Abstract
Aims
To determine whether valsartan improves treadmill exercise time, in patients with symptomatic heart failure with a preserved ejection fraction (HFPEF), compared with placebo.
Methods and results
In this multicentred, double-blind, 14-week study, patients were randomized to receive valsartan (V) 80 mg or placebo (P) once daily on top of background medications. The dose of valsartan was force-titrated up to 320 mg. A total of 152 patients were randomized (V = 70, P = 82). Most patients had well-controlled hypertension (V = 91.2%, P = 89.0%) (mean baseline systolic BP ∼130 mmHg) and >50% were receiving an angiotensin-converting enzyme inhibitor and/or beta-blocker (V = 57.4%, P = 54.9%). The mean ejection fraction at baseline was 70.48% in the placebo group (n = 64) and 71.52% in the valsartan group (n = 79). Valsartan had no significant effect on exercise time (primary variable), gas exchange variables, 6 min walk test distance, exertion-related symptoms, brain natriuretic peptide levels, echocardiographic parameters, or quality-of-life scores. Valsartan significantly lowered peak exercise systolic BP (−13.1 mmHg vs. placebo; P < 0.001) and improved ratings of perceived exertion (Borg score) (−0.69 vs. placebo; P = 0.008).
Conclusion
In this population, which predominantly included patients with well-controlled hypertension and symptomatic HFPEF, addition of valsartan did not increase exercise time within 14 weeks. However, valsartan 320 mg reduced blood pressure and improved symptoms of perceived exertion (Borg score) during exercise and was generally well-tolerated.
Keywords: Heart failure with preserved ejection fraction, Echocardiography, Exercise time, Placebo, Quality of life, Valsartan
Introduction
Heart failure with preserved ejection fraction (HFPEF), (previously known as diastolic heart failure) arises from increased resistance to filling of the ventricles, leading to symptoms of pulmonary and systemic congestion.1 The primary functional abnormalities of HFPEF include impaired active left-ventricular relaxation and increased passive stiffness. Among individuals with heart failure, the prevalence of HFPEF has been reported to range from 13 to 74%, which reflects the lack of a uniform definition of the condition.2,3 Risk factors for HFPEF include increasing age, female gender, hypertension, diabetes, obesity, coronary artery disease, and chronic kidney disease.4 Like systolic heart failure, HFPEF is associated with considerable morbidity and mortality5 and the risk of adverse outcome increases with the severity of diastolic dysfunction.6
Patients with HFPEF have increased activation of the renin–angiotensin–aldosterone system (RAAS), which contributes to the pathogenesis and progression of the condition.7 Angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) have demonstrated beneficial effects in animal models of HFPEF,8,9 but in human studies no medication has shown a mortality benefit. Both ACE-Is and ARBs may decrease hospitalizations in HFPEF, however the benefits with ACE-Is may not be sustained over a year.10,11 Short-duration studies suggest that these agents may have beneficial effects on exercise tolerance, the hypertensive response to exercise, and quality of life (QoL).12–14 These improvements were observed after 2 weeks to 6 months of treatment and are believed to be mediated through blockade of the effects of angiotensin II, the levels of which are elevated during exercise.15 Increases in angiotensin II during exertion may exacerbate diastolic dysfunction, and therefore limit exercise tolerance, by elevating blood pressure and impairing left-ventricular relaxation.16
Valsartan is a highly selective ARB indicated for the treatment of hypertension, heart failure, and left-ventricular failure following myocardial infarction.17 Administration of valsartan to rats with HFPEF improved function and prolonged survival9 and in a small clinical study (n = 24), valsartan improved the impaired left-ventricular diastolic function of hypertensive patients.18
The primary objective of the present study was to determine whether valsartan could improve treadmill exercise time, in patients with symptomatic HFPEF, compared with placebo.
Methods
The study protocol was approved by the Independent Ethics Committee for each centre, and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
Patients
Patients were ≥21 years of age and had the following characteristics: symptoms of breathlessness on exertion (based on patient questioning) with normal lung function at rest, an extrapolated maximum oxygen consumption (EMOC) and/or peak oxygen consumption <85% of the age-corrected normal value on cardiopulmonary exercise testing, preserved systolic function (ejection fraction ≥40%) with evidence of diastolic dysfunction on echocardiography (≥1 of the following: abnormal flow propagation velocity, prolongation of isovolumic relaxation time, E/A ratio reversal, and abnormal E deceleration time), and ability to exercise for ≥3 min on a treadmill. The ejection fraction criterion of ≥40% is similar to that used in other trials in HFPEF including the CHARM-Preserved trial and PEP-CHF.10,11
Exclusion criteria are given in Table 1.
Table 1.
Exclusion criteria
| Uncontrolled hypertension (sitting systolic blood pressure >160 mmHg or sitting diastolic blood pressure >100 mmHg) |
| Presence of clinically significant asthma or chronic obstructive pulmonary disease |
| Abnormal lung function (forced expiratory volume in 1 s [FEV1]/forced vital capacity [FVC] ratio <75%) |
| Treatment with ≥2 bronchodilators |
| Exercise limiting symptomatic angina |
| Haemodynamically significant cardiac valvular disease |
| Documented evidence of systolic heart failure (ejection fraction <40%, fractional shortening <25%) |
| Uncontrolled atrial fibrillation (>100 b.p.m. at rest) |
| History of myocardial infarction, percutaneous transluminal coronary angioplasty, or coronary artery bypass within the previous 3 months |
| Use of ARBs within the previous 1 month |
Study design
This was an investigator-initiated multicentre, randomized, double-blind, placebo-controlled, parallel-group study. Following a screening period of up to 13 weeks duration, eligible patients were randomized at baseline (Visit 2) in a 1:1 ratio to receive either valsartan 80 mg once daily or matching placebo. Use of other ARBs as concomitant medication was prohibited, but other background medications (e.g. diuretics, calcium channel blockers) were allowed and continued throughout the study. Angiotensin-converting enzyme inhibitors and beta-blockers were permitted, although therapy was to be maintained at the same level throughout the study and no new treatment with one of these drugs was permitted during the trial. Eligible patients were allocated to either the active treatment group or the placebo group according to a stratified randomization process in order to minimize the differences between study groups. Stratification was based on exercise test time at Visit 2 divided into sections of: 3–6 min, >6 min to 9 min, and >9 min, each stratum being randomized in blocks of 4.
Study medication was force-titrated between days 5 and 14 (Visit 3) to valsartan 160 mg daily or matching placebo, and between days 10 and 28 (Visit 4) to valsartan 320 mg daily or matching placebo. Up-titration occurred provided the current dose was adequately tolerated. Down-titration occurred for any of the following: evidence of persistent symptomatic hypotension, systolic blood pressure <100 mmHg or decrease of >40 mm Hg from baseline, creatinine increase of >50% from baseline, or if the investigators judged the given dose level as potentially harmful to the patient. A safety evaluation was performed between days 15 and 42 (Visit 5). After the dose-titration period, patients received their maximum tolerated dose through to the end of the study at week 14 (±7 days) (Visit 6). To maintain blinding, valsartan and placebo capsules were identical in appearance.
Efficacy assessments
Exercise testing using a modified Bruce protocol19 was performed during screening (pre-randomization), at baseline (randomization), between days 15 and 42, and at the end of the study. Subjects had a practice treadmill session during the pre-screening visit to demonstrate that they were able to exercise for at least 3 min at baseline (Visit 2). The electrocardiogram (ECG) was monitored continuously. Standing blood pressure was recorded using a mercury sphygmomanometer before starting exercise and immediately after stopping the treadmill. During the exercise test, patients breathed through a valve that separated inspired and expired air. Measurements of oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE BTPS) were obtained and averaged over 10 s. From these measurements, the respiratory exchange ratio (RER) was determined. Ratings of perceived exertion were made during every stage of the exercise test using the Borg scale.20
At baseline and at the end of the study, the 6 min walk test was performed after the exercise testing was complete. The incidence of pre-specified events (i.e. exertion-related symptoms of angina, dizziness, dyspnoea, and fatigue) was determined. At the same timepoints, blood samples were collected to determine the following neurohormone levels: brain natriuretic peptide (BNP),21 noradrenaline and adrenaline,22 plasma renin activity, aldosterone, and procollagen type III amino-terminal peptide.23 Samples for renin, aldosterone, and natriuretic peptides were collected after 10 min of supine rest prior to exercise testing. The Minnesota Living with Heart Failure (MLHF)24 and EuroQol questionnaires25 were completed by patients before any other assessments were done. Echocardiography was performed during screening and at the end of the study. Patient and physician global assessments of efficacy were made at the end of the study (rated as very good, good, moderate, poor, or very poor).
Safety assessments
Safety was assessed via monitoring for adverse events (AEs), standard clinical laboratory testing (haematology, blood chemistry, and urinalysis), ECG recordings, routine physical examinations, and regular measurement of vital signs. Patient and physician global assessments of tolerability were made at the end of the study (rated as very good, good, moderate, poor, or very poor).
Statistical methods
A sample size of 150 patients (128 with data available) was pre-specified to provide 80% power to detect a difference in means of 1.25 min (i.e. an effect size of 0.5) using a two-group t-test with a two-sided significance level of 0.050. Sample-size calculations assumed a common standard deviation (SD) of 2.5 min. The efficacy analysis was performed on the intent-to-treat (ITT) population, which included all patients who received ≥1 dose of study medication and had baseline efficacy data and ≥1 post-baseline measurement. The safety population included all patients allocated to treatment who received ≥1 dose of study medication.
Demographic and baseline characteristics were compared between the two study groups using either the Wilcoxon two-sample test (continuous variables), χ2 test (categorical variables), or Fisher's Exact test (exertion-related symptoms during the 6 min walk test). The hypothesis of no difference in mean change from baseline in exercise time was tested using a two-factor analysis of covariance (ANCOVA), with country, treatment, baseline ACE-Is use (yes/no), and baseline beta-blocker use (yes/no) as factors and with baseline exercise time as a covariate. The ‘endpoint’ measurement for each randomized patient was the last post-randomization measurement carried forward to the end of the study. Secondary efficacy variables were analysed using the same ANCOVA model as described for the primary analysis, with the exception of the incidence of exertion-related symptoms during the 6 min walk test and global assessment results, for which Fisher's Exact test was used. Exploratory analyses were performed to assess the impact of treatment centre and baseline exercise time on the primary efficacy outcome. For these analyses, statistical significance was concluded if the 95% confidence interval did not include 0.
Results
Patients
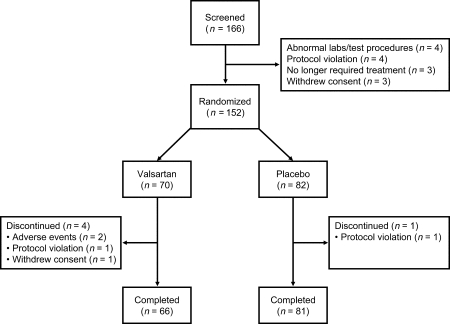
Patient disposition is presented in Figure 1. Of the 166 patients screened, 152 were randomized (70 valsartan, 82 placebo) and 147 completed the study (66 valsartan, 81 placebo). Of the 152 randomized patients, 87 (57.2%) were recruited at German centres and 65 (42.8%) at UK centres. Within each country, recruitment was dominated by one or two centres. At randomization (Baseline Visit 2), 152 patients were stratified according to their exercise time. In the ≥3–6 min stratum there were 19 patients (12.5%), 31 patients (20.4%) in the >6–9 min stratum, and 102 (67.1%) patients in the >9 min stratum. More patients were randomized into the placebo arm than the valsartan arm. It is likely that this was due to the majority of centres enroling only a small number of patients; only three centres enrolled more than seven patients. Consequently there were incomplete blocks, which were exacerbated by the stratification of patients by baseline exercise time. The ITT population comprised 150 patients (68 valsartan, 82 placebo) and the safety population 152 patients (70 valsartan, 82 placebo).
Figure 1.
Patient disposition.
The two study groups were well balanced with respect to demographic and baseline characteristics (Table 2). The only statistically significant difference was a slightly greater mean body mass index in the valsartan group (31.0 vs. 29.3 kg/m2 for placebo; P = 0.010). Half the patients were male, and 94.7% were Caucasian. The mean age was 62.1 years. The majority of patients had a history of hypertension (∼90.0%). However, mean sitting blood pressures were well controlled with a mean baseline of 130.2/76.3 mmHg in the valsartan group and 130.1/75.2 mmHg in the placebo group. Overall 71.4% of patients had cardiac disorders, in particular angina pectoris (15.7% valsartan, 14.6% placebo), myocardial infarction (14.3 vs. 20.7%), and atrial fibrillation (12.9 vs. 8.5%). Other concomitant diseases included diabetes (22.1 vs. 14.6%) and left ventricular hypertrophy (15.7 vs. 13.4%). A substantial proportion of patients were receiving an ACE-I and/or beta-blocker (57.4% valsartan, 54.9% placebo). A history of dihydropyridine calcium channel blocker and thiazide diuretic use was reported for 28.6 and 20.0% of valsartan recipients, respectively, and 26.8 and 30.5% of placebo recipients, respectively. At baseline, findings were similar between the study groups for spirometry, exercise test, 6 min walk test (distance and symptoms), and neurohormone levels. Exercise time at baseline was 11.0 min in the valsartan group and 10.5 min in the placebo group (Table 2). The mean duration of treatment was 13.9 weeks for valsartan recipients and 13.7 weeks for placebo recipients. The maximum dose of study medication was administered to 82.9% of patients in each study group.
Table 2.
Demographic and baseline characteristics
| Variable | Valsartan (n = 68) | Placebo (n = 82) | P-value |
|---|---|---|---|
| Age, years | 61.0 (11.5) | 63.1 (10.3) | 0.444 |
| Age group, n (%) | 0.651 | ||
| <65 years | 39 (57.4) | 44 (53.7) | |
| ≥65 years | 29 (42.6) | 38 (46.3) | |
| Gender, n (%) | 1.000 | ||
| Male | 34 (50.0) | 41 (50.0) | |
| Female | 34 (50.0) | 41 (50.0) | |
| Race, n (%) | 0.886 | ||
| Caucasian | 65 (95.6) | 77 (93.9) | |
| Other | 3 (4.4) | 5 (6.1) | |
| BMI, kg/m2 | 31.0 (4.7) | 29.3 (5.3)a | 0.010 |
| Medical history, n (%) | |||
| Hypertension | 62 (91.2) | 73 (89.0) | 0.662 |
| Diabetes | 15 (22.1) | 12 (14.6) | 0.239 |
| Atrial fibrillation | 11 (16.2) | 8 (9.8) | 0.239 |
| Concomitant medication, n (%) | |||
| ACE-inhibitor | 28 (41.2) | 31 (37.8) | 0.674 |
| Beta-blocker | 23 (33.8) | 28 (34.1) | 0.967 |
| ACE-inhibitor and/or beta-blocker | 39 (57.4) | 45 (54.9) | 0.761 |
| FEV1, L | 2.5 (0.8) | 2.4 (0.7) | 0.493 |
| FVC, L | 2.9 (0.8) | 3.0 (0.9) | 0.914 |
| Exercise time, min | 11.0 (3.7) | 10.5 (3.9) | 0.396 |
| RPE (Borg scale) | 5.2 (1.4) | 5.2 (1.8) | 0.984 |
| Distance walked during 6 min walk test, m | 471.6 (105.3) | 471.6 (114.2) | 0.767 |
Values are expressed as means (standard deviation) unless otherwise stated.
ACE, angiotensin-converting enzyme; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; RPE, rating of perceived exertion.
an = 81.
Exercise time (primary efficacy variable)
None of the study population had inducible ischaemia during exercise. Least-squares mean increases from baseline to endpoint in exercise time, the primary efficacy variable, were observed in both study groups (Table 3), with the improvement on placebo (1.24 min) being slightly better than on valsartan (0.96 min). The between-group difference (valsartan minus placebo) was −0.28 min [95% confidence interval (CI): −0.84, 0.29] (P = 0.336). To explore the effect of unbalanced recruitment, exploratory analyses were performed. These showed that results were consistent across all centres, with the exception of pooled UK centres 4, 5, and 6 (n = 9 patients only) in which the placebo group showed a significantly greater response. Exploratory analysis of the impact of baseline exercise time showed that, for patients with baseline exercise time of ≤9 min, there was a trend (non-significant) toward greater response in the placebo group.
Table 3.
Least-squares mean changes from baseline to endpoint in total exercise test time and gas exchange variables at peak exercise
| Variable | Mean (SD) |
LSM change (SEM) | Difference (SEM)a | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Baseline | Endpoint | |||||
| Exercise time, min | ||||||
| Valsartan (n = 68) | 10.96 (3.73) | 11.80 (3.62) | 0.96 (0.22) | |||
| Placebo (n = 82) | 10.47 (3.88) | 11.70 (3.29) | 1.24 (0.20) | −0.28 (0.29) | −0.84, 0.29 | 0.336 |
| EMOC, mL/kg/minb | ||||||
| Valsartan (n = 38) | 533.71 (245.93) | 566.56 (251.82) | 4.63 (30.83) | |||
| Placebo (n = 48) | 614.04 (285.63) | 642.56 (283.88) | 19.09 (28.19) | −14.46 (39.04) | −92.14, 63.21 | 0.712 |
| VO2, mL/kg/min | ||||||
| Valsartan (n = 68) | 17.83 (4.46) | 17.84 (5.69) | −0.17 (0.53) | |||
| Placebo (n = 82) | 17.68 (5.96) | 18.80 (5.23) | 0.85 (0.49) | −1.02 (0.69) | −2.39, 0.35 | 0.142 |
| VCO2, mL/min | ||||||
| Valsartan (n = 68) | 18.06 (6.87) | 18.59 (7.92) | 0.18 (0.68) | |||
| Placebo (n = 82) | 18.97 (7.87) | 19.71 (7.94) | 0.56 (0.62) | −0.38 (0.88) | −2.13, 1.36 | 0.664 |
| VE BTPS, L/min | ||||||
| Valsartan (n = 68) | 52.37 (15.33) | 53.99 (18.34) | 0.75 (1.83) | |||
| Placebo (n = 82) | 52.14 (16.41) | 54.96 (19.01) | 1.83 (1.69) | −1.08 (2.41) | −5.83, 3.68 | 0.655 |
| RER | ||||||
| Valsartan (n = 68) | 1.06 (0.13) | 1.40 (2.69) | 0.38 (0.37) | |||
| Placebo (n = 82) | 1.09 (0.13) | 1.44 (3.15) | 0.43 (0.34) | −0.05 (0.49) | −1.02, 0.92 | 0.923 |
EMOC, extrapolated maximum oxygen consumption; LSM, least-squares mean; RER, respiratory exchange ratio; SD, standard deviation; SEM, standard error of the mean; VCO2, carbon dioxide production; VE BTPS, minute ventilation; VO2, oxygen consumption.
aValsartan minus placebo.
bEMOC was assessed at German centres only.
Further analysis looking into change from baseline in exercise time against the change from baseline in maximum exercise SBP, the maximum exercise SBP at study end and the maximum exercise SBP at baseline did not show any significant relationship.
During the study, an improvement in exercise time was achieved by 69.1% of valsartan-treated patients and 72.0% of placebo-treated patients. The percentages of patients who had a deterioration in exercise time were also similar (26.5 and 24.4% on valsartan and placebo, respectively).
Other variables derived from exercise test
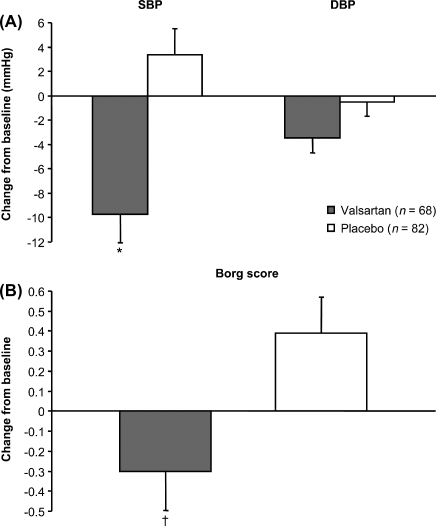
At the end of the study, no significant differences were observed between the study groups in least-squares mean changes from baseline in EMOC, VO2, VCO2, VE BTPS, or RER at peak exercise (Table 3). The least-squares mean reduction from baseline to endpoint in maximum systolic blood pressure was significantly greater in the valsartan group [between-group difference, −13.1 mmHg (95% CI: −19.2, −7.0); P < 0.001] (Figure 2A). No significant between-group differences were found for maximum diastolic blood pressure or maximum pulse rate. Least-squares mean changes from baseline to endpoint in the maximum Borg score showed a reduction (improvement) in the valsartan group and an increase (deterioration) in the placebo group (between-group difference, −0.69 [95% CI: −1.20, −0.18); P = 0.008] (Figure 2B).
Figure 2.
Least-squares mean changes from baseline to endpoint in (A) maximum systolic blood pressure (SBP) and maximum diastolic blood pressure (DBP) and (B) maximum Borg score. Error bars denote standard error of the mean. *P < 0.001 vs. placebo; †P = 0.008 vs. placebo.
6 minute walk test
Treatment with valsartan and placebo was associated with least-squares mean increases from baseline to week 14 in distance on the 6 min walk test (15.6 and 12.7 m, respectively). The between-group difference was 2.9 m (95% CI: −11.7, 17.4; P = 0.698). The incidence of exertion-related symptoms, including angina, dizziness, dyspnoea, and fatigue, did not differ significantly between the study groups (all P ≥ 0.317).
Neurohormone levels
As shown in Table 4, percent least-squares mean changes from baseline to week 14 in plasma BNP and other neurohormone levels did not differ significantly between the study groups, with the exception of the expected greater increase in plasma renin activity and decrease in aldosterone in the valsartan group (P = 0.013 and P = 0.015, respectively, vs. placebo).
Table 4.
Percent least-squares mean changes from baseline to week 14 in neurohormone levels
| Variable | Mean (SD) |
LSM Change (SEM) | Ratio (SEM)a | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Baseline | Endpoint | |||||
| BNP, pg/mL | ||||||
| Valsartan | 93.2 (80.2) (n = 60) | 94.0 (78.6) (n = 61) | 97.5 (110.8) (n = 60) | |||
| Placebo | 120.3 (119.5) (n = 73) | 109.9 (105.0) (n = 74) | 93.9 (109.9) (n = 73) | 103.9 (114.1) | 80.0, 134.9 | 0.774 |
| Noradrenaline, pg/mL | ||||||
| Valsartan (n = 54) | 529.1 (258.9) | 538.5 (282.6) | 94.8 (106.9) | |||
| Placebo (n = 64) | 621.1 (303.9) | 589.1 (254.2) | 100.4 (106.3) | 94.4 (109.0) | 79.6, 112.0 | 0.504 |
| Adrenaline, pg/mL | ||||||
| Valsartan (n = 54) | 91.4 (231.6) | 53.4 (50.7) | 84.4 (108.2) | |||
| Placebo (n = 64) | 60.2 (42.9) | 63.6 (59.3) | 97.3 (107.5) | 86.7 (110.7) | 70.9, 106.0 | 0.161 |
| PRA, ng/mL/h | ||||||
| Valsartan (n = 61) | 4.9 (10.6) | 7.8 (10.9) | 181.8 (122.5) | |||
| Placebo | 4.0 (7.7) (n = 71) | 3.8 (7.6) (n = 72) | 93.1 (121.0) (n = 71) | 195.4 (130.5) | 115.4, 331.0 | 0.013 |
| Aldosterone, pg/mL | ||||||
| Valsartan | 170.2 (101.0) (n = 57) | 134.9 (88.7) (n = 59) | 78.9 (107.3) (n = 57) | |||
| Placebo | 174.6 (113.3) (n = 68) | 169.9 (103.6) (n = 70) | 99.2 (106.7) (n = 68) | 79.5 (109.7) | 66.2, 95.6 | 0.015 |
| PIIINP, µg/mL | ||||||
| Valsartan (n = 60) | 3.3 (1.1) | 3.3 (0.9) | 98.3 (103.0) | |||
| Placebo (n = 72) | 3.5 (1.1) | 3.6 (1.1) | 104.4 (102.7) | 94.1 (103.9) | 87.2, 101.5 | 0.114 |
BNP, brain natriuretic peptide; PIIINP, procollagen type III amino-terminal peptide; PRA, plasma renin activity; SD, standard deviation; SEM, standard error of the mean.
aValsartan relative to placebo.
Other secondary efficacy variables
Echocardiographic parameters and QoL scores did not differ significantly between the study groups, with the exception of a more favourable MLHF emotional score in the placebo group (P = 0.027) (Table 5). The mean ejection fraction at baseline was 70.48% in the placebo group (n = 64) and 71.52% valsartan group (n = 79). There was no significant change compared with baseline in the ejection fraction in either group at the end of the study. The percentage of patients with an overall echocardiographic interpretation of ‘normal,’ in the opinion of the investigators, increased from 1.5% at baseline to 9.0% at the end of the study in the valsartan group compared with 0–1.2% in the placebo group. Patient global assessments of efficacy were comparable (P = 0.202) between the study groups: 58.2% of patients in the valsartan group and 50.0% in the placebo group rated treatment as very good or good. Similar results were reported by physicians.
Table 5.
Least-squares mean changes from baseline to week 14 in echocardiographic parameters and quality-of-life scores
| Variable | Mean (SD) |
LSM change (SEM) | Difference (SEM)a | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Baseline | Endpoint | |||||
| E/A ratio | ||||||
| Valsartan (n = 61) | 1.16 (0.85) | 1.16 (0.73) | 0.05 (0.06) | |||
| Placebo (n = 79) | 1.07 (0.68) | 1.04 (0.44) | −0.03 (0.05) | 0.08 (0.07) | −0.07, 0.23 | 0.277 |
| Flow propagation velocity, cm/s | ||||||
| Valsartan | 37.18 (12.72) (n = 51) | 38.57 (13.42) (n = 52) | 5.00 (7.50) (n = 51) | |||
| Placebo | 37.64 (12.05) (n = 66) | 47.85 (69.44) (n = 69) | 12.11 (7.10) (n = 66) | −7.11 (9.55) | −26.0, 11.82 | 0.458 |
| IVRT, ms | ||||||
| Valsartan | 103.63 (18.84) (n = 64) | 99.62 (17.27) (n = 65) | −4.48 (2.10) (n = 64) | |||
| Placebo | 103.23 (23.06) (n = 81) | 101.38 (20.69) (n = 82) | −2.76 (1.90) (n = 81) | −1.72 (2.72) | −7.10, 3.66 | 0.528 |
| LVIDD, cm | ||||||
| Valsartan | 5.08 (0.88) (n = 64) | 5.08 (0.79) (n = 65) | 0.01 (0.06) (n = 64) | |||
| Placebo | 5.16 (0.71) (n = 79) | 5.16 (0.72) (n = 80) | 0.02 (0.05) (n = 79) | −0.01 (0.07) | −0.16, 0.14 | 0.886 |
| LVIDS, cm | ||||||
| Valsartan | 3.35 (0.85) (n = 64) | 3.32 (0.81) (n = 65) | −0.01 (0.07) (n = 64) | |||
| Placebo | 3.33 (0.75) (n = 79) | 3.46 (0.75) (n = 80) | 0.15 (0.06) (n = 79) | −0.15 (0.09) | −0.33, 0.02 | 0.077 |
| Left atrial size, cm | ||||||
| Valsartan (n = 66) | 4.24 (0.71) | 4.26 (0.66) | 0.02 (0.06) | |||
| Placebo | 4.15 (0.68) (n = 77) | 4.05 (0.69) (n = 79) | −0.12 (0.05) (n = 77) | 0.14 (0.08) | −0.00, 0.29 | 0.055 |
| Left ventricular mass, g | ||||||
| Valsartan | 232.33 (88.45) (n = 55) | 223.74 (74.49) (n = 57) | −8.87 (6.76) (n = 55) | |||
| Placebo | 213.58 (69.45) (n = 70) | 219.87 (75.75) (n = 74) | 0.16 (6.30) (n = 70) | −9.02 (8.88) | −26.6, 8.55 | 0.311 |
| E deceleration time, ms | ||||||
| Valsartan | 248.8 (63.45) (n = 66) | 237.9 (65.13) (n = 67) | −6.51 (5.65) (n = 66) | |||
| Placebo | 230.7 (50.67) (n = 81) | 238.8 (52.32) (n = 82) | 5.10 (5.18) (n = 81) | −11.6 (7.46) | −26.3, 3.13 | 0.122 |
| Ejection fraction % | ||||||
| Valsartan | 70.48 (11.43) (n = 64) | 70.76 (12.19) (n = 65) | −0.39 (1.35) (n = 64) | |||
| Placebo | 71.52 (12.08) (n = 79) | 68.58 (11.77) (n = 80) | −3.14 (1.23) (n = 79) | 2.75 (1.76) | −0.73, 6.22 | 0.120 |
| MLHF | ||||||
| Overall score | ||||||
| Valsartan (n = 67) | 27.02 (20.47) | 23.54 (21.36) | −3.14 (1.58) | |||
| Placebo | 23.49 (19.18) (n = 81) | 19.54 (14.71) (n = 82) | −4.85 (1.45) (n = 81) | 1.71 (2.07) | −2.38, 5.81 | 0.409 |
| Physical dimension score | ||||||
| Valsartan (n = 67) | 13.72 (9.39) | 11.65 (9.66) | −1.72 (0.81) | |||
| Placebo | 12.14 (9.87) (n = 81) | 10.11 (7.71) (n = 82) | −2.28 (0.74) (n = 81) | 0.55 (1.06) | −1.53, 2.64 | 0.601 |
| Emotional score | ||||||
| Valsartan (n = 67) | 5.40 (5.94) | 5.11 (6.55) | −0.26 (0.48) | |||
| Placebo | 5.09 (5.72) (n = 81) | 3.63 (4.18) (n = 82) | −1.67 (0.44) (n = 81) | 1.41 (0.63) | 0.16, 2.66 | 0.027 |
| ED-5D (visual analog scale) | ||||||
| Valsartan (n = 66) | 71.23 (14.43) | 71.06 (16.88) | −0.11 (1.87) | |||
| Placebo | 68.49 (17.40) (n = 81) | 70.99 (17.11) (n = 82) | 1.26 (1.71) (n = 81) | −1.37 (2.45) | −6.21, 3.47 | 0.577 |
A, atrial filling velocity; E, early filling velocity; ED-5D, EuroQol questionnaire; IVRT, isovolumic relaxation time; LVIDD, left-ventricular end diastolic dimension in diastole; LVIDS, left-ventricular end diastolic dimension in systole; MLHF, Minnesota Living with Heart Failure, SD, standard deviation; SEM, standard error of the mean.
aValsartan minus placebo.
Safety
Adverse event were reported for 77.1 and 72.0% of patients in the valsartan and placebo groups, respectively. The most frequently reported events were dizziness (14.3% valsartan, 17.1% placebo) and fatigue (10.0%, 11.0%). Valsartan recipients had a slightly higher incidence of dyspnoea (12.9 vs. 7.3%), cough (7.1 vs. 3.7%), and hypotension (7.1 vs. 1.2%). The majority of AEs were mild or moderate in severity. Adverse events led to premature study discontinuation for two valsartan-treated patients (hypotension, mesothelioma). The patient with hypotension was discontinued when his blood pressure decreased (from 120.0/77.0 mmHg at screening) to 85.3/53.3 mmHg on day 83. This event was believed to be related to the study medication. At the patient's final evaluation on day 118 (after about 1 month off treatment), blood pressure was 107.3/73.0 mmHg. There were no deaths during the study. Ten patients experienced serious AEs (four valsartan, six placebo). However, none was judged by the investigators to be related to study medication. Based on vital signs evaluations, mean systolic blood pressure was reduced from baseline to study end by 4.3 mmHg in the valsartan group while it increased over the same time period by 1.7 mmHg in the placebo group (P = 0.021). Changes in mean pulse rate were negligible. Patient global assessments of tolerability were similar (P = 0.415) for both study groups: 94.0% of patients in the valsartan group and 92.7% in the placebo group rated treatment as very good or good. Similar results were reported by physicians. Other safety findings were unremarkable.
Discussion
Treatment with valsartan did not significantly improve exercise time in this population which predominantly included patients with hypertension (∼90.0%) with good BP control and symptomatic HFPEF. In our study, valsartan reduced mean resting systolic blood pressure from baseline by ∼6 mmHg at study end and peak exercise systolic blood pressure by about 13 mmHg over placebo, despite the extensive use of background medications (e.g. ACE-Is, beta-blockers). We believe that this is an important finding because increases in this parameter are associated with negative long-term cardiovascular outcomes. Specifically, exercise-induced elevations in systolic blood pressure have been associated with cardiac remodelling,26with the development of future hypertension,27 and with increased cardiovascular mortality27,28 and stroke.29
The Borg rating scale results suggest that patients felt better during valsartan treatment, but patient QoL scores did not improve. We cannot explain this inconsistency but it could be due to the small sample size or the timing of data collection. Ratings of perceived exertion were made during every stage of the exercise test, whereas QoL questionnaires were completed before any other assessments. Treatment with valsartan was associated with the expected changes in plasma renin activity and aldosterone levels, providing evidence of angiotensin II type 1 receptor blockade.
There was no BNP requirement for entry into the study. Brain natriuretic peptide is released from the ventricle in response to volume expansion and pressure overload, and levels are elevated in HFPEF.30 Nonetheless, while a low BNP value is highly effective for ruling out heart failure, a high value is only a fair marker of the disease and may be associated with other conditions including renal failure, pulmonary hypertension, and pulmonary embolism. The diagnostic value of BNP testing is likely to be in identifying individuals who require further testing with echocardiography. In our study, the lack of effect of valsartan on lowering BNP is inconsistent with previous findings in patients with heart failure and left ventricular ejection fraction <40%.31,32 However, this may be related to the fact that the baseline BNP levels in those studies were ∼2–2.5-fold greater than those of the valsartan recipients in our study. This again points out that our population had relatively mild HFPEF.33 In addition, the concomitant use of ACE-Is and beta-blockers has been shown to attenuate the effect of valsartan on this parameter.31
Unbalanced recruitment within the study centres did not appear to contribute to our results. Recruitment was dominated by one or two centres in each country, but exploratory analysis indicated that the overall ITT result was not substantially influenced by this factor. One might have expected a greater benefit of valsartan over placebo in patients with lower baseline exercise times (≤9 min), but this was not the case. The reason for the lack of a significant effect appears to be due to the treatment difference being smaller than planned and in the opposite direction to what was anticipated rather than an incorrect assumption of SD at planning.
Valsartan has demonstrated positive effects on diastolic function in two previous randomized, double-blind studies. The earlier study included patients with hypertension and no evidence of left ventricular hypertrophy.18 A subgroup of 12 patients with impaired left-ventricular peak filling rate at baseline had a significant improvement in this parameter, both at rest and at peak exercise (P < 0.05), after 4 weeks of treatment with valsartan 80–160 mg daily. The Valsartan In Diastolic Dysfunction (VALIDD) study included 384 patients with hypertension (mean systolic blood pressure of ∼144 mmHg) and HFPEF.34 Placebo or valsartan 160–320 mg daily were administered for up to 38 weeks, in addition to other antihypertensive agents that did not inhibit the RAAS. The groups demonstrated comparable reductions in blood pressure (P = NS). Significant improvement in diastolic relaxation velocity, the primary endpoint, was observed irrespective of the type of antihypertensive agent used (P < 0.001 for both groups). Improvements in isovolumetric relaxation time and systolic longitudinal velocity were significantly greater with valsartan treatment (P < 0.05).
However, these patients had varying comorbidities (e.g. <2% had atrial fibrillation in VALIDD vs. nearly 13% in the present study) and were receiving different background therapies (e.g. none on ACE-Is in VALIDD vs. ∼39% in the present study).
Limitations
It is possible that the criteria used for patient selection may not have been appropriate for identifying the patients who would benefit most from valsartan therapy. It is also possible that some patients studied may have been in the ‘grey zone’ between symptomatic hypertension and heart failure. However we believe that our patient population satisfied well-defined criteria for heart failure including presence of symptoms of breathlessness and peak VO2 < 85% predicted in the presence of normal lung function.35,36 A specific duration of HFPEF was not required for study entry, and such data were not collected. Patients with a longer history and more severe degree of HFPEF may have been better suited for enrolment as might subjects with an elevated BNP at rest.
The relatively short study period should be taken into consideration. However, there are reports of significant improvement in exercise systolic blood pressure, exercise tolerance (modified Bruce protocol), and QoL (MLHF) in patients with diastolic dysfunction and exaggerated BP response (>200 mmHg), after treatment with other ARBs for durations as short as 2 weeks.12,14 However, these studies were both done in small groups of patients (n = 20 and 40) and the patients had an exaggerated BP (>200 mmHg) response during exercise as a pre-requisite for study entry. In our study population, the mean peak systolic exercise BP was lower (171 mmHg). Moreover, in these studies patients had a higher resting systolic blood pressure at entry (∼140–143 mmHg) compared with our study.
Exercise blood pressure and Borg scale are secondary outcomes. The benefit of valsartan seen on these parameters is not reflected in the other outcomes studied. However, we believe that this data adds important information to the sparse data available in this population of HFPEF.
Conclusion
In this population, which predominantly included patients with well-controlled hypertension who had symptomatic HFPEF, the addition of valsartan did not increase exercise time within a 14 week treatment period. Valsartan significantly improved ratings of perceived exertion (Borg scale), a secondary endpoint of the study, and was well tolerated at the 320 mg dose. Further improvement in exercise capacity in this relatively mild HF population may not be expected by adding an ARB for a short duration.
Funding
The study was funded by Novartis.
Conflicts of interest: H.K.P. and B.P. have no conflicts of interest; C.D.A., M.W., and P.B. remain in the employ of Novartis Pharma and have no other conflicts of interest; A.D.S. received one honorarium (£1000) from Novartis for intellectual input to the rationale and the design of the study; T.M.MacD: competing interests statement Nov 2008: my department has had research grants from GSK, Aventis, Novartis, AstraZeneca, BMS, Bohringer Ingelheim, Pfizer, and Novartis, I am or have been the principal investigator on trials paid for by Pfizer and Novartis, I have been paid Consulting fees by Pfizer, Novartis, Kaiser Permanante, Takeda, Recordati, Quintiles, and Speedel.
List of contributors
List of co-investigators (apart from those mentioned as authors): Dr Robert MacFadyen, Birmingham City Hospital, Main Centre, Birmingham B18 7QH, UK; Prof. Robert Wilcox, Queens Medical Centre, Nottingham NG7 2UH, UK; Dr L. Tan, Leeds General Infirmary, Leeds LS1 3EX, UK; Dr Robert Butler, City General Hospital, Department of Respiratory Medicine, Stoke on Trent, Newcastle Rd ST4 6QG, UK; Prof. Dr Thomas Muenzel, Johannes-Gutenberg-Universitaet Maiz, Mainz 55131,Germany; Prof. Dr Wolfgang von Scheidt, Zentralklinkum Augsburg, Augsburg 86156, Germany; PD Dr Carsten Tschoepe, Freie Universitaet Berlin, Campus Benjamin, Franklin, Berlin 12200, Germany; Prof. Dr Christiane Angermann, Univ.-Klinik Wuerzburg, Wuerzburg 97070, Germany; Prof. Dr Bernhard Maisch, Universitaet Marburg, Kardiologie, Marburg 35043, Germany
References
- 1.Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351:1097–1105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.MacFadyen RJ, MacLeod CM, Shiels P, Russell Smith W, MacDonald TM. Isolated diastolic heart failure as a cause of breathlessness in the community. the Arbroath study. Eur J Heart Fail. 2001;3:243–248. doi: 10.1016/s1388-9842(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 4.Chinnaiyan KM, Alexander D, Maddens M, McCullough PA. Curriculum in cardiology:integrated diagnosis and management of diastolic heart failure. Am Heart J. 2007;153:189–200. doi: 10.1016/j.ahj.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Aurigemma GP. Diastolic heart failure–a common and lethal condition by any name. N Engl J Med. 2006;355:308–310. doi: 10.1056/NEJMe068128. [DOI] [PubMed] [Google Scholar]
- 6.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence:results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan KM, Alexander D, McCullough PA. Role of angiotensin II in the evolution of diastolic heart failure. J Clin Hypertens (Greenwich) 2005;7:740–747. doi: 10.1111/j.1524-6175.2005.04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto E, Kataoka K, Shintaku H, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ichijo H, Ogawa H, Kim-Mitsuyama S. Novel mechanism and role of angiotensin II induced vascular endothelial injury in hypertensive diastolic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2569–2575. doi: 10.1161/ATVBAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Yoshiyama M, Izumi Y, Kawano H, Kimoto M, Zhan Y, Iwao H. Effects of combination of ACE inhibitor and angiotensin receptor blocker on cardiac remodeling, cardiac function, and survival in rat heart failure. Circulation. 2001;103:148–154. doi: 10.1161/01.cir.103.1.148. [DOI] [PubMed] [Google Scholar]
- 10.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 12.Warner JG, Jr, Metzger DC, Kitzman DW, Wesley DJ, Little WC. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol. 1999;33:1567–1572. doi: 10.1016/s0735-1097(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 13.Little WC, Wesley-Farrington DJ, Hoyle J, Brucks S, Robertson S, Kitzman DW, Cheng CP. Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc Pharmacol. 2004;43:288–293. doi: 10.1097/00005344-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Little WC, Zile MR, Klein A, Appleton CP, Kitzman DW, Wesley-Farrington DJ. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383–385. doi: 10.1016/j.amjcard.2006.01.106. [DOI] [PubMed] [Google Scholar]
- 15.Aldigier JC, Huang H, Dalmay F, Lartigue M, Baussant T, Chassain AP, Leroux-Robert C, Galen FX. Angiotensin-converting enzyme inhibition does not suppress plasma angiotensin II increase during exercise in humans. J Cardiovasc Pharmacol. 1993;21:289–295. doi: 10.1097/00005344-199302000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CP, Suzuki M, Ohte N, Ohno M, Wang ZM, Little WC. Altered ventricular and myocyte response to angiotensin II in pacing-induced heart failure. Circ Res. 1996;78:880–892. doi: 10.1161/01.res.78.5.880. [DOI] [PubMed] [Google Scholar]
- 17.Mistry NB, Westheim AS, Kjeldsen SE. The angiotensin receptor antagonist valsartan: a review of the literature with a focus on clinical trials. Expert Opin Pharmacother. 2006;7:575–581. doi: 10.1517/14656566.7.5.575. [DOI] [PubMed] [Google Scholar]
- 18.Cuocolo A, Storto G, Izzo R, Iovino GL, Damiano M, Bertocchi F, Mann J, Trimarco B. Effects of valsartan on left ventricular diastolic function in patients with mild or moderate essential hypertension: comparison with enalapril. J Hypertens. 1999;17:1759–1766. doi: 10.1097/00004872-199917120-00014. [DOI] [PubMed] [Google Scholar]
- 19.Bruce RA. Exercise testing methods and interpretation. Adv Cardiol. 1978:6–15. doi: 10.1159/000401440. [DOI] [PubMed] [Google Scholar]
- 20.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 21.Rana BS, Davies JI, Band MM, Pringle SD, Morris A, Struthers AD. B-type natriuretic peptide can detect silent myocardial ischaemia in asymptomatic type 2 diabetes. Heart. 2006;92:916–920. doi: 10.1136/hrt.2005.071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yee KM, Pringle SD, Struthers AD. Circadian variation in the effects of aldosterone blockade on heart rate variability and QT dispersion in congestive heart failure. J Am Coll Cardiol. 2001;37:1800–1807. doi: 10.1016/s0735-1097(01)01243-8. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90:765–770. doi: 10.1136/hrt.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 25.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Fazio S, Palmieri EA, Izzo R, Affuso F, Romano M, Riccio G, Pilato G, Trimarco B, De Luca N. An exaggerated systolic blood pressure response to exercise is associated with cardiovascular remodeling in subjects with prehypertension. Ital Heart J. 2005;6:886–892. [PubMed] [Google Scholar]
- 27.Palatini P. Exaggerated blood pressure response to exercise: pathophysiologic mechanisms and clinical relevance. J Sports Med Phys Fitness. 1998;38:1–9. [PubMed] [Google Scholar]
- 28.Lund-Johansen P. Blood pressure response during exercise as a prognostic factor. J Hypertens. 2002;20:1473–1475. doi: 10.1097/00004872-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32:2036–2041. doi: 10.1161/hs0901.095395. [DOI] [PubMed] [Google Scholar]
- 30.Doust JA, Glasziou PP, Pietrzak E, Dobson AJ. A systematic review of the diagnostic accuracy of natriuretic peptides for heart failure. Arch Intern Med. 2004;164:1978–1984. doi: 10.1001/archinte.164.18.1978. [DOI] [PubMed] [Google Scholar]
- 31.Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang YT, Bevilacqua M, Salio M, Cardano P, Dunselman PH, Holwerda NJ, Tognoni G, Cohn JN. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2002;106:2454–2458. doi: 10.1161/01.cir.0000036747.68104.ac. [DOI] [PubMed] [Google Scholar]
- 32.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Comparative effects of valsartan and enalapril on cardiac sympathetic nerve activity and plasma brain natriuretic peptide in patients with congestive heart failure. Heart. 2006;92:625–630. doi: 10.1136/hrt.2005.062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 34.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 35.Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83:675–682. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milani RV, Lavie CJ, Mehra MR. Cardiopulmonary Exercise Testing: How Do We Differentiate the Cause of Dyspnea? Circulation. 2004;110:e27–e31. doi: 10.1161/01.CIR.0000136811.45524.2F. [DOI] [PubMed] [Google Scholar]