Abstract
Fgf signaling, mediated in part by the transcription factor Brachyury/Xbra/Ntl, plays important roles in mesoderm formation during the early development of vertebrate embryos. We have identified a zebrafish gene, spr2, which encodes a member of the Sp1-like transcription factor family. spr2 is expressed in both hypoblast and epiblast cells during late blastulation/early gastrulation, and in some mesodermal and neural tissues at later stages. Injection with spr2 mRNA enhances ntl expression and alleviates the inhibitory effect on ntl of XFD, a Xenopus dominant-negative FGF receptor. In contrast, morpholino- mediated knockdown of Spr2 activity inhibits ntl expression and reduces the inductive effect of Fgfs on ntl. We also demonstrate that Fgf signaling relays mesoderm induction activity of Nodal signaling and Spr2 is involved in this signal relay process. Furthermore, the correct spatial expression of spr2 requires Nodal, Fgf and Wnt signals. We suggest that expression of spr2 is an immediate-early response to mesoderm induction by Fgfs, which in turn regulates the expression of effector genes involved in the development of mesodermal tissues.
Keywords: fibroblast growth factor/mesoderm/SP1-like transcription factor/zebrafish
Introduction
The formation and patterning of the three germ layers of vertebrate embryos are highly complex processes that are regulated by extensive interactions between several inductive signals. The mesoderm is induced in amphibians by signals derived from the endoderm (Nieuwkoop, 1969), and in zebrafish by signals produced both by the yolk syncytial layer and the yolk cell itself (Mizuno et al., 1996; Chen and Kimelman, 2000). Molecular and genetic studies have identified VegT, a T-box transcription factor (Zhang et al., 1998; Kofron et al., 1999), as an early endogenous mesoderm inducer in Xenopus, which is maternally produced and vegetally localized. Previous studies in Xenopus suggest that VegT activates the expression of nodal-related genes in the endoderm, which in turn induce the formation of mesoderm (Clements et al., 1999; Kofron et al., 1999; Agius et al., 2000). In addition, mice deficient in Nodal fail to form the primitive streak and lack most mesodermal cells (Zhou et al., 1993; Conlon et al., 1994). Furthermore, in zebrafish, the simultaneous loss-of-function of two nodal genes, squint (sqt) and cyclops (cyc), result in embryos that are missing most of the mesodermal tissues (Feldman et al., 1998). Thus, Nodal signal is believed to be a universal, well conserved, mesoderm inducer during vertebrate embryogenesis.
Fgf signals are also known to play a key role in the induction of the mesoderm and, like Nodal, its role in mesoderm induction appears to be conserved in vertebrates. Mutant mice lacking Fgf8 are unable to undergo normal gastrulation, leading to loss of mesoderm- and endoderm-derived tissues (Sun et al., 1999). In Xenopus, the addition of Fgf causes animal cap explants to form mesoderm tissues (Kimelman and Kirschner, 1987; Slack et al., 1987), while a dominant-negative Fgf receptor (dnFGFR) can inhibit the formation of the posterior and lateral mesoderm (Amaya et al., 1991, 1993). In zebrafish acerebellar mutants, which carry a mutation in the fgf8 locus, a slight reduction of somatic mesoderm is observed (Reifers et al., 1998). In addition, an inhibition of Fgf receptor signaling in the zebrafish embryos leads to complete loss of both the trunk and tail (Griffin et al., 1995), which also supports a role for Fgf signaling in mesoderm induction.
It has been suggested from work in Xenopus that Fgf signals act in a signal relay mechanism to control mesoderm induction, such that the signals enable cells in the marginal zone to be competent for mesoderm induction by TGFβ signals (Cornell and Kimelman, 1994; LaBonne and Whitman, 1994). A well-studied transcription factor that is downstream of Fgf signals is Brachyury, which was first identified in mouse (Herrmann et al., 1990; Wilkinson et al., 1990). Mutant mice homologous for the Brachyury/T locus have insufficient mesoderm and lack a notochord (Herrmann et al., 1990; Wilkinson et al., 1990). The zebrafish homolog of mouse Brachyury is the loss-of-function mutation no tail (ntl) which, like the mouse, lacks a notochord and is also missing a tail (Halpern et al., 1993; Schulte-Merker et al., 1994). The expression of ntl is expanded by the overexpression of eFGF and inhibited by the overexpression of a dnFGFR (Griffin et al., 1995; Rodaway et al., 1999). As in other species, the expression of Xenopus brachyury (Xbra), a pan-mesodermal marker, is regulated by Fgf signals (Smith et al., 1991; Isaacs et al., 1994; Latinkic et al., 1997). The ectopic expression of Xbra leads to the induction of mesoderm in animal cap explants while its loss-of-function results in mesodermal defects (Cunliffe and Smith, 1992; Conlon et al., 1996). The mesoderm inductivity of Xbra can be overcome by overexpression of dnFGFR (Schulte-Merker and Smith, 1995). It has been suggested that Brachyury/ntl is an immediate mediator of Fgf signaling in mesoderm induction.
We are interested in identifying other downstream components of the FGF signaling cascade that regulate mesoderm induction. SP1 is a zinc finger transcription factor that is ubiquitously expressed and binds to GC-rich promoter elements to activate the transcription of target genes (Kadonaga et al., 1987). To date, many SP1-related transcription factors have been identified in a variety of species and these constitute an SP1 family (reviewed in Kaczynski et al., 2003). Unlike SP1, some SP1-like genes are expressed in certain types of cells during the development of vertebrate embryos and are involved in specific developmental processes. For example, mouse Sp5 is a recently identified member that is expressed in the primitive streak during gastrulation and later in the notochord, the neural tube and paraxial mesoderm (Harrison et al., 2000; Treichel et al., 2001). Loss-of-function of Sp5 in the T/+ genetic background can enhance the T/+ phenotype in mice, suggesting a genetic interaction between Sp5 and Brachyury (Harrison et al., 2000). Xenopus XSPR-1 and XSPR-2, and zebrafish Bts1, are closely related to Sp5 (Tallafuss et al., 2001; Ossipova et al., 2002). Both XSPR-2 and bts1 are expressed in the mesoderm precursors. These findings suggest that Sp5 and Sp5-related genes may play a role in mesoderm formation during vertebrate embryogenesis.
In this study we have identified zebrafish spr2, an ortholog of Xenopus XSPR2. Expression of spr2 occurs in both hypoblast and epiblast cells during late blastulation and early gastrulation, and at later stages in several mesoderm and neural tissues. We demonstrate that spr2 expression is dependent on Fgf, Nodal and Wnt signals, and it is implicated in mesoderm induction during early development in zebrafish embryos.
Results
Zebrafish spr2 is a member of the SP1 transcription factor family
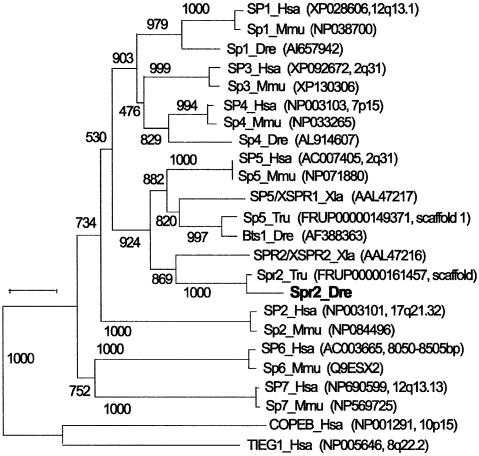
A new SP1-related sequence was initially identified by whole-mount in situ hybridization from a cDNA library as a gene with a restricted expression pattern during early development in zebrafish embryos. An open reading frame of this gene encodes a putative peptide of 357 residues. A BLAST search revealed that the putative peptide had variable degrees of homology with members of the SP1-like transcription factor family. Further BLAST searches of a public database identified seven SP1 family members in the human genome. A phylogenetic analysis of these sequences is shown in Figure 1, which supports a previously published analysis (Kaczynski et al., 2003), but with the addition of two extra human family members. Kaczynski et al. (2003) reported three main clades of SP1/KLF genes, called clades I, II and III. Clade I comprised the SP1 genes, clade II included TIEG1, and clade 3 held COPEB in the topology confirmed in the tree in Figure 1. Because our new SP1-related sequence from zebrafish was grouped in a phylogenetic analysis with XSPR-2 of frog (Ossipova et al., 2002) and a sequence we uncovered in the fugu database, we named this zebrafish gene spr2. The previously described SP1-related factor in zebrafish, Bts1 (Tallafuss et al. 2001), fell into a second strongly supported clade with the frog sequence XSPR-1 (Ossipova et al., 2002) and another fugu sequence (Figure 1). These two clades are both related to the previously described Sp5 gene of mouse (Harrison et al., 2000). By searching the human genome sequence database, we found a previously unannotated human sequence on BAC, which is clearly the human ortholog SP5 as shown by phylogenetic analysis. Because GAD1 is on the same human BAC clone RP11-570C16 and GAD1 maps to Hsa2q31 at nucleotide position 171 637 kb, SP5 should also map just 101 kb away. Supported by chromosomal mapping analysis (data not shown), zebrafish bts1 may prove to be an ortholog of human SP5. The zebrafish Spr2 shares an overall identity in amino acid sequence of 38.5. 56.1, 39.7, 35.8 and 36.6% to XSPR-1, XSPR-2, Bts1, mouse and human SP5, respectively. All of these factors have a conserved btd domain and three zinc finger domains (see Supplementary figure 8 available at The EMBO Journal Online), in which they share a sequence identity of over 88.9%. Phylogenetic analysis showed that Xenopus has an ortholog of both zebrafish genes bts1 and spr2, thus suggesting that the duplication event that produced these two clades occured before the divergence of the Xenopus and teleost lineages, that is, before the divergence of ray-fin fish, which includes teleosts, and lobe-fin fish, which includes tetrapods. Thus, the last common ancestor of frogs and humans had an ortholog of the Spr2 gene, but the gene does not surface in BLAST searches of the human or mouse genomes, and we assume it is either missing or else so diverged that it does not surface as a member of the SP1 gene family.
Fig. 1. Phylogenetic analysis of vertebrate SP1-related factors. Sources of the sequences are indicated in parentheses.
spr2 is expressed in mesodermal and ectodermal tissues during embryogenesis
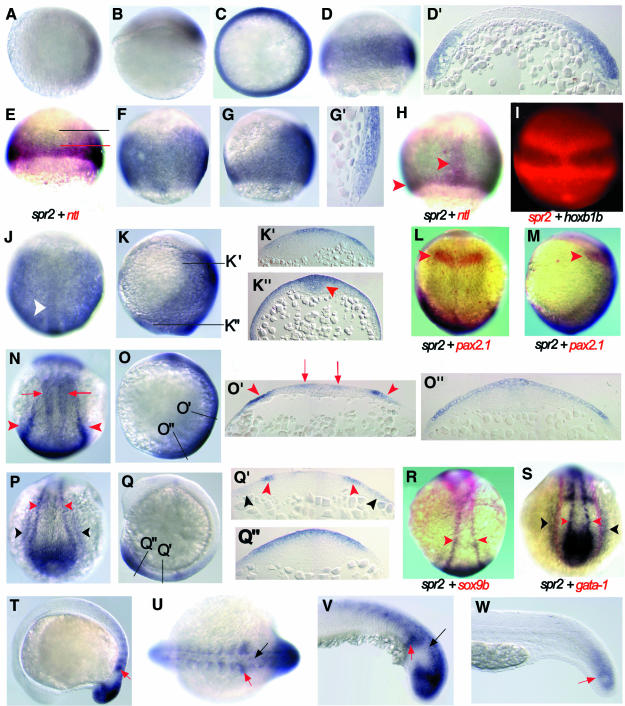
The expression pattern of spr2 in zebrafish embryos was examined by whole-mount in situ hybridization. spr2 transcripts can initially be detected at 30% epiboly on the future dorsal side of the embryo (Figure 2A and B). The expression domain is about four to eight cells in width and occupies ∼30% of the blastodermal circumference. As epiboly proceeds, the expression domain extends ventrally, so that just before the onset of gastrulation an expression domain 16–20 cells wide encircles the whole blastodermal margin (but again with stronger staining at the dorsal side) (Figure 2C and D). Taking a section through the embryo at this stage reveals that spr2 is expressed in both hypoblast and epiblast layers (Figure 2D′). Because ntl is a mesodermal marker (Schulte-Merker et al., 1994), we performed a double in situ hybridization with antisense spr2 and ntl probes to determine the tissue types of spr2-positive cells. As shown in Figure 2E, the marginal region expressing spr2 overlaps with the ntl domain, suggesting the mesodermal fate of these spr2-positive cells. According to the fate map of the early zebrafish gastrula (Kimmel et al., 1990) and the location of spr2 transcripts at later stages, spr2-positive cells that are located anterior to the ntl domain in the animal pole direction should have a neuroectodermal fate.
Fig. 2. Expression pattern of spr2 during the development of zebrafish embryos. Animal-pole (A) and lateral (B) views of an embryo with dorsal to the right at 30% epiboly stage. Animal-pole (C) and lateral (D) views of a shield-stage embryo with dorsal to the right. (D′) A section in parallel to the animal–vegetal plane of the embryo in (D). Embryos simultaneously labeled for ntl (red) and spr2 (blue) at 50% epiboly (E) and 75% epiboly (H). The upper limit of ntl and spr2 domains in (E) is indicated by red and black lines, respectively. The red arrowheads in (H) point at the axial and germ ring domains of ntl. (F, G and G′) Embryos at 75% epiboly. (F) Dorsal view. (G) Lateral view with dorsal to the right. (G′) The shield area of a section along the anteroposterior axis. (I) Fluorescence-microscopic dorsal view of a 60% epiboly-stage embryo simultaneously labeled for hoxb1b (blue/black) and spr2 (red). Dorsal (J) and lateral (K) views at 95% epiboly. The white arrowhead in (J) points at the midline tissues with a higher level of expression, probably including the neural and the notochord precursors. (K′ and K′′) Cross-sections at positions indicated in (K). Expression occurred anteriorly in the outer layer of two to three cell-diameter and posteriorly in the thickening midline tissues (indicated by an arrowhead). Dorsal (L) and lateral (M) views of a bud-stage embryo simultaneously labeled for pax2.1 (red) and spr2 (blue), showing that the anterior border of spr2 domain matched the pax2.1 domain (posterior midbrain, indicated by arrowheads). Dorsal (N) and lateral (O) views of a two-somite stage embryo. (O′ and O′′) Cross-sections at positions indicated in (O). The red arrowheads indicate the posterior border of the neuroectoderm [trunk neural crest (TNC) precursors] and red arrows indicate unidentified neural precursors. (P–S) Emryos at the six-somite stage. (P) Dorsal view of posterior trunk. (Q) Lateral view. (Q′ and Q′′) Cross-sections at positions indicated in (Q). (R) Double staining for sox9b (red) and spr2 (blue). Both genes were expressed in the TNC cells. (S) Double staining for gata-1 (red) and spr2 (blue). The outermost domain (lateral mesoderm) of spr2 partially overlapped the gata-1 domain (hematopoietic progenitors). Red arrowheads in (P–S) point at the TNC and black arrowheads at the lateral mesoderm. Lateral (T) and posterodorsal (U) views at 14-somite stage. Lateral views of posterior trunk at 18-somite stage (V) and 24 h (W). The red and black arrows indicate the newly formed somites and neuronal cells.
During gastrulation the expression of spr2 further expands towards the dorsal side and along the anteroposterior axis as spr2-positive cells proliferate and move dorsally by convergent extension (Figure 2F, G, J and K). At 75% epiboly, expression on the dorsal side extends to an anterior limit presumably marking the midbrain while on the ventral side expression persists only in the blastodermal margin (Figure 2F and G). In contrast, ntl expression during the same period becomes restricted to the blastodermal margin and the notochord precursors (Figure 2H). A sagittal section along the dorsal midline discloses spr2 expression in the hypoblast and epiblast cells (Figure 2G′), suggesting that spr2-positive cells may contribute to both mesodermal and ectodermal tissues. Double labeling with antisense spr2 and hoxb1b probes shows that the anterior border of spr2 expression is several cells ahead of that of hoxb1b expression (Figure 2I). Since the anterior border of the hoxb1b expression domain marks the boundary between rhombomeres 3 and 4 (Alexandre et al., 1996), the most anterior cells that express spr2 may contribute to the more anterior neural tissues. By the end of epiboly, the expression pattern of spr2 is changed little from earlier stages (Figure 2J and K). Sections show that the transcripts are present in both outer and deep layers of the posterior midline tissues (Figure 2K′′) and in the other areas they are mainly restricted to the outer 2–3 layers of cells (Figure 2K′ and K′′). Double labeling with spr2 and pax2.1, a marker for the posterior midbrain (Krauss et al., 1991), indicates that the anterior border of spr2 expression is located in the midbrain (Figure 2L and M).
During the early segmentation period, one of the major tissues expressing spr2 is the trunk neural crest where spr2 expression overlaps that of the neural crest marker sox9b (Li et al., 2002) (Figure 2N–S). A pair of the most lateral bands also covers the expression domain of gata-1 (Figure 2S), a maker for the blood precursor cells (Detrich et al., 1995), suggesting a possible hematopoietic fate for these cells. The other tissues expressing spr2 during segmentation include the tailbud and the developing somites (Figure 2N–V). Later in development the expression of spr2 declines such that at 24 h post-fertilization (hpf), it is detected only in the posterior-most region of the embryo (Figure 2W), and at ∼36 hpf spr2 can no longer be detected by in situ hybridization.
Spr2 positively regulates ntl expression
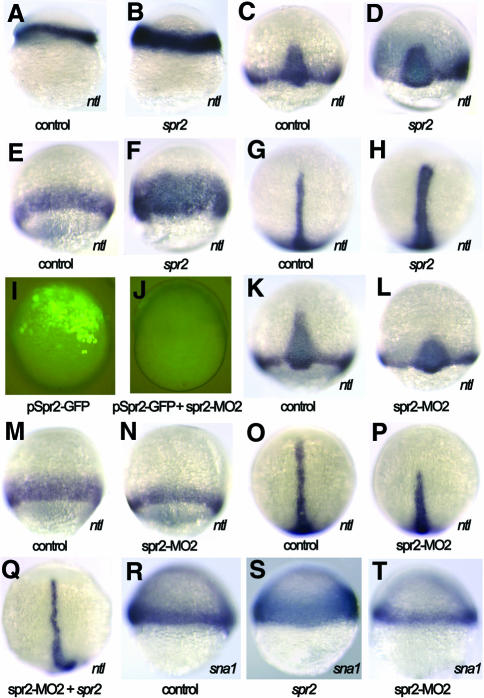
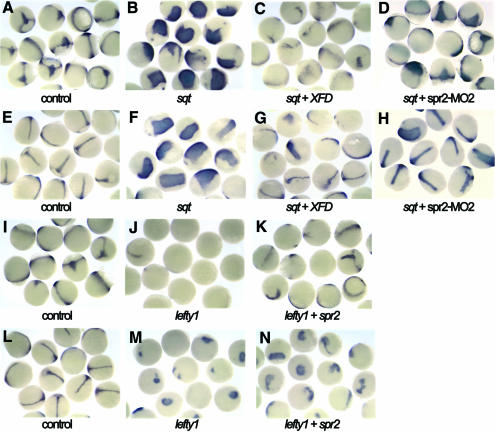
As shown above, the expression domain of the pan-mesodermal marker ntl lies within the spr2 expression domain during late blastulation and early gastrulation. In order to investigate the possibility that spr2 might play a role in mesoderm induction by regulating ntl expression, we explored the potential effect of Spr2 on ntl expression. First we injected synthetic spr2 mRNA into one-cell stage embryos and examined ntl expression at several stages. At the germ ring stage, the expression of ntl in the germ ring was enhanced to a certain extent in the injected embryos (Figure 3B). During gastrulation, phenotypes following spr2 mRNA injection were more readily recognized because the affected embryos showed a significant increase in ntl expression in the axial mesoderm (Figure 3D and H) and in the germ ring (Figure 3F). The inductive effect of spr2 was clearly dose dependent; when injected with 100 pg spr2 mRNA, the percentage of embryos that showed expanded ntl expression was 43.1% (n = 181) at ∼70% epiboly, and when injected with 200 pg spr2 mRNA the percentage increased to 65.9% (n = 41), respectively. At the bud stage, the two doses affected 40% (n = 65) and 51.7% (n = 116) of embryos, respectively. The ectopic expression of ntl at the animal pole or surrounding regions was not observed in the injected embryos, suggesting that Spr2 is a modulator, rather than an activator, of ntl expression.
Fig. 3. Spr2 positively regulates expression of ntl and sna1. (A, C, E, G, K, M, O and R) Control embryos injected with GFP mRNA or the control morpholino. (B, D, F, H–J, L, N, P, Q, S and T) Embryos injected with DNA (100 pg), mRNA (100 pg) and/or morpholino (5 ng) as indicated below each picture. (A and B) Lateral views at 40% epiboly, showing enhanced expression of ntl in the germ ring (B). (C and D) Dorsal views at 70% epiboly, showing ntl expansion in the axial mesoderm (D). (E and F) Ventral views at 70% epiboly, showing ntl expansion in the ventral germ ring (F). (G and H) Dorsal views at the bud stage, showing ntl expansion in the notochord (H). (I) Lateral view of a live embryo at 60% epiboly, showing green fluorescence expressed by pSpr2-GFP. (J) Co-injection with pSpr2-GFP DNA and spr2-MO2 inhibited Spr2-GFP expression. (K and L) Dorsal views at 75% epiboly. The spr2-MO2 injection caused loss of the anteriormost part of ntl expression domain in the axial mesoderm (L). (M and N) Ventral views at 75% epiboly, showing a thinner ntl expression domain in the germ ring (N). (O, P and Q) Dorsal views at the bud stage. The morpholino injection resulted in loss of the anterior notochord domain of ntl (P), which was rescued by co-injection with spr2 mRNA (Q). (R–T) Lateral views at 50% epiboly with dorsal to the right. Overexpression of spr2 expanded sna1 expression (S) while knocking down with spr2-MO2 led to fewer sna1-positive cells (T).
We then studied the effect of Spr2 on ntl expression by blocking the translation of endogenous spr2 mRNA using morpholino-based knockdown technology (Nasevicius and Ekker, 2000). To test the effectiveness of the morpholinos, fertilized eggs were injected with variable amounts of spr2-MO1 or spr2-MO2 in combination with 100 pg of pSpr2-GFP DNA, an expression construct containing a partial 5′ sequence of spr2 cDNA fused in-frame to a GFP coding sequence. At a dose of 5 ng, the spr2-MO2-injected embryos almost lacked green fluorescence from the GFP fusion protein (Figure 3J), while the spr2-MO1-injected embryos retained visible fluorescence (data not shown), suggesting that spr2-MO2 could more effectively block translation of spr2 mRNA. Then we examined ntl expression, by whole-mount in situ hybridization, in the embryos injected with 5 ng of the spr2-MO2. Around the onset of gastrulation, the injected embryos exhibited no obvious changes in ntl expression (data not shown). By 75% epiboly, 52.3% (n = 237) of the injected embryos appeared to lack the anterior-most axial domain of ntl and have a smaller number of ntl-positive cells in the germ ring (Figure 3K–N). The effect of spr2-MO2 was easier to identify at the bud stage when ntl expression was reduced or abolished in the anterior notochord in 43.2% (n = 169) of the injected embryos (Figure 3P). When 100 pg spr2 mRNA and 5 ng spr2-MO2 were co-injected, however, only 7.9% (n = 63) of the embryos showed a loss or significant reduction in ntl expression in the anterior notochord at the bud stage, which was far less than the percentage (43.2%) obtained by injecting with spr2-MO2 alone. The successful rescue of ntl expression (Figure 3Q) means that the effect of blocking the translation of endogenous spr2 mRNA is counteracted by the supply of the synthetic spr2 mRNA. The knockdown results also support the idea that Spr2 is required for maintenance of ntl expression.
To further confirm the mesoderm-promoting activity of Spr2, its impact on the expression of snail1 (sna1), which is a lateral mesoderm marker during early gastrulation (Hammerschmidt and Nusslein-Volhard, 1993; Thisse et al., 1993), was investigated. The overexpression of spr2 enhanced sna1 expression in the lateral germ ring at 50% epiboly stage (Figure 3S) while injection with spr2-MO2 caused a reduction in the number of sna1-positive cells (Figure 3T), implying a general role in mesoderm induction.
Spr2 is involved in Fgf-mediated mesoderm induction
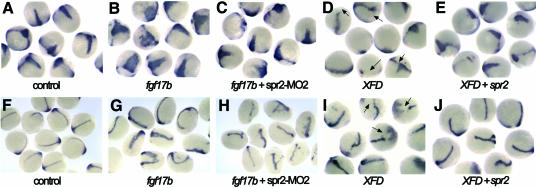
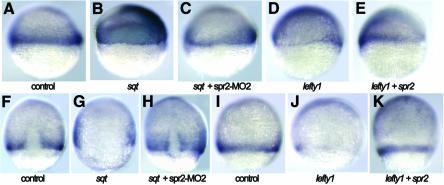
Because Xbra/ntl expression is an immediate-early response to mesoderm induction and Xbra/Ntl mediates mesoderm induction activity of Fgfs, we wondered whether Spr2 regulates ntl expression by mediating Fgf signaling. To test this possibility, we explored the change in ntl expression following co-injection with spr2-MO2 and fgf mRNA. We tested two zebrafish Fgf molecules, Fgf8 (Reifers et al., 1998) and a new Fgf family member Fgf17b (our unpublished data). At 75% epiboly, all of the embryos injected with 10 pg of fgf17b mRNA showed a significant increase in ntl expression in the presumptive notochord (Figure 4B). Injection with 50 pg of fgf8 mRNA also led to the induction of ntl expression in 53.7% (n = 41) of the embryos at the same stage. When the same doses of fgf17b or fgf8 mRNA were combined with 5 ng spr2-MO2, only 35.7% (n = 28) or 34.6% (n = 26) of the embryos, respectively, expressed ntl at a comparably increased level (Figure 4C). At the bud stage, only 3.1% (n = 65) of the embryos co-injected with 2 pg fgf17b mRNA and 5 ng spr2-MO2 exhibited a significant increase in ntl expression (Figure 4H), in contrast to 34.8% (n = 46) for the single mRNA injection (Figure 4G). These results demonstrate that knockdown of Spr2 activity inhibits the inductive effect of Fgf on ntl expression.
Fig. 4. Spr2 mediates Fgf signaling in mesoderm induction. The embryos were examined for ntl expression. (A–E) 75% epiboly stage. (F–J) Bud stage. Injection with 10 pg fgf17b mRNA caused expansion of ntl expression in the axial mesoderm of most of the embryos (B and G), whereas the proportion of the embryos with similarly enhanced ntl expression significantly dropped when co-injected with the same amount of fgf17b mRNA and 5 ng spr2-MO2 (C and H). Injection with 50 pg XFD mRNA led to slightly smaller axial mesoderm domain and interrupted germ ring domain (indicated by arrows) for ntl expression (D and I), while the inhibitory effect of XFD was deterred when co-injected with 200 pg spr2 mRNA (E and J).
The effect of co-injecting spr2 mRNA and XFD mRNA, which encodes a dominant negative form of a Xenopus FGF receptor (Amaya et al., 1991), was also investigated. Overexpression of XFD alone typically led to a slightly smaller axial mesoderm domain and an interrupted germ ring domain (indicated by arrows in Figure 4D and I) for ntl expression during gastrulation. When injected with 50 or 100 pg XFD mRNA, the embryos with a reduction in ntl expression at the 75% epiboly stage accounted for 75% (n = 40) or 76.7% (n = 43) (Figure 4E), respectively. In contrast, the percentage of the embryos with reduced ntl expression, when injected simultaneously with the same two doses of XFD mRNA and 200 pg spr2 mRNA (Figure 4E), was 26.7% (n = 30) and 52.4% (n = 42), respectively. Moreover, the proportion of co-injected embryos with enhanced ntl expression at this stage was <15% for the co-injection, compared to ∼60% for embryos injected with spr2 mRNA alone. At the bud stage, the proportion of the co-injected embryos with enhanced ntl expression dropped to <5% for the co-injection (Figure 4J). These results suggest that the overexpression of spr2 complements reduction of Fgf activity in mesoderm induction.
Spr2 mediates mesoderm induction of the Nodal signal
Nodal signaling is a key player in mesoderm induction (Schier and Shen, 2000). As, in Xenopus, the mesoderm induction activity of Activin, also a TGFβ ligand, is relayed by Fgfs (Cornell and Kimelman, 1994; LaBonne and Whitman, 1994), so Fgfs may also act as a second signal to relay Nodal signaling in mesoderm induction. To test this hypothesis, we coexpressed ectopic zebrafish sqt and Xenopus XFD, and examined ntl expression in the zebrafish embryos at 75% epiboly and the bud stages. At both these stages, expression of ntl was considerably expanded in almost all (n > 32) of the embryos injected with 0.5 pg or 0.25 pg of sqt mRNA alone (Figure 5B and F). When 0.5 pg sqt mRNA and 100 ng XFD mRNA were co-injected, the percentage of embryos with significant induction of ntl decreased to 5.1% (n = 39) and 15% (n = 60) at 75% epiboly and the bud stages (Figure 5C and G), respectively. This suggests that the mesoderm induction activity of Nodal is dependent on Fgf function.
Fig. 5. Fgf signal and Spr2 mediate inductivity of Nodal signal on ntl. The embryos were examined for ntl expression. (A–D and I–K) 75% epiboly stage. (E–H and L–N) Bud stage. Almost all of the embryos injected with 0.5 pg sqt mRNA alone showed a large increase in ntl expression in the axial mesoderm (B and F). Co-injection with 100 pg XFD mRNA effectively inhibited the inductivity of sqt overexpression (C and G). Co-injection with 5 ng spr2-MO2 also inhibited, although less effectively, the inductive activity of sqt overexpression (D and H). Injection with 100 pg lefty1 mRNA almost abolished ntl expression during midgastrulation (J). Co-injection with 50 pg spr2 mRNA rescued ntl expression in some of the embryos (K). At the bud stage, the rescue effect of spr2 mRNA injection was also obvious (M and N).
As demonstrated earlier, Spr2 appears to be an effector of Fgf signaling in mesoderm induction. Thus, we speculated that Spr2 might also mediate mesoderm induction activity of Nodal signal. Therefore, we first tested whether it was possible to inhibit Sqt activity by knocking down Spr2. When co-injected with 0.5 pg sqt mRNA and 5 ng spr2-MO2, the embryos with ntl expansion during gastrulation accounted for ∼78% (n > 36), <100% for the sqt mRNA injection alone. If the dose of sqt mRNA for co-injection was reduced to 0.25 pg, the percentage of the embryos with a comparable level of increase in ntl expression further declined to 41.7% (n = 60) and 38% (n = 71) at 75% epiboly and the bud stages (Figure 5D and H), respectively. This suggests that the inhibition of Spr2 can affect the function of Sqt during mesoderm induction, although this inhibitory effect is not as great as XFD.
Lefty1 is an antagonist of Nodal signaling (Thisse and Thisse, 1999). We injected embryos with 100 pg mRNA of zebrafish lefty1 and found that 71.8% (n = 39) of the embryos had no detectable ntl expression and the remaining embryos only showed weak ntl expression in the ventral germ ring at 75% epiboly stage (Figure 5J). At the bud stage, only 2 out of 47 injected embryos developed a short and thin notochord domain of ntl while the others had a small staining patch in the tailbud (Figure 5M). However, when co-injected with 100 pg lefty1 and 50 pg spr2 mRNA, 70.2% (n = 43) of the embryos retained ntl expression in some domains at 75% epiboly stage (Figure 5K). At the bud stage, 25 out of 44 co-injected embryos (56.8%) expressed ntl at a level higher than that observed with the single injection of lefty1, 7 of which had a notochord expression domain (Figure 5N). Obviously, the overexpression of spr2 restored to a certain extent ntl expression that was suppressed by the ectopic Lefty1. This again supports the hypothesis that Spr2 is able to mediate mesoderm induction activity of Nodal or related TGFβ signals.
The involvement of Spr2 in mesoderm induction by Nodal signal was confirmed using another mesoderm marker, sna1. Compared to dramatic expansion of sna1 towards the animal pole at 50% epiboly stage after injection with sqt mRNA alone (Figure 6B), the expansion was negligible when the embryos were co-injected with sqt mRNA and spr2-MO2 (Figure 6C). At 80% epiboly stage, the embryos injected with sqt mRNA showed an expanded dorsal gap (Figure 6G), whereas the co-injected embryos had a relatively normal dorsal gap (Figure 6H). The lefty1 injection led to fewer sna1-positive cells in the germ ring at the shield stage (Figure 6D), and the number went back to normal when co-injected with spr2 mRNA (Figure 6E). More obviously at 80% epiboly, overexpression of lefty1 almost eliminated sna1 expression in the lateral and ventral germ ring (Figure 6J), which was recovered by co-injection with spr2 mRNA (Figure 6K). These results suggest that induction of sna1 by Nodal signal requires Spr2.
Fig. 6. Spr2 mediates induction of Nodal signal on sna1. The embryos were examined for sna1 expression following injections. (A–E) Lateral views with dorsal to the right at 50% epiboly. (F–H) Dorsal views at 80% epiboly. (I–K) Lateral views at 80% epiboly, with dorsal to the right. Injection doses: sqt mRNA, 0.125 pg; lefty1 mRNA, 100 pg; spr2 mRNA, 100 pg; spr2-MO2, 5 ng.
Expression of Spr2 is dependent on Nodal, Fgf and Wnt signals
Since spr2 is expressed in the mesoderm precursors during early gastrulation and is involved in mesoderm induction by Fgf signals, we asked whether spr2 was regulated by Nodal and Fgf signals and mediated their activities in a feedback fashion. We analysed spr2 expression in the zebrafish embryos in which activities of the signals were altered transiently. When injected with 1 pg sqt mRNA, spr2 was induced throughout the blastoderm at 50% epiboly stage (Figure 7B). Injection with 100 pg lefty1 mRNA completely abolished spr2 expression on the dorsal side of the blastoderm and also, remarkably, reduced expression on the ventral side (Figure 7C). These data suggest that the Nodal signal is essential for the activation of spr2 on the dorsal side and is also required for maintenance of spr2 expression on the ventral side. Likewise, the ectopic expression of fgf17b also induced spr2 expression throughout the blastoderm during early gastrulation (Figure 7E and K). When XFD was overexpressed in the embryos, spr2 expression was almost eliminated in the ventral and ventrolateral domains but retained in the dorsal domain at a lower level (Figure 7F, I and L). Taking a section of an XFD-injected embryo at 75% epiboly revealed that on the dorsal side, only the epiblast cells weakly expressed spr2 whereas the hypoblast cells did not at 75% epiboly (Figure 7N). This suggests that Fgf signaling is absolutely required for spr2 expression in the hypoblast cells that will give rise to the mesodermal tissues, while spr2 expression in the epiblast cells is only partially dependent on Fgf signals. It has been previously demonstrated that in zebrafish the expression of fgf8 and fgf17b in the marginal cells of the late blastulae and early gastrulae is dependent on Nodal signaling (Gritsman et al., 1999; our unpublished data). Therefore, Fgf signaling acts during mesoderm formation, at least in most domains, downstream of Nodal signaling to regulate spr2 expression.
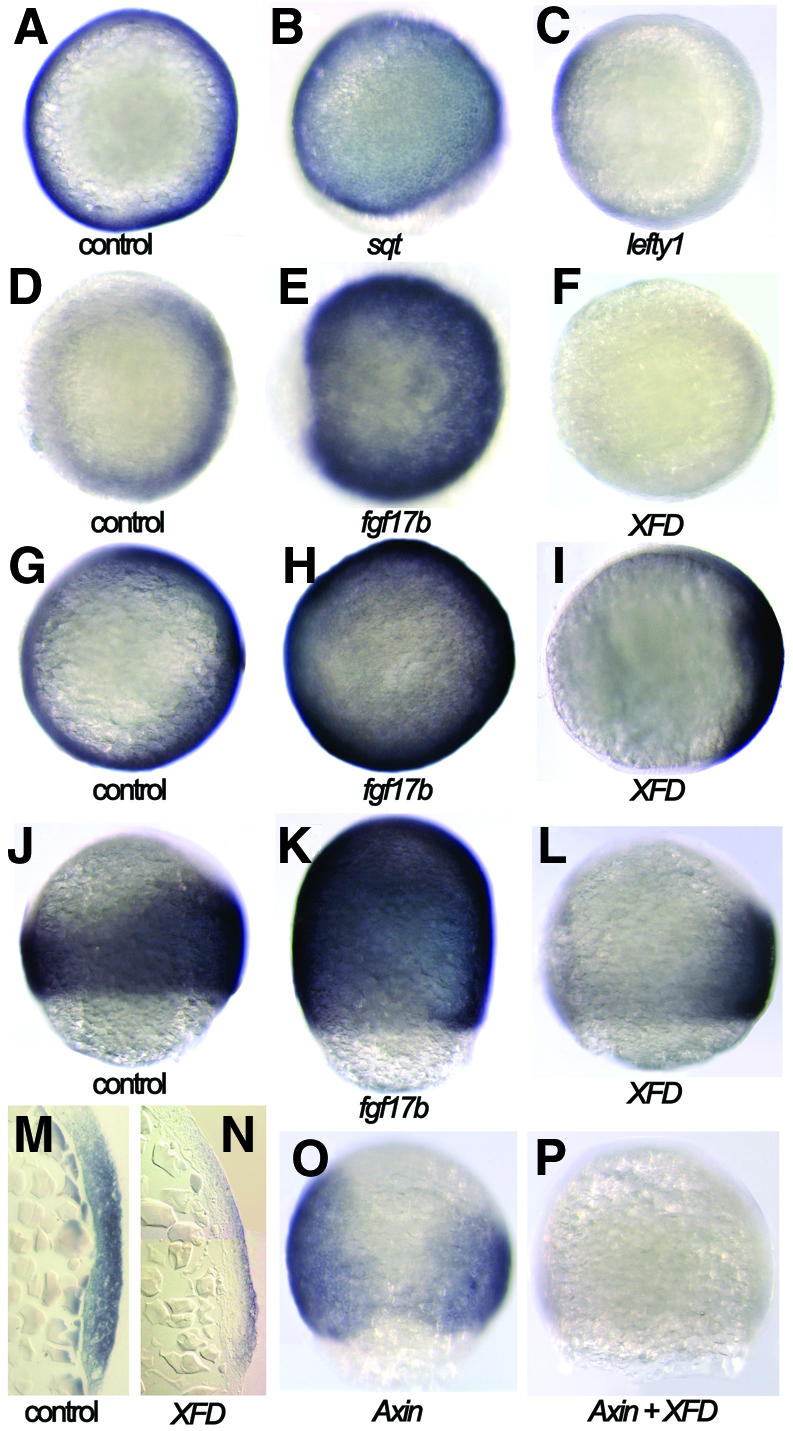
Fig. 7. Dependence of spr2 expression on Nodal, Fgf and Wnt signals. (A–F) 50% epiboly stage. (G–L) 75% epiboly stage. (A–I) Animal-pole views with dorsal to the right. (J–L) Lateral views with dorsal to the right. (O and P) Dorsal views. Injection with 1pg sqt or 10 pg of fgf17b mRNA caused ectopic expression of spr2 (B, E, H and K). When injected with 100 pg of lefty1 mRNA, spr2 expression was abolished on the dorsal side and reduced in the ventral germ ring (C). When injected with 100 pg XFD mRNA, spr2 expression was eliminated on the ventral side and dramatically decreased on the dorsal side (F, I and L). A section revealed that spr2 expression on the dorsal of XFD-injected embryos persisted in the epiblast layer only (N). Injection with mouse Axin mRNA led to suppression of spr2 expression on the dorsal side (O), while co-injection with Axin and XFD mRNAs resulted in elimination of spr2 expression in some of the embryos (P).
The dorsal epiblast cells of the early gastrula are committed to a neuroectodermal fate. Considering that Wnt signaling contributes to dorsal mesoderm and neural induction (Baker et al., 1999; Sokol, 1999; Wilson et al., 2001), we speculated that spr2 expression in the dorsal epiblast cells might be also dependent on Wnt signaling. To test this hypothesis, we injected the zebrafish embryos with an mRNA encoding mouse Axin that is a negative regulator of the canonical Wnt signaling pathway (Kikuchi, 1999). The injected embryos indeed lacked spr2 transcripts in the dorsal midline and adjacent areas at 75% epiboly (Figure 7O). When both XFD and Axin were overexpressed, spr2 expression was almost completely inhibited in some embryos (Figure 7P). This supports the idea that both Fgf and Wnt signaling pathways are essential for the expression of spr2 in the dorsal neuroectoderm.
Discussion
spr2/XSPR2 and bts1/XSPR1/Sp5 share similar expression patterns
Phylogenetic analysis suggests that spr2/XSPR2 and bts1/XSPR1/Sp5 are duplicated from the same ancestor during evolution. Their expression patterns also share a certain degree of similarity. The expression of spr2 during early gastrulation occurs in the marginal cells, including both epiblast and hypoblast cells, and at later stages in some mesodermal and ectodermal tissues. Like spr2, bts1 is also expressed during early gastrulation in zebrafish (Tallafuss et al., 2001). Unlike spr2, however, bts1 expression is mainly restricted to the epiblast cells of the germ ring with its expression in the dorsal hypoblast layer being restricted to very few cells. This suggests that spr2 and bts1 may have distinct functions in development of the dorsal mesoderm. The dynamic expression of mouse Sp5 is also apparent. During early gastrulation Sp5 is expressed in the primitive streak, including both ectodermal and mesodermal precursors, while in development it is expressed in the midbrain, otic vesicle, the spinal cord, the notochord, somites and even in some endodermal tissues (Harrison et al., 2000; Treichel et al., 2001). Xenopus XSPR-2 appears to be predominantly expressed within the presumptive mesoderm during gastrulation, whereas XSPR-1 expression is restricted to the epithelial and subepithelial layers (Ossipova et al., 2002). The expression patterns of these Sp1 family members are indicative of their functions in the formation and patterning of the mesoderm and/or ectoderm.
Spr2 mediates mesoderm induction by Fgf signals
Fgf signaling plays several important roles in mesoderm induction during early development of vertebrate embryos (Kimelman and Kirschner, 1987; Slack et al., 1987; Amaya et al., 1991, 1993; Griffin et al., 1995; Reifers et al., 1998). Expression of Brachyury/Xbra/ntl, a T-box transcription factor and a specific mesoderm marker, is an immediate response to Fgf induction and is thus also implicated in mesoderm induction. We have demonstrated, mainly using ntl as a mesoderm marker, that Spr2 is involved in mesoderm induction via the Fgf signaling pathway. First, the overexpression of Spr2 enhances ntl expression, while knockdown of Spr2 activity inhibits ntl expression to a certain degree. Second, the overexpression of Spr2 is able to release the inhibition of XFD on ntl expression. Third, induction of ntl expression by ectopic Fgf is blocked by the simultaneous knockdown of Spr2 activity. However, the extent of increase or decrease in ntl expression caused by overexpression or knockdown of spr2 is not as great as when Fgf or XFD are ectopically expressed, suggesting that multiple effectors may mediate mesoderm induction via Fgf signaling.
Individual Sp1-like transcription factors function as activators or repressors, depending on which promoter they bind and the coregulators with which they interact (reviewed by Kaczynski et al., 2003). We have found that the overexpression of spr2 enhances ntl expression in the germ ring at the onset of gastrulation and in the presumptive notochord during epiboly, which excludes the possibility that Spr2 acts as a repressor on ntl promoter. However, the overexpression of spr2 is unable to induce the ectopic expression of ntl in domains where ntl is not normally expressed, and when Spr2 is knocked down then ntl expression is not completely blocked. This implies that Spr2 is involved in the maintenance rather than activation of ntl expression.
Mouse embryos that are homozygous for a targeted mutation in Sp5 show no obvious phenotype (Harrison et al., 2000). Nevertheless, the homozygous mutant mice in a genetic background with a deletion of one Brachyury allele have defects in the mesoderm-derived vertebrae, which are not observed in mice with a single mutation. This observation at least suggests that Sp5 genetically interacts with Brachyury to affect development of the mesodermal tissues.
Zebrafish spr2 is expressed in the epiblast layer during gastrulation and in some neuronal cells at later stages. Our preliminary study has found that spr2 positively regulates transcription of a posterior neuroectodermal marker hoxb1b and might mediate posteriorization of the neuroectoderm during gastrulation via the Fgf signaling pathway (data not shown).
Brachyury/Xbra/ntl may be a direct target of Spr2
Analysis of the mouse Brachyury promoter has found binding sites for Sp1 and Sp4, and the deletion of these sites together with the GATA and Pea3 sites reduces reporter gene expression in embryonal carcinoma P19 cells (Yamaguchi et al., 1999). In the Xbra2 promoter a GC box (GCTGGGGGGGGGGGGGTG), a potential cis-element recognized by Sp1 family members, can be found between –250 and –267. Latinkic et al. (1997) have reported that the 381 bp proximal region of the Xbra2 promoter can elicit responses to Fgf and Activin, whereas the 231 bp promoter, which loses the GC box, fails to respond to Fgf and Activin induction, suggesting requirement of the GC box for such induction. However, further biochemical and molecular studies are needed to confirm the role of Sp1-like factors in transcriptional regulation of Brachyury/Xbra/ntl.
Nodal-mediated mesoderm induction is dependent on the action of Fgfs
Nodal proteins have been found to be essential for the development of mesoderm in vertebrates (Schier and Shen, 2000). In this study, we have demonstrated that, like Fgf, the overexpression of sqt greatly induces the ectopic expression of ntl and this induction can be effectively inhibited by the coexpression of XFD, suggesting that action of Nodal signaling depends on the action of Fgf signaling. This is consistent with the findings in Xenopus that mesoderm induction by Activin depends on Fgf (Cornell and Kimelman, 1994; LaBonne and Whitman, 1994). The fact that induction of ntl and sna1 expression by ectopic Sqt can be reduced by knocking down Spr2 activity, and that the inhibition of ntl and sna1 expression by the nodal-antagonist lefty1 can be restored by the overexpression of spr2, supports the idea that Spr2 mediates mesoderm induction of Nodal signaling by acting downstream of Fgf signaling during early embryogenesis.
Materials and methods
Isolation of spr2 cDNAs
spr2 was first identified from a zebrafish cDNA library as described in Zhao et al. (2002). The sequence of spr2 was deposited in GenBank with an accession number AY338748.
Generation of constructs
The coding sequence of spr2 was amplified by PCR with a pair of specific primers and cloned into an expression vector pXT7 to generate construct pXT7-spr2 for in vitro synthesis of spr2 mRNA. The coding sequence of fgf17b, a new member of Fgf family identified in our laboratory (unpublished results), was similarly cloned to generate pXT7-fgf17b. A recombinant pSpr2-GFP plasmid was constructed by inserting a 787 bp fragment of spr2, which contains a 325 bp 5′ UTR and its adjacent coding sequence encoding the first 124 amino acids, in-frame into vector peGFP-N2. This plasmid was used to test the effectiveness of spr2-MO. A GFP coding sequence plus the SV40 polyadenylation signal sequence were cloned into pBluescript KS(–). GFP mRNA was synthesized from the resulting plasmid and was used as an internal injection control.
Morpholino oligonucleotides
Two antisense morpholino oligonucleotides, spr2-MO1 (5′-CCG CGCTGTTGCTCCTGTTTTTCTG-3′) and spr2-MO2 (5′- CCCCCT TACACAGCCAGGTGCGTAC-3′), were designed to target spr2 mRNA and synthesized by Gene Tools, LLC. Co-injection of spr2-MO1 or spr2-MO2 with pSpr2-GFP DNA revealed that spr2-MO2 was much more effective and so this alone was used for all subsequent experiments. Another morpholino oligonucleotide with the sequence 5′- CTG CTGTAACTACGACCATTTTTGT-3′, which is unrelated in sequence to spr2 and produces no morphological or molecular changes after injection, was used as a control.
In vitro synthesis of mRNA
Linearized plasmids were used as templates for in vitro transcription using an appropriate Cap-Scribe Kit (Roche). The synthesized mRNA was purified using the RNAeasy@ Mini Kit (Qiagen) after treatment with RNase-free DNase and dissolved in nuclease-free water.
Injection
DNA or mRNA was diluted in 0.1 M KCl to an appropriate concentration prior to injection, while the morpholino oligonucleotides were diluted in 1× Danieau’s buffer. DNA was injected into the cytoplasm of embryos at the one-cell stage, while the RNAs and morpholinos were injected into the yolk or cytoplasm between the one- and two-cell stages. Injection with GFP mRNA was performed to confirm the effects of overexpression of spr2 or other genes. The injection dose was an estimated amount received by a single embryo. For co-injection of two mRNAs, they were mixed prior to injection. For co-injection of an mRNA with a morpholino (not RNase-free), an embryo was first injected with the mRNA, followed by a second injection with the morpholino. Data obtained from independent micro-injections were pooled.
Whole-mount in situ hybridization and histological sectioning
Digoxigenin-UTP- or fluorescein-UTP-labeled antisense RNA probes were generated by in vitro transcription. Whole-mount in situ hybridizations essentially followed the standard protocol with minor modifications. Some of the embryos were sectioned at a thickness of ∼10 µm.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Drs S.Lin, M.R.Rebagliati, B.Thisse, S.C.Lin and J.S.Eisen for kindly providing plasmids. We are also grateful to Dr Sarah Webb for helpful comments. This work was supported by NSFC grants (30270690, 30221003 and 30025020), the ‘863’ Program (2001AA221241), and TRAPOYT of the MOE.
References
- Agius E., Oelgeschlager,M., Wessely,O., Kemp,C. and De Robertis,E.M. (2000) Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development, 127, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre D., Clarke,J.D.W., Oxtoby,E., Yan,Y.L., Jowett,T. and Holder,N. (1996) Ectopic expression of Hoxa-1 in the zebrafish alters the fate of the mandibular arch neural crest and phenocopies a retinoic acidinduced phenotype. Development, 122, 735–746. [DOI] [PubMed] [Google Scholar]
- Amaya E., Musci,T.J. and Kirschner,M.W. (1991) Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in the Xenopus embryo. Cell, 66, 257–270. [DOI] [PubMed] [Google Scholar]
- Amaya E., Stein,P.A., Musci,T.J. and Kirschner,M.W. (1993) FGF signalling in the early specification of mesoderm in Xenopus. Development, 118, 477–487. [DOI] [PubMed] [Google Scholar]
- Baker J.C., Beddington,R.S. and Harland,R.M. (1999) Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev., 113, 3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. and Kimelman,D. (2000) The role of the yolk syncytial layer in germ layer patterning in zebrafish. Development, 127, 4681–4689. [DOI] [PubMed] [Google Scholar]
- Clements D., Friday,R.V. and Woodland,H.R. (1999) Mode of action of VegT in mesoderm and endoderm formation. Development, 126, 4903–4911. [DOI] [PubMed] [Google Scholar]
- Conlon F.L., Lyons,K.M., Takaesu,N., Barth,K.S., Kispert,A., Herrmann,B. and Robertson,E.J. (1994) A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development, 120, 1919–1928. [DOI] [PubMed] [Google Scholar]
- Conlon F.L., Sedgwick,S.G., Weston,K.M. and Smith,J.C. (1996) Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development, 122, 2427–2435. [DOI] [PubMed] [Google Scholar]
- Cornell R.A. and Kimelman,D. (1994) Activin-mediated mesoderm induction requires FGF. Development, 120, 453–462. [DOI] [PubMed] [Google Scholar]
- Cunliffe V. and Smith,J.C. (1992) Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature, 358, 427–430. [DOI] [PubMed] [Google Scholar]
- Detrich H.W. III, Kieran,M.W., Chan,F.Y., Barone,L.M., Yee,K., Rundstadler,J.A., Pratt,S., Ransom,D. and Zon,L.I. (1995) Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl Acad. Sci. USA, 92, 10713–10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B., Gates,M.A., Egan,E.S., Dougan,S.T., Rennebeck,G., Sirotkin,H.I., Schier,A.F. and Talbot,W.S. (1998) Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature, 395, 181–185. [DOI] [PubMed] [Google Scholar]
- Griffin K., Patient,R. and Holder,N. (1995) Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development, 121, 2983–2994. [DOI] [PubMed] [Google Scholar]
- Gritsman K., Zhang,J., Cheng,S., Heckscher,E., Talbot,W.S. and Schier,A.F. (1999) The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell, 97, 121–132. [DOI] [PubMed] [Google Scholar]
- Halpern M.E., Ho,R.K., Walker,C. and Kimmel,C.B. (1993) Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell, 75, 99–112. [PubMed] [Google Scholar]
- Hammerschmidt M. and Nusslein-Volhard,C. (1993) The expression of a zebrafish gene homologous to Drosophila snail suggests a conserved function in invertebrate and vertebrate gastrulation. Development, 119, 1107–1118. [DOI] [PubMed] [Google Scholar]
- Harrison S.M., Houzelstein,D., Dunwoodie,S.L. and Beddington,R.S. (2000) Sp5, a new member of the Sp1 family, is dynamically expressed during development and genetically interacts with Brachyury. Dev. Biol., 227, 358–372. [DOI] [PubMed] [Google Scholar]
- Herrmann B.G., Labeit,S., Poustka,A., King,T.R. and Lehrach,H. (1990) Cloning of the T gene required in mesoderm formation in the mouse. Nature, 343, 617–622. [DOI] [PubMed] [Google Scholar]
- Isaacs H.V., Pownall,M.E. and Slack,J.M. (1994) eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J., 13, 4469–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczynski J., Cook,T. and Urrutia,R. (2003) Sp1- and Kruppel-like transcription factors. Genome Biol., 4, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J.T., Carner,K.R., Masiarz,F.R. and Tjian,R. (1987) Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell, 51, 1079–1090. [DOI] [PubMed] [Google Scholar]
- Kikuchi A. (1999) Roles of Axin in the Wnt signalling pathway. Cell Signal, 11, 777–788. [DOI] [PubMed] [Google Scholar]
- Kimelman D. and Kirschner,M. (1987) Synergistic induction of mesoderm by FGF and TGF-β and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell, 51, 869–877. [DOI] [PubMed] [Google Scholar]
- Kimmel C.B., Warga,R.M. and Schilling,T.F. (1990) Origin and organization of the zebrafish fate map. Development, 108, 581–594. [DOI] [PubMed] [Google Scholar]
- Kofron M. et al. (1999) Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFβ growth factors. Development, 126, 5759–5770. [DOI] [PubMed] [Google Scholar]
- Krauss S., Johansen,T., Korzh,V. and Fjose,A. (1991) Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development, 113, 1193–1206. [DOI] [PubMed] [Google Scholar]
- LaBonne C. and Whitman,M. (1994) Mesoderm induction by activin requires FGF-mediated intracellular signals. Development, 120, 463–472. [DOI] [PubMed] [Google Scholar]
- Latinkic B.V., Umbhauer,M., Neal,K.A., Lerchner,W., Smith,J.C. and Cunliffe,V. (1997) The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev., 11, 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhao,C., Wang,Y., Zhao,Z. and Meng,A. (2002) Zebrafish sox9b is an early neural crest marker. Dev. Genes Evol., 212, 203–206. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Yamaha,E., Wakahara,M., Kuroiwa,A. and Takeda,H. (1996) Mesoderm induction in zebrafish. Nature, 383, 131–132. [Google Scholar]
- Nasevicius A. and Ekker,S.C. (2000) Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet., 26, 216–220. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D. (1969) The formation of the mesoderm in urodelean amphibians. I. Induction by the endoderm W. Roux’s Archiv. Entw. Mech. Org., 162., 341–373. [DOI] [PubMed] [Google Scholar]
- Ossipova O., Stick,R. and Pieler,T. (2002) XSPR-1 and XSPR-2, novel Sp1 related zinc finger containing genes, are dynamically expressed during Xenopus embryogenesis. Mech. Dev., 115, 117–122. [DOI] [PubMed] [Google Scholar]
- Reifers F., Bohli,H., Walsh,E.C., Crossley,P.H., Stainier,D.Y. and Brand,M. (1998) Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development, 125, 2381–2395. [DOI] [PubMed] [Google Scholar]
- Rodaway A., Takeda,H., Koshida,S., Broadbent,J., Price,B., Smith,J.C., Patient,R. and Holder,N. (1999) Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived TGF-β family signals and discrimination of mesoderm and endoderm by FGF. Development, 126, 3067–3078. [DOI] [PubMed] [Google Scholar]
- Schier A.F. and Shen,M.M. (2000) Nodal signaling in vertebrate development. Nature, 403, 385–389. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S. and Smith,J.C. (1995) Mesoderm formation in response to Brachyury requires FGF signalling. Curr. Biol., 5, 62–67. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., van Eeden,F., Halpern,M.E., Kimmel,C.B. and Nusslein-Volhard,C. (1994) no tail (ntl) is the zebrafish homologue of the mouse T (brachyury) gene. Development, 120, 1009–1015. [DOI] [PubMed] [Google Scholar]
- Slack J.M., Darlington,B.G., Heath,J.K. and Godsave,S.F. (1987) Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature, 326, 197–200. [DOI] [PubMed] [Google Scholar]
- Smith J.C., Price,B.M., Green,J.B., Weigel,D. and Herrmann,B.G. (1991) Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell, 67, 79–87. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. (1999) Wnt signaling and dorso–ventral axis specification in vertebrates. Curr. Opin. Genet. Dev., 9, 405–410. [DOI] [PubMed] [Google Scholar]
- Sun X., Meyers,E.N., Lewandoski,M. and Martin,G.R. (1999) Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev., 13, 1834–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallafuss A., Wilm,T.P., Crozatier,M., Pfeffer,P., Wassef,M. and Bally-Cuif,L. (2001) The zebrafish buttonhead-like factor Bts1 is an early regulator of pax2.1 expression during mid-hindbrain development. Development, 128, 4021–4034. [DOI] [PubMed] [Google Scholar]
- Thisse C. and Thisse,B. (1999) Antivin, a novel and divergent member of the TGFβ superfamily, negatively regulates mesoderm induction. Development, 126, 229–240. [DOI] [PubMed] [Google Scholar]
- Thisse C., Thisse,B., Schilling,T.F. and Postlethwait,J.H. (1993) Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development, 119, 1203–1215. [DOI] [PubMed] [Google Scholar]
- Treichel D., Becker,M.B. and Gruss,P. (2001) The novel transcription factor gene Sp5 exhibits a dynamic and highly restricted expression pattern during mouse embryogenesis. Mech. Dev., 101, 175–179. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.G., Bhatt,S. and Herrmann,B.G. (1990) Expression pattern of the mouse T gene and its role in mesoderm formation. Nature, 343, 657–659. [DOI] [PubMed] [Google Scholar]
- Wilson S.I., Rydstrom,A., Trimborn,T., Willert,K., Nusse,R., Jessell,T.M. and Edlund,T. (2001) The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature, 411, 325–330. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Tanaka,K., Kitagawa,Y. and Miki,K. (1999) A PEA3 site flanked by SP1, SP4 and GATA sites positively regulates the differentiation-dependent expression of Brachyuryin embryonal carcinoma P19 cells. Biochem. Biophys. Res. Commun., 254, 542–547. [DOI] [PubMed] [Google Scholar]
- Zhang J., Houston,D.W., King,M.L., Payne,C., Wylie,C. and Heasman,J. (1998) The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell, 94, 515–524. [DOI] [PubMed] [Google Scholar]
- Zhao C.T., Zhang,Y., Su,Y. and Meng,A.M. (2002) Somite-specific expression of a novel fibronectin variant FN3 is negatively regulated by SHH. Chin. Sci. Bull., 47, 1807–1811. [Google Scholar]
- Zhou X., Sasaki,H., Lowe,L., Hogan,B.L. and Kuehn,M.R. (1993) Nodal is a novel TGF-β-like gene expressed in the mouse node during gastrulation. Nature, 361, 543–547. [DOI] [PubMed] [Google Scholar]