Abstract
Aims
Hawthorn's efficacy when added to contemporary evidence-based heart failure therapy is unknown. We aimed to determine whether hawthorn increases submaximal exercise capacity when added to standard medical therapy.
Methods and results
We performed a randomized, double-blind, placebo-controlled trial in 120 ambulatory patients aged ≥18 years with New York Heart Association (NYHA) class II-III chronic heart failure. All patients received conventional medical therapy, as tolerated, and were randomized to either hawthorn 450 mg twice daily or placebo for 6 months. The primary outcome was change in 6 min walk distance at 6 months. Secondary outcomes included quality of life (QOL) measures, peak oxygen consumption, and anaerobic threshold during maximal treadmill exercise testing, NYHA classification, left ventricular ejection fraction (LVEF), neurohormones, and measures of oxidative stress and inflammation. There were no significant differences between groups in the change in 6 min walk distance (P = 0.61), or on measures of QOL, functional capacity, neurohormones, oxidative stress, or inflammation. A modest difference in LVEF favoured hawthorn (P = 0.04). There were significantly more adverse events reported in the hawthorn group (P = 0.02), although most were non-cardiac.
Conclusion
Hawthorn provides no symptomatic or functional benefit when given with standard medical therapy to patients with heart failure.
This trial is registered in ClinicalTrials.gov ID: NCT00343902.
Keywords: Hawthorn, Crataegus, Heart failure, Systolic
Introduction
Contemporary therapies [i.e. angiotensin-converting enzyme- (ACE) inhibitors, beta-blockers, spironolactone, implantable cardioverter–defibrillators, and biventricular pacemakers] have produced remarkable reductions in heart failure-related morbidity and mortality. Despite these advances, both the length and quality of life (QOL) for those with heart failure remain impaired and improved treatment regimens are still needed.
Crataegus monogyna or hawthorn has been used for cardiac and circulatory disorders since the first century AD.1 Hawthorn demonstrates numerous properties that may be beneficial in heart failure including anti-arrhythmic activities,2,3 and the ability to increase coronary blood flow,4,5 and cardiac output.6 These effects may be mediated by inhibition of phosphodiesterase types III and IV,7,8 antioxidant activities9,10 and anti-inflammatory effects.3,11 A meta-analyses of clinical trials concluded that hawthorn may be a safe and effective treatment for chronic heart failure.12
Although most previous trials of hawthorn have reported modest improvements in exercise capacity, QOL, and heart failure-related symptoms,12 these studies are limited by their short duration, lack of meaningful clinical outcomes for CHF, unclear severity of study patients, and frequent absence of conventional, evidence-based, concomitant medical therapy. Using a well-defined patient sample, meaningful standardized clinical outcomes, longer study duration, and contemporary evidence-based medical therapy, we conducted a randomized, placebo controlled, double-blind clinical trial to determine the efficacy of a hawthorn extract for the treatment of ambulatory patients with New York Heart Association (NYHA) class II to III chronic heart failure.
Methods
The study protocol and all procedures were approved by the University of Michigan Medical School Institutional Review Board and were overseen by an independent Data Safety Monitoring Board. All participants provided written informed consent. Ambulatory patients aged 18 years of age and older with a ≥3 months history of HF (NYHA classes II-III) and a left ventricular ejection fraction (LVEF) ≤40% (by radionuclide or contrast ventriculography or by echocardiography), as assessed during usual clinical care within the 12 months prior to randomization, were eligible for enrolment. Patients had to be receiving standard medical therapy (in the absence of a contraindication or intolerance), defined as an angiotensin converting enzyme inhibitor or angiotensin receptor antagonist, a beta-blocker and a diuretic. Potential subjects had to walk between 150 and 450 m during two 6 min walk tests, conducted 2 weeks apart (screening and baseline visits, respectively), to be deemed eligible for the study. Eligible subjects were randomly assigned to receive either C. oxycantha extract, Crataegus Special Extract WS 1442 (Crategutt forte, Willmar Schwabe Pharmaceuticals, Karlsruhe, Germany), 450 mg orally twice daily, or a matching placebo. Patients were seen at the study clinic 3 months and 6 months after the baseline visit. A detailed description of the study inclusion and exclusion criteria, entry screening process, and the study intervention has been published recently.13
Objectives and outcomes
Our primary objective was to test the effect of Crataegus Special Extract WS 1442 on submaximal exercise capacity at 6 months as determined by the 6 min walk test. The 6 min walk test is a validated measure of submaximal exercise capacity in which a study participant is asked to walk at their best pace for 6 min along a designated 20 m straight path. During the test, pre-specified verbal encouragements (e.g. ‘you are doing very well’) were given at 30 s and 2 min intervals to increase the likelihood that participants would achieve and maintain their best walking pace.
The secondary objectives included: (i) physician and patient global assessment of the change in heart failure symptoms from baseline on a 7-point Likert scale (markedly, moderately, or mildly better; same; mildly, moderately, or markedly better); (ii) disease-specific QOL as determined by the Minnesota Living with Heart Failure Questionnaire (MLHFQ); (iii) functional capacity as accessed by peak exercise oxygen consumption (peak VO2) and anaerobic threshold during maximal cardiopulmonary treadmill exercise testing using a modified Naughton ramp protocol; (iv) functional capacity as subjectively assessed by NYHA functional classification; (v) LVEF; (vi) mortality risk as determined by the Heart Failure Survival Score (HFSS);14 (vii) neurohormone profile (plasma norepinephrine and brain natriuretic peptide); (viii) oxidative stress (plasma F2α 8-isoprostane); (ix) inflammation (plasma high-sensitivity C-reactive protein); (x) global QOL [as determined by the EuroQol-5D questionnaire (EQ5D)15]; (xi) utility for health status [as determined by the utility component of the EQ5D (Visual Analogue Scale)15], and (xii) hospitalizations (heart failure-related and total) during the 6 month study period.
Randomization, blinding, and allocation
Eligible participants were randomized equally to either placebo or hawthorn groups. The randomization code was computer-generated in blocks of size 6 by the study biostatistician. The randomization list was then given to the research pharmacist who was not associated with the study. The research pharmacist dispensed the study medication, which was in blister packs provided by the manufacturer, and enclosed it in numbered boxes as per the randomization scheme. All study participants as well as all study personnel who assessed outcomes worked with study data or administered tests or questionnaires were unaware of the randomization list or treatment assignment.
Statistical methods and sample size
Baseline characteristics are reported, stratified by treatment group, using means and SDs for continuous variables, and counts and percentages for categorical variables. Balance between treatment groups on baseline characteristics was tested using independent samples t-tests for continuous variables and Fisher exact tests for categorical variables. For continuous variables, the assumption of normality was checked using the Shapiro–Wilks test. To investigate a change from baseline in 6 min walk distance or secondary continuous outcomes, the difference between the 6 months and baseline measure was calculated. Analyses on these differences were performed using independent samples t-tests between treatment groups.
Despite the entry criteria of an LVEF ≤40% on a clinically indicated study obtained within the prior 12 months (see above), the baseline study radionuclide ventriculogram performed after randomization revealed an LVEF of greater than 40% in 38 study participants. Since the baseline assessment of LVEF occurred after randomization, we were not able to exclude these patients. Consequently, the effects of study drug vs. placebo on 6 min walk distance and secondary outcomes at 6 months were also evaluated by ANCOVA, adjusting for baseline value and baseline LVEF. For physician and patient global assessments and NYHA, the change over time was tested using the Wilcoxon rank-sum test. For categorical secondary variables, Fisher exact tests were first performed, followed by adjusted analyses using Cochran–Mantel–Haenzel tests stratifying by LVEF (≤40 and >40%). For ranked data (i.e. NYHA class), a Cochran–Armitage χ2 for linear trend was performed. Analyses were conducted according to the intention-to-treat principle when possible (i.e. for deaths, hospitalizations and adverse events), however no imputation was performed for missing values at 6 months. Data were entered into SPSS Windows version 11 (SPSS, Chicago, IL, USA) and analysed using SAS version 9 (Cary, NC: SAS Institute Inc.). For all analyses, two-sided tests and a significance level of 0.05 were used. The experiment-wise Type I error rate was protected only for the principal outcome measure. No adjustments were made for multiple hypotheses testing as the secondary outcomes were viewed as hypothesis generating.
Since the effect of hawthorn on 6 min walk distance had not been studied previously, no such information was available to guide sample size considerations. We selected 40 m (≥10% improvement) as a clinically meaningful improvement in 6 min walk distance, based on our anticipation of a mean baseline walk distance of 350–400 m and no change in the control arm. The study was therefore designed to have 80% power to detect a treatment difference of 39 m (85% power to detect a treatment difference of 41 m) in 6 min walk distance, based on published standard deviations of 64 to 74 in similar populations walking between 150 and 450 m,16 assuming a two-sided alpha level of 0.05 and n = 60 patients per treatment group. We calculated retrospective power by substituting the observed pooled standard deviation of the change in 6 min walk distance from baseline to 6 months to the hypothesized 40 m improvement, 120 patient total sample size, and two-sided alpha level of 0.05. A similar retrospective power analysis was done for the 82 patient subsample with baseline LVEF ≤40%.
Results
Screening, enrolment, and withdrawals
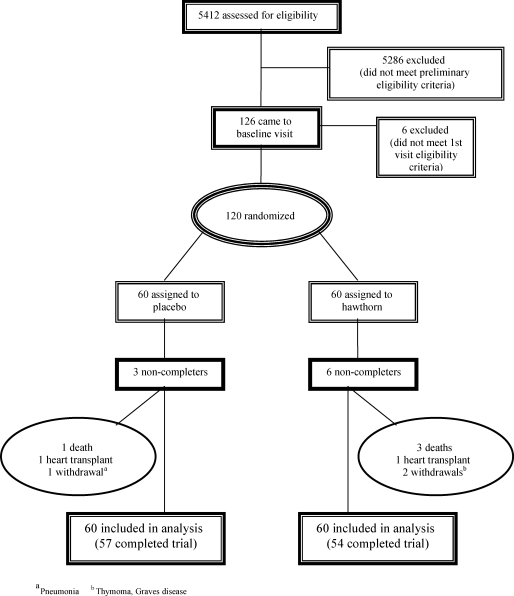
We screened 5412 patients, of whom 120 met all eligibility criteria and were randomized, 60 to the placebo and 60 to the hawthorn group. Figure 1 document sources of recruitment for potential participants, reasons for exclusions, and reasons for discontinuing the intervention. The low proportion of recruited patients reflects the broad screening of unselected patients presenting to our cardiology clinics. Fifty-seven participants in the placebo group and 54 participants in the hawthorn arm completed all study visits. Adherence to study medications was high with 98% of all participants taking greater than 95% of all study medication and with no significant differences between groups.
Figure 1.
Patient Flow in the Randomized Controlled Trial.
Sociodemographic and clinical characteristics
In Table 1, we present the sociodemographic and clinical characteristics of participants by treatment group. There were no significant differences between treatment groups for any demographic or clinical characteristics.
Table 1.
Baseline characteristics
| Characteristics | Placebo (n = 60), n (%) or mean ± SD | Hawthorn (n = 60), n (%) or mean ± SD | P-value* |
|---|---|---|---|
| Sex | 0.83 | ||
| Men | 44 (73) | 46 (77) | |
| Women | 16 (27) | 14 (23) | |
| Age | 57.8 (±9.0) | 54.4 (±12.6) | 0.10 |
| Race | 0.37 | ||
| White | 50 (83) | 45 (75) | |
| Blood pressure, mm Hg | |||
| Systolic | 113 (±19) | 109 (±15) | 0.25 |
| Diastolic | 66 (±10) | 66 ± 10 | 0.76 |
| Heart rate, bpm | 71 ± 11 | 68 ± 11 | 0.14 |
| NYHA class | 0.72 | ||
| II | 33 (55) | 30 (50) | |
| III | 27 (45) | 30 (50) | |
| LVEF, % | 34.8 (±14.5) | 36.2 (±15.1) | 0.59 |
| LVEF ≤40% | 44 (73) | 38 (63) | 0.33 |
| Six-minute walk test, m | 374.0 (±52.4) | 358.2 (±59.2) | 0.13 |
| Peak oxygen consumption, mL\kg\min | 14.6 (±3.8) | 14.7 (±3.5) | 0.79 |
| Medications | |||
| ACE inhibitor | 46 (77) | 48 (80) | 0.82 |
| ARB | 11 (18) | 9 (15) | 0.81 |
| ACE inhibitor or ARB | 57 (95) | 57 (95) | 1.00 |
| Beta-blocker | 52 (87) | 54 (90) | 0.78 |
| Digoxin | 49 (82) | 47 (78) | 0.82 |
| Loop diuretic | 57 (95) | 55 (92) | 0.72 |
| Spironolactone | 39 (65) | 39 (65) | 1.00 |
| Thiazide diuretic | 10 (17) | 11 (18) | 1.00 |
NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ACE, angiotensin-converting enzyme; ARB, angiotensin-II receptor blockers.
*P-value based on independent sample t-test for continuous variables and Fisher exact tests for categorical variables.
Effects on blood pressure and heart rate
There were no significant between group differences for changes in blood pressure [ΔSBPhawthorn –2.1 ± 13.3 (mean ± standard deviation), ΔSBPplacebo 0.8 ± 13.2, P = 0.26; ΔDBPhawthorn −0.8 ± 10.7, ΔDBP 0.9 ± 11.4, P = 0.45], or heart rate (ΔHRhawthorn 1.9 ± 11.0, ΔHRplacebo 0.7 ± 8.4, P = 0.51) at rest over the 6 month study period.
Clinical and quality of life outcomes
Tables 2–4 present the results for the primary and secondary study endpoints. In Table 2, we present all continuous outcomes (mean difference at 6 months and adjusted mean 6 month difference by LVEF and baseline variable of interest), in Table 3, physician and patient global assessments and in Table 4, data on NYHA classifications.
Table 2.
HERB CHF results
| Outcome | Baseline |
Six month visit |
P-valuea | P-valueb | ||
|---|---|---|---|---|---|---|
| Placebo (N = 60), Mean ± SD | Hawthorn (N = 60), Mean ± SD | Placebo (N = 57), Mean ± SD | Hawthorn (N = 54), Mean ± SD | |||
| Six minute walk test, m | 374 ± 52 | 358 ± 59 | 379 ± 52 | 371 ± 89 | 0.35 | 0.61 |
| MLHFQ | ||||||
| Total | 47 ± 21 | 49 ± 20 | 40 ± 20 | 43 ± 23 | 0.62 | 0.46 |
| Emotional | 8.9 ± 6.5 | 9.7 ± 5.4 | 7.4 ± 5.3 | 8.2 ± 5.3 | 0.83 | 0.61 |
| Physical | 22.3 ± 9.3 | 23.6 ± 9.7 | 18.9 ± 8.4 | 20.3 ± 11.3 | 0.53 | 0.45 |
| EQ-5D | ||||||
| Questionnaire score | 8.90 ± 2.1 | 9.4 ± 1.9 | 8.6 ± 1.9 | 9.3 ± 2.0 | 0.51 | 0.18 |
| Visual analogue scale | 68 ± 18.8 | 60 ± 18.5 | 73 ± 13.8 | 70 ± 20.4 | 0.07 | 0.58 |
| LVEF, % | 35 ± 14 | 37 ± 15 | 33 ± 13 | 37 ± 16 | 0.06 | 0.04 |
| Peak exercise test | ||||||
| Peak O2 consumption, mL/kg/min | 14.6 ± 3.8 | 14.7 ± 3.5 | 14.7 ± 4.4 | 15.7 ± 3.5 | 0.22 | 0.22 |
| Anaerobic threshold, mL/kg/min | 10.0 ± 3.2 | 10.4 ± 2.6 | 10.1 ± 2.8 | 10.1 ± 2.5 | 0.49 | 0.67 |
| HFSS | 8.77 ± 1.1 | 8.90 ± 1.2 | 8.62 ± 1.1 | 9.02 ± 1.3 | 0.13 | 0.22 |
| BNP, pg/mL | 260 ± 446 | 232 ± 347 | 281 ± 495 | 243 ± 519 | 0.47 | 0.25 |
| NE, pg/mL | 445±231 | 388 ± 194 | 512 ± 447 | 444 ± 312 | 0.38 | 0.50 |
| hs-C-reactive protein, pg/mL | 8.9 ± 6.6 | 9.3 ± 6.5 | 8.5 ± 6.1 | 9.2 ± 6.9 | 0.86 | 0.77 |
| 8-isoprostanes, pg/mL | 174 ± 87 | 173 ± 85 | 183 ± 89 | 170 ± 93 | 0.14 | 0.12 |
MLHFQ, Minnesota Living with Heart Failure Questionnaire; EQ-5D, (EuroQol) European quality of life instrument; LVEF, left ventricular ejection fraction; HFSS, Heart Failure Survival Score; BNP, B-type natriuretic peptide; NE, norepinephrine.
aIndependent sample t-test of mean difference.
bANCOVA adjusted for baseline value of the variable of interest and for baseline LVEF (as a continuous measure) except for HFSS and LVEF which are only adjusted for baseline variable.
Table 4.
HERB CHF NYHA results
| Outcome | Baseline visit |
Six month visit |
P-value* | P-value** | ||
|---|---|---|---|---|---|---|
| Placebo, n (%)a | Hawthorn, n (%)a | Placebo, n (%)a | Hawthorn, n (%)a | |||
| NYHA Class | 0.56 | 0.64 | ||||
| I | 0 (0) | 0 (0) | 4 (7) | 8 (15) | ||
| II | 33 (55) | 30 (50) | 31 (54) | 23 (43) | ||
| III | 27 (45) | 30 (50) | 22 (29) | 21 (39) | ||
| IV | 0 (0) | 0 (0) | 0 (0) | 2 (4) | ||
NYHA, New York Heart Association classification.
aPercentages may not add up to 100 due to rounding of digits.
*P-value based on Wilcoxon rank-sum test on the change in NYHA from baseline to 6 months after study entry.
**P-value based on Cochran–Armitage χ2 for linear trend on the change in NYHA from baseline to 6 months after study entry.
Table 3.
HERB CHF global assessment results
| Outcome | Six month visit |
P-value(1)* | P-value(2)* | |
|---|---|---|---|---|
| Placebo, n (%)a | Hawthorn, n (%)a | |||
| Patient global assessment | 0.55 | 0.58 | ||
| Markedly better | 9 (16) | 10 (19) | ||
| Moderately better | 14 (25) | 10 (19) | ||
| Mildly better | 11 (20) | 8 (15) | ||
| No change | 17 (30) | 18 (34) | ||
| Mildly worse | 4 (7) | 4 (8) | ||
| Moderately worse | 1 (2) | 3 (6) | ||
| Markedly worse | 0 (0) | 0 (0) | ||
| Physician global assessment | 0.76 | 0.59 | ||
| Markedly better | 4 (7) | 7 (13) | ||
| Moderately better | 14 (25) | 7 (13) | ||
| Mildly better | 10 (18) | 14 (26) | ||
| No change | 22 (39) | 14 (26) | ||
| Mildly worse | 4 (7) | 6 (11) | ||
| Moderately worse | 1 (2) | 2 (4) | ||
| Markedly worse | 1 (2) | 4 (7) | ||
aPercentages may not add up to 100 due to rounding of digits.
*P-value based on Wilcoxon rank-sum test comparing placebo to hawthorn at 3 months (1) or 6 months (2) after study entry. Ratings were converted to ordinal values from 1 (markedly worse) to 7 (markedly better) for calculation.
We found no significant difference between the placebo and hawthorn groups in our primary outcome, change in 6 min walk distance over the 6 month study period (P = 0.61). This reflected an absence of any statistically significant within group effect of hawthorn (14 ± 62 m, 95% CI 3–31 m) or placebo (5 ± 32 m, 95% CI 3–14 m) on 6 min walk distance. Using the observed pooled standard deviation of 49 m for the change in 6 min walk distance, this study had greater than 99% power to have detected the hypothesized 40 m difference in 6 min walk distance between treatment groups. Moreover, using the observed pooled standard deviation of 51.04 m for the change in 6 min walk distance for the 82 patients with LVEF ≤40%, results in 93% power to detect the same 40 m difference.
Other evaluations of functional capacity were similarly unimpressive, with no significant between group differences for changes in peak exercise oxygen consumption (P = 0.22) or anaerobic threshold (P = 0.67) by maximal cardiopulmonary testing and no significant linear trend in the change in NYHA (P = 0.64).
There were no significant differences between any measure of QOL or health utility—neither global nor disease-specific (MLHFQ, patient or physician global assessment, EQ5D)—nor in measures of neurohormonal activation (NE, BNP), oxidative stress (8-isoprostane) or inflammation (hs-C-reactive protein).
We did find a difference (P = 0.04, no adjustment for multiple testing) in favour of hawthorn for LVEF in the analysis adjusting for baseline LVEF. This resulted from a slight drop in LVEF in the placebo group with no change in the hawthorn group. Stratification by LVEF (≤40 vs.>40%) revealed a decrease in LVEF in both LVEF strata of the placebo group with a 3 to 4 EF unit relatively more favourable change in each LVEF strata of the hawthorn group.
Moreover, when the primary and secondary outcomes were restricted to the 82 patients with LVEF ≤40% the differences between the hawthorn and placebo groups remained statistically insignificant (Table 5).
Table 5.
HERB CHF results for patients with left ventricular ejection fraction ≤40%
| Outcome | Baseline |
Six month visit |
P-valuea | P-valueb | ||
|---|---|---|---|---|---|---|
| Placebo (N = 44), mean ± SD | Hawthorn (N = 38), mean ± SD | Placebo (N = 41), mean ± SD | Hawthorn (N = 33), mean ± SD | |||
| Six minute walk test, m | 373 ± 51 | 347 ± 54 | 377 ± 51 | 352 ± 88 | 0.88 | 0.96 |
| MLHFQ | ||||||
| Total | 43 ± 20 | 52 ± 19 | 38 ± 18 | 47 ± 23 | 0.53 | 0.20 |
| Emotional | 7.8 ± 6.1 | 9.5 ± 5.5 | 6.3 ± 4.6 | 8.7 ± 5.4 | 0.44 | 0.10 |
| Physical | 21.7 ± 8.7 | 24.7 ± 9.0 | 18.8 ± 8.2 | 22.6 ± 10.7 | 0.43 | 0.21 |
| EQ-5D | ||||||
| Questionnaire score | 8.6 ± 1.9 | 9.3 ± 2.0 | 8.3 ± 2.0 | 9.3 ± 1.8 | 0.70 | 0.15 |
| Visual analogue scale | 68 ± 19.1 | 60 ± 18.6 | 73 ± 14.7 | 66 ± 22.0 | 0.18 | 0.72 |
| LVEF, % | 28 ± 8 | 27 ± 8 | 27 ± 8 | 29 ± 12 | 0.09 | 0.13 |
| Peak exercise test | ||||||
| Peak O2 consumption, mL/kg/min | 13.7 ± 3.1 | 13.8 ± 3.1 | 14.0 ± 4.2 | 15.0 ± 3.6 | 0.18 | 0.18 |
| Anaerobic threshold, mL/kg/min | 9.4 ± 3.2 | 9.9 ± 2.6 | 9.8 ± 2.2 | 9.8 ± 2.7 | 0.88 | 0.83 |
| HFSS | 8.3 ± 0.9 | 8.2 ± 0.7 | 8.2 ± 0.9 | 2.3 ± 1.0 | 0.24 | 0.25 |
| BNP, pg/mL | 305 ± 490 | 340 ± 395 | 346 ± 561 | 345 ± 630 | 0.72 | 0.63 |
| NE, pg/mL | 455±240 | 427 ± 197 | 500 ± 399 | 517 ± 352 | 0.83 | 0.81 |
| hs-C-reactive protein, pg/mL | 9.1 ± 6.8 | 9.9 ± 6.3 | 8.8 ± 6.4 | 9.5 ± 6.5 | 0.60 | 0.75 |
| 8-isoprostanes, pg/mL | 179 ± 84 | 169 ± 88 | 174 ± 79 | 167 ± 102 | 0.60 | 0.59 |
aIndependent sample t-test of mean difference.
bANCOVA adjusted for baseline value of the variable of interest.
MLHFQ, Minnesota Living with Heart Failure Questionnaire; EQ-5D, (EuroQol) European quality of life instrument; LVEF, left ventricular ejection fraction; HFSS, Heart Failure Survival Score; BNP, B-type natriuretic peptide; NE, norepinephrine.
Adverse events and hospitalizations
Adverse events and hospitalizations are displayed in Table 6. There were no significant differences in total deaths, deaths due to CHF, or deaths due to cardiovascular disease (CVD) between treatment groups. We divided adverse events into cardiac categories (e.g. angina and atrial fibrillation) and those that most commonly occurred in the trial (e.g. infections). While there were significantly more total adverse events in the hawthorn group (36 vs. 23, P = 0.02), there was no significant difference in total cardiac events or in any category of cardiac events, nor was there another specific category of adverse events that differed in frequency between placebo and hawthorn groups. There were 37 participants who were hospitalized during the study period (range of one to four hospitalizations) for non-elective reasons. We found no significant difference in total hospitalizations between groups with 23 participants hospitalized in the hawthorn group and 14 participants hospitalized in the placebo group (P = 0.11). Of the 37 participants, 22 were hospitalized for a reason directly related to their CHF. We observed no significant difference in hospitalizations due to CHF (P = 0.24), for which 14 participants in the hawthorn groups were hospitalized vs. 8 in the placebo group. We also found no significant difference between either total (P = 0.07) or CHF-related (P = 0.11) hospitalizations after adjusting for baseline LVEF.
Table 6.
Death, hospitalization, and other adverse events in HERB CHF
| Adverse event | Placebo (n = 60), n (%) | Hawthorn (n = 60), n (%) | P-Value(1)a | P-Value(2)a |
|---|---|---|---|---|
| All deaths (within 6 months) | 2 (3) | 5 (8) | 0.26 | 0.22 |
| Deaths due to CHFb | 1 (2) | 1 (2) | 1.0 | 0.92 |
| Deaths due to CVDb | 2 (3) | 2 (3) | 1.0 | 0.99 |
| All hospitalizations (within 6 months) | 14 (23) | 23 (38) | 0.11 | 0.07 |
| Hospitalizations due to CHFb | 8 (13) | 14 (23) | 0.24 | 0.11 |
| All patients with any adverse events | 23 (38) | 36 (60) | 0.02 | 0.02 |
| All cardiac-related adverse events | 12 (20) | 12 (20) | 1.0 | 0.98 |
| Worsening CHF | 5 (8) | 5 (8) | 1.0 | 0.81 |
| Angina/chest pain | 3 (5) | 2 (3) | 1.0 | 0.93 |
| Syncopal event | 2 (3) | 3 (5) | 0.65 | 0.61 |
| Atrial fibrillation | 2 (3) | 2 (3) | 1.0 | 0.98 |
| Infectionsc | 9 (15) | 7 (12) | 0.59 | 0.68 |
| Headache | 1 (2) | 1 (2) | 1.0 | 0.95 |
| Rash | 1 (2) | 2 (3) | 0.57 | 0.57 |
| GI symptomsd | 2 (3) | 5 (8) | 0.47 | 0.59 |
| Musculoskeletale | 1 (2) | 2 (3) | 0.59 | 0.48 |
| Other adverse eventf | 10 (17) | 18 (30) | 0.13 | 0.08 |
aP-values based on (1) Fisher exact tests comparing adverse or (2) Cochran–Mantel–Haenzel tests, stratified by LVEF (≤40 and >40%) events in hawthorn vs. placebo.
bOne death in the placebo group is due to both CHF and CVD; causes of death in the hawthorn group other than CVD or CHF are aplastic anaemia, renal failure, and unknown causes.
cInfections include styes, URI, pneumonia, conjunctivitis, cellulitis, infected pilonidal cyst, dental abscess, and urosepsis.
dGI symptoms include: constipation, diarrhea, loose stool, nausea and vomiting.
eMusculoskeletal includes: sprain/strain of back, degenerative joint disease in knee.
fOther includes: pancreatitis, lightheadedness and hypotension, complication of diabetes, onset of diabetes, renal insufficiency with multiple cysts found on kidney, discharge of defibrillator, elevated transaminases, narcotic dependency, thymus gland with diffuse nodular lymphoid hyperplasia, femoral artery stent for PVOD, superficial thrombophlebitis, hyperthyroidism, dysphasia, prerenal azotemia, thrombocytopenia, replacement of defibrillator; small lesions (on pancreas, liver and kidneys), increased serum levels of AST and total bilirubin, gout, leg ulcer from PVOD, plantar keratosis, venous thrombosis of the right great saphenous vein, phlebitis, lower extremity deep venous thrombosis. CVD, cardiovascular disease.
Discussion
We found no benefit of a hawthorn extract, in the dose and formulation used, on our primary endpoint, the 6 min walk test distance, when added to contemporary standard therapy in patients with systolic chronic heart failure. Likewise, hawthorn extract caused no difference in any index of QOL including heart failure related or global QOL, patient and physician assessments, or health utility measures. These results are consistent whether evaluated in unadjusted analyses or in analyses that adjusted for patients’ baseline LVEF and baseline value for each measure. We also found no differences between hawthorn and placebo for any secondary outcome except for LVEF, for which we observed a modest difference in its change over the 6 month study period in favour of hawthorn. As we did not adjust for multiple hypothesis testing in secondary analyses, this should not be interpreted as substantial evidence for a positive effect of hawthorn on left ventricular function but rather as worthy of further study. Overall, the difference in LV function resulted from a decrease in LVEF in the placebo group and maintenance of LVEF in the hawthorn group. The drop in LVEF in the placebo group was driven by a substantial reduction in those patients whose initial LVEF was greater than 40%. Since all study subjects had clinical measurements of LVEF of 40% or less prior to study entry, some of the reduction of LVEF in these patients may have reflected a regression to the mean.
In general, hawthorn appeared to be well tolerated. There were no differences between placebo and hawthorn for cardiac-related adverse events or in common AE categories including infections, rashes, gastrointestinal complaints, or headaches. However, we observed significantly more total adverse events in the hawthorn group. The increased number of adverse events in the hawthorn group was driven by miscellaneous events. The wide variety of events in the hawthorn group appears unlikely to be explainable by a pharmacologic effect of hawthorn and may have occurred by chance alone. In addition, there were no significant differences between hawthorn and placebo in the number of deaths (all or CHF related) nor in study participants hospitalized overall or for CHF. Given the modest sample size of the present study, the possibility of a type II error must be considered. In this regard, the statistically insignificant increase in hospitalizations (total and CHF) in the analysis stratified by baseline LVEF should be explored in future studies.
Our results are in contrast to numerous earlier RCTs examining the safety and efficacy of hawthorn extracts in systolic chronic heart failure. A meta-analysis12 examining these RCTs concluded that, ‘there is a significant benefit from hawthorn extract as an adjunctive treatment for chronic heart failure.’ Overall, hawthorn extracts were found to significantly decrease pressure–heart rate product and maximal workload and to improve heart failure related symptoms.12 There are numerous possible explanations for the different findings in our trial. Previous RCTs with hawthorn have failed to mandate contemporary, evidence-based, concomitant medical therapy for heart failure in their control and active treatment arms. In several studies, it is unclear or unspecified which medications were allowed.17,18 Other studies have not allowed any concomitant medications,19 or allowed only diuretics as concomitant heart failure therapy,18,20,21 while remaining studies have allowed, but not required (absent contraindications or intolerances), an ACE inhibitor.22–24 No studies have required use of a beta-blocker (absent contraindications or intolerances). When concomitant therapies were allowed, no period of dose stabilization was required. Hawthorn's mechanism of action may have considerable pharmacological overlap with several medications regularly prescribed for heart failure such as beta-blockers or ACE inhibitors. One trial has been conducted in which a hawthorn extract (LI 132 at a dose of 300 mg three times daily) was directly compared with the ACE inhibitor captopril (12.5 mg three times daily) in 132 patients. Both groups experienced improved exercise tolerance and a decrease in frequency of heart failure-related symptoms. There were no significant differences in the responses.25 Thus, hawthorn could have had clinical effects in studies in which these concomitant medications were not used that were masked by the effects of such medications in our study.
Earlier studies enrolled samples that were potentially less ill than our study sample. Most prior trials did not specify how long a study participant had to have heart failure before study entry. These studies may have enrolled patients with recent acute heart failure who were destined for recovery over the next few months regardless of medical interventions. All but one prior study24 enrolled only NYHA class II patients, excluding patients with more advanced heart failure. By enrolling patients who walked between 150 and 450 m on a screening 6 min walk test, and by excluding patients with comorbidities (e.g. arthritis, peripheral vascular disease, etc.) that could limit exercise performance, we identified a group of stable CHF patients that were neither too ill nor too well to achieve measurable improvements in exercise capacity with a successful intervention.
Differences between our results and previous hawthorn RCTs could also be due to different exercise and QOL measures. The principal exercise outcome in earlier studies was the change in pressure-rate product (PRP) at constant, low-level bicycle exercise.17,22 The smaller increase in PRP for the same level of external work for hawthorn vs. placebo patients found in other RCTs represents greater exercise efficiency. However, this outcome measure, the change in PRP, has minimal clinical relevance and no known prognostic significance in patients with CHF.26 On the other hand, the 6 min walk test distance is a surrogate measure that provides relevant information about death27 and QOL,28 arguably the two most clinically relevant outcome measures in heart failure. Further, peak VO2 is an objective, reproducible measure of maximal functional capacity, and is a strong, consistent, and well-validated predictor of mortality in heart failure.29 No other trial examining hawthorn for heart failure had a submaximal exercise outcome or assessed peak VO2.
Many hawthorn trials have used patient and physician assessments, but few have used either global or disease-specific QOL measures that have been validated within the CHF population. Without these measures, it is difficult to assess the impact of hawthorn on QOL (disease-specific and global), and impossible to compare the QOL impact of hawthorn to other heart failure therapies. In contrast, the HERB-CHF study utilized both global (EQ5D) and disease-specific (MLHFQ) QOL scales. Both are well-validated measures, and the MLHFQ has been used in numerous clinical heart failure drug trials to evaluate clinical response.
It is possible that the negative results we observed in this study are due to incorrect preparation or too low a dose of hawthorn extract. Most studies examining Crataegus Special Extract WS 1442 have used similar or lower doses. However, one study24 examined the difference between a 900 and 1800 mg daily dose of Crataegus Special Extract WS 1442 in patients with NYHA class III CHF on maximal work load (bicycle exercise test), both patients and physicians QOL assessments and safety. Both doses were found to be statistically superior to placebo. The 1800 mg dose was found to be superior to the 900 mg dose for both patient and physician assessment of efficacy and tolerability. However, there was no difference between doses for maximal workload tolerated or a score of typical heart failure symptoms. These results would appear to indicate that little is gained by substantially increasing the dose. Also, as with any herbal product hawthorn extracts can be made in numerous fashions leading to different constituent blends with potentially different medicinal effects. We chose to use the Crataegus Special Extract WS 1442 preparation because it was widely utilized in Europe, had been used the most in previous studies in chronic heart failure,12 and was manufactured with high levels of quality control. As such, by utilizing the Crataegus Special Extract WS 1442 preparation we were able to compare our results to the majority of other studies, have clinical relevance to patients and clinicians, and offer considerable prior information on safety and tolerability. However, other hawthorn preparations could produce other outcomes and adverse effects than those presented in this study.
Limitations
Our study has several limitations. We did not assess baseline study LVEF until after randomization and instead relied on LVEF measures obtained for clinical indications within 12 months of enrolment to determine LVEF for study entry. Consequently, some participants may have improved in the interim with corresponding increases in their LVEF, although all continued to have substantial clinical heart failure. The subsequent decline in LVEF in those with baseline LVEF >40% suggests that some of the baseline measurements overestimated LV function. We were limited also by inadequate power to detect small effect sizes for secondary outcomes. However, effect sizes observed for these outcomes were all so small that clinically significant differences appear unlikely.
Our study is much too small to provide a reliable estimate of the effect of this hawthorn extract on mortality. The lack of an effect on the 6 min walk test, peak VO2, and the Heart Failure Survival Score, each a well-validated prognostic marker in chronic heart failure, suggests that a mortality benefit would not be expected. Our results appear to complement the results of SPICE, a randomized clinical trial comparing the same hawthorn extract used in HERB CHF to placebo in 2681 patients with NYHA class II-III systolic heart failure and LVEF ≤35%, as reported in the European Journal of Heart Failure. No effect of hawthorn was observed on the principal outcome of time until first cardiac event, defined as a composite of cardiac death, non-fatal myocardial infarction, and hospitalization due to progressive heart failure.30
There is weak evidence from this study that hawthorn may have a favourable effect on LVEF. A preparation that increases LVEF may appear attractive to heart failure patients and their physicians. However, without a proven mortality benefit, a heart failure medication should provide clear evidence of an improvement in symptoms, QOL, or functional capacity. As hawthorn—or more specifically, this hawthorn extract at the dose studied—provided none of these benefits, its use outside the context of a clinical trial cannot be recommended.
Conclusion
In summary, the data from this study indicate that a hawthorn extract, Crataegus Special Extract WS 1442, provides no clinical benefit, at the dosing regimen evaluated, when given in addition to standard evidence-based contemporary medical therapy to patients with ambulatory symptomatic chronic heart failure. Hawthorn extract may be associated with an increase in non-cardiac adverse events.
Funding
This research was supported by a grant to the University of Michigan Complementary and Alternative Medicine Research Center from the National Center for Complementary and Alternative Medicine (P50 HL 061202 01). Research resources were also provided by the General Clinical Research Center of the University of Michigan (M01-RR00042).
Acknowledgements
We are grateful for the work of the members of the Data Safety Monitoring Board (Drs Gary Chase, Jonathan Sackner Bernstein, Allen Sedman, Cynthia Long, and Shan Wong), and for the contributions of our study team: Drs Robert Cody, Todd Koelling, David Bradley Dyke, Audrey Wu, Ragaven Baliga (UM Heart Failure and Transplant Management Program); Brian Nordin (exercise physiology); Dr Roberta Tankanow (research pharmacy); E. Mitchell Seymour (laboratory sciences); Dr James Corbett (nuclear medicine); Margaret Ann Murphy (data management); Alexis Zirpoli and Amie Litzinger (manuscript preparation); Katherine Rice, Patricia Stimac, Robert Adwere-Boamah, Amy Blume, and Fayeannette Pierce (clinical research assistants); Drs Sara Warber and Steven Bolling (U-M Complementary and Alternative Medicine Research Center). We would also like to thank Dr Willmar Schwabe Pharmaceuticals and Dr Günter Meng for generously providing the Crataegus Special Extract WS 1442 and matching placebo. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Conflict of interest: none declared.
References
- 1.Weihmayr T, Ernst E. Therapeutic effectiveness of Crataegus. Fortschr Med. 1996;114:27–29. [PubMed] [Google Scholar]
- 2.Garjani A, Nazemiyeh H, Maleki N, Valizadeh H. Effects of extracts from flowering tops of Crataegus meyeri A. Pojark. on ischaemic arrhythmias in anaesthetized rats. Phytother Res. 2000;14:428–431. doi: 10.1002/1099-1573(200009)14:6<428::aid-ptr618>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee SS, Koch E, Jaggy H, Krzeminski T. In vitro and in vivo studies on the cardioprotective action of oligomeric procyanidins in a Crataegus extract of leaves and blooms. Arzneimittel-Forschung. 1997;47:821–825. [PubMed] [Google Scholar]
- 4.Occhiuto F, Circosta C, Briguglio F, Tommasini A, de Pasquale A. Comparative study of the cardiovascular activity of shoots, leaves and flowers of Crataegus oxyacantha: 1. Electrical activity and arterial pressure in the rat. Plantes medicinales et phytotherapie. 1986;20:37–51. [Google Scholar]
- 5.Occhiuto F, Circosta C, Costa R, Briguglio F, Tommasini A. Comparative study of the cardiovascular activity of shoots, leaves and flowers of Crataegus oxyacantha: 2. Action of extracts and isolated pure active principles on the isolated rabbit heart. Plantes medicinales et phytotherapie. 1986;20:52–63. [Google Scholar]
- 6.Brixius K, Frank K, Munch G, Muller-Ehmsen J, Schwinger RHG. WS 1442 (Crataegus-Special Extract) increases contractile force in the myocardium of humans with congestive heart failure. Herz-Kreislauf. 1998;30:28–33. [Google Scholar]
- 7.Ruchstuhl M, Beretz A, Anton R, Landry Y. Flavonoids are selective cyclic GMP phosphodiesterase inhibitors. Biochem Pharmacol. 1979;28:535–538. doi: 10.1016/0006-2952(79)90249-1. [DOI] [PubMed] [Google Scholar]
- 8.Schussler M, Holzl J, Fricke U. Myocardial effects of flavonoids from Crataegus species. Arzneimittelforschung. 1995;45:842–845. [PubMed] [Google Scholar]
- 9.Periera da Silva A, Rocha R, Silva CM, Mira L, Duarte MF, Florencio MH. Antioxidants in medicinal plant extracts. A research study of the antioxidant capacity of Crataegus, Hamamelis and Hydrastis. Phytother Res. 2000;14:612–616. doi: 10.1002/1099-1573(200012)14:8<612::aid-ptr677>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Bahorun T, Aumjaud E, Ramphul H, Rycha M, Luximon-Ramma A, Trotin F, Aruoma OI. Phenolic constituents and antioxidant capacities of Crataegus monogyna (Hawthorn) callus extracts. Nahrung. 2003;47:191–198. doi: 10.1002/food.200390045. [DOI] [PubMed] [Google Scholar]
- 11.Masquelier J. Pycnogenols: recent advances in the therapeutical activity of procyanidins. In: Beal JL, Reinhard E, editors. Natural Products as Medicinal Agents: Plenary Lectures of the International Research Congress on Medicinal Plant Research, Stuttgart, July 1980. Strasbourg: Hippokrates Verlag; 1981. [Google Scholar]
- 12.Pittler MH, Schmidt K, Ernst E. Hawthorn extract for treating chronic heart failure: meta-analysis of randomized trials. Am J Med. 2003;114:665–674. doi: 10.1016/s0002-9343(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 13.Zick SM, Gillespie B, Aaronson KD. The effect of Crataegus oxycantha Special Extract WS 1442 on clinical progression in patients with mild to moderate symptoms of heart failure. Eur J Heart Fail. 2008;10:587–593. doi: 10.1016/j.ejheart.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 15.Group TE. EuroQoL-A new facility for the measurement of health related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, Colucci WS, Sackner-Bernstein JD, Liang CS, Goldscher DA, Freeman I, Kukin ML, Kinhal V, Udelson JE, Klapholz M, Gottlieb SS, Pearle D, Cody RJ, Gregory JJ, Kantrowitz NE, LeJemtel TH, Young ST, Lukas MA, Shusterman NH. Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE trial. Circulation. 1996;94:2793–2799. doi: 10.1161/01.cir.94.11.2793. [DOI] [PubMed] [Google Scholar]
- 17.Leuchtgens H. Crataegus Special Extract WS 1442 in NYHA II heart failure. A placebo controlled randomized double-blind study. Fortschr Med. 1993;111:352–354. [PubMed] [Google Scholar]
- 18.O'Connolly M, Jansen W, Bernhoft G, Bartsch G. Treatment of decreasing cardiac performance. Therapy using standardized Crataegus extract in advanced age. Fortschritte der Medizin. 1986;104:805–808. [PubMed] [Google Scholar]
- 19.Hanack T, Bruckel MH. The treatment of mild stable forms of angina pectoris using Crategutt novo. Therapiewoche. 1983;33:4331–4333. [Google Scholar]
- 20.Zapfe G. Clinical efficacy of Crataegus Extract WS 1442 in congestive heart failure NYHA Class II. Phytomedicine. 2001;8:262–266. doi: 10.1078/0944-7113-00041. [DOI] [PubMed] [Google Scholar]
- 21.Bodigheimer K, Chasa D. Effectiveness of hawthorn extract at a dosage of 3 × 100 mg per day. Multicentre double-blind trial with 85 NYHA stage II heart failure patients. Munch Med Wschr. 1994;136:S7–S11. [Google Scholar]
- 22.Weikl A, Assmus KD, Neukum-Schmidt A, Schmitz J, Zapfe G, Noh HS, Siegrist J. Crataegus Special Extract WS 1442. Assessment of objective effectiveness in patients with heart failure (NYHA II) Fortschr Med. 1996;114:291–296. [PubMed] [Google Scholar]
- 23.O'Connolly VM, Jansen W, Bernhoft G, Bartsch G. Treatment of heart failure. Fortschr Med. 1986;104:805–808. [PubMed] [Google Scholar]
- 24.Tauchert M. Efficacy and safety of Crataegus Extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association Class-III heart failure. Am Heart J. 2002;143:910–915. doi: 10.1067/mhj.2002.121463. [DOI] [PubMed] [Google Scholar]
- 25.Tauchert M, Ploch M, Hubner W-D. Effectiveness of the hawthorn extract LI 132 compared with the ACE inhibitor captopril. Multicentre double-blind study with 132 NYHA stage II heart failure patients. Munchener Medizinische Wochenschrift. 1994;136:S27–S33. [Google Scholar]
- 26.Strauss P, Ietta M, Thomas S, Fichman B. The mean blood pressure—heart rate quotient and systolic blood pressure—heart rate product are not useful indicators of myocardial ischemia. Anesthesiology. 1989;71 [Google Scholar]
- 27.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M. Prediction of mortality and morbidity with a 6 min walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 28.Demers C, McKelvie R, Negassa A, Yusuf S. RESOLVD Pilot Study Investigators. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142:698–703. doi: 10.1067/mhj.2001.118468. [DOI] [PubMed] [Google Scholar]
- 29.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 30.Holubarsch C, Colucci W, Meinertz T, Gaus W, Tendera M. Crataegus extract WS® 1442, congestive heart failure, survival, efficacy, safety, controlled randomized trial. Eur J Heart Failure. 2008;10:1255–1263. [Google Scholar]