Abstract
Objective
Describe a novel approach to comprehensively summarize medication adherence.
Data Sources/Study Setting
Kaiser Permanente Northern California Diabetes Registry (n∼220,000)
Study Design
In a new prescription cohort design (27,329 subjects prescribed new medications), we used pharmacy utilization data to estimate adherence during 24 months follow-up. Proportion of time without sufficient medications (medication gaps) was estimated using a novel measure (New Prescription Medication Gaps [NPMG]) and compared with a traditional measure of adherence.
Data Collection/Extraction Methods
Data derived from electronic medical records and survey responses.
Principal Findings
Twenty-two percent of patients did not become ongoing users (had zero or only one dispensing of the new prescription). The proportion of newly prescribed patients that never became ongoing users was eightfold greater than the proportion who maintained ongoing use, but with inadequate adherence. Four percent of those with at least two dispensings discontinued therapy during the 24 months follow-up. NPMG was significantly associated with high out-of-pocket costs, self-reported adherence, and clinical response to therapy.
Conclusions
NPMG is a valid adherence measure. Findings also suggest a larger burden of inadequate adherence than previously thought. Public health efforts have traditionally focused on improving adherence in ongoing users; clearly more attention is needed to address nonpersistence in the very first stages after a new medication is prescribed.
Keywords: Medication adherence, primary adherence, diabetes mellitus, antihypertensive medication, cholesterol-lowering medication, antihyperglycemic medication, continuous multiple-interval measure of gaps (CMG)
Medication nonadherence has well-established clinical consequences. In diabetes, poorer medication adherence has been associated with poorer glycemic, blood pressure, and lipid control (Morris et al. 1997; Ho et al. 2006a;), higher rates of acute metabolic events (Morris et al. 1997), and increased risk of hospitalization and mortality (Ho et al. 2006a,b; Simpson et al. 2006; Jackevicius, Li, and Tu 2008;). There are well-established and validated methods to quantify secondary adherence for ongoing, long-term (prevalent) medication use (Steiner et al. 1988; Steiner and Prochazka 1997;). However, these secondary adherence measures give biased estimates for newly prescribed therapies because the methodologies exclude significant periods of nonadherence by (1) excluding patients who never initiated therapy, (2) excluding patients who never obtained a refill (because at least two dispensings are required to determine a secondary adherence rate), and (3) censoring follow-up time at the last fill, thus ignoring nonadherence after discontinuation. Conventional adherence measures therefore systematically underestimate the public health burden of poor medication adherence for newly prescribed medications (DiMatteo et al. 2002; Sabate 2003;).
The recent introduction of electronic medical records with electronic recording of physician orders for new prescriptions (as well as dosage changes for prevalent prescriptions, discontinuation orders, and notation of drug-related side effects) now facilitates a more detailed and comprehensive assessment of adherence. We can identify cohorts of patients who have been prescribed new medications (new prescription cohorts) and determine whether medication was ever dispensed (primary adherence), was refilled at least once (early persistence), used as prescribed (secondary adherence), or subsequently discontinued without clinical advice or recognition. Adherence during the initial stages following a new prescription, particularly primary adherence, has been studied in only a few populations, despite the obvious public health implications of not initiating newly prescribed therapy for a chronic condition.
In this paper, we describe the stages of adherence for newly prescribed medications and present and validate a novel, comprehensive adherence metric, New Prescription Medication Gap (NPMG), which summarizes gaps in medication supplies during a defined period following a new prescription. This new measure more comprehensively summarizes adherence than existing secondary (ongoing) adherence measures because it captures additional, critical periods of nonadherence. We will compare NPMG to a widely used conventional measure of secondary adherence (continuous, multiple-interval measure of gaps [CMG]; Steiner and Prochazka 1997) in a study of medication adherence observed over the 2 years following a new prescription for any cardiometabolic (antihypertensive, cholesterol lowering, or antihyperglycemic) therapy in a cohort of diabetic patients. NPMG is not meant to replace existing, validated measures of secondary adherence (e.g., CMG), but rather to provide an additional and more comprehensive assessment of adherence in patients who are prescribed a new medication for a chronic condition.
METHODS
The study setting was Kaiser Permanente Northern California (Kaiser), a large, integrated health care delivery system that provides comprehensive medical services to ∼3.2 million members (∼one-third of insured adults in the geographic region) in the San Francisco and greater Bay area through 39 medical facilities.
The study sampling frame was the Kaiser Permanente Northern California Diabetes Registry, a large, well-characterized, and ethnically diverse diabetic population receiving care in a managed care setting (Karter et al. 2002, 2005). This diabetes registry has been in existence and updated annually since 1993, by flagging diabetes-related indicators from Kaiser's electronic laboratory, pharmacy, outpatient encounter, and hospitalization records. More extensive descriptions of the registry methods are available elsewhere (Karter et al. 1998, 2002, 2005; Selby et al. 2001). Kaiser Permanente members can only use their prescription benefits at the Kaiser pharmacies (closed system) located on-site at all outpatient and hospital facilities, so pharmacy utilization is most reliably captured after excluding members who lacked drug benefits during the study period (∼4 percent) and thus lacked incentive to use Kaiser pharmacies (Karter et al. 2005).
We identified 49,428 electronic prescriptions for new glycemic-lowering, antihypertensive, or lipid-lowering (cardiometabolic) medications ordered during the first half of 2006 (January 1, 2006 to June 30, 2006) for 36,712 of the 163,448 diabetic patients ages 19 years and older with continuous health plan membership for at least 2 years prior and after the prescription date. To be considered a new therapy, we required that there was no evidence of pharmacy dispensing of the drug during the previous 2 years. To simplify the illustration of our proposed adherence methodology, we focused analyses only on the first cardiometabolic medication prescribed (index therapy) even if multiple new medications were prescribed for a given subject. We excluded the following subjects for whom there were periods for which it would be difficult to detect utilization or changes to their medication regimen: 2,125 subjects lacking continuous pharmacy benefits or health plan membership from 2 years before baseline through the end of follow-up; 7,228 subjects with a hospitalization anytime during the period from 1 month before the index date through the end of follow-up. We also excluded 30 women with a positive pregnancy test at any time from 9 months before the first prescription of the index therapy through the end of follow-up. The remaining 27,329 patients comprised our “new prescription cohort.”
The baseline (index date) was defined as the date of the initial dispensing or, if no dispensing, then the date a health care provider submitted the electronic order for the new prescription. In some cases, patients may fail to initiate the index therapy because they were switched by the pharmacist or their physician to an alternative medication to treat the same condition (e.g., switched to a generic due to cost). When the index therapy was never filled, we searched for alternative prescriptions filled within 1 month following the original index date. If such alternatives were identified, we reset the baseline index date and evaluated adherence starting with the dispensing of the new, alternative medication (the new index therapy), in lieu of the initial prescription. We then characterized demographics, socioeconomic status, clinical profile, access to care, health behaviors, pharmacy benefits, and cost sharing for the new prescription cohort using the latest data available during the 12 months before the index date.
Cohort Follow-Up
Our goal was to estimate pharmacy utilization as a measure of adherence subsequent to a new prescription order. A follow-up period of 24 months was assumed to be sufficient for patients to establish a utilization pattern and included a sufficient number of dispensings to generate stable adherence estimates during ongoing use. On average, this 2-year follow-up window included 6.4 refill intervals among the 18,770 early persistent users.
We downloaded all electronic pharmacy utilization files and medical records, physician's notes, and dates relevant to the index therapy for the entire follow-up period. Follow-up of the index therapy continued until the first of the following: end of follow-up (24 months after the index date), the patient discontinued the therapy without clinical advice (nonpersistence), or the health care provider ordered the therapy to be discontinued or switched the patient to alternative therapy (clinically recognized discontinuation). Among subjects who initiated therapy, “nonpersistence” was defined as no dispensing of the index therapy within a grace period of 90 days after exhaustion of the days' supply from the last dispensing, plus the estimated stockpile of medication (medication accumulation from all previous dispensings). The estimated “discontinuation date” marking the beginning of nonpersistence was defined as the date the stockpiled supply was available after the last dispensing should have run out if used as prescribed. We assumed that not refilling the index therapy was in consultation with the health care provider (rather than nonadherence) under the following two conditions: (1) if there were medical records indicating a stop therapy order any time up to 1 month after the estimated time when a patient was estimated to have discontinued, or (2) if the patient was switched to an alternative therapy between 100 days (maximum days supply) before 30 days after the date when a patient was estimated to have stopped therapy. If either of these conditions for clinically recognized discontinuation was met, follow-up was censored and the subject was considered persistent up until the date of censoring.
Discrete Measures of Persistence
We evaluated adherence at several critical stages. “Primary nonadherence” was defined as the patient having no dispensing of the index therapy in a timely fashion (within 60 days of the index date). In a sensitivity analysis, we found that among primary adherent patients, 50 percent were dispensed their first prescription on the same day as it was prescribed, while 90, 95, and 99 percent were dispensed within 6, 12, and 89 days, respectively. “Early-stage nonpersistence” was defined as a patient failing to obtain at least one refill within the period defined by the number of days worth of medication dispensed (days' supply) at the first dispensing plus a 90 days grace period. We note that patients who failed to obtain refills may or may not have ever started the medication; however, for our analyses, we assume that each patient took all medications dispensed. Among patients with early-stage persistence, “second-stage nonpersistence” was defined as later discontinuation (i.e., failure to refill within 90 days of end of days' supply of previous dispensings) before the end of follow-up.
Continuous Adherence Measures
We compared NPMG with a standard, refill-based estimate of secondary adherence, CMG (Steiner and Prochazka 1997). CMG is a well-validated method to evaluate “secondary” adherence (adherence among ongoing users) for periods bounded by medication dispensings; the proportion of time for which a patient received insufficient medication supplies (also called “gap measure”). CMG sums the proportion of days without sufficient medication supply across refill intervals, starting with the first and ending with the last (terminal) dispensing before censoring or the end of follow-up, which ever comes first. Recent modifications of CMG have been proposed that more carefully account for accumulating medications (stockpiling) using a time-forward algorithm (Bryson et al. 2007). We use this modified approach given stockpiling can be accurately accounted for when starting with the first prescription. By definition, CMG excludes those without at least two fills (i.e., primary nonadherent and early-stage nonpersistent). Because discontinuation is assumed to occur after the last dispensing and stockpiled medications have been exhausted, person-time is censored and nonadherence subsequent to discontinuation is not captured by CMG.
Like CMG, the novel, NPMG measure estimates gaps in medication supplies. NPMG extends the period for which adherence gaps are captured by estimating all nonadherent time starting with the index date (time the index therapy was prescribed or first filled) until the point of clinically recognized discontinuation or the end of follow-up. NPMG will equal CMG for persistent subjects who continue filling the medication through the end of follow-up. Gap measures are continuous measures, theoretically ranging from 100 percent for patients who obtain no medication (i.e., lack supplies for the entire follow-up) to 0 percent for those who consistently refilled in a timely fashion and thus maintained adequate supplies. Following convention, these measures were categorized into “adequate” and “inadequate” adherence based on commonly used cutpoints in gaps in medication supplies (i.e., <20 percent versus≥20 percent of the time, respectively). This study was approved by the Kaiser Foundation Research Institute's Institutional Review Board.
Validation Study
We evaluated NPMG's construct validity (ability to predict something that a measure should theoretically be able to predict) by specifying it as either an outcome or an exposure, and comparing its performance with that of CMG. We hypothesized that NPMG should be associated with (1) self-reported adherence, (2) out-of-pocket costs, and (3) clinical response to therapy. First, we evaluated the distribution of adequate versus poor adherence (<20 percent versus ≥20 percent gap) based on our two objective measures (CMG and NPMG) compared with self-reported days missed for cholesterol-lowering medications using a χ2 statistic. The sample used for this analysis included respondents to the DISTANCE Survey (Moffet et al. 2009) who had self-reported adherence to these medications (in the past week, none versus 1 day versus 2+ days). Second, we evaluated the association of adequate versus poor adherence measures with out-of-pocket costs (>$30 versus≤$30 per fill) using a χ2 statistic. Third, we used an ANCOVA difference-in-differences model (Meyer 1995) to test the association of adherence and response to therapy. In this model, we adjusted for baseline LDL (prestatin initiation), age, and gender to evaluate how better LDL lowering (pre–post change) was associated with having adequate (versus poor) adherence in early persistent patients who had newly initiated statins. As a second test of this clinical validation, we specified an analogous model, which adjusted for baseline hemoglobin A1c (HbA1c) (premetformin initiation), age, and gender and then evaluated how pre–post changes in HbA1c were associated with having adequate (versus poor) adherence in early persistent patients who had newly initiated metformin.
RESULTS
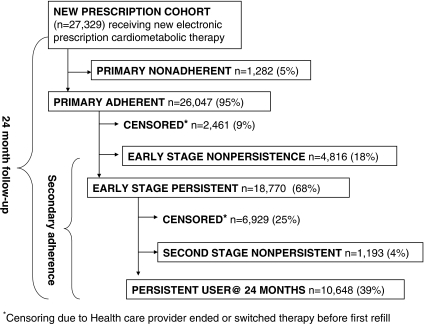
Among 163,357 adults with diabetes and continuous health plan membership with prescription benefits, we identified 27,329 patients with at least one electronic prescription for a new antihyperglycemic (n=8,191), cholesterol-lowering (n=6,426), or antihypertensive (n=12,712) medication during January 1, 2006 to June 30, 2006 (Figure 1). Subjects in this new prescription cohort included ethnically diverse, older diabetic patients (Table 1). As would be expected in patients just prescribed cardiometabolic therapies, subjects had fairly poor risk factor control and commonly exceeded recommended cardiometabolic risk factor targets (59 percent had systolic blood pressure ≥130 mmHg; 30 percent had diastolic blood pressure≥80 mmHg; 44 percent had LDL cholesterol≥100 mg/dl; 53 percent had HgA1c≥7 percent; and 28 percent had CKD [glomerular filtration rate <60 ml/min/1.73m2]).
Figure 1.
Flowchart Depicting the 24-Month Observation Period for 27,329 Diabetic Patients Newly Prescribed a Single Cardiometabolic Therapy
Table 1.
Patient Characteristics (n=27,329)*
| Demographic characteristics | |
| Mean ± SD, age (years) | 61.2 ± 12.4 |
| Age (years) | |
| <45 | 2,515 (9.2) |
| 45–64 | 13,861 (50.7) |
| 65+ | 10,953 (40.1) |
| Women | 12,989 (47.5) |
| Ethnicity | |
| White | 11,225 (41.1) |
| Black/African American | 2,561 (9.4) |
| Latino | 2,547 (9.3) |
| Asian | 3,154 (11.5) |
| Other/multiethnic or unknown | 7,842 (28.7) |
| Clinical profile | |
| Systolic hypertension (≥130 mmHg) | |
| Yes | 16,218 (59.3) |
| No | 10,461 (38.3) |
| Missing data | 650 (2.4) |
| Diastolic hypertension (>80 mmHg) | |
| Yes | 8,152 (29.8) |
| No | 18,527 (67.8) |
| Missing data | 650 (2.4) |
| Hyperlipidemia (LDL cholesterol≥100 mg/dl) (≥2.6 mmol/l) | |
| Yes | 11,895 (43.5) |
| No | 13,149 (48.1) |
| Missing | 2,285 (8.4) |
| Hyperglycemia (hemoglobin A1c≥7%) | |
| Yes | 14,366 (52.6) |
| No | 10,749 (39.3) |
| Missing | 2,204 (8.1) |
| Chronic renal disease (CKD) (MDRD estimate of glomerular filtration rate <60 ml/min/1.73 m2) | |
| Yes | 7,699 (28.2) |
| No | 16,913 (61.9) |
| Missing | 2,712 (10.0) |
| Access to care | |
| Primary care visit in prior year | |
| Yes | 24,928 (91.2) |
| No | 2,401 (8.8) |
| Ophthalmology exam in prior year | |
| Yes | 12,248 (44.8) |
| No | 15,081 (55.2) |
| Cholesterol check in prior year | |
| Yes | 25,044 (91.6) |
| No | 2,285 (8.4) |
| Health behaviors | |
| Current smoker | |
| Yes | 2,288 (8.4) |
| No | 21,381 (78.2) |
| Missing data | 3,660 (13.4) |
| Practices self-monitoring blood glucose | |
| Yes | 16,626 (60.8) |
| No | 10,703 (39.2) |
| Pharmacy benefits and cost sharing | |
| Pharmacy copayment | |
| Free medications ($0) | 1,838 (6.7) |
| $1–$10 | 16,607 (60.8) |
| $11–$20 | 3,711 (13.6) |
| $21–$30 | 3,407 (12.5) |
| >$30 | 1,766 (6.5) |
| Pharmacy benefit with medication cap | |
| Yes | 193 (0.7) |
| No | 27,136 (99.3) |
| Type of plans | |
| Commercial | 16,406 (60.0) |
| Medicare | 10,611 (38.8) |
| Medicaid/other subsidized plans | 312 (1.1) |
Among 27,329 patients with type 2 diabetes prescribed a new antihypertensive (n=12,712), cholesterol-lowering (n=6,426), and antihyperglycemic (n=8,191) therapy. For all other variables, values are given as numbers and percentages of column totals unless otherwise indicated.
During the 24 months following a new cardiometabolic prescription, 4.7 percent (95 percent CI ± 0.25 percent; 1,282/27,329) of patients were primary nonadherent (zero dispensings) and 17.6 percent (95 percent CI ± 0.45 percent; 4,816/27,329) were early nonpersistent because they obtained only one dispensing (i.e., no refills) (Table 2). Thus, 22.3 percent (95 percent CI ± 0.49 percent; 6,098/27,329) of patients prescribed new cardiometabolic medications never became ongoing users. An additional 2,461 (9.0 percent) patients were switched to another therapy or told by their provider to discontinue the index therapy before the first refill. Of the remaining 18,770 early persistent patients, 6.4 percent (95 percent CI ± 0.47 percent; 1,193/18,770 or 4.4 percent [95 percent CI ± 0.37 percent; 1,193/27,329] of the total new prescription cohort) discontinued the index therapy within 24 months without apparent consultation with their provider or switching to an alternative therapy. We censored follow-up of 37 percent (6,929/18,770) of early persistent subjects because the provider discontinued the therapy or switched the patient to an alternative therapy for the same condition. Of the 27,329 patients originally prescribed cardiometabolic therapies, there were only 10,648 (39 percent) persistent patients at 24 months, and only 744 (2.7 percent) of these had inadequate adherence (gaps>20 percent).
Table 2.
Differences in Medication Adherence across Index Therapies*
| Type of Index Therapy |
|||||
|---|---|---|---|---|---|
| Adherence across the Stages of Medication Taking | Antihypertensive Therapy n=12,712 | Cholesterol-Lowering Therapy n=6,426 | Antihyperglycemic Therapy n=8,191 | p Value | All n=27,329 |
| Primary nonadherence (index therapy never dispensed) | 407 (3.2) | 548 (8.5) | 327 (4.0) | <.0001 | 1,282 (4.7) |
| Early-stage nonpersistence (index therapy dispensed once, but never refilled)† | 2,116 (16.7) | 1,403 (21.8) | 1,297 (15.8) | <.0001 | 4,816 (17.6) |
| Second-stage nonpersistence (index therapy refilled at least once, but discontinued‡ before 24 months)† | 462 (3.6) | 355 (5.5) | 376 (4.6) | <.0001 | 1,193 (4.4) |
| Censored due to a timely therapy switch or physician directed discontinuation before 24 months | 4,451 (35.0) | 1,988 (30.9) | 2,951 (36.0) | <.0001 | 9,390 (34.4) |
| Second-stage persistent† | 5,276 (41.5) | 2,132 (33.2) | 3,240 (39.6) | <.0001 | 10,648 (39.0) |
For all other variables, values are given as numbers and percentages of column totals unless otherwise indicated. Follow-up ended with which ever came earliest, 24 months after initial prescription or the date that the medical records indicated a medication stop order or that the patient initiated an alternate therapy for the same condition.
Excludes subjects from these categories who were censored due to a timely therapy switch or physician-directed discontinuation.
Discontinuation defined as failing to receive a dispensing of the index therapy within 90 days after exhaustion of the days' supply at the last dispensing, plus the estimated stockpile of medication (accumulation from previous dispensings).
The distribution of adherence patterns across the observation period varied by type of cardiometabolic therapy prescribed. Patients prescribed cholesterol-lowering therapies were more than twice as likely to never obtain the medication compared with those prescribed antihypertensive or antihyperglycemic therapies (8.5 percent primary nonadherent versus 3.2 and 4.0 percent, respectively). There was also a slightly greater rate of not obtaining the first refill for cholesterol-lowering therapies relative to antihypertensive and antihyperglycemic therapies (21.8 percent early nonpersistent versus 16.7 and 15.8 percent, respectively). Interestingly, this pattern was also seen for secondary adherence, albeit to a lesser extent. Among the 69 percent of remaining patients who were early persistent, the rate of discontinuing before the end of follow-up (second-stage nonpersistence) was slightly higher for cholesterol-lowering medications (5.5 percent [95 percent CI ± 0.55 percent; 355/6,424]) as compared with antihyperglycemics (4.6 percent [95 percent CI ± 0.45 percent; 376/8,191]) or antihypertensives (3.6 percent [95 percent CI ± 0.33 percent; 462/12,712]).
Comparing adherence estimates resulting from CMG and NPMG methods reveals the differences that result from their distinct inclusion criteria and assumptions (Table 3). CMG measures adherence only among ongoing users (those with at least two dispensings), while NPMG includes all patients prescribed a new medication. Thus, the proportion of time that patients had inadequate supply was 15.5 percent (95 percent CI ± 0.29 percent) based on CMG, but 29.3 percent (95 percent CI ± 0.39 percent) based on NPMG. Similarly, 28.9 percent (95 percent CI ± 0.55 percent; 5,422/18,770) of patients were classified as having inadequate adherence (≥20 percent gap in medication) based on CMG, while 47.4 percent (95 percent CI ± 0.59 percent; 12,954/27,329) received that classification based on NPMG.
Table 3.
Comparison of Adherence Definitions and Results for Continuous Medication Gaps (CMG) and New Prescription Medication Gaps (NPMG)
| CMG | NPMG | |
|---|---|---|
| Definitions | ||
| Population | Patients from a “new user cohort” with two or more dispensings for medication (i.e., early persistent) n=18,770 | All patients for whom medication has been prescribed. (new prescription cohort) n=27,329 |
| Follow-up time | Time between the first and last fill in the observation period. Time after discontinuation is not included in the measure | Time between the date the provider first prescribes the medication until what ever comes first: end of follow-up, censoring due to the patient being switched to an alternate therapy or the doctor orders the medication to be stopped |
| Results | % (± 95% CI) | % (± 95% CI) |
| Gaps in medication supply as a continuous measure: average percent of time without sufficient supplies | 15.5% (± 0.29%) (percent time with insufficient medication supplies among ongoing users) | 29.3% (± 0.39%) (percent time with insufficient medication supplies from new prescription until end of follow-up or censoring) |
| Nonadherence rates as a discrete measure: number and percent of subjects with inadequate adherence defined as having gaps in medication supplies for ≥20% of follow-up time | n=5,422/18,770; 28.9% (± 0.55%) (number and percent of new user cohort with inadequate adherence) | n=12,954/27,329; 47.4% (± 0.59%) (number and percent of new prescription cohort with inadequate adherence) |
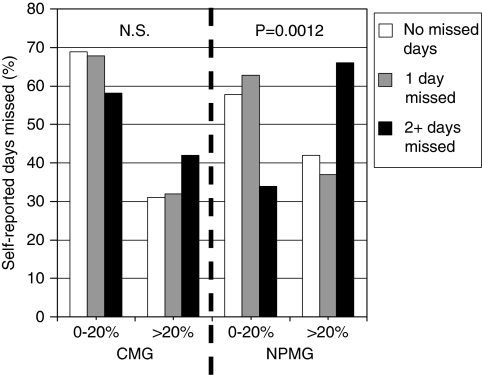
In the validation study, we detected a significant (p=.0012), discriminating relationship between the distribution of NPMG's classification of adequate (<20 percent gap) versus poor adherence (≥20 percent gap in medication) and self-reported adherence (0, 1, or≥2 days missed in past week) for cholesterol-lowering medications (Figure 2). The same relationship between CMG and self-report was not significant (p=.49). Additionally, the proportion with poor adherence (≥20 percent gap) based on NPMG was slightly greater (p<.0001) for those paying $30 or more for each fill (50.5 percent had poor adherence) versus paying <$30 (46.9 percent with poor adherence). In the analogous assessment, CMG and out-of-pocket costs were not significantly related (p=.34). As would be expected among early persistent patients, NPMG and CMG were both similarly and significantly predictive of LDL response to new initiated statins. Relative to poor adherence, adequate adherence for statins based on NPMG was associated with a 11.4 mg/dl (95 percent CI: −13.9, −9.0) greater lowering of LDL (−39.9 versus −28.5 mg/dl drop in LDL; p<.0001) after adjusting for baseline LDL (prestatin initiation), age, and gender. Using the same difference-in-differences model for CMG, adequate adherence for statins was associated with the identical 11.4 mg/dl (95 percent CI:−13.9,−8.9) greater LDL response to statin initiation (−40.0 versus −28.6 mg/dl drop in LDL; p<.0001) relative to poor adherence. Using the same method for early persistent metformin users, relative to poor adherence, adequate adherence was associated with 0.48 point greater A1c decrease in response to initiating metformin (95 percent CI:−0.59,−0.36) when based on NPMG compared with 0.51 point greater A1c decrease in response to initiating metformin (95 percent CI:−0.63, −0.39) when based on CMG.
Figure 2.
Self-Reported Adherence (Days Missed out of Seven) for Cholesterol-Lowering Medications Relative to Adequate (<20 Percent Gap) versus Inadequate (≥20 Percent Gap) Adherence in New Prescription Medication Gaps (NPMG) and Continuous Medication Gaps (CMG). DISTANCE Study
DISCUSSION
A systematic review of published work on adherence of oral medications for diabetes reported that 15–33 percent of patients failed to have adequate adherence for oral agent doses as prescribed (Cramer 2004). Other meta-analytic and systematic reviews suggest similar estimates that are primarily based on secondary adherence measures (Brown et al. 1999; Donnan, MacDonald, and Morris 2002; Ray 2003; Cramer 2004; Odegard and Capoccia 2007;). In our cohort, 28.9 percent (95 percent CI ± 0.55 percent; 5,422/18,770) were classified as having inadequate adherence (≥20 percent gap in medication) based on CMG, a conventional measure of secondary adherence, while 47.4 percent (95 percent CI ± 0.59 percent; 12,954/27,329) received that classification based on NPMG. Thus, our findings suggest that the current public health burden associated with inadequate medication adherence may be larger than previously thought. Poor adherence among ongoing users was low (2.7 percent), but failure to initiate or obtain even one refill is a substantial problem (22.3 percent).
A substantially poorer medication adherence rate is observed when using a new prescription cohort, and accounting for those who fail to initiate the new medication (primary nonadherence), fail to ever refill (early nonpersistence), and time after discontinuation, rather than the more commonplace approach of only observing ongoing users. Because researchers were previously incapable of constructing large, new prescription cohorts, the public health burden attributable to poor medication adherence has been significantly underappreciated. The new prescription cohort design has the added advantage of enabling researchers to distinguish failure to initiate therapy (primary nonadherence) from the failure to prescribe appropriate therapy (clinical inertia), as well as facilitating better accounting of medication stockpiling (which can bias adherence estimates).
Although Kaiser Permanente physicians would likely have obtained a patient's agreement about starting new medication before writing a prescription, 3–9 percent of the patients prescribed new cardiometabolic therapies never filled the new prescription. In one of the few large studies of primary adherence, Canadian researchers evaluated primary adherence for cardiac and noncardiac discharge medications prescribed for secondary prevention after hospitalization for an acute myocardial infarction (Jackevicius, Li, and Tu 2008). These authors reported similar rates of primary nonadherence for antihypertensives (e.g., 3.8 percent for ACE inhibitors) and cholesterol-lowering medications (e.g., 5.2 percent for statins) as in the present study, although higher primary nonadherence rates for diabetes medications (14.6 percent for insulin and 13.3 percent for oral agents). A study in another managed care organization (Harvard Vanguard Medical Associates) recently reported low primary nonadherence rates for oral diabetes medications (under 10 percent) (Trinacty et al. 2009), which are closer to our observations of 4 percent. Differences may be attributable to the case mix and type of prescriptions. The Kaiser sample included prevalent diabetes patients, whereas the Harvard sample included only new onset diabetes cases. However, both groups focused on ambulatory prescriptions, whereas the Canadian study included prescriptions first issued during a hospitalization. Primary nonadherence may reflect inadequate communication or shared decision making (failure of the provider to assess patient readiness to initiate a new therapy and gain a patient's buy-in) (Heisler et al. 2007). It could also reflect a lack of trust in the provider or unaddressed patient concerns (e.g., side effects, inability to pay).
While few studies have evaluated primary nonadherence, several studies have evaluated early nonpersistence (i.e., filling once, but never refilling) in new user cohorts. In this study, 16–22 percent of the cohort obtained the initial dispensing but never refilled despite easy access to a pharmacy (every Kaiser medical facility has an onsite pharmacy and a mail order pharmacy service is available). While one earlier study of diabetic patients (1997–2000), suggested lower rates of early nonpersistence for antihyperglycemics (Hertz, Unger, and Lustik 2005), most studies are consistent with ours in reporting high rates of early discontinuation of oral diabetes medications (Trinacty et al. 2009), statins (Avorn et al. 1998; Benner et al. 2002; Jackevicius, Mamdani, and Tu 2002;), and antihypertensives (Elliott et al. 2007; Siegel, Lopez, and Meier 2007;). Even when cardiometabolic medications were prescribed at discharge following hospitalizations for myocardial infarction (Prospective Registry Evaluating Myocardial Infarction Study or PREMIER), the largest decline in medication use was observed within 1 month of discharge (one in five discontinued use of β-blockers or statins); thereafter medication use remained relatively stable (Ho et al. 2006b). These findings are qualitatively consistent with our findings.
Estimates from this study are not readily compared with the existing literature given the differing methodologies used. The one-fifth of diabetes patients who never become ongoing users of newly prescribed cardiometabolic medications would be excluded from summary measures of secondary adherence. Moreover, our ability to censor patient follow-up when their discontinuation was clinically recognized (34 percent of total new prescriptions) rather than misclassifying it as a form of nonadherence likely explains the low (4 percent) rate of discontinuation at later stages (“second-stage nonpersistence,” i.e., discontinuation after the initial two fills and during the 24-month follow-up).
The validation study suggests that, among ongoing users, NPMG performed similarly to a well-accepted, objective secondary adherence measure (CMG) in predicting response to newly initiated statin therapy. Moreover, NPMG showed a significant relationship to self-reported adherence and out-of-pocket costs, while CMG did not, although the differences between the measures were not clinically relevant. Findings suggest that NPMG is a valid objective, summary adherence measure and, because it is numerically identical to CMG for persistent users, recommend it as a viable alternative when the necessary data are available.
Pharmacy-based measures have limitations. While medications gaps, strictly speaking, indicate pharmacy underutilization, they provide strong evidence of nonadherence; patients cannot take medications that are not dispensed. However, these measures of adherence average gaps over the entire follow-up and we are not able to pinpoint when the nonadherence occurred. Moreover, we are unable to verify whether patients actually took dispensed medications; therefore, levels of nonadherence estimated from pharmacy utilization data must be viewed as lower bounds. While we set generous time limits to allow dispensings to occur (e.g., remaining days supply plus 90 days grace period) to identify discontinuation, dispensings may occur after follow-up ended for a few, exceedingly nonadherent patients. We have conducted a sensitivity analysis and found that only∼3 percent of patients had a subsequent refill after we determined them to have discontinued based on our decision rule. We did search electronic medical records for evidence that a physician initiated a stop medication order or prescribed an alternative medication as an indication that ending therapy (during the same time period) was clinically recognized. However, attributing the remaining discontinuations as strictly a patient decision may somewhat overestimate discontinuation by the patients without apparent consultation with their provider. Finally, because both CMG and NPMG integrate adherence over an extended observation window for their respective populations (ongoing users and all patients prescribed a new medication, respectively), they are imperfect predictors of clinical effectiveness. Ideally, the timing of the adherence assessment should immediately precede the follow-up clinical measurements for response to therapy initiation (e.g., postinitiation LDL measure). Unfortunately, stockpiling and variations in timing of refills make it difficult, if not intractable, to reliably estimate adherence for short periods proximal to the clinical outcome of interest. For that reason, self-report at the time of the clinical outcome measure might be the most relevant adherence measure despite the potential shortcomings (e.g., social desirability and white coat compliance) for the purpose of linking adherence to clinical response.
We cannot entirely rule out some pharmacy utilization at non-Kaiser pharmacies, which is not captured in our databases. Kaiser members with pharmacy benefits have a financial incentive to use only Kaiser pharmacies because the benefits are not honored at non-Kaiser pharmacies. In 2005–2006, we evaluated survey responses from 20,188 Kaiser diabetic patients involved in the NIDDK-funded Diabetes Study of Northern California (DISTANCE) (Moffet et al. 2009) regarding their out-of-plan pharmacy utilization in the previous 12 months. Of the 96 percent who had a pharmacy benefit, non-Kaiser pharmacies were used less than a single time (0.4 times) during the previous year. Because we excluded the 4 percent lacking prescription benefits, the underascertainment of pharmacy utilization in this present study should be minimal.
In some settings, pharmaceutical distributors provide free samples to physicians. Patients receiving such free samples might try the medication but quit before ever obtaining the first recorded dispensing at the pharmacy. However, pharmaceutical representatives have no direct access to Kaiser providers, who are not permitted to receive or provide patients with free samples. We excluded patients who had a hospitalization during follow-up to avoid misclassifying early nonpersistence as primary nonadherence, for patients never refilling a new prescription order issued after discharge, when an initial course of a new medication was administered in the hospital.
NPMG provides a valid, comprehensive measure of adherence, which can be used in addition to existing metrics currently available to study medication adherence. Secondary adherence measures (e.g., CMG, as a proxy for current adherence) and self-reported recent adherence are the most appropriate exposures to estimate adherence for currently persistent patients. The advantages of using NPMG come into play when it is used as an outcome measure because it more comprehensively summarizes overall adherence among patients newly prescribed a medication, without excluding particularly nonadherent subgroups. NPMG should be an appropriate tool for the evaluation of the effectiveness of population-level interventions aiming to improve adherence, or summarize overall adherence to a clinical trial medication protocol. It may help explain lower than expected effectiveness of population-based pharmacotherapy interventions (e.g., prescribing all high-risk patients the polypill; Reddy 2007). By capturing initiation and early adherence, NPMG may be sensitive to disadvantageous characteristics of a new medication, which may be barriers to ongoing use such as high out-of-pocket costs, poor side effect profile, and low patient acceptability. NPMG may also be useful as a quality improvement measure or as a feedback measure for providers or at the health care facility level. While not appropriate for linkage to clinical outcomes when medication taking has only short-term effects (e.g., blood pressure), NPMG may be useful to establish an integrated measure of total drug exposure (to adjust prescribed drug dosages) in studies of adverse drug effects with long lag times (e.g., cancer risk with past drug exposures). While efficient monitoring of primary nonadherence may require electronic prescribing data (not yet available at most health care facilities), early nonpersistence can be identified using pharmacy utilization data; NPMG can be easily modified for use among primary adherent cohorts (new user cohorts) as long as it is understood that it slightly overestimates total adherence because it will ignore primary nonadherence. Finally, although this study used cardiometabolic therapies in diabetes to illustrate the methods, our findings likely have relevance for adherence in other chronic diseases.
The need for therapy intensification reoccurs over the course of diabetes (United Kingdom Prospective Diabetes Study [UKPDS] 1995) and other chronic diseases, and thus the failure of patients to become persistent users may occur repeatedly, with cumulative negative health consequences. Using electronic prescribing records, we can now capture potential nonadherence associated with each intensification attempt, estimate adherence in a more comprehensive way, and intervene in a timely fashion in the patients who fail to become ongoing users. A prescription for a new cardiometabolic medication implies a long-term commitment to therapy, and providers need to recognize that there may be considerable reluctance on the part of patients to make such a commitment. Interventions to address adherence need to be multifaceted (technical, behavioral, and educational) and sensitive to the myriad causes for such patient resistance, including provider, medication-specific, and system-level factors (Pound et al. 2005; Odegard and Capoccia 2007; Polonsky 2007; Huang et al. 2009;). The relative importance of these factors likely differs across the stages of medication taking; thus, barriers and promoters of adherence need to be evaluated separately for each stage of adherence. A surprising finding in this study was that the proportion of newly prescribed patients that never became ongoing users (n=6,098; 22 percent) was eightfold greater than the proportion who maintained ongoing use, but with inadequate adherence (n=744; 3 percent). Moreover, observed rates of discontinuation at the later stages of medication taking were much lower than previously reported and likely due to our ability to censor follow-up at the point a provider stopped or switched the patient's medication. While public health efforts have primarily focused on improving adherence and reducing nonpersistence among ongoing users, clearly more attention is needed to address these issues at the earliest stages after a new medication is prescribed.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK065664). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Avorn J, Bohn RL, Lacour A, Monane M, Mogun H, LeLorier J. Persistence of Use of Lipid-Lowering Medications. Journal of the American Medical Association. 1998;279(18):1458–62. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-Term Persistence in Use of Statin Therapy in Elderly Patients. Journal of the American Medical Association. 2002;288(4):455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- Brown JB, Nichols GA, Glauber HS, Bakst A. Ten-Year Follow-Up of Antidiabetic Drug Use, Nonadherence, and Mortality in a Defined Population with Type 2 Diabetes Mellitus. Clinical Therapeutics. 1999;21(6):1045–57. doi: 10.1016/S0149-2918(99)80023-X. [DOI] [PubMed] [Google Scholar]
- Bryson CL, Au DH, Young B, McDonell MB, Fihn SD. A Refill Adherence Algorithm for Multiple Short Intervals to Estimate Refill Compliance (ReComp) Medical Care. 2007;45(6):497–504. doi: 10.1097/MLR.0b013e3180329368. [DOI] [PubMed] [Google Scholar]
- Cramer JA. A Systematic Review of Adherence with Medications for Diabetes. Diabetes Care. 2004;27(5):1218–24. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient Adherence and Medical Treatment Outcomes. A Meta-Analysis. Medical Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Donnan PT, MacDonald TM, Morris AD. Adherence to Prescribed Oral Hypoglycaemic Medication in a Population of Patients with Type 2 Diabetes: A Retrospective Cohort Study. Diabetic Medicine. 2002;19(4):279–84. doi: 10.1046/j.1464-5491.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- Elliott WJ, Plauschinat CA, Skrepnek GH, Gause D. Persistence, Adherence, and Risk of Discontinuation Associated with Commonly Prescribed Antihypertensive Drug Monotherapies. Journal of the American Board of Family Medicine. 2007;20(1):72–80. doi: 10.3122/jabfm.2007.01.060094. [DOI] [PubMed] [Google Scholar]
- Heisler M, Cole I, Weir D, Kerr EA, Hayward RA. Does Physician Communication Influence Older Patients' Diabetes Self-Management and Glycemic Control? Results from the Health and Retirement Study (HRS) Journal of the Gerontology. Series A, Biological Sciences and Medical Sciences. 2007;62(12):1435–42. doi: 10.1093/gerona/62.12.1435. [DOI] [PubMed] [Google Scholar]
- Hertz RP, Unger AN, Lustik MB. Adherence with Pharmacotherapy for Type 2 Diabetes: A Retrospective Cohort Study of Adults with Employer-Sponsored Health Insurance. Clinical Therapy. 2005;27(7):1064–73. doi: 10.1016/j.clinthera.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of Medication Nonadherence on Hospitalization and Mortality among Patients with Diabetes Mellitus. Archives of Internal Medicine. 2006a;166(17):1836–41. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of Medication Therapy Discontinuation on Mortality after Myocardial Infarction. Archives of Internal Medicine. 2006b;166(17):1842–7. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- Huang ES, Brown SE, Thakur N, Carlisle L, Foley E, Ewigman B, Meltzer DO. Racial/Ethnic Differences in Concerns about Current and Future Medications among Patients with Type 2 Diabetes. Diabetes Care. 2009;32(2):311–6. doi: 10.2337/dc08-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackevicius CA, Li P, Tu JV. Prevalence, Predictors, and Outcomes of Primary Nonadherence after Acute Myocardial Infarction. Circulation. 2008;117(8):1028–36. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- Jackevicius CA, Mamdani M, Tu JV. Adherence with Statin Therapy in Elderly Patients with and without Acute Coronary Syndromes. Journal of the American Medical Association. 2002;288:462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- Karter AJ, Ahmed AT, Liu J, Moffet HH, Parker MM. Pioglitazone Initiation and Subsequent Hospitalization for Congestive Heart Failure. Diabetic Medicine. 2005;22(8):986–93. doi: 10.1111/j.1464-5491.2005.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic Disparities in Diabetic Complications in an Insured Population. Journal of the American Medical Association. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- Karter AJ, Moffet HH, Liu J, Parker MM, Ahmed AT, Ferrara A, Selby JV. Achieving Good Glycemic Control: Initiation of New Antihyperglycemic Therapies in Patients with Type 2 Diabetes from the Kaiser Permanente Northern California Diabetes Registry. American Journal of Managed Care. 2005;11(4):262–70. [PMC free article] [PubMed] [Google Scholar]
- Karter AJ, Newman B, Rowell S, Harrison R, Birner CR, St Pierre DJ, Joslyn G, Selby JV. Large-Scale Collection of Family History Data and Recruitment of Informative Families for Genetic Analysis. Journal of Registry Management. 1998;25(1):7–12. [Google Scholar]
- Meyer BD. Natural and Quasi-Experiments in Economics. Journal of Business and Economic Statistics. 1995;13(2):151–61. [Google Scholar]
- Moffet HH, Adler N, Schillinger D, Ahmed AT, Laraia B, Selby JV, Neugebauer R, Liu JY, Parker MM, Warton M, Karter AJ. Cohort Profile: The Diabetes Study of Northern California (DISTANCE)—Objectives and Design of a Survey Follow-Up Study of Social Health Disparities in A Managed Care Population. International Journal of Epidemiology. 2009;38(1):38–47. doi: 10.1093/ije/dyn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AD, Boyle DI, McMahon AD, Greene SA, MacDonald TM, Newton RW. Adherence to Insulin Treatment, Glycaemic Control, and Ketoacidosis in Insulin-Dependent Diabetes Mellitus. The DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland. Medicines Monitoring Unit. Lancet. 1997;350:1505–10. doi: 10.1016/s0140-6736(97)06234-x. [DOI] [PubMed] [Google Scholar]
- Odegard PS, Capoccia K. Medication Taking and Diabetes: A Systematic Review of the Literature. Diabetes Educator. 2007;33(6):1014–29. doi: 10.1177/0145721707308407. [DOI] [PubMed] [Google Scholar]
- Polonsky W. Psychological Insulin Resistance: The Patient Perspective. Diabetes Educator. 2007;33(suppl 7):241S–4S. doi: 10.1177/0145721707305701. [DOI] [PubMed] [Google Scholar]
- Pound P, Britten N, Morgan M, Yardley L, Pope C, Ker-White G, Campbell R. Resisting Medicines: A Synthesis of Qualitative Studies of Medicine Taking. Social Science and Medicine. 2005;61(1):133–55. doi: 10.1016/j.socscimed.2004.11.063. [DOI] [PubMed] [Google Scholar]
- Ray WA. Evaluating Medication Effects outside of Clinical Trials: New-User Designs. American Journal of Epidemiology. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- Reddy KS. The Preventive Polypill–Much Promise, Insufficient Evidence. New England Journal of Medicine. 2007;356(3):212. doi: 10.1056/NEJMp068219. [DOI] [PubMed] [Google Scholar]
- Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Switzerland: World Health Organization; 2003. [Google Scholar]
- Selby JV, Karter AJ, Ackerson LM, Ferrara A, Liu J. Developing a Prediction Rule from Automated Clinical Databases to Identify High-Risk Patients in a Large Population with Diabetes. Diabetes Care. 2001;24:1547–55. doi: 10.2337/diacare.24.9.1547. [DOI] [PubMed] [Google Scholar]
- Siegel D, Lopez J, Meier J. Antihypertensive Medication Adherence in the Department of Veterans Affairs. American Journal of Medicine. 2007;120(1):26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A Meta-Analysis of the Association between Adherence to Drug Therapy and Mortality. British Medical Journal. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JF, Koepsell TD, Fihn SD, Inui TS. A General Method of Compliance Assessment Using Centralized Pharmacy Records. Description and Validation. Medical Care. 1988;26:814–23. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- Steiner JF, Prochazka AV. The Assessment of Refill Compliance Using Pharmacy Records: Methods, Validity, and Applications. Journal of Clinical Epidemiology. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- Trinacty CM, Adams AS, Soumerai SB, Zhang F, Meigs JB, Piette JD, Ross-Degnan D. Racial Differences in Long-Term Adherence to Oral Antidiabetic Drug Therapy: A Longitudinal Cohort Study. BMC Health Services Research. 2009;9(1):24. doi: 10.1186/1472-6963-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative Efficacy of Randomly Allocated Diet, Sulphonylurea, Insulin, or Metformin in Patients with Newly Diagnosed Non-Insulin Dependent Diabetes Followed for Three Years. British Medical Journal. 1995;310(6972):83–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.