Abstract
AIM: To analyze the risk factors for central port failure in cancer patients administered chemotherapy, using univariate and multivariate analyses.
METHODS: A total of 1348 totally implantable venous access devices (TIVADs) were implanted into 1280 cancer patients in this cohort study. A Cox proportional hazard model was applied to analyze risk factors for failure of TIVADs. Log-rank test was used to compare actuarial survival rates. Infection, thrombosis, and surgical complication rates (χ2 test or Fisher’s exact test) were compared in relation to the risk factors.
RESULTS: Increasing age, male gender and open-ended catheter use were significant risk factors reducing survival of TIVADs as determined by univariate and multivariate analyses. Hematogenous malignancy decreased the survival time of TIVADs; this reduction was not statistically significant by univariate analysis [hazard ratio (HR) = 1.336, 95% CI: 0.966-1.849, P = 0.080)]. However, it became a significant risk factor by multivariate analysis (HR = 1.499, 95% CI: 1.079-2.083, P = 0.016) when correlated with variables of age, sex and catheter type. Close-ended (Groshong) catheters had a lower thrombosis rate than open-ended catheters (2.5% vs 5%, P = 0.015). Hematogenous malignancy had higher infection rates than solid malignancy (10.5% vs 2.5%, P < 0.001).
CONCLUSION: Increasing age, male gender, open-ended catheters and hematogenous malignancy were risk factors for TIVAD failure. Close-ended catheters had lower thrombosis rates and hematogenous malignancy had higher infection rates.
Keywords: Central venous port, Chemotherapy, Risk factor, Cancer patient, Multivariate analysis
INTRODUCTION
Central venous access is necessary for patients who require long-term intravenous chemotherapy, parenteral nutrition, transfusion of blood components and repetitive blood sampling. Techniques for external cannulation of the subclavian and internal jugular veins were described by Broviac et al[1] and Hickman et al[2] in the 1970s. In 1982, Niederhuber et al[3] introduced the totally implantable access port. The principal advantages of these access ports are; no external dressing, lower infection rates than non-totally implantable devices and allowance of patients to perform normal physical activities. It is common practice to insert totally implantable venous access devices (TIVADs) in cancer patients beginning a course of chemotherapy to eliminate potential peripheral venous access problems[4,5]; however, risk factors impacting the survival of TIVADs remain unclear. Accordingly, this retrospective cohort study analyzed the risk factors for failure of the TIVADs and compared adverse events among risk factors.
MATERIALS AND METHODS
Patient population
Between January 1, 2003 and December 31, 2006, this retrospective study enrolled 1280 cancer patients who underwent an operation for placement of TIVAD at Chang Gung Memorial Hospital, Chiayi, Taiwan. All devices were indicated for administering prolonged antineoplastic chemotherapy. Medical records for 1280 cancer patients provided data for age, sex, disease, catheter type, surgical procedure, origin of patients, reason of catheter failure, surgical complications and length of implantation. All patients were followed through December 31, 2006, until death or catheter removal due to complications.
Surgical procedure and device care
All procedures were conducted in an operating room by surgeons under fluoroscopic control for correct positioning of the catheter tip in the superior vena cava. The implantation of TIVAD required only local anesthesia (usually 10 mL of 2% mepivacaine hydrochloride) in almost all patients; general anesthesia was used in combination with major surgical procedures for 10 devices. Perioperative antibiotic prophylaxis consisted of a single dose of cephalosporin during the surgical procedure.
Surgical approaches were cephalic cut-down or subclavian vein puncture on either side depending on the surgeon’s preference. The right side was usually selected for the TIVAD implantation due to shorter access route to superior vena cava than left side. Implantation from the jugular vein or femoral vein system was considered when there was failure of the cephalic vein and subclavian vein system. The TIVAD system was fixed to the underlying pectoral muscle fascia with one non-absorbable suture. Filling the port system with diluted heparin saline was performed routinely at the end of each procedure.
The TIVAD system was routinely flushed with diluted heparin saline by trained oncologic nurses, following each administration of chemotherapy agents. The device was maintained by flushing with a heparinized solution every 4 wk. Non-coring Huber needles were utilized for all injections into the TIVAD system.
Two catheter tips for port systems are used at our institution: the Groshong valved close-ended catheter (Bard, Salk Lake City, UT, USA), and the open-ended catheter. Port systems were assigned to patients depending on surgeon’s preference during the review period.
Study outcomes
The primary endpoint was failure of the TIVAD and the analysis of risk factors affecting device failure among the variables of age, gender, origin of patients, type of catheter, insertion site and type of malignancy using univariate and multivariate methods. The secondary endpoint was to compare the adverse events in relation to the risk factors.
The number of catheter-indwelling-days of TIVAD was calculated from day of insertion to one of the following observation end points: December 31, 2006; date of death; date of catheter removal due to adverse events.
Adverse events of the TIVAD system were divided into three categories: infection events (local erythematous skin change or catheter-related systemic febrile); thrombosis events (intraluminal thrombosis); and surgical complications (pneumothorax, hemothorax, distortion of port system, catheter fracture or malposition). The incidence rate of adverse event was defined as number of events per 1000 catheter-days.
Statistical analysis
The numeric variables were presented as mean ± SD. The number of catheter-indwelling-days was presented as median with inner quartile range (IQR). Log-rank test was conducted for time of death, device removal due to adverse events. For other analyses, independent proportions were compared by using the chi-square test or Fisher’s exact test. Using catheter-indwelling-days of TIVAD as a dependent variable and age, sex, catheter type, tumor type, origin of patients and insertion site as independent variables, Cox proportional hazards modeling with forward selection was performed using a two-step technique. First, univariate analysis was performed and included any potential prognostic factor. Thereafter, only variables with a value of P < 0.10 by univariate analysis were introduced in the Cox model. P < 0.05 indicated a significant statistical difference. All statistical analyses were performed using Stata Statistical Software version 9.2. (StataCorp. 2005. Stata Statistical Software: Release 9.2. College Station, TX, USA).
RESULTS
Distribution of device and device life
From January 1, 2003 and December 31, 2006, 1348 TIVADs were implanted into 1280 consecutive patients. Of the study population, 796 (62%) (842 TIVADs) were male and 484 (38%) (506 TIVADs) were female. The mean age of the subjects was 60.13 ± 13.06 years (range, 13-91 years). Patient origins for insertion of TIVADs were 967 (72%) inpatients and 381 (28%) outpatients. The devices were inserted into 1272 (94%) patients for treatment of solid tumors and 76 (6%) patients for hematogenous tumors. The catheters used were 830 (61%) Groshong catheters and 518 (39%) open-ended catheters (Table 1). Table 2 lists the distribution of primary malignancies and TIVADs.
Table 1.
Distribution of 1348 TIVADs and average catheter-indwelling-days
| n (%) | Median (range) | |
| Sex | ||
| Male | 842 (62) | 151 (60 337) |
| Female | 506 (38) | 228 (88 473) |
| Origin of patient | ||
| Inpatients | 967 (72) | 157 (60 358) |
| Outpatients | 381 (28) | 217 (93 455) |
| Type of malignancy | ||
| Hematogenous | 76 (6) | 148 (61 303) |
| Solid | 1272 (94) | 180 (70 403) |
| Type of catheter | ||
| Groshong | 830 (61) | 218 (81 478) |
| Open-ended | 518 (39) | 143 (55 280) |
| Total | 1348 (100) | 178 (70 399) |
Table 2.
Primary malignancy in 1280 patients with 1348 TIVADs for long-term intravenous chemotherapy n (%)
| Malignancy | Patients | TIVADs |
| Colorectal | 354 (27.7) | 359 (26.6) |
| Lung | 348 (27.2) | 367 (27.2) |
| Head and Neck | 139 (10.8) | 150 (11.1) |
| Breast | 103 (8.0) | 109 (8.1) |
| Gastric | 78 (6.1) | 91 (6.8) |
| Hematogenous | 70 (5.5) | 76 (5.6) |
| H-B-P | 69 (5.5) | 72 (5.4) |
| Esophageal | 41 (3.2) | 43 (3.2) |
| Urologic | 40 (3.1) | 40 (3.0) |
| Gynecologic | 12 (0.9) | 14 (1.0) |
| Others1 | 26 (2.0) | 27 (2.0) |
| Total | 1280 (100) | 1348 (100) |
Others include skin, brain, bone, sarcoma and unknown primary origin. H-B-P: Hepato-biliary-pancreatic.
Table 3 lists the insertion sites, surgical procedures and catheter type used. Of the 1280 consecutive patients who required 1348 TIVADs, 1100 (81.6%) were suited to a cephalic vein cut-down approach and 196 (14.6%) to a subclavian vein puncture procedure. Of the remaining 52 devices, 23 (1.7%) were placed via the jugular vein system due to difficulty approaching the subclavian vein system. The final 29 (2.1%) devices utilized femoral vein placement with or without a saphenous vein approach due to previous neck/thorax radiotherapy or superior vena cava syndrome.
Table 3.
Insertion site, surgical procedure and catheter type n (%)
| Site | Surgical procedure | Open-ended catheter | Groshong catheter | Total |
| Cephalic vein | Cutdown | |||
| Right | 287 | 620 | 907 (67.3) | |
| Left | 111 | 82 | 193 (14.3) | |
| Subclavian vein | Puncture | |||
| Right | 54 | 87 | 141 (10.5) | |
| Left | 38 | 17 | 55 (4.1) | |
| Internal jugular vein | ||||
| Right | 8 | 1 | 9 (0.7) | |
| Left | 0 | 1 | 1 (0.1) | |
| External jugular vein | ||||
| Right | 4 | 7 | 11 (0.8) | |
| Left | 0 | 2 | 2 (0.1) | |
| Femoral vein | ||||
| Right | 14 | 9 | 23 (1.7) | |
| Left | 2 | 4 | 6 (0.4) | |
| Total | 518 | 830 | 1348 |
The median (IQR) number of catheter-indwelling-days was 178 (70 399) d and total number of catheter-indwelling-days was 368 373 d. There were 563 device expires in this study, including 461 deaths (331 males and 130 females) and catheters removed due to 102 adverse events.
Comparisons of risk factors and adverse events
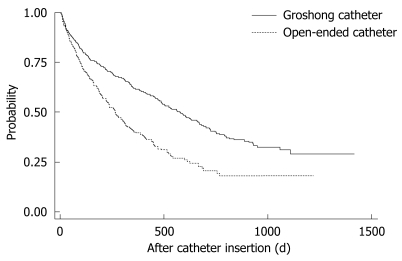
Univariate analysis demonstrated that the following were significant risk factors for TIVAD failure: increasing age; male gender; and use of an open-ended catheter (Table 4). The remaining variables, such as patient origin, insertion site and malignancy type were not statistically significant. Increasing age, male gender, open-ended catheter and hematogenous malignancy [hazard ratio (HR) = 1.499, 95% CI: 1.079-2.083, P = 0.016] were significant risk factors by multivariate analysis for reduced TIVAD survival, although hematogenous malignancy (HR = 1.336, 95% CI: 0.966-1.849, P = 0.080) was not statistically significant by univariate analysis. The median numbers of catheter-indwelling-days for patients inserted with a Groshong or open-ended tube were 218 and 143 d, respectively. The log-rank test showed highly significant statistical differences between these survival curves (P < 0.0001) (Figure 1). Clearly, the patients inserted with open-ended catheter type had much lower survival rates than those with Groshong catheters.
Table 4.
Univariate and multivariate analyses of risk factors for TIVAD failure
| Univariate hazard ratio | 95% CI | P value | Multivariate hazard ratio | 95% CI | P value | |
| Age (yr) | 1.009 | 1.002-1.015 | 0.006 | 1.009 | 1.003-1.016 | 0.003 |
| Sex | ||||||
| Male | 1.63 | 1.373-1.936 | < 0.001 | 1.566 | 1.318-1.861 | < 0.001 |
| Female | 1 | |||||
| Origin of patients | ||||||
| Inpatient | 1 | |||||
| Outpatient | 0.926 | 0.779-1.100 | 0.385 | |||
| Type of catheter | ||||||
| Groshong | 1 | |||||
| Open-ended | 1.719 | 1.461-2.023 | < 0.001 | 1.689 | 1.435-1.989 | < 0.001 |
| Insertion site | ||||||
| RCV | 0.891 | 0.754-1.053 | 0.179 | |||
| Others1 | 1 | |||||
| Type of malignancy | ||||||
| Solid | 1 | |||||
| Hematogenous | 1.336 | 0.966-1.849 | 0.080 | 1.499 | 1.079-2.083 | 0.016 |
Others include left cephalic vein, right subclavian vein, left subclavian vein, femoral vein and jugular vein. RCV: Right cephalic vein.
Figure 1.
Kaplan-Meier survival curve showing that the Groshong catheter of the TIVAD had better survival time than open-ended catheters by log-rank test (P < 0.001).
The overall complications were 102 events (7.5%): 40 infection events; 47 thrombosis events; and 15 surgical complications. The overall infection rate was 0.108 events per 1000 catheter-days (40 cases, 2.96%), the thrombosis rate was 0.127 events per 1000 catheter-days (47 cases, 3.48%), and the surgical complication rate was 0.04 events per 1000 catheter-days (15 cases, 1.1%).
Table 5 presents comparisons of adverse events for open-ended vs Groshong catheters and solid vs hematogenous malignancies. Open-ended catheter devices had a higher thrombosis rate than Groshong catheter devices (5% vs 2.5%, P = 0.015). Hematogenous malignancies had a higher infection rate (10.5% vs 2.5%, P < 0.001) and surgical complication rate (3.9% vs 0.9%, P = 0.048) than solid malignancies.
Table 5.
Comparisons of adverse events for open-ended vs Groshong catheter and solid vs hematogenous malignancy
| Open-ended catheter (n = 518) | Groshong catheter (n = 830) | P value1 | Solid malignancy (n = 1272) | Hematogenous malignancy (n = 76) | P value1 | |
| Infection | 14 , 2.7% (0.130) | 26, 3.1% (0.099) | 0.651 | 32, 2.5% (0.091) | 8, 10.5% (0.456) | < 0.001 |
| Thrombosis | 26, 5% (0.242) | 21, 2.5% (0.080) | 0.015 | 46, 3.6% (0.131) | 1, 1.3% (0.057) | 0.515 |
| Surgical complication | 9, 1.7% (0.083) | 6, 0.7% (0.022) | 0.084 | 12, 0.9% (0.034) | 3, 3.9% (0.171) | 0.048 |
χ2 test or Fisher’s exact test. Parenthesis indicated events per 1000 catheter-days.
DISCUSSION
Notably, the TIVAD is designed to be a reliable, safe and dependable means of long-term venous access for administering chemotherapy or hyperalimentation nutrition. Several studies have documented the relative superiority of TIVADs over non-totally implanted devices[3-6]. Nevertheless, factors affecting device survival remain a major concern. This study analyzed several risk factors including age, sex, patient origin, catheter type, insertion site and malignancy type to assess whether these factors influence the failure of TIVADs.
According to the literature, the average indwelling duration of the implanted devices varies from 61 to 512 d[7-11]. In this series, the total number of catheter-indwelling-days was 368 373 d and the median duration was 178 d.
The variable of age was a significant risk factor for TIVAD failure by univariate and multivariate analyses (HR = 1.009, 95% CI: 1.003-1.016, P = 0.003), signifying that risk of TIVAD failure will increase 1.009 times for each additional year of age. Puig-la Calle et al[11] reported an average of 512 catheter-indwelling-days for 123 patients with a mean age of 37 years. Hou et al[10] identified an average of 395 catheter-indwelling-days for 298 patients with a mean age of 55 years. In this series, the median of catheter-indwelling-days was 178 d and mean age of 1280 patient was 60 years. Taken together, these three studies indicate that age affects TIVAD survival.
Male gender was another risk factor for TIVAD failure by univariate and multivariate analyses (HR = 1.566, 95% CI: 1.318-1.861, P < 0.001). Average number of catheter-indwelling-day for females (228 d) was longer than that for males (151 d). This finding may be due to a higher TIVAD failure rate for males (69%, 417/607 catheter) than females (31%, 190/607) in this series. The causes of TIVAD failure for males were 331 deaths and catheter removal due to 68 adverse events. Advanced stage of cancer with reduced life expectancy for male patients is the major reason for the decreased survival of the devices (data not shown).
The third significant risk factor was catheter type. Open-ended catheters (HR = 1.689, 95% CI: 1.435-1.989, P < 0.001) had shorter TIVAD survival time than close-ended catheters (Groshong catheter). We utilized the log-rank test to compare the survival distributions of open-ended and closed-ended catheters. The results, depicted in Figure 1, show that the survival time for close-ended catheters (Groshong catheter) is significantly better than open-ended catheters using log-rank test (P < 0.001). Hou et al[10] reported these two catheter types had similar actuarial survival rates in their study. The Groshong catheter[12] has a unique three-way, pressure-sensitive valve that allows infusion and blood aspiration while reducing risk of an air embolism, blood reflux, and clotting. This special design is supposed to increase TIVAD survival, which is confirmed in our study.
Hematogenous malignancy was the last risk factor analysed here. By univariate analysis, hematogenous malignancy decreased TIVAD survival, but was not statistically significant (HR = 1.336, 95% CI: 0.966-1.849, P = 0.080). Hematogenous malignancy became a significant risk factor by multivariate analysis (HR = 1.499, 95% CI: 1.079-2.083, P = 0.016) when correlated with age, sex and catheter type variables. Hematogenous malignancy has a hypercoagulable status when compared to solid tumors that is considered to be an etiology for shorter device life.
The reported total adverse event rate was 5.1% (13/296 catheters) in the study by Dillon et al[13], 11% (33/298 catheters) in the study by Hou et al[10], 12.8% (192/1500 catheters) in the study by Kock et al[14] and 21% (14/66 catheters) in the study by Grannan et al[15]. There was a 7.5% (102/1348 catheters) total adverse event rate in the present series. Groshong catheters (6.3%, 53/830 catheters) had a lower total adverse rate than the open-ended group (9.4%, 49/518 catheters), especially for thrombotic events (2.5% vs 5%, P = 0.015). This result supports the proposition that the valved tip of Groshong catheters prevents thrombotic events and prolongs catheter survival. Hematogenous malignancies had higher infection and surgical complication rates than solid malignancies (P < 0.001, P = 0.048, respectively). Vescia et al[16] mentioned that sterile precautions are essential when implanting and accessing port systems; infections must be treated with adequate antimicrobial therapy and catheter-related thromboembolic complications constitute a major problem during the device life, but routine anticoagulation cannot be recommended based on the results of four clinical trials[17-20].
Patients from an outpatient background for insertion of TIVAD had a lower risk for device failure than inpatients; however, the difference between the two groups was not statistically significant (HR = 0.926, 95% CI: 0.779-1.100, P = 0.385). The insertion site, i.e. right cephalic vein site or other sites, was not a significant risk factor.
In conclusion, increasing age, male gender, open-ended catheter use and hematogenous malignancy were all risk factors for reduced actuarial device survival time in this study by univariate and multivariate analyses. Close-ended (Groshong) catheters had a lower thrombotic rate than open-ended catheters. Hematogenous malignancies had higher infection and surgical complication rates than solid malignancies.
COMMENTS
Background
Central venous access is crucial for patients who have need of long-term intravenous chemotherapy, parenteral nutrition, transfusion of blood components and repetitive blood sampling. The main advantages of the totally implantable venous access device (TIVAD) are no external dressing, lower infection rates than non-totally implantable devices and allowance of patients to achieve normal physical activities.
Research frontiers
It is common practice to insert the TIVAD in cancer patients beginning a course of chemotherapy to eliminate potential peripheral venous access problems; however, risk factors impacting the survival of TIVADs remain unclear. Accordingly, the research hotspot is to investigate the risk factors for failure of the TIVAD and correlate adverse events with risk factors.
Innovations and breakthroughs
Recent reports have highlighted the relative superiority of TIVADs over non-totally implantable devices. Nevertheless, factors influencing device survival are a major concern. In this study, the authors found that increasing age, male gender, open-ended catheter use and hematogenous malignancy were significant risk factors reducing survival of TIVAD by multivariate analysis. Close-ended catheters (Groshong) had a lower thrombosis rate than open-ended catheters; hematogenous malignancies had higher infection rates than solid malignancies.
Applications
By understanding the risk factors that affect the survival of TIVADs and adverse events relating to the risk factors, this study may represent a future strategy for prolonging the survival time of TIVADs and help prevent the occurrence of adverse events when cancer patients need central venous ports for long-term chemotherapy.
Terminology
TIVAD is a port with a central venous line that does not have an external connector, instead, it has a small reservoir that is covered with silicone rubber and is implanted under the skin. Medication is administered intermittently by placing a small needle through the skin, piercing the silicone and into the reservoir.
Peer review
This is a large-scale analytic study in which authors investigated risk factors and their impact on failure of TIVADs in cancer patients receiving long-term chemotherapy. The findings suggest that increasing age, male gender, open-ended catheter and hematogenous malignancy are the factors that reduce the survival of devices. Additionally, the authors depict that close-ended catheters had lower thrombosis rates and hematogenous malignancies had higher infection rates. The results are interesting and informative, adding to existing literature.
Acknowledgments
The authors thank Mrs. Peggy Liu for data collection and management.
Footnotes
Peer reviewers: Ross C Smith, Professor, Department of Surgery, University of Sydney, Royal North Shore Hospital, St Leonards, New South Wales 2065, Australia; Klaus Thaler, PhD, University of Missouri-Columbia, Columbia 65212, United States
S- Editor Li LF L- Editor Logan S E- Editor Zheng XM
References
- 1.Broviac JW, Cole JJ, Scribner BH. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet. 1973;136:602–606. [PubMed] [Google Scholar]
- 2.Hickman RO, Buckner CD, Clift RA, Sanders JE, Stewart P, Thomas ED. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet. 1979;148:871–875. [PubMed] [Google Scholar]
- 3.Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706–712. [PubMed] [Google Scholar]
- 4.Biffi R, Corrado F, de Braud F, de Lucia F, Scarpa D, Testori A, Orsi F, Bellomi M, Mauri S, Aapro M, et al. Long-term, totally implantable central venous access ports connected to a Groshong catheter for chemotherapy of solid tumours: experience from 178 cases using a single type of device. Eur J Cancer. 1997;33:1190–1194. doi: 10.1016/s0959-8049(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 5.Biffi R, De Braud F, Orsi F, Pozzi S, Arnaldi P, Goldhirsch A, Rotmensz N, Robertson C, Bellomi M, Andreoni B. A randomized, prospective trial of central venous ports connected to standard open-ended or Groshong catheters in adult oncology patients. Cancer. 2001;92:1204–1212. doi: 10.1002/1097-0142(20010901)92:5<1204::aid-cncr1439>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Stanislav GV, Fitzgibbons RJ Jr, Bailey RT Jr, Mailliard JA, Johnson PS, Feole JB. Reliability of implantable central venous access devices in patients with cancer. Arch Surg. 1987;122:1280–1283. doi: 10.1001/archsurg.1987.01400230066012. [DOI] [PubMed] [Google Scholar]
- 7.Gyves JW, Ensminger WD, Niederhuber JE, Dent T, Walker S, Gilbertson S, Cozzi E, Saran P. A totally implanted injection port system for blood sampling and chemotherapy administration. JAMA. 1984;251:2538–2541. [PubMed] [Google Scholar]
- 8.Bothe A Jr, Piccione W, Ambrosino JJ, Benotti PN, Lokich JJ. Implantable central venous access system. Am J Surg. 1984;147:565–569. doi: 10.1016/0002-9610(84)90023-0. [DOI] [PubMed] [Google Scholar]
- 9.Harvey WH, Pick TE, Reed K, Solenberger RI. A prospective evaluation of the Port-A-Cath implantable venous access system in chronically ill adults and children. Surg Gynecol Obstet. 1989;169:495–500. [PubMed] [Google Scholar]
- 10.Hou SM, Wang PC, Sung YC, Lee HH, Liu HT, Chen YH. Comparisons of outcomes and survivals for two central venous access port systems. J Surg Oncol. 2005;91:61–66. doi: 10.1002/jso.20264. [DOI] [PubMed] [Google Scholar]
- 11.Puig-la Calle J Jr, López Sánchez S, Piedrafita Serra E, Allende Honorato L, Artigas Raventós V, Puig la Calle J. Totally implanted device for long-term intravenous chemotherapy: experience in 123 adult patients with solid neoplasms. J Surg Oncol. 1996;62:273–278. doi: 10.1002/(SICI)1096-9098(199608)62:4<273::AID-JSO9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Central venous catheters. Groshong catheter. Available from: URL: http://www.bardaccess.com/picc-grosh-cath.php. [Google Scholar]
- 13.Dillon PA, Foglia RP. Complications associated with an implantable vascular access device. J Pediatr Surg. 2006;41:1582–1587. doi: 10.1016/j.jpedsurg.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Kock HJ, Pietsch M, Krause U, Wilke H, Eigler FW. Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg. 1998;22:12–16. doi: 10.1007/s002689900342. [DOI] [PubMed] [Google Scholar]
- 15.Grannan KJ, Taylor PH. Early and late complications of totally implantable venous access devices. J Surg Oncol. 1990;44:52–54. doi: 10.1002/jso.2930440112. [DOI] [PubMed] [Google Scholar]
- 16.Vescia S, Baumgärtner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, Harbeck N. Management of venous port systems in oncology: a review of current evidence. Ann Oncol. 2008;19:9–15. doi: 10.1093/annonc/mdm272. [DOI] [PubMed] [Google Scholar]
- 17.Verso M, Agnelli G, Bertoglio S, Di Somma FC, Paoletti F, Ageno W, Bazzan M, Parise P, Quintavalla R, Naglieri E, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol. 2005;23:4057–4062. doi: 10.1200/JCO.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 18.Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21:3665–3675. doi: 10.1200/JCO.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Couban S, Goodyear M, Burnell M, Dolan S, Wasi P, Barnes D, Macleod D, Burton E, Andreou P, Anderson DR. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23:4063–4069. doi: 10.1200/JCO.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 20.Karthaus M, Kretzschmar A, Kröning H, Biakhov M, Irwin D, Marschner N, Slabber C, Fountzilas G, Garin A, Abecasis NG, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006;17:289–296. doi: 10.1093/annonc/mdj059. [DOI] [PubMed] [Google Scholar]