Abstract
Inhibition of the c-Jun N-terminal kinase (JNK) pathway by glucocorticoids (GCs) results in AP-1 repression. GC antagonism of AP-1 relies mainly on the transrepression function of the GC receptor (GR) and mediates essential physiological and pharmacological actions. Here we show that GCs induce the disassembly of JNK from mitogen-activated protein kinase kinase 7 (MKK7) by promoting its association with GR. Moreover, we have characterized a hormone-regulated JNK docking site in the GR ligand-binding domain that mediates GR–JNK interaction. The binding of GR to JNK is required for inhibition of JNK activation and induction of inactive JNK nuclear transfer by GCs. The dissociation of these two hormone actions shows that JNK nuclear transfer is dispensable for the downregulation of JNK activation by GCs. Nonetheless, nuclear accumulation of inactive JNK may still be relevant for enhancing the repression of AP-1 activity by GCs. In this regard, chromatin immunoprecipitation assays show that GC-induced GR–JNK association correlates with an increase in the loading of inactive JNK on the AP-1-bound response elements of the c-jun gene.
Keywords: AP-1 antagonism/cell signaling/cross-talk/MAPK docking site/MAPK pathway
Introduction
Glucocorticoids (GCs) play key physiological roles in development, cellular proliferation and differentiation. In addition, the prominent pharmacological actions of these hormones have prompted their widespread medical use to treat diverse pathological conditions such as asthma, allergic rhinitis, rheumatoid arthritis and leukemia (Barnes, 1998). GCs exert most of their actions by binding to an intracellular GC receptor (GR), a ligand-activated transcriptional regulator that belongs to the nuclear receptor (NR) superfamily (Beato et al., 1995).
In most circumstances, hormone-free GR is associated with heterotypic complexes that contain chaperones, such as Hsp90, and co-chaperones, and is retained in the cytoplasm. Upon ligand binding, the chaperone complex is released and hormone-bound GR is rapidly transferred into the nucleus. Hormone-activated GR regulates gene transcription, either positively or negatively, by two major modes of action. The most well known involves the binding of GR homodimers to the GC response elements (GREs) found in the regulatory sequences of GC target genes. A second and more elusive mode of action is independent of the direct interaction of GR with DNA and relies on the interference (thus, also known as cross-talk or transrepression) with the activity of other transcriptional regulators by mechanisms based on protein–protein interactions. In contrast to the former, transrepression is apparently mediated by GR monomers. In fact, transactivation-defective mutants of GR, which cannot dimerize (GRdim) or bind DNA (GRLS7), are fully competent in transrepression (Heck et al., 1994; Helmberg et al., 1995). Remarkably, the in vivo relevance of the DNA binding-independent actions of GR has been evidenced by the generation of mice that harbor the GRdim mutation (Reichardt et al., 1998). Unlike the GR-deficient mice, which show severe abnormalities and die shortly after birth (Cole et al., 1995), GRdim mice are viable, indicating that the DNA binding-dependent activities of GR are dispensable for survival (Reichardt et al., 1998). Important sets of genes transrepressed by GR are those which are under the positive control of AP-1 and/or NF-κB. As these transcriptional regulators play critical roles in controlling the expression of many proinflammatory genes, GR antagonism with AP-1 and NF-κB is believed to underlie the anti-inflammatory and immune-suppressive actions of GCs (Göttlicher et al., 1998; Herrlich, 2001). The maintenance of GC anti-inflammatory activity together with the ability to transrepress AP-1 and NF-κB in GRdim mice indicate that this pharmacological action is mostly mediated by the transrepression function of the GR (Reichardt et al., 2001).
The antagonism between GR and AP-1 was described in the early 1990s, and several underlying mechanisms have since been proposed (reviewed in Herrlich, 2001). While early studies suggested a mutual inhibition to bind to DNA because of the formation of a GR–AP-1 complex, genomic footprinting (Konig et al., 1992) and chromatin immunoprecipitation (ChIP) assays (Rogatsky et al., 2001) showed that GC repression occurs with promoter-bound AP-1, suggesting that interaction with GR prevents AP-1 from proper interactions with the transcriptional machinery or with a co-activator complex (Saatcioglu et al., 1994) and/or recruits co-repressor complexes (Rogatsky et al., 2001).
An alternative mechanism by which GCs may exert their antagonistic action on AP-1 is through GR-mediated interference of the signaling pathways that activate AP-1, in particular the mitogen-activated protein kinase (MAPK) pathways (Caelles et al., 2002). MAPKs contribute to AP-1 induction in response to a series of extracellular stimuli. Interestingly, AP-1 induction by distinct MAPKs is usually mediated by phosphorylation of a particular set of substrates and therefore involves distinct mechanisms (Karin, 1995). In recent years, compelling evidence has highlighted the relevance of MAPK pathways as targets of GC action. Indeed, GC-induced repression of AP-1 activation may be achieved by GR-mediated inhibition of the c-Jun N-terminal kinase (JNK) pathway. This GC action leads to the inhibition of phosphorylation, and concomitant activation, of JNK-targeted transcriptional activators, such as c-Jun, ATF-2 or Elk-1, which are involved in the induction of AP-1 activity by different mechanisms (Caelles et al., 1997). Other MAPK pathways, such as the extracellular signal-regulated protein kinase (ERK) and p38 MAPK cascades, are also targets for repression by GCs (Caelles et al., 2002). GCs may interfere with these signaling pathways through the expression of the dual-specificity MAPK phosphatase-1 (MKP-1). This mechanism has been shown to account for the inhibition of ERK (Kassel et al., 2001) and p38 MAPK pathways (Imasato et al., 2002; Lasa et al., 2002) in a number of cell types. Although MKP-1 induction may also be involved in GC-induced inhibition of the JNK pathway, alternative mechanisms should exist since GCs can inhibit JNK activation even in the absence of de novo gene expression (Caelles et al., 1997; Ventura et al., 1999).
Here we report that GR physically interacts with JNK through a hormone-regulated JNK docking site located in the ligand-binding domain (LBD) of GR. This GC action mediates the inhibition of JNK pathway activation and the induction of inactive JNK nuclear transfer. Remarkably, both hormone effects may separately contribute to AP-1 transrepression. GR–JNK interaction constitutes a novel mechanism by which GCs regulate cellular signaling and gene expression. Additionally, as this GC action targets the AP-1 complex, this mechanism may also conduct some of the pharmacological actions of GCs.
Results
GCs reduce the number of MKK7–JNK complexes
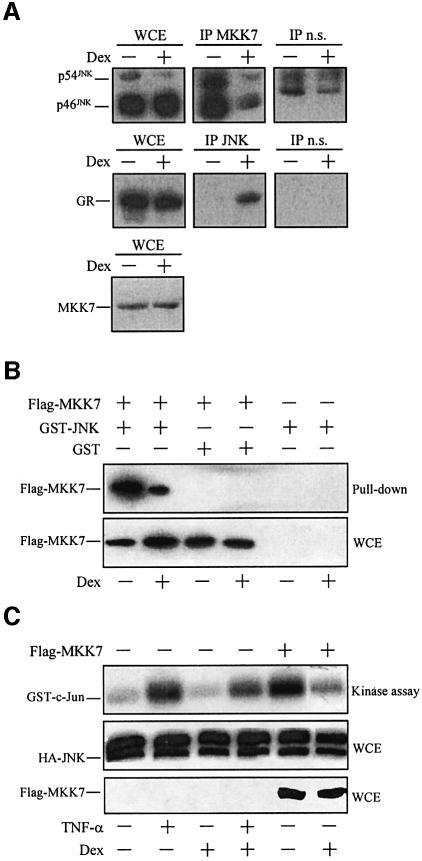
In HeLa cells, a short GC treatment inhibits the JNK pathway and c-Jun N-terminal phosphorylation in a GR-dependent manner (Caelles et al., 1997; González et al., 2000; Caelles et al., 2002). In parallel, this same GC treatment induces inactive JNK to accumulate in the nucleus (González et al., 2000). In the light of these observations, we proposed that this latter hormone action may be involved in the interference of GCs with activation of the JNK pathway and the AP-1 complex (Caelles et al., 2002). To study the molecular mechanism that underlies this interference further, we analyzed the effect of GC treatment on the integrity of mitogen-activated protein kinase kinase 7 (MKK7)–JNK complexes by co-immunoprecipitation assays of endogenous proteins performed in extracts from HeLa cells. In agreement with previous reports, we found that JNK was associated with the MKK7 immunocomplexes in unstimulated cells (Tournier et al., 1999). Remarkably, treatment with dexamethasone (Dex) clearly reduced the amount of JNK bound to MKK7 without altering the overall amount of JNK or MKK7 in the whole-cell extracts (Figure 1A, upper and lower panels, respectively). These results were corroborated further by GST pull-down assays performed in extracts from HeLa cells transiently co-transfected with expression vectors for Flag-MKK7 and/or GST–JNK or, as a negative control, GST (Figure 1B). Moreover, the decrease in the MKK7–JNK complexes induced by Dex correlated with its inhibitory action on the JNK activity triggered by tumor necrosis factor (TNF)-α stimulation or MKK7 overexpression (Figure 1C).
Fig. 1. GCs induce reduction of the MKK7–JNK complexes and promote GR–JNK association. (A) Extracts from serum-starved HeLa cells incubated with Dex (or vehicle) were immunoprecipitated with antibodies to MKK7 (IP MKK7), JNK (IP JNK) and, as a non-specific antibody, GST (IP n.s.), respectively. Immunoblots were performed to analyze the presence of JNK and GR in the immunocomplexes and the JNK, GR and MKK7 content in the whole-cell extracts (WCE), as indicated. (B) HeLa cells were transiently co-transfected with either pCM15-Flag-MKK7 or pCM15 along with pEBG-JNK or pEBG, serum starved and treated with Dex (or vehicle). GST or GST–JNK was precipitated from cell extracts by glutathione–Sepharose beads and the associated Flag-MKK7 was examined by immunoblotting using an anti-Flag antibody (upper panel). The lower panel shows the protein level of Flag-MKK7 in the cell extracts assessed by immunoblotting using an anti-Flag antibody. (C) HeLa cells were transiently co- transfected with pCEFL-KZ-HA-JNK together with pCM15-Flag-MKK7 or empty vector, serum starved and treated with Dex (or vehicle). When indicated, extracts were prepared after stimulation with TNF-α. The activity of HA-JNK was determined by immunocomplex assay (upper panel) and the amount of HA-JNK (middle panel) and Flag-MKK7 (lower panel) by immunoblotting using anti-HA and anti-Flag antibodies, respectively.
GR binds to JNK through a hormone-regulated JNK docking site
The results above, together with those previously reported for GC-induced nuclear transfer of inactive JNK (González et al., 2000), suggested that GR might physically interact with JNK in response to hormone. Additionally, this hypothesis was supported by previous data that showed that the kinetics of GC inhibitory action on the JNK pathway correlated with the nuclear entry of the GR (Caelles et al., 1997; Ventura et al., 1999). To pursue this idea further, we analyzed GR–JNK interaction in response to Dex treatment by co-immunoprecipitation assays of endogenous proteins using extracts from HeLa cells. In contrast to the GC action on the MKK7–JNK complexes, GR co-immunoprecipitated with JNK specifically in extracts from GC-treated cells (Figure 1A, middle panels). Unfortunately, in these same assays, we did not detect JNK in GR immunoprecipitates (not shown), which may be due to the interference of the GR antibody with the GR–JNK complex by either masking the interaction surface or promoting an inadequate conformation of GR. Hormone-induced GR–JNK interaction could also be evidenced by GST pull-down assays performed with extracts from cells overexpressing GST–JNK (see below).
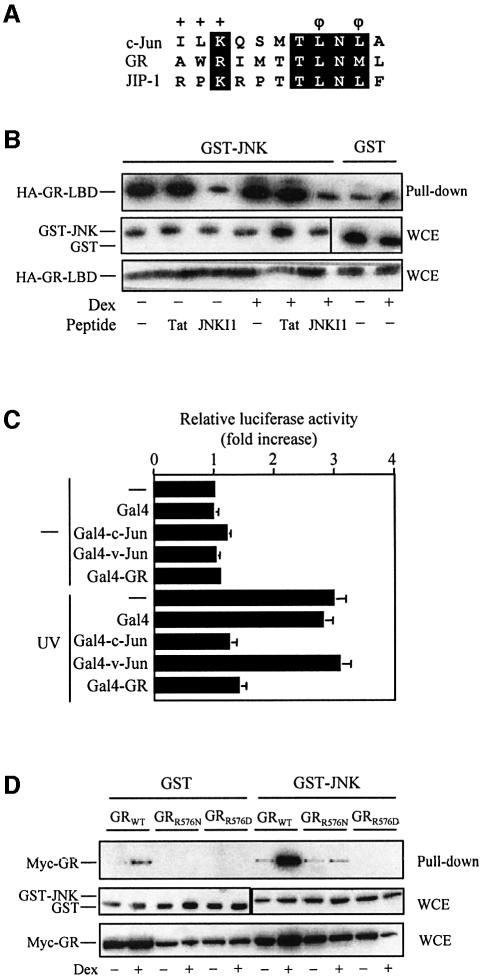
Most MAPK-interacting proteins contain discrete amino acid motifs that function as MAPK docking sites (Jacobs et al., 1999). Thus, we searched the primary sequence of GR for potential MAPK docking sites. We found that the GR LBD contains an amino acid sequence that significantly resembles a D-box, a MAPK docking site which is characterized by a basic and a hydrophobic–X–hydrophobic submotif separated by a short spacer and found in other JNK-interacting proteins, such as c-Jun and JIP-1 (Hibi et al., 1993; Dickens et al., 1997) (Figure 2A). Indeed, an 11 residue peptide corresponding to the JIP amino acid sequence shown in Figure 2A is sufficient for binding to and inhibiting JNK (Barr et al., 2002). Consistent with these observations, a truncated version of GR, GR-LBD, comprising 25 amino acids of the hinge region plus the whole LBD, effectively binds JNK when transiently expressed in vivo (Figure 2B). Moreover, this interaction is competed specifically in vivo by the JNKI1 peptide, a cell-permeable peptide which contains the JNK docking site of JIP –1157–176 covalently linked to the 10 amino acid human immunodeficiency virus (HIV)-Tat48–57 sequence, which acts as a carrier peptide (Bonny et al., 2001). Interestingly, although the GR-LBD still exhibits ligand modulation in relation to JNK interaction to some extent, it binds JNK even in the absence of hormone (Figure 2B). Although we do not have a conclusive explanation for the partial loss of hormone regulation of this truncated GR protein, co-immunoprecipitation assays comparing the amount of Hsp90 associated with the full-length GR versus the GR-LBD showed that the latter associates with Hsp90 very poorly (data not shown). This result suggests that conformational differences between these two GR versions might exist and/or could support the hypothesis that Hsp90 might be somehow involved in masking the JNK docking site.
Fig. 2. GR harbors a hormone-regulated docking site for JNK. (A) Alignment of amino acid sequences of rat GR (574–584) with the JNK docking site found in human c-Jun (33–43) and JIP-1 (157–167). The characteristic features of the D-box MAPK docking site which are a basic (+) and a hydrophobic (φ)–X–hydrophobic submotif separated by a short spacer are indicated. Identical or conserved residues among all sequences are boxed in black. (B) Cos-7 cells were transiently co-transfected with pCEFL-KZ-HA-GR-LBD along with pEBG-JNK or pEBG, serum starved and treated with Dex (or vehicle). Thereafter, vehicle, Tat or JNKI1 peptides (1 µM) were added as indicated and cells further incubated for 30 min. Cell extracts were prepared, glutathione–Sepharose precipitated and the presence of HA-GR-LBD associated with GST–JNK or GST was analyzed by immunobloting using an anti-HA antibody (upper panel). The protein level of GST–JNK or GST (middle panels) and HA-GR-LBD (lower panel) in cell extracts was analyzed by immunoblotting using anti-GST and anti-HA antibodies, respectively. (C) HeLa cells were transiently co-transfected with the –73col-luciferase reporter (3 µg) along with pSG424, pSG424-Gal4–c-Jun(1–116), pSG424-Gal4–v-Jun(1–89) or pSG424-Gal4–GR(540–738) (2 µg), as indicated, and pCH110 (0.5 µg). After serum starvation, cells were UV stimulated, as indicated, and luciferase and β-galactosidase activities were measured after 8 h. Relative luciferase activity corresponds to the β-galactosidase-normalized luciferase activity. The fold increase compared with the activity in non-irradiated cells transfected with the reporter construct alone, which was set to 1, is shown (mean ± SD of three independent experiments run in triplicate). (D) Cos-7 cells were transiently co-transfected with pEBG-JNK or pEBG along with pMTG-myc-GR, pMTG-myc-GRR576N or pMTG-myc-GRR576D, serum starved and treated with Dex (or vehicle) as indicated. Thereafter, GST pull-down assays were performed and the GST precipitates were analyzed by immunoblotting using an anti-myc antibody (upper panel). The protein levels in the whole-cell extracts of GST–JNK or GST (middle panels) and myc-GR (lower panel) were determined by immunoblotting using anti-GST or anti-myc antibodies, respectively.
Additionally, we tested the ability of a region of GR that encompasses this putative JNK docking site to repress JNK pathway-dependent transcription as evidence that this interaction takes place in living cells. As shown in Figure 2C, a Gal4–GR(540–738) fusion protein inhibits the JNK-mediated activation of an AP-1-dependent reporter as efficiently as Gal4–c-Jun(1–116), which contains the JNK docking site of c-Jun. In contrast, Gal4–v-Jun(1–89), which is defective in the JNK-docking site and, hence, in interaction with JNK, or the Gal4 DNA-binding domain alone have no effect. None of these constructs had any significant effect on the basal expression of the AP-1-dependent reporter in unstimulated cells.
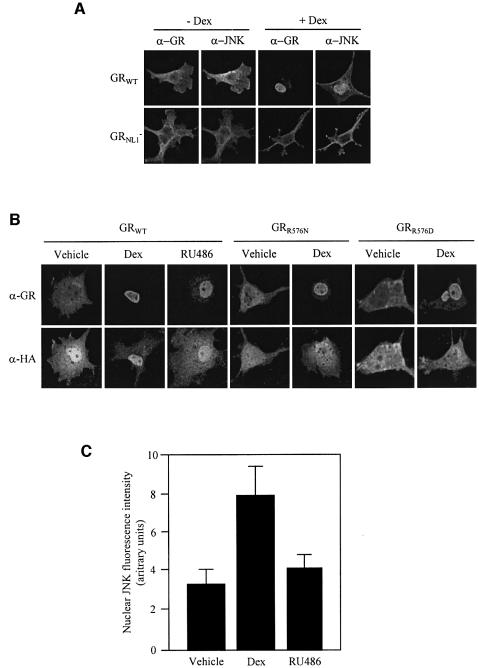
Notably, the amino acid sequence of this putative JNK docking site of GR is not fully conserved in any other member of the NR superfamily, not even in its closest relative, the mineralocorticoid receptor (MR) in which several non-conservative amino acid changes affect, respectively, the basic and the hydrophobic–X–hydrophobic submotifs. In particular, the basic motif of GR is not conserved in either human or murine MR due to the substitution of Arg576 by an asparagine or serine, respectively. Since it has been shown that the basic submotif is critical for JNK binding (Ho et al., 2003), to abrogate GR interaction with JNK we mutated Arg576 of GR to asparagine (GRR576N) or aspartate (GRR576D). At the Dex concentration tested (10–6 M), both GR mutants trigger transcription to the same level as the wild-type receptor, as shown by transient transfection assays using a 2xGRE-luciferase reporter (data not shown). In contrast, GST pull-down assays showed that both GR mutants are defective in hormone-induced binding to JNK (Figure 2D).
GR–JNK interaction correlates with inhibition of JNK activation
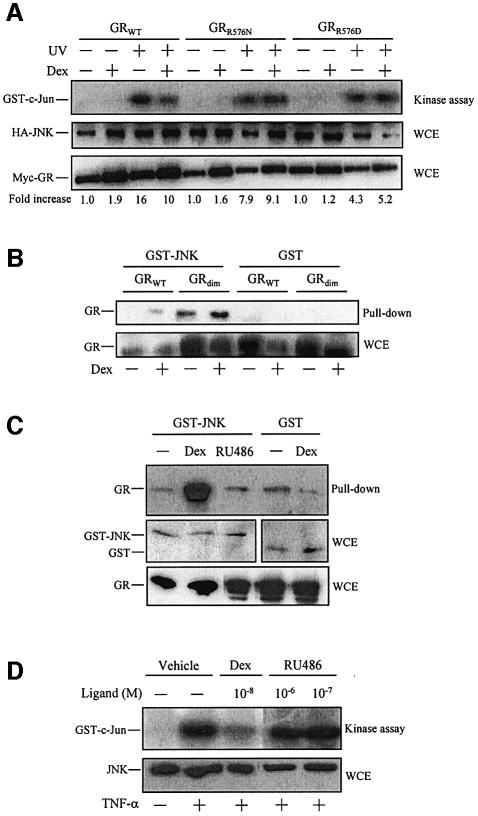
Next, we used several approaches to study whether GC-induced inhibition of JNK correlates with JNK binding to GR. First, we tested whether the JNK interaction-defective mutants of GR, GRR576N and GRR576D, failed to mediated GC-induced inhibition of JNK activation. As shown in Figure 3A, in contrast to the wild-type receptor, both JNK interaction-defective mutants of GR are also defective in JNK inhibition.
Fig. 3. GR binding to JNK mediates downregulation of JNK activation by GCs and does not require GR dimerization. (A) Cos-7 cells were transiently co-transfected with pCEFL-KZ-HA-JNK along with pMTG-myc-GR, pMTG-myc-GRR576N or pMTG-myc-GRR576D, serum starved, pre-treated with Dex (or vehicle) and stimulated with UV, as indicated. Thereafter, cells were harvested and the activity of HA-JNK determined (upper panel). The expression level of HA-JNK (middle panel) and myc-GR (lower panel) in each cell extract was analyzed by immunoblotting using anti-HA and anti-myc antibodies, respectively. The numbers below each lane indicate the fold increase of HA-JNK activity relative to the untreated/unstimulated condition. (B) Cos-7 cells were transiently co-transfected with pSB-GR or pSB-GR(A458T) along with pEBG-JNK or pEBG, serum starved and treated with Dex (or vehicle), as indicated. Thereafter, GST pull-down assays were performed and the GST precipitates (upper panel) and cell extracts (lower panel) were analyzed by immunoblotting using an antibody to GR. (C) HeLa cells were transiently transfected with pEBG-JNK or pEBG, serum starved and treated with Dex (10–8 M), RU486 (10–6 M) or vehicle, as indicated. Thereafter, GST pull-down assays were performed and the presence of GR in glutathione–Sepharose precipitates was analyzed by immunoblotting using an antibody to GR. The protein level in the cell extracts of GST–JNK or GST (middle panels) and GR (lower panel) was measured by immunoblotting, using antibodies to GST or GR, respectively (D) Serum-starved HeLa cells were pre-treated with Dex, RU486 or vehicle at the doses indicated and then stimulated with TNF-α. Cell extracts were tested for JNK activity by immunocomplex assay (upper panel) and JNK protein level by immunoblotting (lower panel) using an antibody to JNK.
Previously, we have shown that the dimerization-defective GR mutant GRdim is as efficient as the wild-type receptor in mediating downregulation of JNK activity by GCs (González et al., 2000; Caelles et al., 2002). Therefore, here we tested the GRdim mutant for hormone-induced binding to JNK. For this purpose, GST pull-down assays were performed in extracts from Cos-7 cells overexpressing GST–JNK or, as a negative control, GST together with wild-type GR (GRWT) or GRdim. As shown in Figure 3B, GRdim is fully competent at mediating GC-induced binding to JNK.
Additionally, we also compared the ability of the GR agonist Dex versus the antagonist RU486 to induce binding to and inhibition of JNK. Unlike Dex, RU486 failed to induce GR to either interact with or inhibit activation of JNK (Figure 3C and D, respectively). Altogether, these results strongly support the correlation between GR–JNK interaction and inhibition of JNK activation by GCs. Furthermore, the results obtained with GRdim indicate that GR dimerization is dispensable for GR binding to JNK.
GC-induced nuclear transfer of JNK is mediated by binding to GR but is dispensable for inhibition of JNK activation by GCs
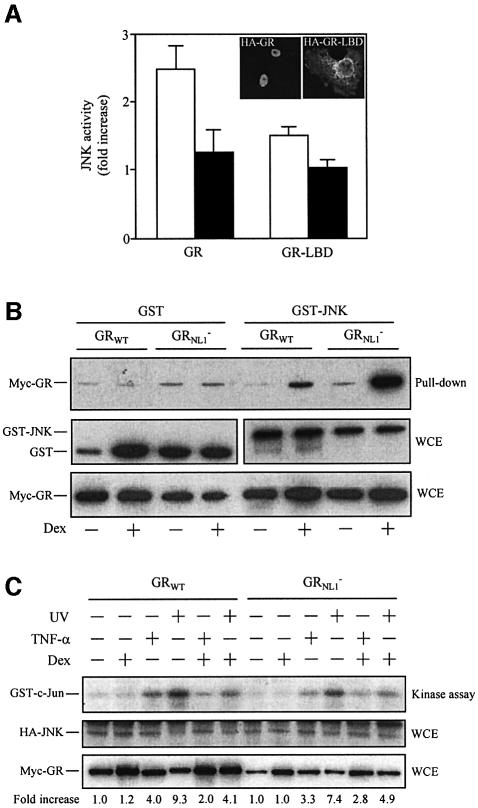
The GR-LBD truncated protein, which is fully competent in binding to JNK, lacks the nuclear localization signal 1 (NL1). Consistently, GR-LBD is not translocated efficiently into the nucleus upon ligand activation, as shown by immunocytochemistry analysis (inset in Figure 4A), even though expression of the GR-LBD efficiently inhibits the TNF-α-induced activity of JNK when overexpressed in Cos-7 cells (Figure 4A). In fact, similarly to the binding to JNK, the GR-LBD inhibits JNK activation even in the absence of hormone. Hormone addition only weakly, though significantly, improved JNK inhibition by this truncated form of GR. On the basis of this observation, we examined whether these two hormone actions, inhibition of JNK activation and induction of inactive JNK nuclear transfer, could be dissociated in the context of the full-length receptor. For this purpose, we took advantage of the GRNL1– mutant, which, because of three point mutations in the NL1, is highly defective in hormone-induced transfer into the nucleus (Savory et al., 1999). First, the GRNL1– mutant was tested for its ability to interact with JNK. Ligand-induced binding of GRNL1– to JNK was evidenced by GST pull-down assays performed with extracts from Cos-7 cells overexpressing either GRWT or GRNL1– along with GST–JNK or GST (Figure 4B). We next compared GC action on the UV- and TNF-α-induced activation of JNK mediated by either wild-type or NL1-defective GR. Hormone-activated GRNL1– was as efficient as the wild-type receptor in inhibiting the activation of JNK (Figure 4C). In a manner similar to GR-LBD, this mutated version of GR, engineered to remain in the cytoplasmic compartment upon hormone activation, can mediate inhibition of the JNK pathway.
Fig. 4. Hormone-induced nuclear transfer of GR is dispensable for binding to and downregulation of JNK. (A) Cos-7 cells were transiently co-transfected with pCEFL-KZ-HA-JNK together with pEBG-GR or pEBG-GR-LBD, serum starved and treated with Dex (or vehicle) for 45 min before TNF-α stimulation. Cell extracts were prepared 20 min after stimulation and the HA-JNK activity determined by immunocomplex assay. The graphic shows the increase of JNK activity in response to TNF-α stimulation in the absence (white bars) or presence (back bars) of Dex. The average results from three independent experiments are shown. The inset shows an anti-HA immunocytochemical analysis by confocal microscopy of Dex-treated Cos-7 cells transiently transfected with pCEFL-KZ-HA-GR or pCEFL-KZ-HA-GR-LBD, as indicated. (B) Cos-7 cells were transiently co-transfected with pMTG-myc-GR or pMTG-myc-GRNL1– along with pEBG or pEBG-JNK, serum starved and treated with Dex (or vehicle), as indicated. GST pull-down assays were performed and precipitates analyzed for the presence of myc-GR by immunoblotting with an anti-myc antibody (upper panel). The GST or GST–JNK and myc-GR protein level in each cell extract was analyzed by immunoblotting using specific anti-GST or anti-myc antibodies, respectively (middle and lower panel, respectively). (C) Cos-7 cells were transiently co-transfected with pCEFL-KZ-HA-JNK along with pMTG-myc-GR or pMTG- myc-GRNL1–, serum starved, pre-treated with Dex (or vehicle) and stimulated with UV or TNF-α, as indicated. Thereafter, cells were harvested and the activity of HA-JNK determined (upper panel). The expression level of HA-JNK (middle panel) and myc-GR (lower panel) in each cell extract was analyzed by immunoblotting using anti-HA and anti-myc antibodies, respectively. The numbers below each lane indicate the fold increase of HA-JNK activity relative to the untreated/unstimulated condition.
We also used this NL1-defective GR mutant to assess whether JNK binding to GR dictates JNK subcellular trafficking in response to GCs. Immunofluorescence analysis by confocal microscopy showed that while the GRWT translocates into the nucleus and induces nuclear accumulation of JNK in response to Dex, GRNL1– did not mediate either of these two hormone actions (Figure 5A). To support this argument further, we took advantage of the JNK interaction-defective mutants GRR576N and GRR576D, as well as the fact that RU486 is fully competent in inducing GR nuclear translocation (Htun et al., 1996) whereas it did not induce either binding to or inhibition of JNK. We performed immunocytochemical analysis by confocal microscopy of GR and JNK in Cos-7 cells overexpressing hemagglutinin (HA)-JNK along with GRWT, GRR576N or GRR576D, respectively, and treated with vehicle, Dex or, when relevant, RU486. GRWT as well as both GR mutants translocate into the nuclear compartment upon Dex addition. However, unlike GRWT, both JNK interaction-defective mutants failed to induce the accumulation of JNK inside the nucleus (Figure 5B). In relation to the GR antagonist RU486, it induced nuclear transfer of GR which was comparable with Dex, but failed to stimulate JNK nuclear accumulation (Figure 5B). Similar results were obtained by immunocytochemical analysis of the endogenous GR and JNK proteins in HeLa cells (Figure 5C).
Fig. 5. JNK binding to GR mediates GC-induced nuclear transfer of JNK. (A) Immunofluorescence analysis by confocal microscopy of JNK and GR in Cos-7 cells transiently transfected with pMTG-myc-GR or pMTG-myc-GRNL1–. After transfection, cells were serum starved and fixed after treatment with Dex (or vehicle). Cells were double immunostained by using primary antibodies to JNK and GR, as described in Materials and methods. (B) Cos-7 cells were transiently co-transfected with pMTG-myc-GR, pMTG-myc-GRR576N or pMTG-myc-GRR576D along with pCEFL- KZ-HA-JNK, serum starved and treated with Dex (10–8 M), RU486 (10–7 M) or vehicle, as indicated. Thereafter, immunocytochemical analysis was performed by double staining with anti-GR and anti-HA antibodies and confocal microscopy. (C) Serum-starved HeLa cells were treated with Dex (10–8 M), RU486 (10–7 M) or vehicle, as indicated, and analyzed by immunocytochemistry using antibodies to GR and JNK. Immunofluorescence was analyzed by confocal microscopy. For each condition, at least 300 nuclei were analyzed and quantified as described in Materials and methods.
These results support our initial hypothesis that inhibition of the JNK pathway by GCs is likely to occur at the cytoplasmic level and strongly indicate that GC-induced dissociation of JNK from the MAPK module, by binding to the GR, suffices to inhibit the activation of JNK. Conversely, JNK nuclear translocation, while also being mediated by binding to GR, may be dispensable for this particular inhibitory action.
GC-induced nuclear transfer of JNK increases loading of JNK on the AP-1-bound response elements of the c-jun gene
Although JNK nuclear transfer does not appear to be essential for GC-induced downregulation of JNK activation, we reasoned that it could still be relevant for AP-1 transrepression. Indeed, in a previous study, we proposed that inactive JNK accumulated in the nucleus in response to GCs may act as an inhibitor of AP-1 activity by binding to the c-Jun transactivation domain and, consequently, blocking further interaction with activated JNK (González et al., 2000; Caelles et al., 2002). To gain evidence to support this idea, we performed ChIP assays to monitor the JNK associated in vivo, and in response to GCs, with the AP-1 response elements of the c-jun gene. In these assays, we amplify a DNA fragment (5′-c-jun) containing both the proximal and distal AP-1 response elements of the c-jun gene (Angel et al., 1988). Importantly, both AP-1 sites mediate transrepression of the c-jun gene by GCs (Wei et al., 1998). ChIP assays using antibodies to c-Jun showed that AP-1 complexes containing c-Jun are bound in vivo to the AP-1 sites of the c-jun gene in both non- and GC-treated HeLa cells (not shown). In agreement with previous reports showing that c-Jun and JNK co-immunoprecipitate in resting cells (Dai et al., 1995), we also found JNK specifically associated with this region in unstimulated cells. Remarkably, Dex treatment produces a modest but reproducible enrichment of JNK associated with the AP-1 response elements of the c-jun gene (Figure 6). ChIP assays also showed that GR is tethered to the AP-1 sites of the c-jun gene in response to Dex or, to a lesser extent, RU486, a result which is consistent with a previous study on the AP-1 response element of the collagenase 3 gene (Rogatsky et al., 2001). However, the most striking difference between the GR agonist (Dex) and antagonist (RU486) is the efficiency of the former in tethering JNK to the AP-1 sites (Figure 6). Significantly, this divergence draws a parallel with the differences observed between these GC analogs in inducing the binding of GR to JNK.

Fig. 6. GC-induced nuclear transfer of JNK increases JNK associated with the AP-1 response elements of the c-jun gene. Serum-starved HeLa cells were treated with Dex (D), RU486 (R) or vehicle (–), as indicated. Thereafter, cells were processed for ChIP assays using antibodies to JNK (IP JNK), GR (IP GR) or, as a non-specific antibody (IP n.s.), GST, and primers pairs that amplify DNA fragments containing either the distal and proximal AP-1 sites of the c-jun promoter region (5′-c-jun) or, as a measure of PCR amplification due to non- specific immunoprecipitation, a region corresponding to the 3′ end of the c-jun gene (3′-c-jun). Equal amounts of total genomic DNA (INPUT) were used for immunoprecipitation in each condition. For each sample, the radioactive labeling of the 5′-jun and 3′-c-jun PCR products was quantified and expressed as a 5′-c-jun:3′c-jun ratio. The ratio obtained with GST antibody in untreated cells was arbitrarily set as 1 (values shown below the gels).
Discussion
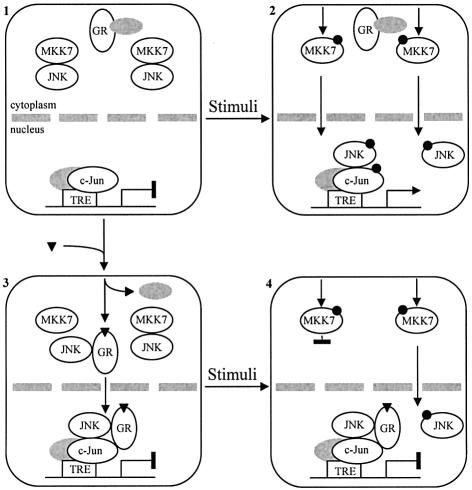
In recent years, several studies have described the negative regulation of MAPK pathways by GCs and have proposed its involvement in carrying out some of the physiological and pharmacological actions of these hormones. Here we show that GCs reduce the amount of JNK associated with the MAPK module by promoting its binding to the GR. Consistently, we have identified a hormone-regulated JNK docking site located within the GR LBD. We also show that GR binding to JNK mediates the inhibition of the JNK pathway and the induction of JNK nuclear transfer in response to GCs. Although JNK binding to GR is sufficient for the former GC action, we provide evidence that the latter may synergize in AP-1 transrepression by inducing the loading of inactive JNK onto the AP-1-bound response elements (see model in Figure 7).
Fig. 7. GC action on the JNK pathway. (1) In the absence of GCs, GR is located in the cytoplasmic compartment and associated with multiprotein complexes. Likewise, in non-stimulated cells, JNK associated with MKK7 is tied by scaffold proteins into specific signaling modules. In the nuclear compartment, AP-1 complexes containing c-Jun are bound to the TPA response elements (TREs) found in the regulatory sequences of the AP-1 target genes, although the AP-1-dependent transcription is turned off. (2) Upon stimulation, the signal is transduced along the JNK pathway signaling modules by a cascade of phosphorylation events (filled circles) that ends in the activation of JNK. Active JNK dissociates from the signaling module and translocates into the nuclear compartment where it associates with and phosphorylates the c-Jun N-terminal domain. Thereafter, c-Jun phosphorylation induces the dissociation of a repressor complex resulting in AP-1 activation and, in consequence, triggers transcription of AP-1-responsive genes. (3) In the presence of GCs (inverted triangles), hormone-bound GR dissociates from the multiprotein complexes and exposes a JNK docking site. Some JNK molecules dissociate from the signaling modules, bind to GR and travel together with GR into the nuclear compartment where they associate with c-Jun. (4) In these conditions, upon stimulation, JNK-deficient signaling modules fail to transduce the signal, resulting in the GC-induced inhibition of JNK pathway activation. In consequence, fewer molecules of active JNK are produced and, hence, enter into the nucleus. In addition, these active JNK molecules have to compete for the c-Jun docking sites with the already c-Jun-bound inactive JNK molecules.
Induction of MKP-1 expression contributes to the downregulation of the ERK and p38 MAPK pathways by GCs (Kassel et al., 2001; Imasato et al., 2002; Lasa et al., 2002). While this mechanism may also be suitable to mediate GC-induced inhibition of the JNK pathway, alternative modes of action should exist since the downregulation of this pathway by GCs proceeds even in the presence of actinomycin D and can be mediated by transactivation-defective mutants of GR such as GRdim or GRLS7 (Caelles et al., 1997; Ventura et al., 1999; González et al., 2000). In this regard, our results support the hypothesis of an alternative mechanism based on protein–protein interactions.
We had reported previously that the step along the JNK pathway targeted by GCs is situated downstream from the MAP3K level (Caelles et al., 1997). Our present data are consistent with this finding since GCs reduce the overall amount of MKK7–JNK complexes, and hence inhibit MKK7-induced activation of JNK. Moreover, we show that GC interference with the MKK7–JNK complexes correlates with the induction of GR to bind to JNK by these hormones. In summary, our results support a novel mechanism responsible for the GC inhibitory action on the JNK cascade: the disruption of the pathway by the GR-mediated sequestration of JNK. This mechanism may affect all JNK signaling pathways since this MAPK would be unavailable not only to MKK7 but also to its other MAP2K, SEK1. In this regard, we already showed that GCs inhibit SEK1-dependent activation of JNK (Caelles et al., 1997).
Here we demonstrate that GR binding to JNK is mediated by docking interactions analogous to those described to underlie other relevant traits of MAPK signaling pathways, such as sequential and specific activation and inactivation, substrate recognition and subcellular location of MAPKs (Tanoue and Nishida, 2002). In the LBD of GR, we have identified an amino acid motif that greatly resembles a D-box (Jacobs et al., 1999), the type of MAPK docking site found in JNK-interacting proteins such as c-Jun or JIP-1. Within this amino acid sequence, GR, c-Jun and JIP-1 display identical or highly conserved residues in positions that are critical for binding to JNK (Kallunki et al., 1996; Bonny et al., 2001; Barr et al., 2002; Sprowles and Wisdom, 2003). Consistently, the GR-LBD is sufficient to mediate binding to JNK and, most significantly, this binding is competed specifically in vivo by a short peptide already validated as a JNK docking site, JNKI1 (Bonny et al., 2001). Functionally, we show that this region of GR inhibits the JNK-induced transcription of an AP-1-dependent reporter in a similar way to the c-Jun N-terminal domain, thus further supporting its interaction with JNK in vivo. Remarkably, the amino acid sequence corresponding to this JNK docking site is highly conserved along GRs from a number of species, suggesting that the ability of this receptor to bind to JNK has been conserved during evolution. On the contrary, complete conservation of this docking site is not found in any other member of the NR superfamily, not even in its closest relative MR, suggesting that this may be a particular attribute of GR. In this regard, the inhibitory action on the JNK pathway of other NRs, such as the retinoic acid receptor, may be restricted to alternative mechanisms such as the induction of MKP-1 expression (Lee et al., 1999; Xu et al., 2002). It remains an open question as to whether the specific ability of GR to bind to JNK is related to its higher capacity, compared with other NRs, to inhibit the JNK pathway and transrepress AP-1. Taking advantage of the non-conservative amino acid exchanges, with respect to GR, in this region of the MR, we have disrupted the basic submotif of the JNK docking site of GR without significantly affecting hormone binding and shown that the single substitution of Arg576 by asparagine, the amino acid found in the same position in human MR, or by an acidic residue such as aspartate abolishes hormone-induced binding to and inhibition of JNK. Experiments are in progress to test if the poor AP-1 transrepression activity of MR (Pearce and Yamamoto, 1993; Heck et al., 1994) might be improved by exchanging the amino acids of MR which are different from GR within this region, with the aim of providing MR with a JNK docking site and, eventually, with the ability to bind to and inhibit JNK.
Using several mutants of GR, such as GR-LBD, GRdim, GRNL1–, GRR576N and GRR576D, as well as GC analogs such as RU486, we show that GR binding to JNK mediates two actions of GCs: inhibition of JNK pathway activation and induction of inactive JNK nuclear transfer. Dissociation of these two hormone actions demonstrated that nuclear accumulation of JNK is not required for downregulation of the JNK pathway. Therefore, we conclude that JNK activation is inhibited simply by its binding to the docking site offered by hormone-bound GR, in a similar manner to the JIP-1-based JNK inhibitor peptides (Bonny et al., 2001; Barr et al., 2002). Moreover, the results obtained with GR mutants with a constitutive cytoplasmic location indicate that binding, and hence inhibition, occurs in the cytoplasm. Additionally, by binding to GR, JNK may be provided with a nuclear localization signal and hence would be transferred to the nuclear compartment together with GR. Furthermore, we show that receptor dimerization is not required for binding to JNK, further confirming the association of JNK pathway inhibition with the transrepressive and DNA binding-independent function of GR.
We propose that once inside the nucleus via the action of GCs, inactive JNK is recruited to AP-1-bound response elements, such as those in the c-jun gene. Moreover, we show that the GR agonist Dex increases loading of both GR and JNK onto these AP-1 response elements, while the GR antagonist RU486 is less effective in inducing GR and completely fails to induce JNK to associate with these AP-1 sites. Significantly, the failure of RU486 in binding to, inhibiting and inducing nuclear transfer of JNK is consistent with its weak AP-1 transrepressive activity (Vayssière et al., 1997; Rogatsky et al., 2001). Inactive JNK bound to the c-Jun N-terminal domain may block further interaction with and phosphorylation by activated JNK and thereby keep AP-1-dependent transcription repressed (Weiss et al., 2003).
The negative regulation of MAPK pathways by GCs is achieved by alternative non-exclusive mechanisms and, most importantly, appears to mediate relevant physiological and pharmacological actions of GCs. Our data reveal a novel mechanism by which GCs target the JNK cascade, a major MAPK signal transduction pathway involved in AP-1 activation. Therefore, our results give further support to and rationale for pharmacological intervention in MAPK pathways.
Materials and methods
Plasmid constructs
To construct pSG424-Gal4–GR(540–738), the PstI–HindIII DNA fragment from rat GR encoding amino acids 540–738 was cloned into pBS, excised with BamHI–KpnI and inserted into pSG424. To construct the plasmids pcDNA3-GR, pCEFL-KZ-HA-GR, pEBG-GR, pCEFL-KZ-HA-GR-LBD and pEBG-GR-LBD, the nucleotide sequence of rat GR encoding amino acids 1–795 or 525–795 was PCR-amplified using the following primer pairs: 5′-AGCTGGATCCACCATGGACTCCAAAG AATCCTTA-3′/5′-AGCTACGCGGCCGTCCATTTTTGATGAAACA GAAGCTT-3′ and 5′-AGCTGGATCCACCATGGGAGTCTCACAAG ACACT-3′/5′-AGCTACGCGGCCGCTCATTTTTGATGAAACAGA AGCTT-3′, respectively. Restricted PCR fragments were cloned into pCEFL-KZ-HA and pEBG between the BamHI and NotI restriction sites. pMTG-myc-GRR576N and pMTG-myc-GRR576D were obtained with the QuikChange Site-Directed Mutagenesis kit (Stratagene) using the following primer pairs: 5′-GTTCCAGATTACGCATGGAATATTATG ACCACACTC-3′/5′-GAGTGTGGTCATAATATTCCATGCTGAATC TGGAAC-3′ and 5′-CAGCATGGGACATTATGACCACACTCAAC-3′/5′-GTCATAATGTCCCATGCTGAATCTGGAAC-3′, respectively. All constructs were analyzed by DNA sequencing. Other plasmids have been described elsewhere (Heck et al., 1994; Caelles et al., 1997; Savory et al., 1999; Chadee et al., 2002).
Cell culture and transfection
Cos-7 and HeLa cells were grown in Dulbeco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). Cells were serum starved in DMEM plus 0.5% FCS for 16 h. Unless indicated, Dex (10–6 M) or vehicle (ethanol) was added for 45 min before the cells were harvested, or stimulated with TNF-α (10 ng/ml) or UV (30 J/m2) and harvested 20 min later. Cells seeded in 100 mm tissue culture plates were transiently transfected by lipofection following the manufacturer’s recommendations (Invitrogen) and using the following amounts of expression vectors: 0.5 µg of pEBG, pEBG-JNK, pCEFL-KZ-HA-JNK, pEBG-GR or pEBG-GR-LBD and 1 µg of pcDNA3-GR, pSB-GR, pSB-GR(A458T), pMTG-myc-GR, pMTG-myc-GRNL1–, pMTG-myc-GRR576N, pMTG-myc-GRR576D, pCEFL-KZ-HA-GR pCEFL-KZ-HA-GR-LBD or pCM15-Flag-MKK7, as indicated. Cells were harvested 24 h after transfection. Gene reporter assays were performed as described (Caelles et al., 1997).
GST pull-down and co-immunoprecipitation assays
Cells were suspended in low-salt buffer [20 mM HEPES pH 7.4, 2 mM EGTA and 2 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 20 µg/ml aprotinin] and lysed mechanically. Immunoprecipitation of HA-tagged proteins and endogenous JNK and MKK7 was performed using the antibodies 12CA5 (BabCO), sc-474 and sc-7104 (Santa Cruz Biotechnology Inc.), respectively. For the GST pull-down assays, cell lysates were incubated with 20 µl of glutathione–Sepharose beads. After three washes with low-salt buffer, immunocomplexes or glutathione–Sepharose precipitates were subjected to SDS–PAGE and analyzed by immunoblotting.
Immunoblotting
JNK, GR, MKK7, GST-, Flag-, HA- and myc-tagged proteins were detected by immunoblotting using the antibodies sc-474, sc-8992, sc-7104 and sc-138 from Santa Cruz Biotechnology, Inc., M2 antibody from Kodak, 12CA5 from BabCO, and 9E10 (a kind gift from Dr J.Ayté), respectively. Immunoblots were performed according to the enhanced chemiluminescence (ECL) detection system from Amersham Pharmacia.
Immunofluorescence analysis
Serum-starved HeLa or transiently transfected Cos-7 cells were treated with Dex, RU486 or vehicle. Thereafter, cells were processed as described (González et al., 2000). Primary antibodies against GR (sc-8992, Santa Cruz Biotechnology, Inc.), JNK (15701A, Pharmingen) and HA tag (BabCO, 12CA5) were used. Immunofluorescence was analyzed by laser scanning confocal microscopy, and nuclear fluorescence quantified by the Metamorph Imaging System (Universal Imaging Corporation).
ChIP assay
ChIP assays were performed in serum-starved HeLa cells (2.5 × 107 cells per condition) treated with vehicle or hormone (Dex or RU486, 10–7 M) and following the method described by Rogatsky et al. (2001). Immunoprecipitation was performed using 2.5 µg of anti-JNK, anti-GR or anti-GST antibody (sc-474, sc-8992 or sc-138, respectively, from Santa Cruz Biotechnology, Inc.) Immunoprecipitated DNA was suspended in 50 µl of 0.1× TE. Aliquots of 5 µl of each sample were PCR-amplified in the presence of 2.5 µCi of [α-32P]dCTP with either the primer pair 5′-CAAGGACGTCAGCCCACAATG-3′/5′-ACACTCAGTGCAACTCT GAG-3′, which flanks the two AP-1 response elements found in the 5′-regulatory region of the c-jun gene and gives rise to a 347 bp fragment, or the primer pair 5′-CCAGCGTATCTATATGGAATTG-3′/5′-AAAGA TGGCCTTTGTCTTA-3′, which amplifies a 287 bp fragment corresponding to the 3′ end of c-jun. One-fifth of each PCR was electrophoresed onto a 6% polyacrylamide gel in 0.5× TBE and radioactive labeling was quantified using a PhosphorImager.
JNK inmunocomplex assay
JNK and HA-JNK were immunoprecipitated from cell extracts using anti-JNK (sc-474 from Santa Cruz Biotechnology) and anti-HA antibodies (12CA5 from BabCO), respectively, and the JNK activity associated with immunoprecipitates was determined and quantified as described (Caelles et al., 1997).
Acknowledgments
Acknowledgements
We thank Drs A.C.B.Cato, R.J.G.Haché, J.Ayté and G.Gil for providing the plasmids and reagents used in this study, C.Vila for her excellent technical assistance, the Confocal microscopy service of the Scientific and Technical Services of the University of Barcelona for their help with the confocal analysis, and Tanya Yates for style correction. This work was supported by grants from the Plan Nacional de I+D+I (SAF2001-3347) from the Ministerio de Ciencia y Tecnología and the Fundació ‘La Caixa’ (99/032-00). A.B. and M.N. were supported by a fellowship from Ministerio de Educación y Cultura and a grant from the Generalitat de Catalunya, Spain, respectively.
References
- Angel P., Hattori,K., Smeal,T. and Karin,M. (1988) The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell, 55, 875–885. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. (1998) Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci., 94, 557–572. [DOI] [PubMed] [Google Scholar]
- Barr R.K., Kendrick,T.S. and Bogoyevitch,M.A. (2002) Identification of the critical features of a small peptide inhibitor of JNK activity. J. Biol. Chem., 277, 10987–10997. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich,P. and Schutz,G. (1995) Steroid hormone receptors: many actors in search of a plot. Cell, 83, 851–857. [DOI] [PubMed] [Google Scholar]
- Bonny C., Oberson,A., Negri,S., Sauser,C. and Schorderet,F. (2001) Cell-permeable peptide inhibitors of JNK. Diabetes, 50, 77–82. [DOI] [PubMed] [Google Scholar]
- Caelles C., González-Sancho,J.M. and Muñoz,A. (1997) Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev., 11, 3351–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caelles C., Bruna,A., Morales,M., González-Sancho,J.M., González,M.V., Jiménez,B. and Muñoz,A. (2002) Glucocorticoid receptor antagonism of AP-1 activity by inhibition of MAPK family. In Cato,A.C.B., Schäcke,H. and Asadullah,K. (eds), Recent Advances in Glucocorticoid Receptor Action. Ernst Schering Research Foundation Workshop. Springer-Verlag, Berlin, Vol. 40, pp. 131–152. [DOI] [PubMed] [Google Scholar]
- Cole T.J. et al. (1995) Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maduration. Genes Dev., 9, 1608–1621. [DOI] [PubMed] [Google Scholar]
- Chadee D.N., Yuasa,T. and Kyriakis,J.M. (2002) Direct activation of mitogen-activated protein kinase kinase MEKK1 by the Ste20p homologue GCK and the adapter protein TRAF2. Mol. Cell. Biol., 22, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T., Rubie,E., Franklin,C.C., Kraft,A., Gillespie,D.A.F., Avruch,J., Kyriakis,J.M. and Woodgett,J.R. (1995) Stress-activated protein kinases bind directly to the δ domain of c-Jun in resting cells: implications for repression of c-Jun function. Oncogene, 10, 849–855. [PubMed] [Google Scholar]
- Dickens M., Rogers,J.S., Cavanagh,J., Raitano,A., Xia,Z., Halpern,J.R., Greenberg,M.E., Sawyers,C.L. and Davis,R.J. (1997) A cytoplasmic inhibitor of the JNK signal transduction pathway. Science, 277, 693–696. [DOI] [PubMed] [Google Scholar]
- González M.V., Jiménez,B., Berciano,M.T., González-Sancho,J.M., Caelles,C., Lafarga,M. and Muñoz,A. (2000) Glucocorticoids antagonize AP-1 by inhibiting the activation/phosphorylation of JNK without affecting its subcellular distribution. J. Cell Biol., 150, 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlicher M., Heck,S. and Herrlich,P. (1998) Transcriptional cross-talk, the second mode of steroid hormone receptor action. J. Mol. Med., 76, 480–489. [DOI] [PubMed] [Google Scholar]
- Heck S., Kullmann,M., Gast,A., Ponta,H., Rahmsdorf,H.J., Herrlich,P. and Cato,A.C.B. (1994) A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J., 13, 4087–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmberg A., Auphan,N., Caelles,C. and Karin,M. (1995) Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J., 14, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P. (2001) Cross-talk between glucocorticoid receptor and AP-1. Oncogene, 20, 2465–2475. [DOI] [PubMed] [Google Scholar]
- Hibi M., Lin,A., Smeal,T., Minden,A. and Karin,M. (1993) Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev., 7, 2135–2148. [DOI] [PubMed] [Google Scholar]
- Ho D.T., Bradwell,A.J., Abdollahi,M. and Bardwell,L. (2003) A docking site in MKK4 mediates high affinity binding to JNK MAPKs and competes with similar docking sites in JNK substrates. J. Biol. Chem., 278, 32662–32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun H., Barsony,J., Renyi,I., Gould,D.L. and Hager,G.L. (1996) Visualization of glucocorticoid receptor translocation and intracellular organization in living cells with a green fluorescent protein chimera. Proc. Natl Acad. Sci. USA, 93, 4845–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imasato A., Desbois-Mouthon,C., Han,J., Kai,H., Cato,A.C.B., Akira,S. and Li,J.D. (2002) Inhibition of p38 MAPK by glucocorticoids via induction of MAPK phosphatase-1 enhances nontypeable Haemophilus influenzae-induced expression of Toll-like receptor 2. J. Biol. Chem., 277, 47444–47450. [DOI] [PubMed] [Google Scholar]
- Jacobs D., Glossip,D., Xing,H., Muslin,A.J. and Kornfeld,K. (1999) Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev., 13, 163–175. [PMC free article] [PubMed] [Google Scholar]
- Kallunki T., Deng,T., Hibi,M. and Karin,M. (1996) c-Jun recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell, 87, 929–939. [DOI] [PubMed] [Google Scholar]
- Karin M. (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem., 270, 16483–16486. [DOI] [PubMed] [Google Scholar]
- Kassel O., Sancono,A., Krätzschmar,J., Kreft,B., Stassen,M. and Cato,A.C.B. (2001) Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J., 20, 7108–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig H., Ponta,H., Rahmsdorf,H.J. and Herrlich,P. (1992) Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J., 11, 2241–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa M., Abraham,S.M., Boucheron,C., Saklatvala,J. and Clark,A.R. (2002) Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol., 22, 7802–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.Y., Sueoka,N., Hong,W.K., Mangelsdorf,D.J., Claret,F.X. and Kurie,J.M. (1999) All-trans-retinoic acid inhibits Jun N-terminal kinase by increasing dual-specificity phosphatase activity. Mol. Cell. Biol., 19, 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce D. and Yamamoto,K.R. (1993) Mineralocorticoid and glucocorticoid receptor activities distinguished by nonreceptor factors at a composite response element. Science, 259, 1161–1165. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M. et al. (1998) DNA binding of the glucocorticoid receptor is not essential for survival. Cell, 93, 531–541. [DOI] [PubMed] [Google Scholar]
- Reichardt H.M., Tuckermann,J.P., Göttlicher,M., Vujic,M., Weih,F., Angel,P., Herrlich,P. and Schütz,G. (2001) Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J., 20, 7168–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I., Zarember,K.A. and Yamamoto,K.R. (2001) Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J., 20, 6071–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatcioglu F., Claret,F.X. and Karin,M. (1994) Negative transcriptional regulation by nuclear receptors. Semin. Cancer Biol., 5, 347–359. [PubMed] [Google Scholar]
- Savory J.G.A., Hsu,B., Laquian,I.R., Giffin,W., Reich,T., Haché,R.J.G. and Lefebvre,Y.A. (1999) Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol. Cell. Biol., 19, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowles A. and Wisdom,R. (2003) Oncogenic effect of delta deletion in v-Jun does not result from uncoupling Jun from JNK signaling. Oncogene, 22, 498–506. [DOI] [PubMed] [Google Scholar]
- Tanoue T. and Nishida,E. (2002) Docking interactions in the mitogen-activated protein kinase cascades. Pharmacol. Ther., 93, 193–202. [DOI] [PubMed] [Google Scholar]
- Tournier C., Whitmarsh,A.J., Cavanagh,J., Barret,T. and Davis,R.J. (1999) The MKK7 gene encodes a group of c-Jun NH2-terminal kinase kinases. Mol. Cell. Biol. 19, 1569–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssière B.M., Dupont,S., Choquart,A., Petit,F., Garcia,T., Marchandeau,C., Gronemeyer,H. and Resche-Rigon,M. (1997) Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo. Mol. Endocrinol., 11, 1245–1255. [DOI] [PubMed] [Google Scholar]
- Ventura J.J., Roncero,C., Fabregat,I. and Benito,M. (1999) Glucocorticoid receptor down-regulates c-Jun amino terminal kinases induced by tumor necrosis factor α in fetal rat hepatocyte primary cultures. Hepatology, 29, 849–857. [DOI] [PubMed] [Google Scholar]
- Wei P., Inamdar,N. and Vedeckis,W.V. (1998) Transrepression of c-jun gene expression by glucocorticoid receptor requires both AP-1 sites in the c-jun promoter. Mol. Endocrinol., 12, 1322–1333. [DOI] [PubMed] [Google Scholar]
- Weiss C., Schneider,S., Wagner,E.F., Zang,X., Seto,E. and Bohmann,D. (2003) JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. EMBO J., 22, 3686–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Konta,T., Furusu,A., Nakayama,K., Lucio-Cazana,J., Fine,L.G. and Kitamura,M. (2002) Transcriptional induction of mitogen-activated protein kinase phosphatase 1 by retinoids. J. Biol. Chem., 277, 41693–41700. [DOI] [PubMed] [Google Scholar]