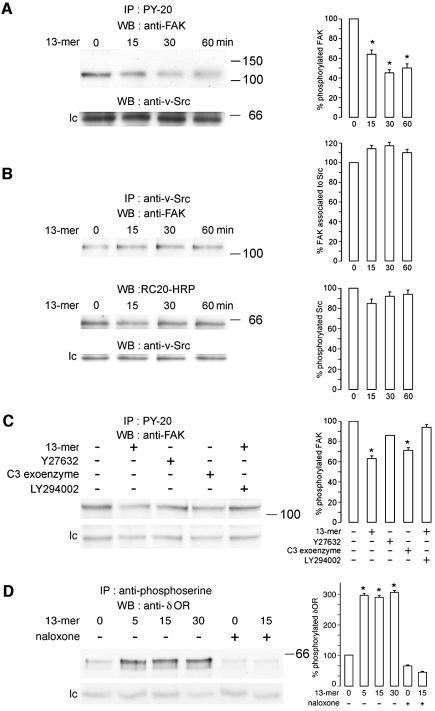
To detect more elements of the 13mer peptide invasion pathway, cell lysates were immunoprecipitated with an anti-phosphotyrosine antibody (PY-20). We found decreased phosphorylation of a 125 kDa protein identified, by immunoblot, as focal adhesion kinase (FAK) (Figure 6A). Immunoprecipitation with an anti-v-Src antibody, followed by immunoblotting with anti-FAK or with anti-phosphotyrosine recombinant (RC20-HRP) antibodies, showed that FAK dephosphorylation, induced by the 13mer peptide, occurs in an Src-independent manner. Treatment with the 13mer peptide affected neither the association between Src and FAK nor the expression or phosphorylation levels of Src (Figure 6B). Furthermore, we show that FAK does not function upstream of PI3K since the PI3K inhibitor LY294002 prevented dephosphorylation of FAK (Figure 6C). Since RhoA inactivation by C3 exoenzyme caused dephosphorylation of FAK, like the pro-invasive 13mer peptide, we presume that, in our system, FAK regulates focal adhesion assembly downstream of RhoA. This is in line with the decrease in focal adhesion plaques as evidenced by FAK and vinculin immunostaining in 13mer peptide-treated cells (data not shown).
Fig. 6. The pro-invasive 13mer peptide causes FAK dephosphorylation and δOR serine phosphorylation. (A) HCT-8/E11 cells were left untreated or treated with the 13mer peptide (10–7 M) for 15, 30 or 60 min, followed by solubilization in lysis buffer. Cell lysates were immunoprecipitated with an antibody against tyrosine-phosphorylated residues (PY-20). The immunoprecipitates were immunoblotted with anti-FAK (upper panel) or anti-v-Src (lower panel; lc, loading control) antibodies. Immunoblots were quantified as in Figure 4B (right panel). *Significantly different from untreated at p < 0.005. (B) HCT-8/E11 cells were treated as in (A). Cell lysates were immunoprecipitated with an anti-v-Src antibody. The immunoprecipitates were immunoblotted with an anti-FAK antibody (upper panel), a recombinant antibody, specific for tyrosine-phosphorylated residues (RC20-HRP) (middle panel) or an anti-v-Src antibody (lower panel; lc, loading control). Immunoblots were quantified as in Figure 4B (right panel). (C) HCT-8/E11 cells were left untreated (–) or treated for 60 min (+) with the 13mer peptide (10–7 M), Y27632 (10–6 M), C3 exoenzyme (6.6 µg/ml) or LY294002 (10–5 M), followed by solubilization in lysis buffer. Immunoprecipitates were prepared and probed as in (A) (upper panel). A non-identified tyrosine phosphorylated protein crossreacting with the anti-FAK antibody is presented as loading control (lc). Immunoblots were quantified as in Figure 4B (right panel). *Significantly different from untreated (–) cells at p < 0.005. (D) HCT-8/E11 cells were left untreated (–) or treated (+) for 5, 15 or 30 min with the 13mer peptide (10–7 M) and/or with naloxone (10–5 M). Cell lysates were immunoprecipitated using an anti-phosphoserine antibody and immunoblotted with an anti-δOR antibody. A non-identified serine phosphorylated protein, crossreacting with the anti-δOR antibody, is presented as loading control (lc). Immunoblots were quantified as in Figure 4B (right panel). *Significantly different from untreated (–) cells at p < 0.005.
The δ opioid receptor (δOR) is a candidate receptor for the pro-invasive 13mer peptide
In silico analysis revealed sequence homology between the C-terminus sequence of the 13mer peptide and opiate receptor ligands such as human β-casomorphin. We found that, within 5 min, the 13mer peptide increases the serine phosphorylation level of the δOR (Figure 6D). Naloxone (10–5 M), an δOR antagonist, inhibited 13mer peptide-mediated invasion (see Supplementary figure 2) and serine phosphorylation of the receptor, pointing to δOR as a candidate receptor for the pro-invasive 13mer peptide.
Discussion
A new pro-invasive peptide was isolated from an in vitro collagen type I coculture system with Listeria and colon cancer cells. The interaction of Listeria with invasion-suppressor molecules provides a paradigm for the participation of bacteria at the ecosystem of colon cancer. We do not, however, want to infer from our experiments that Listeria constitutes a specific pro-invasive element in the human colon cancer ecosystem. The experiments with tumour biopsies isolated from colon cancer patients demonstrate that the bacterial flora may also participate in the production of pro-invasive factors (the same or other peptides) and that antibiotics interfere with this process. The microenvironment needs to provide extrinsic pro-invasive factors in order to make cancer cells invade the surrounding tissues once their genetic invasion program has been switched on (Liotta and Kohn, 2001; Trusolino et al., 2001; Egeblad and Werb, 2002; Mareel and Leroy, 2003). Generation of the new pro-invasive peptide needs, next to collagen, elements from the diet, and from the enteric flora both being the major determinants of the colon microenvironment (Hill et al., 1978; Swidsinski et al., 1998).
β-casein is present as undefined fragments in the culture medium of the bacteria used in our experiments. It is the most abundant protein in milk and milk-derived products, leaving peptides that are resistant to further proteolysis in the colon (Corpet et al., 1995). β-casein is a rich source of peptides, some of which exert relevant biological functions, such as activation of opioid receptors (Meisel and FitzGerald, 2000). The capacity of the pro-invasive 13mer peptide to stimulate δOR serine phosphorylation together with the inhibition of 13mer-mediated invasion by naloxone, an δOR antagonist, point to δOR as a putative 13mer peptide receptor. Further experiments need to be performed to confirm this hypothesis. Putative participation of β-casein peptides in cancer development is suggested by the finding that oral administration of heat-treated casein to rats promoted the formation of colon tumours (Zhang et al., 1992; Corpet et al., 1995). Listeria monocytogenes participates in the generation of the 13mer peptide through Mpl, a 35 kDa thermolysin-like metalloprotease, described as degrading β-casein over a wide range of temperatures and pH values (Coffey et al., 2000). It remains to be elucidated whether the other bacteria, obtained from laboratory stock cultures or directly from tumour biopsies of colon cancer patients, generated their pro-invasive activity by the same mechanisms. It cannot be excluded that other enzymatic combinations may generate pro-invasive peptides. Bacterial proteases have multiple functions: producing nutrients, stimulating bacterial invasion, activating eukaryotic proteolytic cascades and, in our present experiments, assisting cancer cell invasion. The latter is compatible with the presence of bacteria at the front of invasion in human colon cancer (Swidsinski et al., 1998). Whereas others have shown that bacteria promote colon cancer development at the earlier stages of aberrant crypt and adenoma formation (Ellmerich et al., 2000; Kado et al., 2001; Newman et al., 2001), our present observations suggest that they also act at the later stages of invasive carcinoma. The collagen gel contains the enzyme that shapes the N-terminus of the 13mer peptide. This enzyme is a putative trypsin-like serine protease as it cleaves between the amino acids lysine and histidine and is inhibited by TLCK. In our system, this protease might have been attracted to the complex collagen matrix in vivo or added to the collagen solution during its purification in vitro. Taken together, we infer that the elements needed for the generation of the pro-invasive peptides in our in vitro system are present in the human colon.
Formation of filopodia, as evidenced by phalloidin staining of the actin cytoskeleton and other motility assay (see Supplementary figure 1), indicated that the 13mer peptide is a motility factor. Bacterial motility factors that stimulate invasion, in line with their chemotactic effect on leukocytes, have been described earlier. N-formylmethionyl-leucyl-phenylalanine at 10–7 M stimulated the invasion of sarcoma cells through native connective tissue in vitro (Thorgeirsson et al., 1982; Leiper et al., 2001). Lipopolysaccharides of Gram-negative bacteria stimulate angiogenesis and tumour invasion in BALB/c mice (Harmey et al., 2002). CagA (cytotoxin-associated gene A) of H.pylori causes the transition from an epithelioid to a fibroblastic morphotype of gastric cancer cells through activation of the motility factor receptor c-Met (Churin et al., 2003). In contrast with these bacterial motility factors, our 13mer peptide constitutes a product generated by bacteria through interaction with other elements of the microenvironment.
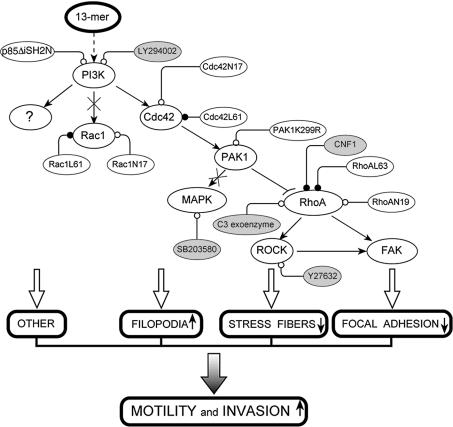
Our current model of the 13mer peptide-mediated invasion pathway is presented in Figure 7. In this pathway inactivation of RhoA and activation of Cdc42 are necessary but not sufficient, as evidenced by the use of dominant negative or constitutively active transfectants and of pharmacological inhibitors or activators. Activation of Cdc42 explains the formation of filopodia (Nobes and Hall, 1995; Jiménez et al., 2000), whereas inactivation of RhoA explains the release of stress fibers (Itoh et al., 1999). The serine/threonine kinase PAK1 is the obvious link between the two relevant small GTPases as it is activated by Cdc42 (Manser et al., 1994) and inactivates RhoA and its downstream effectors (Zhao et al., 1998). In line with the observations of others on the spontaneous invasion of human breast cancer cells through Matrigel® (Adam et al., 2000), we found that the kinase dead PAK1 mutant is resistant to the pro-invasive effect of the 13mer peptide, only becoming sensitive when RhoA is inhibited by C3 exoenzyme or by a dominant-negative construct. PI3K is the element upstream of Cdc42 (Jiménez et al., 2000) that is essential for the 13mer peptide invasion pathway, as it was found also for other positive invasion pathways (Barbier et al., 2001). In the context of PDGF-stimulated migration of cells through a collagen-coated filter, PI3K activates Cdc42 through its p85α regulatory subunit independent of its kinase activity (Jiménez et al., 2000). In our present experiments, the 13mer peptide-mediated invasion depends on both the regulatory and the kinase subunits, as evidenced by the dominant-negative transfectant p85ΔiSH2N as well as by the neutralizing effect of LY294002, an inhibitor of the ATP-binding site of the kinase. It is our hypothesis that PI3K serves as the branching point for a complementary pathway.
Fig. 7. Model of the 13mer peptide-mediated invasion pathway. Inactivation of RhoA and activation of PAK1 and Cdc42, both upstream of RhoA, are necessary but not sufficient for invasion. We explained this through a putative pathway branching upstream of Cdc42 at PI3K (question mark). Arrows and arcs indicate activation and inactivation, respectively. Crossed arrows indicate dispensable pathways. Open or closed circles indicate, respectively, inactivation or activation by pharmacological modulators (shaded) or by dominant-negative or constitutively active constructs (unshaded). Definitions are: 13mer, 13mer β-casein-derived peptide; PI3K, phosphoinositide 3 kinase; Rac1, Ras related C3 botulinum toxin substrate 1; Cdc42, cell division cycle 42; PAK1, p21-activated kinase 1; MAPK, mitogen-activated protein kinase; RhoA, Ras homolog; ROCK, Rho-associated coil-coiled kinase; FAK, p125 focal adhesion kinase; CNF1, Escherichia coli cytotoxic necrotizing factor 1; C3 exoenzyme, Clostridium botulinum exoenzyme C3 transferase.
Inactivation of RhoA may influence invasion through ROCK (Amano et al., 1997) and FAK (Ridley and Hall, 1994). In contrast with our observations, invasion of rat MM1 hepatoma cells through a mesothelial cell monolayer is prevented by the ROCK inhibitor Y27632 or by transfection of dominant-negative ROCK (Itoh et al., 1999). Here also the effect on invasion may depend upon the cellular context. The decrease in FAK phosphorylation may result from RhoA inactivation either directly (Flinn and Ridley, 1996) or via ROCK (Sinnett-Smith et al., 2001). In HCT-8/E11 cells this is confirmed by the dephosphorylation of FAK upon RhoA inactivation by C3 exoenzyme. Consistently, in Swiss 3T3 cells, the RhoA activator CNF1 induces phosphorylation of FAK (Lacerda et al., 1997).
In conclusion, our initial observations with Listeria, confirmed with other enteric bacteria isolated from laboratory stock cultures or from tumour biopsies of colon cancer patients, show that the combined action of diet, bacteria and host elements produce specific pro-invasive and motility-promoting β-casein-derived peptides.
Materials and methods
Cell and bacterial culture
Human colon cancer HCT-8/E11 cells were grown in RPMI 1640 medium (Invitrogen, Merelbeke, Belgium) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 2.5 µg/ml Fungizone® (Bristol-Myers Squibb, Brussels, Belgium), 200 µg/ml streptomycin and 200 IU/ml penicillin (Invitrogen).
Listeria monocytogenes wild-type EGD and mutant strains (ΔinlAB, Δmpl, ΔplcB and ΔactA) were kindly provided by P. Cossart (Pasteur Institute, Paris) and grown as described (Kocks et al., 1992; Mengaud et al., 1996; Schlüter et al., 1998). Salmonella typhimurium (ATCC 14028) was obtained from the American Type Culture Collection (Rockville, MD) and the other bacterial strains, including EPEC (0128K67), from our Hospital Culture Collection. Prior to each experiment, one bacterial colony, growing in tryptic soy agar (TSA) (Oxoid, Hampshire, UK) with 5% sheep blood (for hemolytic strains), was incubated overnight at 37°C, in 5 ml of TSB (Oxoid). This culture was then diluted (1:20) and incubated for an additional 4 h. Bacterial density was measured as described (Mengaud et al., 1996).
Isolation of bacteria from human colon biopsies
Extracellular bacteria were isolated from tumour colon biopsies in direct contact with MacConkey II, Schaedler or 5% sheep blood supplemented TSA plates (Oxoid). Intracellular bacteria were isolated as described (Swidsinski et al., 1998), plated out and incubated at 37°C. Extracellular and intracellular bacteria were mixed before CM preparation.
Human colon biopsies
Surgical biopsies of colon cancer patients were kept at 4°C to minimize proteolysis and washed once in cold phosphate-buffered saline. Biopsies were then maintained, for 1 h, at 37°C and 5% CO2, in serum-free medium without or with the synthetic 33mer peptide (0.5 µg/µl) in the presence or not of antibiotics. Culture supernatants were centrifuged at 3220 g for 2 min, passed through a 0.2 µm pore-size filter (CMTumour, CMTumour+antibiotics+33mer or CMTumour+33mer, respectively), analysed by RP-HPLC-MS/MS or tested in the collagen invasion assay. For antibiotic treatment, colon biopsies were incubated overnight in serum-free medium with 25 µg/ml Fungizone®, 2 mg/ml streptomycin and 2000 IU/ml penicillin, prior to addition of the 33mer peptide.
Collagen invasion assay
Collagen invasion assays were performed as described (Vleminckx et al., 1991). Briefly, gels were prepared in a 6-well plate (Nunc, Roskilde, Denmark), from a collagen type I solution (Upstate Biotechnology, Lake Placid, NY). HCT-8/E11 cells (1 × 105) were incubated on top of the gels, for 24 h at 37°C, with bacteria (∼2 × 104/ml) or with 300 µl CM. To restrain growth of Listeria, 5 µg/ml gentamicin (Geomycine®; Schering-Plough, Kenilworth, NJ) was added. Invasion indices (%) are ratios between the number of invasive cells inside the gel and the total number of cells, counted in at least 12 microscopic fields.
CM preparation
To prepare CMCollListeria/TSB or CMCollbacteria/TSB, ∼1 × 107 bacteria growing in TSB were washed three times in 1 ml serum-free medium (moderately washed), added to 1.5 ml of medium containing 1 mM sodium pyruvate and 5 µg/ml gentamicin, and incubated on collagen gels for 48 h at 37°C. Similarly, we prepared CMCollbacteriaTumour/TSB using bacteria isolated from colon cancers. CMCollListeria, CMColl Listeria+TSB, CMCollListeria+β-casein or CMCollListeria+33mer were prepared with ∼1 × 107 bacteria washed 10 times in 15 ml serum-free medium (exhaustively washed). This procedure removed TSB remnants, as confirmed by RP-HPLC-MS/MS. TSB, β-casein (Sigma, St Louis, MO) or the 33mer peptide (0.5 µg/µl) were exogenously supplied. CMCollTSB, CMCollβ-casein or CMColl33mer were prepared for 48 h on collagen gels without bacteria. All CM were centrifuged at 3220 g for 2 min and passed through a 0.2 µm pore-size filter (Schleicher & Schüell, Dassel, Germany).
RP-HPLC-MS and RP-HPLC-MS/MS analysis
See Supplementary data.
Peptide synthesis
See Supplementary data.
β-casein zymography
Collagen type I was loaded (144 µg/lane) on a 12% SDS gel with 1% of bovine β-casein as substrate (Invitrogen). After electrophoresis, gels were washed twice for 30 min in 2% Triton X-100 (Bio-Rad, Hercules, CA), and incubated overnight at 37°C in a serine-protease buffer (0.025 M EDTA in 0.1 M glycine buffer, pH 7.8) with or without 1 µM TLCK (Sigma). Gels were then stained with 0.1% Coomassie® Brilliant Blue R-250 (Bio-Rad), 50% methanol and 10% acetic acid and destained with 20% methanol and 10% acetic acid.
Cancer cell motility
HCT-8/E11 cells (3 × 104), incubated for 24 h on glass coverslips in a 24-well plate (Nunc), were washed three times in serum-free medium and incubated with synthetic peptides. Cultures were monitored, under 5% CO2 at 37°C, with an inverted time-lapse video Axiovert 200 microscope (Carl-Zeiss, Göttingen, Germany). Cultures were fixed in 3% paraformaldehyde and permeabilized with 0.2% Triton X-100 prior to FITC-phalloidin (Sigma) staining.
Drugs
LY294002, a PI3K inhibitor, was obtained from Sigma. The ROCK inhibitor Y27632 was kindly provided by Yoshitomi Pharmaceutical Industries (Osaka, Japan). The C3 exoenzyme, which ADP-ribosylates and inactivates the small GTPase Rho, and CNF1, which activates small GTPases of the Rho family, were kindly provided by Dr C.Gespach (INSERM U482, France). The p38 specific MAPK inhibitor SB203580 was from Alexis Corporation (Lausen, Switzerland), and the MLCK inhibitor (ML-7) from Calbiochem (San Diego, CA).
Determination of Rac1, Cdc42 and RhoA activation states by pull-down assays
GTP-bound Rac1 or GTP-bound Cdc42 were precipitated by PAK167–150 while GTP-bound RhoA was precipitated by the C21 domain of Rhotekin, each fused to GST and precoupled to glutathione–Sepharose beads (Sander et al., 1998). Bound proteins were eluted with sample buffer, subjected to SDS–PAGE and immunoblotted with antibodies against Rac1, Cdc42 or RhoA, also used to analyse the total amount of small GTPases present in whole-cell lysates. Quantification was performed with the Quantity One Software (Bio-Rad).
Transfections
See Supplementary data.
Immunoblot analysis
See Supplementary data.
Immunoprecipitation
See Supplementary data.
Statistical analysis
Data are expressed as mean values of at least three independent experiments ± SD. Student’s t-tests were used to determine statistically significant differences (p < 0.005).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
The authors thank Professor Piet Pattyn (Department of Surgery, Ghent University Hospital) and Dr Pieter Demetter (Department of Pathology, Ghent University Hospital) for human colon tumour biopsies, and M.Vlerickx, E.Bruyneel and S.Derveaux for excellent technical assistance. This work was supported by the Portuguese Foundation for Science and Technology (FCT) BD 15980, the ‘Stichting Emmanuel van der Schueren’, the GOA 2002, Gent University and the Fund for Scientific Research-Flanders. V.D.C. and A.L. are postdoctoral fellows from the FWO Vlaanderen, Belgium.
References
- Adam L., Vadlamudi,R., Mandal,M., Chernoff,J. and Kumar,R. (2000) Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J. Biol. Chem., 275, 12041–12050. [DOI] [PubMed] [Google Scholar]
- Amano M., Chihara,K., Kimura,K., Fukata,Y., Nakamura,N., Matsuura,Y. and Kaibuchi,K. (1997) Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science, 275, 1308–1311. [DOI] [PubMed] [Google Scholar]
- Barbier M. et al. (2001) Tumour biology. Weakening link to colorectal cancer? Nature, 413, 796. [DOI] [PubMed] [Google Scholar]
- Churin Y., Al-Ghoul,L., Kepp,O., Meyer,T.F., Birchmeier,W. and Naumann,M. (2003) Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol., 161, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey A., van den Burg,B., Veltman,R. and Abee,T. (2000) Characteristics of the biologically active 35-kDa metalloprotease virulence factor from Listeria monocytogenes. J. Appl. Microbiol., 88, 132–141. [DOI] [PubMed] [Google Scholar]
- Corpet D.E., Yin,Y., Zhang,X.-M., Rémésy,C., Stamp,D., Medline,A., Thompson,L., Bruce,W.R. and Archer,M.C. (1995) Colonic protein fermentation and promotion of colon carcinogenesis by thermolyzed casein. Nutr. Cancer, 23, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Corte V., Bruyneel,E., Boucherie,C., Mareel,M., Vandekerckhove,J. and Gettemans,J. (2002) Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J., 21, 6781–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M. and Werb,Z. (2002) New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer, 2, 161–174. [DOI] [PubMed] [Google Scholar]
- Ellmerich S., Schöller,M., Duranton,B., Gossé,F., Galluser,M., Klein,J.-P. and Raul,F. (2000) Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis, 21, 753–756. [DOI] [PubMed] [Google Scholar]
- Emami S., Le Floch,N., Bruyneel,E., Thim,L., May,F., Westley,B., Rio,M.-C., Mareel,M. and Gespach,C. (2001) Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J., 15, 351–361. [DOI] [PubMed] [Google Scholar]
- Flinn H.M. and Ridley,A.J. (1996) Rho stimulates tyrosine phosphorylation of focal adhesion kinase, p130 and paxillin. J. Cell Sci., 109, 1133–1141. [DOI] [PubMed] [Google Scholar]
- Gaillard J.L., Berche,P. and Sansonetti,P. (1986) Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect. Immun., 52, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J.-L., Berche,P., Frehel,C., Gouin,E. and Cossart,P. (1991) Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell, 65, 1127–1141. [DOI] [PubMed] [Google Scholar]
- Harmey J.H., Bucana,C.D., Lu,W., Byrne,A.M., McDonnell,S., Lynch,C., Bouchier-Hayes,D. and Dong,Z. (2002) Lipopoly saccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumour cell invasion. Int. J. Cancer, 101, 415–422. [DOI] [PubMed] [Google Scholar]
- Higashi H., Tsutsumi,R., Muto,S., Sugiyama,T., Azuma,T., Asaka,M. and Hatakeyama,M. (2002) SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science, 295, 683–686. [DOI] [PubMed] [Google Scholar]
- Hill M.J., Morson,B.C. and Bussey,H.J. (1978) Aetiology of adenoma–carcinoma sequence in large bowel. Lancet, 1, 245–247. [DOI] [PubMed] [Google Scholar]
- Itoh K., Yoshioka,K., Akedo,H., Uehata,M., Ishizaki,T. and Narumiya,S. (1999) An essential part for Rho-associated kinase in the transcellular invasion of tumour cells. Nat. Med., 5, 221–225. [DOI] [PubMed] [Google Scholar]
- Jiménez C., Portela,R.A., Mellado,M., Rodríguez-Frade,J.M., Collard,J., Serrano,A., Martínez-A,C., Avila,J. and Carrera,A.C. (2000) Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J. Cell Biol., 151, 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado S. et al. (2001) Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor β chain and p53 double-knockout mice. Cancer Res., 61, 2395–2398. [PubMed] [Google Scholar]
- Kocks C., Gouin,E., Tabouret,M., Berche,P., Ohayon,H. and Cossart,P. (1992) L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell, 68, 521–531. [DOI] [PubMed] [Google Scholar]
- Lacerda H.M., Pullinger,G.D., Lax,A.J. and Rozengurt,E. (1997) Cytotoxic necrotizing factor 1 from Escherichia coli and dermonecrotic toxin from Bordetella bronchiseptica induce p21rho-dependent tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 cells. J. Biol. Chem., 272, 9587–9596. [DOI] [PubMed] [Google Scholar]
- Leiper K., Campbell,B.J., Jenkinson,M.D., Milton,J., Yu,L.-G., Democratis,J. and Rhodes,J.M. (2001) Interaction between bacterial peptides, neutrophils and goblet cells: a possible mechanism for neutrophil recruitment and goblet cell depletion in colitis. Clin. Sci. (Lond.), 101, 395–402. [PubMed] [Google Scholar]
- Liotta L.A. and Kohn,E.C. (2001) The microenvironment of the tumour-host interface. Nature, 411, 375–379. [DOI] [PubMed] [Google Scholar]
- Manser E., Leung,T., Salihuddin,H., Zhao,Z.-S. and Lim,L. (1994) A brain serine/theonine protein kinase activated by Cdc42 and Rac1. Nature, 367, 40–46. [DOI] [PubMed] [Google Scholar]
- Mareel M. and Leroy,A. (2003) Clinical, cellular, and molecular aspects of cancer invasion. Physiol. Rev., 83, 337–376. [DOI] [PubMed] [Google Scholar]
- Marquis H., Goldfine,H. and Portnoy,D.A. (1997) Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol., 137, 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel H. and FitzGerald,R.J. (2000) Opioid peptides encrypted in intact milk protein sequences. Br. J. Nutr., 84, S27–S31. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Ohayon,H., Gounon,P., Mège,R.-M. and Cossart,P. (1996) E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell, 84, 923–932. [DOI] [PubMed] [Google Scholar]
- Newman J.V., Kosaka,T., Sheppard,B.J., Fox,J.G. and Schauer,D.B. (2001) Bacterial infection promotes colon tumourigenesis in ApcMin/+ mice. J. Infect. Dis., 184, 227–230. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.-D., Faivre,S., Bruyneel,E., Rivat,C., Seto,M., Endo,T., Mareel,M., Emami,S. and Gespach,C. (2002) RhoA- and RhoD-dependent regulatory switch of Gα subunit signaling by PAR-1 receptors in cellular invasion. FASEB J., 16, 565–276. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Parsonnet J. (1995) Bacterial infection as a cause of cancer. Environ. Health Perspect., 103(Suppl. 8), 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J. and Hall,A. (1994) Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J., 13, 2600–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander E.E., van Delft,S., ten Klooster,J.P., Reid,T., van der Kammen,R.A., Michiels,F. and Collard,J.G. (1998) Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol., 143, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter D., Domann,E., Buck,C., Hain,T., Hof,H., Chakraborty,T. and Deckert-Schlüter,M. (1998) Phosphatidylcholine-specific phospho lipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect. Immun., 66, 5930–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith J., Lunn,J.A., Leopoldt,D. and Rozengurt,E. (2001) Y-27632, an inhibitor of Rho-associated kinases, prevents tyrosine phosphorylation of focal adhesion kinase and paxillin induced by bombesin: dissociation from tyrosine phosphorylation of p130cas. Exp. Cell Res., 266, 292–302. [DOI] [PubMed] [Google Scholar]
- Swidsinski A., Khilkin,M., Kerjaschki,D., Scheiber,S., Ortner,M., Weber,J. and Lochs,H. (1998) Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology, 115, 281–286. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson U.P., Liotta,L.A., Kalebic,T., Margulies,I.M., Thomas,K., Rios-Candelore,M. and Russo,R.G. (1982) Effect of natural protease inhibitors and a chemoattractant on tumour cell invasion in vitro. J. Natl Cancer Inst., 69, 1049–1054. [PubMed] [Google Scholar]
- Trusolino L., Bertotti,A. and Comoglio,P.M. (2001) A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell, 107, 643–654. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet,L.,Jr, Mareel,M., Fiers,W. and Van Roy,F. (1991) Genetic manipulation of E-cadherin expression by epithelial tumour cells reveals an invasion suppressor role. Cell, 66, 107–119. [DOI] [PubMed] [Google Scholar]
- Zhang X.-M., Stamp,D., Minkin,S., Medline,A., Corpet,D.E., Bruce,W.R. and Archer,M.C. (1992) Promotion of aberrant crypt foci and cancer in rat colon by thermolyzed protein. J. Natl Cancer Inst., 84, 1026–1030. [DOI] [PubMed] [Google Scholar]
- Zhao Z.-S., Manser,E., Chen,X.-Q., Chong,C., Leung,T. and Lim,L. (1998) A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol. Cell. Biol., 18, 2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]