Abstract
Objective
To evaluate the impact of offering US$100 each to patients and their obstetricians or midwives for timely and comprehensive prenatal care on low birth weight, neonatal intensive care admissions, and total pediatric health care spending in the first year of life.
Data Sources/Study Setting
Claims and enrollment profiles of the predominantly low-income and Hispanic participants of a union-sponsored, health insurance plan from 1998 to 2001.
Study Design
Panel data analysis of outcomes and spending for participants and nonparticipants using instrumental variables to account for selection bias.
Data Collection/Abstraction Methods
Data provided were analyzed using t-tests and chi-squared tests to compare maternal characteristics and birth outcomes for incentive program participants and nonparticipants, with and without instrumental variables to address selection bias. Adjusted variables were analyzed using logistic regression models.
Principle Findings
Participation in the incentive program was significantly associated with lower odds of neonatal intensive care unit admission (0.45; 95 percent CI, 0.23–0.88) and spending in the first year of life (estimated elasticity of −0.07; 95 percent CI, −0.12 to −0.01), but not low birth weight (0.53; 95 percent CI, 0.23–1.18).
Conclusion
The use of patient and physician incentives may be an effective mechanism for improving use of recommended prenatal care and associated outcomes, particularly among low-income women.
Keywords: Prenatal care, financial incentives, managed care, birth outcomes
In the health care sector, command and control mechanisms for influencing practice patterns have given way to a reliance on financial incentives for both physicians and patients to encourage adherence to evidence-based and recommended care (Rosenthal et al. 2006). Health care services typically targeted by such incentives include routine testing for people with chronic disease, cancer screening, and immunizations. In the reproductive age population, use of recommended prenatal care services may also be a salient target for quality improvement, particularly in disadvantaged populations, where adherence is lower and birth outcomes poorer (National Center for Health Statistics [NCHS] 2006).
Prenatal care offers maternal and infant health benefits by supporting optimal pregnancy health, reducing high-risk behaviors, and providing entry into the health care system for both mother and infant. While prenatal care has been reported to improve outcomes and offer cost savings in postnatal care in some contexts, the evidence for both its effectiveness and cost-effectiveness is inconclusive (Institute of Medicine 1985; Grossman and Joyce 1990; Currie and Gruber 1996; Kaestner 1999; Conway and Deb 2005; Russell et al. 2007;).
Financial incentives to physicians have been shown to maintain participation in prenatal care programs (Krieger, Connell, and LoGerfo 1992). Patient incentives for adherence to recommended prenatal care, however, have yielded mixed results (McQuide, Delvaux, and Buekens 1998). It should be noted, however, that patient incentives for prenatal care adherence have generally been studied in the context of Medicaid, where regulation limits the type and value of incentives that can be offered to enrollees. In this study, we examine the impact on low birth weight, neonatal intensive care unit (NICU) use, and health care spending of a private insurer program that pairs physician and patient incentives to increase timely and comprehensive prenatal care.
SETTING
Our study relies on data from the Culinary Health Fund (hereafter, the Fund) of Las Vegas, a union-sponsored (i.e., Taft–Hartley) health plan. The Fund covers approximately 56,000 primary subscribers and their dependents (a total of roughly 135,000 lives) in a single metropolitan area. The individuals covered by the Fund are 40 percent Hispanic and typically employed in food service and hospitality occupations. In November 1999, the Fund introduced a program to encourage members to seek prenatal care in the first trimester of pregnancy to complement its traditional high-risk maternity management program. The program offers US$100 to both the pregnant member and the member's network obstetrician or midwife after delivery upon verification that the patient entered care during the first trimester and completed regular visits thereafter. Verification, at the time the Healthy Pregnancy Program (HPP) was introduced, of medical management data indicated that only a small minority of members (14 percent) received prenatal care in the first trimester. At the same time, the rate of NICU use was reported to be above 10 percent—in excess of the national Medicaid average (a low benchmark since the Fund covers a general employed population, albeit of low average socioeconomic status). The HPP was intensively advertised to patients and providers by mail, telephone, and newsletters.
DATA AND METHODS
We used administrative data to evaluate the impact of the HPP on three target outcomes of the intervention: (1) rate of low birth weight, (2) NICU admission rates, and (3) spending in the first year of life.
Data
Data for the study were obtained from the Fund's claims and enrollment profiles from 1998 to 2001. CPT-4 (Current Procedural Terminology, 4th Edition) codes and ICD-9 (International Classification of Diseases, 9th Edition) diagnosis codes were used to identify deliveries, low birth weight, and both pregnancy-related and unrelated comorbid conditions. NICU admissions were identified based on room type, CPT codes, revenue codes, and diagnosis-related group codes, corroborated by the dates of birth and admission. Claims data were also the source of information about participation in HPP; a claim (with the patient as payee) was generated for each participating delivery as the basis of tracking incentive payments. All delivery claims linked to member enrollment profiles were then matched with both inpatient and outpatient pediatric claims for babies to evaluate the outcome measures of interest.
Outcome Measures
We selected low birth weight rates, NICU admission rates, and total pediatric spending in the first year of life as the primary outcome measures for the analysis. These outcomes capture the objectives of the Fund in implementing HPP—to improve neonatal health and reduce the spending associated with NICU admissions and the sequelae of low birth weight (NCHS 2006; Behrman and Stith Butler 2007;). NICU admissions with a length of stay <1 day, which were assumed to be related to evaluation only or data errors, were excluded from the measure of NICU admissions.
We could not directly analyze changes in the use of prenatal care, the means by which the HPP sought to improve outcomes and lower costs, because obstetrical care is paid using bundled case rates that only vary by the type of delivery. For women who received an incentive payment, we can infer that they received timely and comprehensive prenatal care, but we cannot ascertain whether nonparticipants received such care. Finally, we examine the correlates of participation in HPP, both as a secondary outcome of interest and as a means of understanding patterns of self-selection into the program.
Analytic Approach
Analyses were restricted to cases where we could match claims and enrollment data for mothers with claims and enrollment data for infants. We first plotted the trends between 1998 and 2001 in annual participation in the HPP, low birth weight, and NICU admissions. We next summarized maternal characteristics and outcomes associated with deliveries paid for by the Fund, stratifying our results by HPP participation. t-Tests and chi-squared tests were used to examine significance of differences in these bivariate analyses. p-Values <.05 were considered to be statistically significant. Thirdly, we examined birth outcomes and spending as a function of maternal characteristics and risk factors for poor birth outcomes using multiple regression analysis. Logistic regression models with adjustments for clustering by provider were estimated to examine low birth weight and NICU admissions. Odds and 95 percent confidence intervals (CI) are reported for these regressions.
To analyze spending in the first year of life for infants born to HPP participants and nonparticipants we used a generalized linear model with a log link function and the assumption of a gamma error distribution (Buntin and Zaslavsky 2004). Coefficients from the spending model were converted to elasticities for ease of interpretation. For dichotomous variables (including the indicator for HPP), these elasticities can be interpreted as the percent change in spending associated with the presence of a positive indicator.
In all models, the key variable of interest was an indicator that the mother and her physician had participated in the HPP. Explanatory variables included in the regressions were selected based on the clinical literature on maternal factors associated with birth outcomes (Iams 2002). In particular, models included major obstetrical complications of pregnancy that could be identified in claims data and a measure of comorbidity. Obstetrical complications included in the analysis were preeclampsia, gestational diabetes, multiple births, and placenta previa. In the final estimates, we excluded placenta previa because it occurred extremely infrequently and perfectly predicted some outcomes. The comorbidity measure used was the Elixhauser index, which counts the 30 different comorbid conditions using secondary diagnoses reported on provider claims (Elixhauser et al. 1998). In our application of the index, we include diagnoses from both inpatient and outpatient sources. Other important maternal risk factors, including, for example, smoking status and substance use, could not be observed. We account for secular trends in birth outcomes and spending unrelated to the HPP through a common (linear) time trend.
Because the HPP is voluntary and we identify its effect through a comparison of participants with nonparticipants, our results may be affected by selection bias. Specifically, women may choose to participate in HPP based on factors that are also associated, either positively or negatively, with outcomes. To address this issue, we re-estimate our models of health outcomes and spending using an instrumental variables approach that allows us to make more robust causal inferences from our analyses. To instrument for individual maternal participation in HPP, we use the rate of HPP participation in that year for the physician or midwife who billed for the delivery. This instrument was selected because we observe substantial variation over time and between health care providers in the rate of participation in HPP and we surmise that some providers have greater awareness and interest in the program (possibly as a function of the share of their panel accounted for by the Fund's patients). Unlike the individual indicator of participation in HPP, we do not expect the provider's rate of participation in HPP to be associated with unobserved patient factors that influence birth outcomes—that is, it is plausibly exogenous. We use a two-stage approach to implement the instrumental variables estimation, where the first stage is a logistic regression on HPP participation with the instrument and all other explanatory variables included as regressors. The second stage models are the same as those described above, with the exception that the indicator for HPP participation is replaced by the individual's predicted value from the first-stage logistic model. We report estimates from both the ordinary and instrumental variables models but base our conclusions on the latter set of estimates because we believe that self-selection among participants is theoretically likely.
The instrument we have selected may leave residual bias if provider (physician or hospital) differences that affect outcomes are associated with the rates of provider HPP participation and the distribution of patients among providers is changing over time. As a check on our instrumental variables estimates we ran two sets of alternative models that include both the instrumented HPP variables and either a hospital or physician fixed effect. While these models more effectively eliminate potential bias, they do so at the cost of statistical power because they allow only within-provider variation to be used to identify the effects.
RESULTS
Trend Analysis and Sample Description
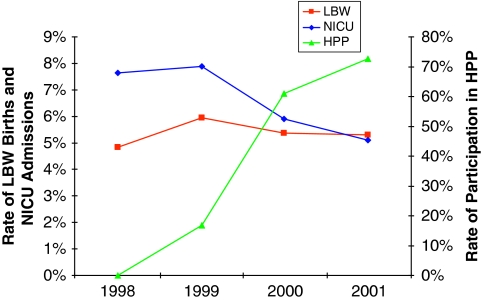
Participation in the HPP increased substantially over the study period (Figure 1). In 2001, HPP participants accounted for 73 percent of deliveries paid for by the Fund. Over the same period, rates of both low birth weight and NICU admissions among Fund-covered deliveries increased initially, and then declined after 1999. The decline in NICU admissions was particularly steep between 1999 and 2000.
Figure 1.
Annual Trends in HPP Participation, Low Birthweight, and NICU Admissions
There were a total of 3,590 deliveries in the sample, 1,436 of which were to HPP participants (Table 1). HPP participants had more comorbid health conditions as measured by the Elixhauser index, although overall rates were low (0.09 vs. 0.12; p<.0001). Other maternal characteristics did not differ significantly between the two groups. Unadjusted comparisons of birth outcomes and spending in the first year of life for babies delivered to Fund enrollees indicated that HPP participants had significantly lower rates of low birth weight (4.5 vs. 6.0 percent; p=.04) and NICU admissions (5.2 vs. 7.5 percent; p=.01) than nonparticipants.
Table 1.
Maternal Characteristics and Birth Outcomes for HPP Participants and Nonparticipants
| Characteristics | All Deliveries | HPP Participants | Non- Participants | p-Value of Difference |
|---|---|---|---|---|
| Number of deliveries | 3,590 | 1,436 (40%) | 2,154 (60%) | — |
| Mean maternal age (SD) | 29.7 (6.0) | 29.9 (6.0) | 29.6 (6.0) | .15 |
| Mean Elixhauser index (SD) | 0.09 (0.37) | 0.12 (0.42) | 0.05 (0.29) | <.0001 |
| Preeclampsia | 59 (1.6%) | 28 (2.0%) | 31 (1.4%) | .27 |
| Gestational diabetes | 18 (0.5%) | 10 (1.0%) | 8 (0.4%) | .19 |
| Multiple births | 32 (0.90%) | 11 (1.0%) | 21 (1.0%) | .48 |
| Placenta previa | 8 (0.2%) | 4 (0.3%) | 4 (0.2%) | .58 |
| Outcomes | ||||
| Low birth weight | 193 (5.0%) | 64 (4.4%) | 129 (6.0%) | .04 |
| NICU admissions | 235 (7.0%) | 75 (5.2%) | 160 (7.5%) | .01 |
| Mean spending in first year of life | US$3,363.51 (US$388.80) | US$3,109.52 (US$522.50) | US$3,535.76 (US$576.40) | .59 |
Source: Authors' calculations from claims data.
HPP, Healthy Pregnancy Program; NICU, neonatal intensive care unit; SD, standard deviation.
Regression Analysis of Birth Outcomes and Spending
In the first set of models in which HPP participation was treated as exogenous, it was associated with significantly reduced odds of low birth weight (0.61; 95 percent CI, 0.43–0.85) (Table 2). The effect of HPP participation on NICU admissions (0.76; 95 percent CI, 0.55–1.04) and spending (−0.02; 95 percent CI, −0.04 to 0.00) was also negative but not significant. Other explanatory variables had the expected signs (i.e., odds >1 and positive effects on spending), although many were insignificant due to the low frequency of risk factors in the study population.
Table 2.
Ordinary and Instrumental Variables Estimates of Impact of HPP on Low Birth Weight, NICU Admissions, and Spending in the First Year of Life
| Outcome |
Low Birth Weight |
NICU Admissions |
Spending* |
|||
|---|---|---|---|---|---|---|
| Model Type | Ordinary Logistic Regression | IV Logistic Regression | Ordinary Logistic Regression | IV Logistic Regression | GLM | IV-GLM |
| Explanatory variables | ||||||
| Maternal age (continuous) | 1.03 (1.01, 1.06) | 1.04 (1.01, 1.06) | 1.02 (1.00, 1.05) | 1.02 (1.00, 1.05) | 0.05 (−0.01, 0.09) | 0.05 (−0.01, 0.11) |
| Elixhauser index≥2 | 2.43 (0.51, 11.54) | 2.65 (0.58, 12.04) | 2.45 (0.37, 16.18) | 2.96 (0.47, 18.51) | 0.10 (−0.05, 0.27) | 0.12 (−0.03, 0.29) |
| Preeclampsia | 7.79 (4.26, 14.27) | 7.76 (4.14, 14.44) | 3.33 (1.82, 6.12) | 3.42 (1.85, 6.30) | 0.27 (0.17, 0.37) | 0.27 (0.17, 0.37) |
| Gestational diabetes | 1.06 (0.14, 7.82) | 1.07 (0.15, 7.80) | 4.37 (1.76, 10.84) | 4.45 (0.90, 4.01) | 0.06 (−0.05, 0.19) | 0.07 (−0.05, 0.20) |
| Multiple birth | 18.52 (9.20, 37.26) | 17.89 (9.01, 35.54) | 4.08 (1.65, 10.07) | 3.85 (1.57, 9.42) | 0.37 (0.25, 0.49) | 0.36 (0.24, 0.48) |
| HPP participation | 0.61 (0.43, 0.85) | 0.53 (0.23, 1.18) | 0.76 (0.55, 1.04) | 0.45 (0.23, 0.88) | −0.02 (−0.04, 0.00) | −0.07 (−0.12, −0.01) |
Spending was modeled using a generalized linear model with a log link and gamma distribution. Coefficients have been converted to elasticities for ease of interpretation.
Other Statistical Notes: All models included a linear time trend and adjustment for clustering at the provider level. Placenta previa was not included in the final models because it occurred too infrequently and was perfectly correlated with some outcomes. 95% confidence intervals reported in parentheses. Odds reported for ordinary and IV logistic regressions
N=3,590 for all models.
GLM, generalized linear model; IV, instrumental variables; HPP, Healthy Pregnancy Program; NICU, neonatal intensive care unit.
The instrument, the annual rate of a patient's physician's or midwife's patients' participation in HPP, was strongly associated with individual participation in the program (data not shown). The incremental R2 associated with inclusion of the instrument in the first stage regression was approximately 0.20 (about 50 percent of total R2) and thus overcomes any concern about spurious results deriving from weak instruments (Staiger and Stock 1997).
The instrumental variables results for the impact of HPP participation on birth outcomes and spending were directionally consistent with the first set of models, although there were differences in the statistical significance of the HPP effect (Table 3). In particular, HPP participation was significantly associated with lower odds of NICU admission (0.45; 95 percent CI, 0.23–0.88) and spending in the first year of life (an elasticity of −0.07; 95 percent CI, −0.12 to −0.01), but not low birth weight (0.53; 95 percent CI, 0.23–1.18).
Table 3.
Instrumental Variables Estimates of Impact of HPP with Hospital or Physician Fixed Effects
| Outcome |
Low Birth Weight |
NICU Admissions |
Spending* |
|||
|---|---|---|---|---|---|---|
| Model Type | IV with Hospital Fixed Effects N=3,295 | IV with Physician Fixed Effects N=3,497 | IV with Hospital Fixed Effects N=3,295 | IV with Physician Fixed Effects N=3,497 | IV with Hospital Fixed Effects N=3,295 | IV with Physician Fixed Effects N=3,497 |
| Explanatory variables | ||||||
| Maternal age (continuous) | 1.03 (1.00, 1.06) | 1.04 (1.00, 1.07) | 1.02 (1.00, 1.05) | 1.03 (1.00, 1.05) | 0.05 (0.00, 0.10) | 0.04 (0.00, 0.10) |
| Elixhauser index≥2 | 2.55 (0.46, 14.17) | 2.33 (0.41, 13.33) | 2.96 (0.58, 15.05) | 1.09 (0.13, 9.26) | 0.10 (−0.06, 0.28) | 0.08 (−0.09, 0.27) |
| Preeclampsia | 7.08 (3.83, 13.07) | 7.44 (3.81, 14.50) | 3.16 (1.59, 6.29) | 3.19 (1.55, 6.58) | 0.25 (0.16, 0.34) | 0.27 (0.17, 0.35) |
| Gestational diabetes | 1.10 (0.14, 8.54) | 0.71 (0.08, 6.11) | 4.00 (1.67, 9.57) | 4.37 (1.30, 14.70) | 0.05 (−0.07, 0.20) | 0.07 (−0.07, 0.22) |
| Multiple birth | 19.76 (9.22, 42.36) | 14.24 (6.09, 33.35) | 4.08 (1.65, 10.07) | 2.42 (0.89, 6.56) | 0.34 (0.22, 0.48) | 0.27 (0.14, 0.40) |
| HPP participation | 0.58 (0.29, 1.17) | 0.63 (0.28, 1.39) | 0.56 (0.29, 1.05) | 0.56 (0.27, 1.17) | −0.05 (−0.09, −0.01) | −0.04 (−0.08, 0.00) |
Spending was modeled using a generalized linear model with a log link and gamma distribution. Coefficients have been converted to elasticities for ease of interpretation.
Other Statistical Notes: All models included a linear time trend. Placenta previa was not included in the final models because it occurred too infrequently and was perfectly correlated with some outcomes. 95% confidence intervals reported in parentheses. Odds reported for logistic regressions.
GLM, generalized linear model; HPP, Healthy Pregnancy Program; IV, instrumental variables.
Inclusion of hospital or physician fixed effects in the instrumental variables models had little effect on the point estimates of the impact of the HPP on all outcomes but produced larger confidence intervals for all estimated coefficients, as expected. In these models, HPP participation was significantly associated only with reduced spending in the first year of life (an elasticity of −0.05; 95 percent CI, −0.09 to −0.01).
DISCUSSION
In a union-sponsored health plan, we found substantial take up of an incentive program designed to improve prenatal care that targeted both patients and health care providers. Uptake of the program suggests adherence to recommended prenatal care reached 76 percent, a nearly fivefold increase over baseline estimates produced by medical management. Moreover, the increased use of prenatal care was associated with reductions in adverse birth outcomes and the cost of care in the first year of life.
Our findings have several important implications for policy and research. First, we find that a financial incentive of US$100 for patients combined with US$100 for health care providers can significantly increase timely and comprehensive prenatal care use as reported by physicians and patients. The role of the financial incentive offered to patients is of particular importance, since patients play a key role in determining whether prenatal care is initiated in the first trimester. Patient incentives in this natural experiment were of a more substantial magnitude (and paid in cash, rather than in kind) than those described in previous studies, which may explain the high rate of the observed uptake.
Second, we find moderately strong evidence that the prenatal care incentive program improved birth outcomes. Across models, all of our estimates were directionally consistent and similar in magnitude. Not surprisingly, given the relative infrequency of the outcomes of interest (<10 percent of births are admitted to the NICU or are low birth weight), however, inclusion of hospital or physician fixed effects results in lower levels of statistical significance. The literature suggests that the likely mechanism for this risk reduction is through effects on maternal smoking behavior and management of medical comorbidities, although the rates of such comorbidities—measured through billing data alone—were low in the study population. It may also be the case that infants delivered to mothers who receive complete prenatal care are less likely to require NICU admission for prophylactic measures against neonatal sepsis. Likewise, preterm infants delivered to mothers receiving optimal prenatal care may have fewer neonatal respiratory problems because of antenatal steroids administered at the first sign of possible preterm labor.
Finally, while we do not have data on the cost of administering the program (i.e., collecting documentation and paying out bonuses), the estimates of health care spending reductions associated with HPP participation suggest that the overall financial consequences of the program may have been favorable from a payer's perspective. The HPP paid out US$200 per participating delivery and our instrumental variables results indicate a reduction in spending in the first year of life of about US$235. Beyond the first year of life, payers may also benefit from higher rates of well-child visits and immunizations, which have been associated with prenatal care use in previous studies (Kogan et al. 1998). These cost savings estimates, however, are subject to the limitations of our quasi-experimental analysis (noted below) and are necessarily speculative because we lack data on administrative costs of the program.
Our findings, which are somewhat more positive than the literature on prenatal care as a whole suggests, may be viewed by some with skepticism. A number of similarly positive studies published over the previous several decades have been criticized for selection bias (Alexander and Kotelchuck 2001). On the other hand, some of the negative findings in the previous literature have also been challenged (Conway and Deb 2005). Because prenatal care was established as the standard of practice before studies demonstrating the efficacy of its components, effectively precluding a randomized-controlled trial, this debate is likely to continue (Alexander and Kotelchuck 2001). We have several reasons to believe that selection bias does not play a major role in our results, however. First, on observable characteristics, participants in the program differed from nonparticipants only in the Elixhauser index and this was higher for participants (indicating more comorbid conditions). Second, we were able to take advantage of a highly predictive instrumental variable to address potential selection bias directly. Third, because the HPP achieved 76 percent participation by the end of the study period, there should be less concern about the participating group being atypical of the general population than in a program that attracted only a small share of potential participants. Finally, the inclusion of provider fixed effects in our models had little impact on the point estimates, although it decreased their precision.
Our conclusions are tempered by several important limitations of the study. First, because we rely on billing data alone, we are missing a number of important pieces of information. Most centrally, because obstetrical care is paid for using a case rate, we cannot measure prenatal care use for non-HPP-participants. This omission should bias our estimates downward because we essentially assume that all nonparticipants have not received comparable prenatal care. We are also unable to assess race, ethnicity, smoking status, substance use, and parity, all of which are known to be associated with birth outcomes. Likewise, we are limited to the diagnosis codes available on outpatient and inpatient claims to identify clinical conditions that may be associated with the outcomes we examine. If these omitted factors are associated with participation in the HPP, our results may be biased. The instrumental variables estimates should, however, overcome these data limitations unless patients sort themselves among health care providers along these dimensions.
Our study takes place in the context of a privately insured but relatively low-income population that is disproportionately Hispanic. These characteristics as well as the relatively low rate of prenatal care use and high rates of poor birth outcomes at baseline may limit generalizability of our findings to other settings. In particular, our findings might be more positive than would be found in higher income groups both because lower-income women have more to gain from prenatal care and because they might be more likely to be influenced by a US$100 incentive. In addition, an incentive as large as US$100 (for both mothers and physicians) would be infeasible in some settings, such as traditional Medicaid, due to regulatory restrictions, fairness concerns, or budgetary constraints. Finally, a program such as this, which leverages not only patient but also provides incentives, might be less effective in cases where the payer covers a relatively small share of patients.
Based on previous literature, we surmise that the size and form of incentive offered to patients were important determinants of impact of the HPP. In particular, we hypothesize that while employers tend to favor tax-advantaged incentives such as premium discounts for wellness or disease management program participation, cash incentives will elicit greater response, particularly in low-income populations. Unfortunately, our study design does not permit separate examination of either the effect of the physician incentive vs. the patient incentive or the dose–response relationship.
The use of financial incentives for providers and patients, aligned with common goals of improved adherence to recommended care, has become a core element of value-based purchasing in the United States (Landon et al. 2008). Our results offer some encouragement for these efforts, both in terms of the potential for improving health outcomes and avoiding high-cost adverse events. Moreover, while individual payers would have to weigh the importance of prenatal care relative to other population health priorities, our findings suggest that improving such care may be a worthy investment. Important scientific and policy questions remain about the extent to which these results can be generalized to other populations, types of incentive arrangements, and quality improvement targets such as chronic disease management, which requires long-term behavioral change on the part of patients.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: Financial support for the project was provided by the Commonwealth Fund, a national, private foundation based in New York City that supports independent research on health care issues and makes grants to improve health care practice and policy. We are grateful to the Culinary Health Fund and Elizabeth Gilbertson in particular for providing us with access to data and information to undertake the study. Leigh Kost and Tom Mayer also provided valuable insights and technical assistance.
Disclosures: Dr. Milstein has served as a paid clinical consultant to the Culinary Health Fund.
Disclaimers: The views presented here are those of the authors and not necessarily those of the Commonwealth Fund, their directors, officers, or staff.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Alexander GR, Kotelchuck M. Assessing the Role and Effectiveness of Prenatal Care: History, Challenges, and Directions for Future Research. Public Health Report. 2001;116(4):306–16. doi: 10.1016/S0033-3549(04)50052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, Committee on Understanding Premature Birth and Assuring Healthy Outcomes Board on Health Sciences Policy: Preterm Birth—Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [Google Scholar]
- Buntin MB, Zaslavsky AM. Too Much Ado about Two-Part Models and Transformation? Comparing Methods of Modeling Medicare Expenditures. Journal of Health Economics. 2004;23(3):525–42. doi: 10.1016/j.jhealeco.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Conway KS, Deb P. Is Prenatal Care Really Ineffective? Or, Is the ‘Devil’ in the Distribution? Journal of Health Economics. 2005;24(3):489–513. doi: 10.1016/j.jhealeco.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Currie J, Gruber J. Saving Babies: The Efficacy and Cost of Recent Expansions of Medicaid Eligibility for Pregnant Women. Journal of Political Economy. 1996;104(6):1263–96. [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Medical Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Grossman M, Joyce TJ. Unobservables, Pregnancy Resolutions, and Birthweight Production Functions in New York City. Journal of Political Economy. 1990;98(5):983–1007. [Google Scholar]
- Iams JD. Preterm Birth. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 4th Edition. New York: Churchill Livingstone; 2002. pp. 755–826. [Google Scholar]
- Institute of Medicine. Committee to Study the Prevention of Low Birth Weight: Preventing Low Birthweight. Washington, DC: National Academy Press; 1985. [Google Scholar]
- Kaestner R. Health Insurance, the Quantity and Quality of Prenatal Care, and Infant Health. Inquiry. 1999;36(2):162–75. [PubMed] [Google Scholar]
- Kogan MD, Alexander GR, Jack BW, Allen MC. The Association between Adequacy of Prenatal Care Utilization and Subsequent Pediatric Care Utilization in the United States. Pediatrics. 1998;102(1 Pt 1):25–30. doi: 10.1542/peds.102.1.25. [DOI] [PubMed] [Google Scholar]
- Krieger JW, Connell FA, LoGerfo JP. Medicaid Prenatal Care: A Comparison of Use and Outcomes in Fee-For-Service and Managed Care. American Journal of Public Health. 1992;82(2):185–90. doi: 10.2105/ajph.82.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Rosenthal MB, Normand SL, Frank RG, Epstein AM. Quality Monitoring and Management in Commercial Health Plans. American Journal of Management Care. 2008;14(6):377–86. [PubMed] [Google Scholar]
- McQuide PA, Delvaux T, Buekens P. Study Group on Barriers and Incentives to Prenatal Care in Europe. Journal of Public Health Policy. 1998;19(3):331–49. [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2006 with Chartbook on Trends in the Health of Americans. Hyattsville, MD: U.S. Government Printing Office; 2006. [PubMed] [Google Scholar]
- Rosenthal MB, Landon BE, Normand SL, Frank RG, Epstein AM. Pay for Performance in Commercial HMOs. New England Journal of Medicine. 2006;355(18):1895–902. doi: 10.1056/NEJMsa063682. [DOI] [PubMed] [Google Scholar]
- Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, Dias T, Potetz L, Davidoff MJ, Damus K. Cost of Hospitalization for Preterm and Low Birth Weight Infants in the United States. Pediatrics. 2007;120(1):e1–9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- Staiger D, Stock JH. Instrumental Variables Regression with Weak Instruments. Econometrica. 1997;65:557–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.